A Kinetic Study of Photocatalytic Degradation of Phenol over Titania–Silica Mixed Oxide Materials under UV Illumination
Abstract
:1. Introduction
2. Results and Discussion
2.1. Photocatalytic Degradation
2.2. HPLC Analysis
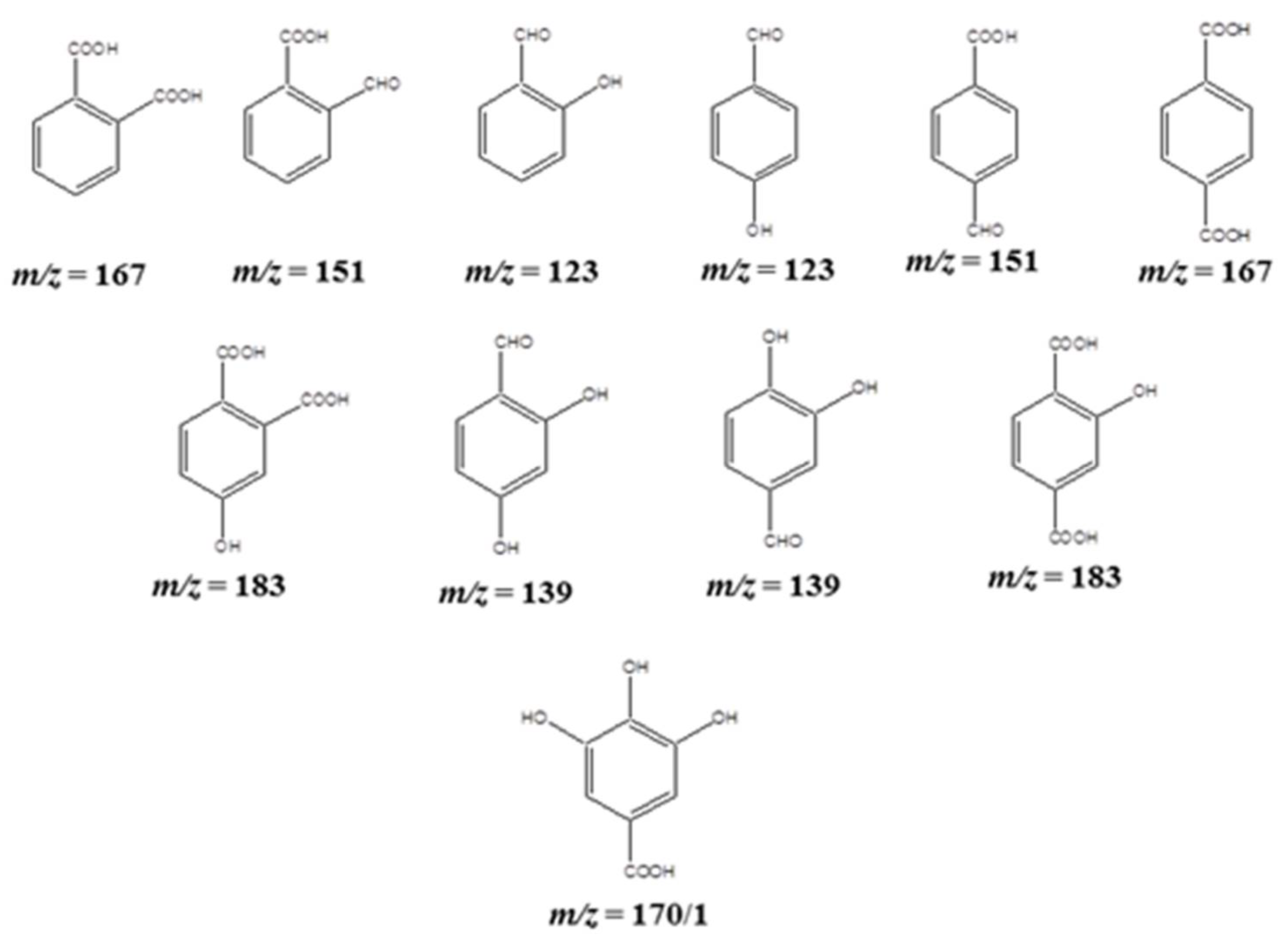
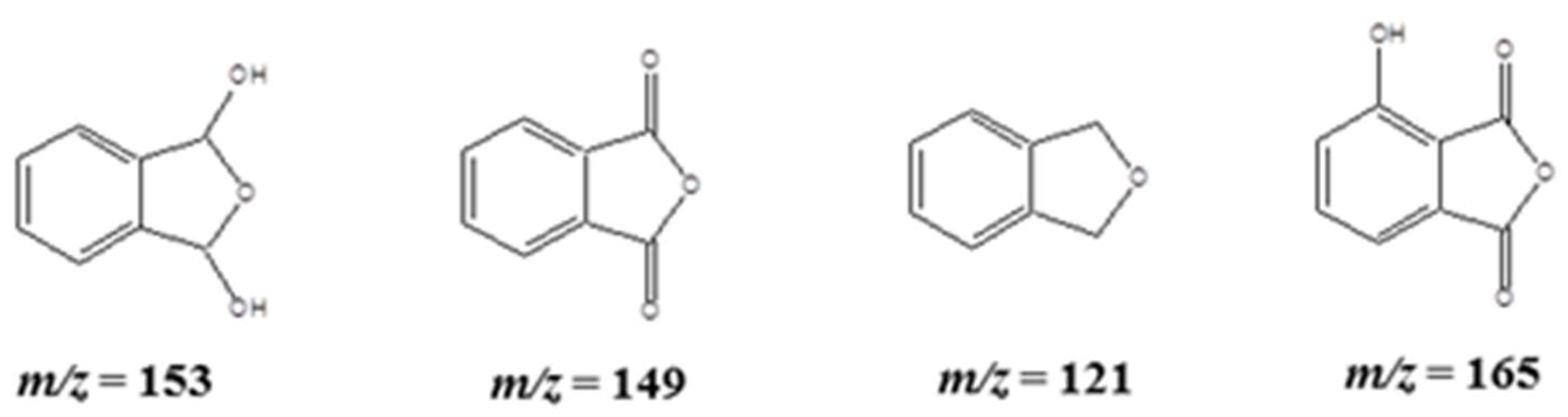
2.3. APCI-MS Analysis for the Fragmented Products
3. Materials and Methods
3.1. Materials
3.2. Synthesis of TiO2–SiO2 Mixed Oxides
3.3. Characterization
3.4. Photo Degradation
3.5. Analytical Techniques
3.6. Scavenging Experiment
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bahnemann, D. Photocatalytic water treatment: Solar energy applications. Sol. Energy 2004, 77, 445–459. [Google Scholar] [CrossRef]
- Vidal, A. Developments in solar photocatalysis for water purification. Chemosphere 1998, 36, 2593–2606. [Google Scholar] [CrossRef]
- Schröder, H.F. Pollutants in drinking water and waste water. J. Chromatogr. A 1993, 643, 145–161. [Google Scholar] [CrossRef]
- Barceló, D. Environmental Protection Agency and other methods for the determination of priority pesticides and their transformation products in water. J. Chromatogr. A 1993, 643, 117–143. [Google Scholar] [CrossRef]
- Tyas, M.J. A histochemical study of the effect of phenol on the mitochondria and lysosomes of cultured cells. Histochem. J. 1978, 10, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Beloborodova, N.; Bairamov, I.; Olenin, A.; Shubina, V.; Teplova, V.; Fedotcheva, N. Effect of phenolic acids of microbial origin on production of reactive oxygen species in mitochondria and neutrophils. J. Biomed. Sci. 2012, 19, 89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michałowicz, J.; Duda, W. Phenols—Sources and toxicity. Pol. J. Environ. Stud. 2007, 16, 347–362. [Google Scholar]
- Tian, L.H.; Liu, H.T.; Gao, Y. Degradation and adsorption of rhodamine B and phenol on TiO2/MCM-41. Kinet. Catal. 2012, 53, 554–559. [Google Scholar] [CrossRef]
- Rasalingam, S.; Peng, R.; Koodali, R.T. An investigation into the effect of porosities on the adsorption of rhodamine B using titania–silica mixed oxide xerogels. J. Environ. Manag. 2013, 128, 530–539. [Google Scholar] [CrossRef]
- Kibombo, H.S.; Rasalingam, S.; Koodali, R.T. Facile template free method for textural property modulation that enhances adsorption and photocatalytic activity of aperiodic titania supported silica materials. Appl. Catal. B-Environ. 2013, 142, 119–128. [Google Scholar] [CrossRef]
- Guo, N.; Liang, Y.; Lan, S.; Liu, L.; Ji, G.; Gan, S.; Zou, H.; Xu, X. Uniform TiO2–SiO2 hollow nanospheres: Synthesis, characterization and enhanced adsorption–photodegradation of azo dyes and phenol. Appl. Surf. Sci. 2014, 305, 562–574. [Google Scholar] [CrossRef]
- Brigante, M.; Schulz, P.C. Adsorption of paraquat on mesoporous silica modified with titania: Effects of pH, ionic strength and temperature. J. Colloid Interface Sci. 2011, 363, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lebrun, R.E.; Gallo, P.-J.; Blond, P. Treatment of textile dye plant effluent by nanofiltration membrane. Sep. Sci. Technol. 1999, 34, 2501–2519. [Google Scholar] [CrossRef]
- Won, S.W.; Choi, S.B.; Chung, B.W.; Park, D.; Park, J.M.; Yun, Y.S. Biosorptive decolorization of reactive orange 16 using the waste biomass of Corynebacterium glutamicum. Ind. Eng. Chem. Res. 2004, 43, 7865–7869. [Google Scholar] [CrossRef]
- Chong, M.N.; Jin, B.; Chow, C.W.K.; Saint, C. Recent developments in photocatalytic water treatment technology: A review. Water Res. 2010, 44, 2997–3027. [Google Scholar] [CrossRef]
- Honglan, S.; Xiaoliang, C.; Qihua, W.; Ruipu, M.; Yinfa, M. Assessment and removal of emerging water contaminants. J. Environ. Anal. Toxicol. 2012, 2, 1–14. [Google Scholar]
- Riegel, G.; Bolton, J.R. Photocatalytic efficiency variability in TiO2 particles. J. Phys. Chem. 1995, 99, 4215–4224. [Google Scholar] [CrossRef]
- Zou, J.; Gao, J. H2O2-sensitized TiO2/SiO2 composites with high photocatalytic activity under visible irradiation. J. Hazard. Mater. 2011, 185, 710–716. [Google Scholar] [CrossRef]
- Shifu, C.; Gengyu, C. Photocatalytic degradation of organophosphorus pesticides using floating photocatalyst TiO2·SiO2/beads by sunlight. Sol. Energy 2005, 79, 1–9. [Google Scholar] [CrossRef]
- Marugán, J.; López-Muñoz, M.-J.; Gernjak, W.; Malato, S. Fe/TiO2/pH interactions in solar degradation of imidacloprid with TiO2/SiO2 photocatalysts at pilot-plant scale. Ind. Eng. Chem. Res. 2006, 45, 8900–8908. [Google Scholar] [CrossRef]
- Mahyar, A.; Behnajady, M.A.; Modirshahla, N. Enhanced photocatalytic degradation of CI Basic Violet 2 using TiO2–SiO2 composite nanoparticles. Photochem. Photobiol. 2011, 87, 795–801. [Google Scholar] [CrossRef]
- Aguado, J.; van Grieken, R.; López-Muñoz, M.-J.; Marugán, J. A comprehensive study of the synthesis, characterization and activity of TiO2 and mixed TiO2/SiO2 photocatalysts. Appl. Catal. A: Gen. 2006, 312, 202–212. [Google Scholar] [CrossRef]
- Davis, R.J.; Liu, Z. Titania-silica: A model binary oxide catalyst system. Chem. Mater. 1997, 9, 2311–2324. [Google Scholar] [CrossRef]
- Cetinkaya, T.; Neuwirthová, L.; Kutláková, K.M.; Tomášek, V.; Akbulut, H. Synthesis of nanostructured TiO2/SiO2 as an effective photocatalyst for degradation of acid orange. Appl. Surf. Sci. 2013, 279, 384–390. [Google Scholar] [CrossRef]
- Malinowska, B.; Walendziewski, J.; Robert, D.; Weber, J.V.; Stolarski, M. The study of photocatalytic activities of titania and titania–silica aerogels. Appl. Catal. B Environ. 2003, 46, 441–451. [Google Scholar] [CrossRef]
- Ismail, A.A.; Ibrahim, I.A.; Ahmed, M.S.; Mohamed, R.M.; El-Shall, H. Sol–gel synthesis of titania-silica photocatalyst for cyanide photodegradation. J. Photochem. Photobiol. A Chem. 2004, 163, 445–451. [Google Scholar] [CrossRef]
- Klein, S.; Thorimbert, S.; Maier, W.F. Amorphous microporous titania–silica mixed oxides: Preparation, characterization, and catalytic redox properties. J. Catal. 1996, 163, 476–488. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. An improved photocatalyst of TiO2/SiO2 prepared by a sol-gel synthesis. J. Phys. Chem. 1995, 99, 9882–9885. [Google Scholar] [CrossRef]
- Hutter, R.; Mallat, T.; Baiker, A. Titania silica mixed oxides: II. Catalytic behavior in olefin epoxidation. J. Catal. 1995, 153, 177–189. [Google Scholar] [CrossRef]
- Kibombo, H.S.; Zhao, D.; Gonshorowski, A.; Budhi, S.; Koppang, M.D.; Koodali, R.T. Cosolvent-induced gelation and the hydrothermal enhancement of the crystallinity of titania—Silica mixed oxides for the photocatalytic remediation of organic pollutants. J. Phys. Chem. C 2011, 115, 6126–6135. [Google Scholar] [CrossRef]
- Rasalingam, S.; Kibombo, H.S.; Wu, C.-M.; Budhi, S.; Peng, R.; Baltrusaitis, J.; Koodali, R.T. Influence of Ti–O–Si hetero-linkages in the photocatalytic degradation of Rhodamine B. Catal. Commun. 2013, 31, 66–70. [Google Scholar] [CrossRef]
- Rasalingam, S.; Kibombo, H.S.; Wu, C.-M.; Peng, R.; Baltrusaitis, J.; Koodali, R.T. Competitive role of structural properties of titania–silica mixed oxides and a mechanistic study of the photocatalytic degradation of phenol. Appl. Catal. B Environ. 2014, 148–149, 394–405. [Google Scholar] [CrossRef]
- Xu, N.P.; Shi, Z.F.; Fan, Y.Q.; Dong, J.H.; Shi, J.; Hu, M.Z.C. Effects of particle size of TiO2 on photocatalytic degradation of methylene blue in aqueous suspensions. Ind. Eng. Chem. Res. 1999, 38, 373–379. [Google Scholar] [CrossRef]
- Takeda, N.; Torimoto, T.; Sampath, S.; Kuwabata, S.; Yoneyama, H. Effect of inert supports for titanium dioxide loading on enhancement of photodecomposition rate of gaseous propionaldehyde. J. Phys. Chem. 1995, 99, 9986–9991. [Google Scholar] [CrossRef]
- Torimoto, T.; Ito, S.; Kuwabata, S.; Yoneyama, H. Effects of adsorbents used as supports for titanium dioxide loading on photocatalytic degradation of propyzamide. Environ. Sci. Technol. 1996, 30, 1275–1281. [Google Scholar] [CrossRef]
- Alemany, L.J.; Banares, M.A.; Pardo, E.; Martin, F.; Galán-Fereres, M.; Blasco, J. Photodegradation of phenol in water using silica-supported titania catalysts. Appl. Catal. B Environ. 1997, 13, 289–297. [Google Scholar] [CrossRef]
- Vasconcelos, D.C.L.; Costa, V.C.; Nunes, E.H.M.; Sabioni, A.C.S.; Gasparon, M.; Vasconcelos, W.L. Infrared spectroscopy of titania sol-gel coatings on 316L stainless steel. Mater. Sci. Appl. 2011, 2, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Kooyman, P.; Waal, P.; Verdaasdonk, P.J.; Jansen, K.; Bekkum, H. Titanium deposited from TiCl4 on amorphous silica and silicalite-1 as catalyst in aromatic hydroxylation reactions. Catal. Lett. 1992, 13, 229–238. [Google Scholar] [CrossRef]
- Anderson, C.; Bard, A.J. Improved photocatalytic activity and characterization of mixed TiO2/SiO2 and TiO2/Al2O3 materials. J. Phys. Chem. B 1997, 101, 2611–2616. [Google Scholar] [CrossRef]
- Erdem, B.; Hunsicker, R.A.; Simmons, G.W.; Sudol, E.D.; Dimonie, V.L.; El-Aasser, M.S. XPS and FTIR surface characterization of TiO2 particles used in polymer encapsulation. Langmuir 2001, 17, 2664–2669. [Google Scholar] [CrossRef]
- Fukushima, M.; Zhou, Y.; Yoshizawa, Y.-I.; Hirao, K. Water vapor corrosion behavior of porous silicon carbide membrane support. J. Eur. Ceram. Soc. 2008, 28, 1043–1048. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, K.-D.; Seo, H.O.; Luo, Y.; Dey, N.K.; Kim, Y.D. Improvement in the photocatalytic activity of TiO2 by the partial oxidation of the C impurities. Appl. Surf. Sci. 2011, 257, 2489–2493. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Li, R.; Qiao, P.; Xiao, L.; Fan, J. TiO2 nanoparticles with increased surface hydroxyl groups and their improved photocatalytic activity. Catal. Commun. 2012, 19, 96–99. [Google Scholar] [CrossRef]
- Gaya, U.I.; Abdullah, A.H.; Zainal, Z.; Hussein, M.Z. Photocatalytic degradation of 2, 4-dichlorophenol in irradiated aqueous ZnO suspension. Int. J. Chem. 2010, 2, 180–193. [Google Scholar] [CrossRef]
- Zenkevich, I.G.; Kochetova, M.V.; Larionov, O.G.; Revina, A.A. Retention indices as the best reproducible chromatographic parameters for the characterization of phenolic compounds in reversed-phase high-performance liquid chromatography. J. Anal. Chem. 2005, 60, 655–667. [Google Scholar] [CrossRef]
- Theurich, J.; Lindner, M.; Bahnemann, D.W. Photocatalytic degradation of 4-chlorophenol in aerated aqueous titanium dioxide suspensions: A kinetic and mechanistic study. Langmuir 1996, 12, 6368–6376. [Google Scholar] [CrossRef]
- Devlin, H.R.; Iestyn, H.J. Mechanism of the oxidation of aqueous phenol with dissolved oxygen. Ind. Eng. Chem. Fundam. 1984, 23, 387–392. [Google Scholar] [CrossRef]
- Alnaizy, R.; Akgerman, A. Advanced oxidation of phenolic compounds. Adv. Environ. Res. 2000, 4, 233–244. [Google Scholar] [CrossRef]
- Gopalan, S.; Savage, P.E. Reaction mechanism for phenol oxidation in supercritical water. J. Phys. Chem. 1994, 98, 12646–12652. [Google Scholar] [CrossRef]
- Chen, J.; Eberlein, L.; Langford, C.H. Pathways of phenol and benzene photooxidation using TiO2 supported on a zeolite. J. Photochem. Photobiol. A Chem. 2002, 148, 183–189. [Google Scholar] [CrossRef]
- Zazo, J.A.; Casas, J.A.; Mohedano, A.F.; Gilarranz, M.A.; Rodríguez, J.J. Chemical pathway and kinetics of phenol oxidation by Fenton’s reagent. Environ. Sci. Technol. 2005, 39, 9295–9302. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Ma, R.; Li, G. Degradation of phenol by nanomaterial TiO2 in wastewater. Chem. Eng. J. 2006, 119, 55–59. [Google Scholar] [CrossRef]

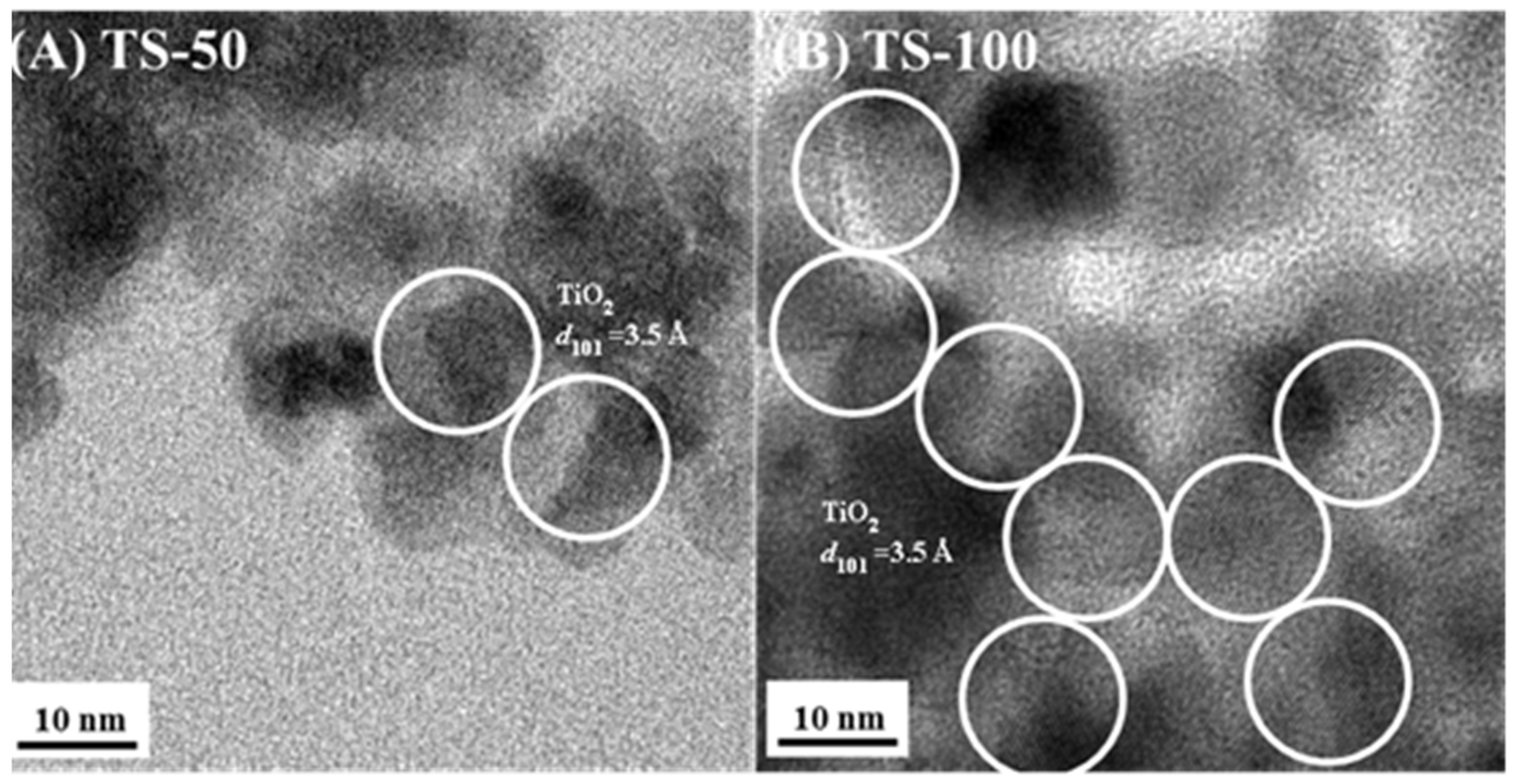

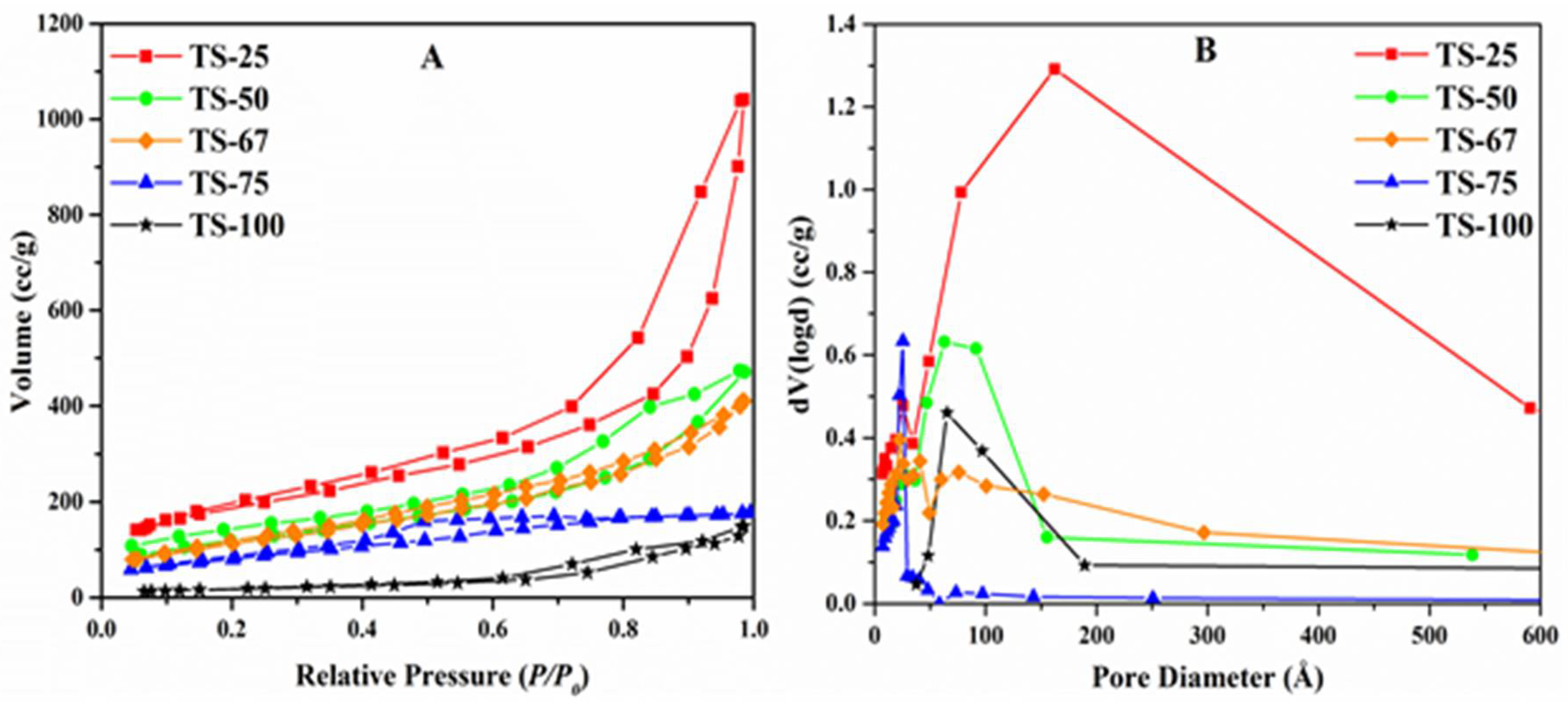
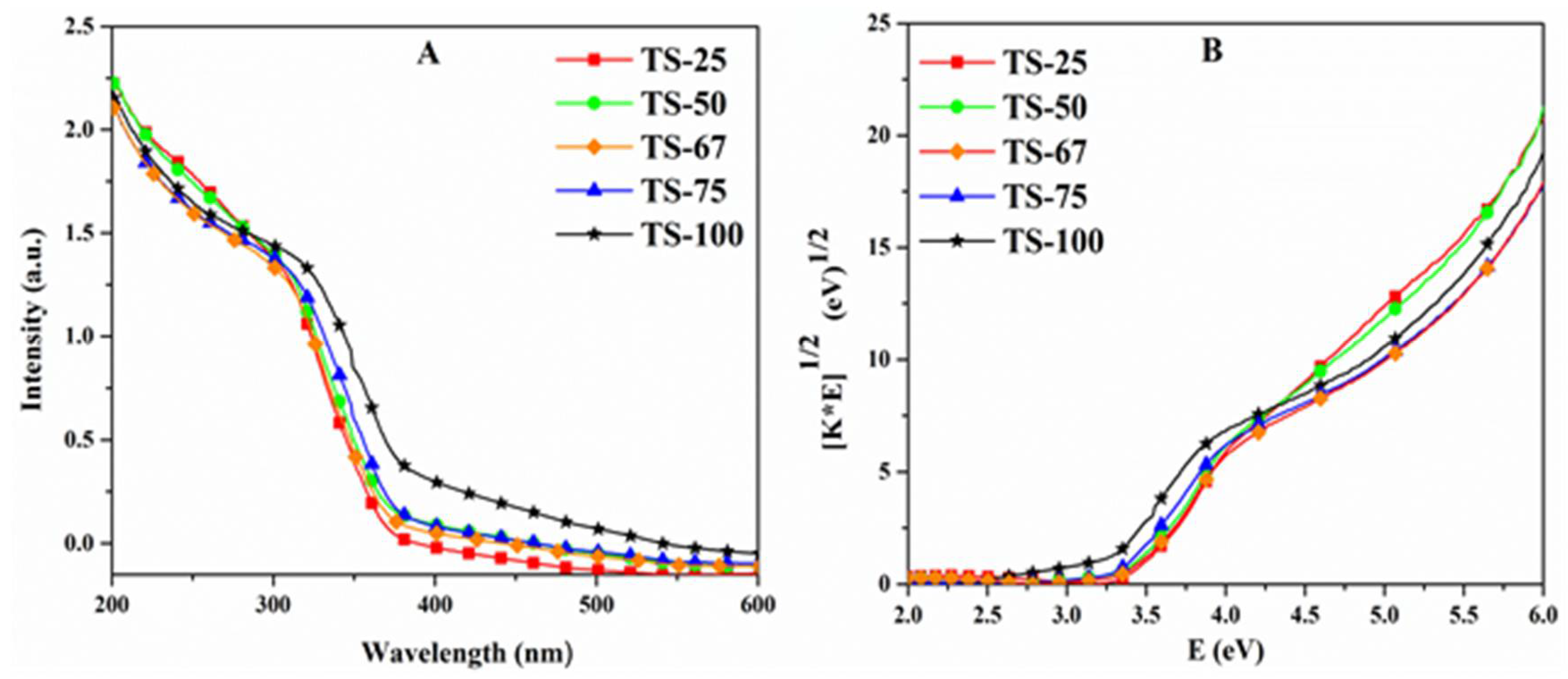
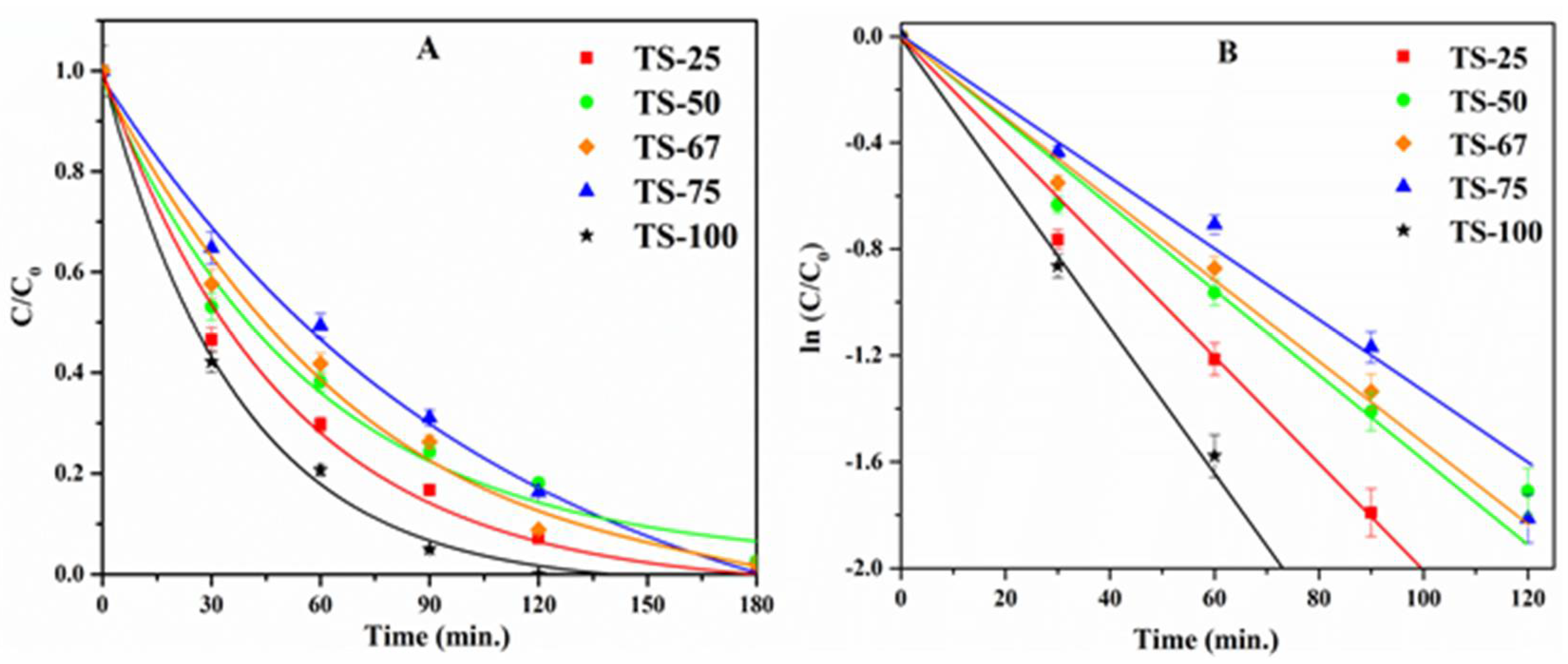
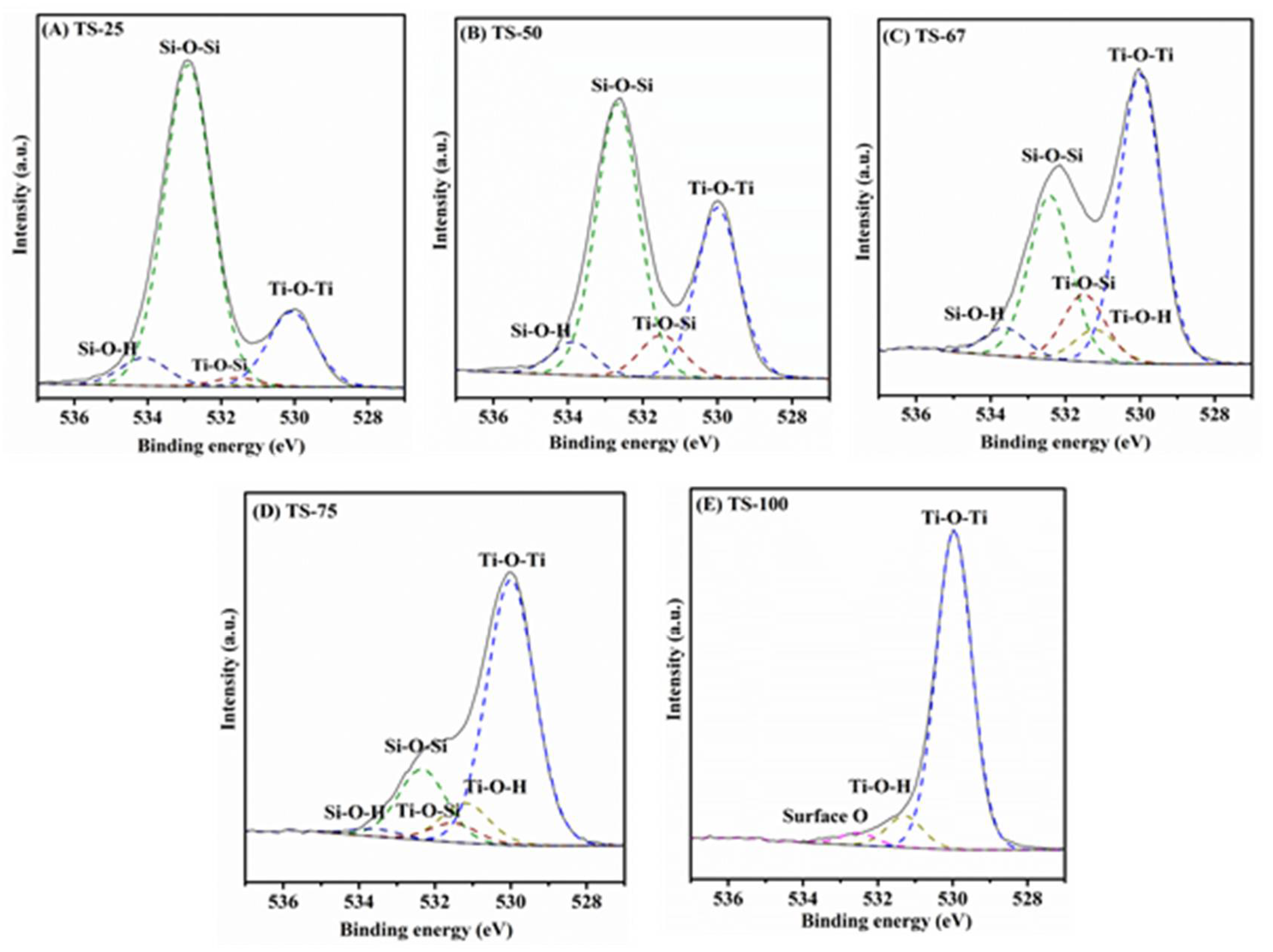



| Sample | % Ti | SSA (m2/g) | PV (cm3/g) | PD (Å) | Crystallinity (Intensity of d101) | Particle Size (nm) | Rate (×10−4) (s−1) |
|---|---|---|---|---|---|---|---|
| TS-25 | 25 | 672 | 1.61 | 96 | 610 | 2.7 | 4.59 |
| TS-50 | 50 | 423 | 0.73 | 68 | 551 | 3.3 | 2.54 |
| TS-67 | 67 | 409 | 0.64 | 62 | 505 | 3.2 | 2.41 |
| TS-75 | 75 | 295 | 0.27 | 37 | 431 | 4.2 | 2.10 |
| TS-100 | 100 | 65 | 0.23 | 141 | 729 | ND | 5.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yohi, S.; Wu, C.-M.; Koodali, R.T. A Kinetic Study of Photocatalytic Degradation of Phenol over Titania–Silica Mixed Oxide Materials under UV Illumination. Catalysts 2022, 12, 193. https://doi.org/10.3390/catal12020193
Yohi S, Wu C-M, Koodali RT. A Kinetic Study of Photocatalytic Degradation of Phenol over Titania–Silica Mixed Oxide Materials under UV Illumination. Catalysts. 2022; 12(2):193. https://doi.org/10.3390/catal12020193
Chicago/Turabian StyleYohi, Shivatharsiny, Chia-Ming Wu, and Ranjit T. Koodali. 2022. "A Kinetic Study of Photocatalytic Degradation of Phenol over Titania–Silica Mixed Oxide Materials under UV Illumination" Catalysts 12, no. 2: 193. https://doi.org/10.3390/catal12020193
APA StyleYohi, S., Wu, C.-M., & Koodali, R. T. (2022). A Kinetic Study of Photocatalytic Degradation of Phenol over Titania–Silica Mixed Oxide Materials under UV Illumination. Catalysts, 12(2), 193. https://doi.org/10.3390/catal12020193









