Recent Progress on Sulfated Nanozirconia as a Solid Acid Catalyst in the Hydrocracking Reaction
Abstract
:1. Introduction
2. Zirconium Dioxide (ZrO2)
3. ZrO2-SO4 Catalyst
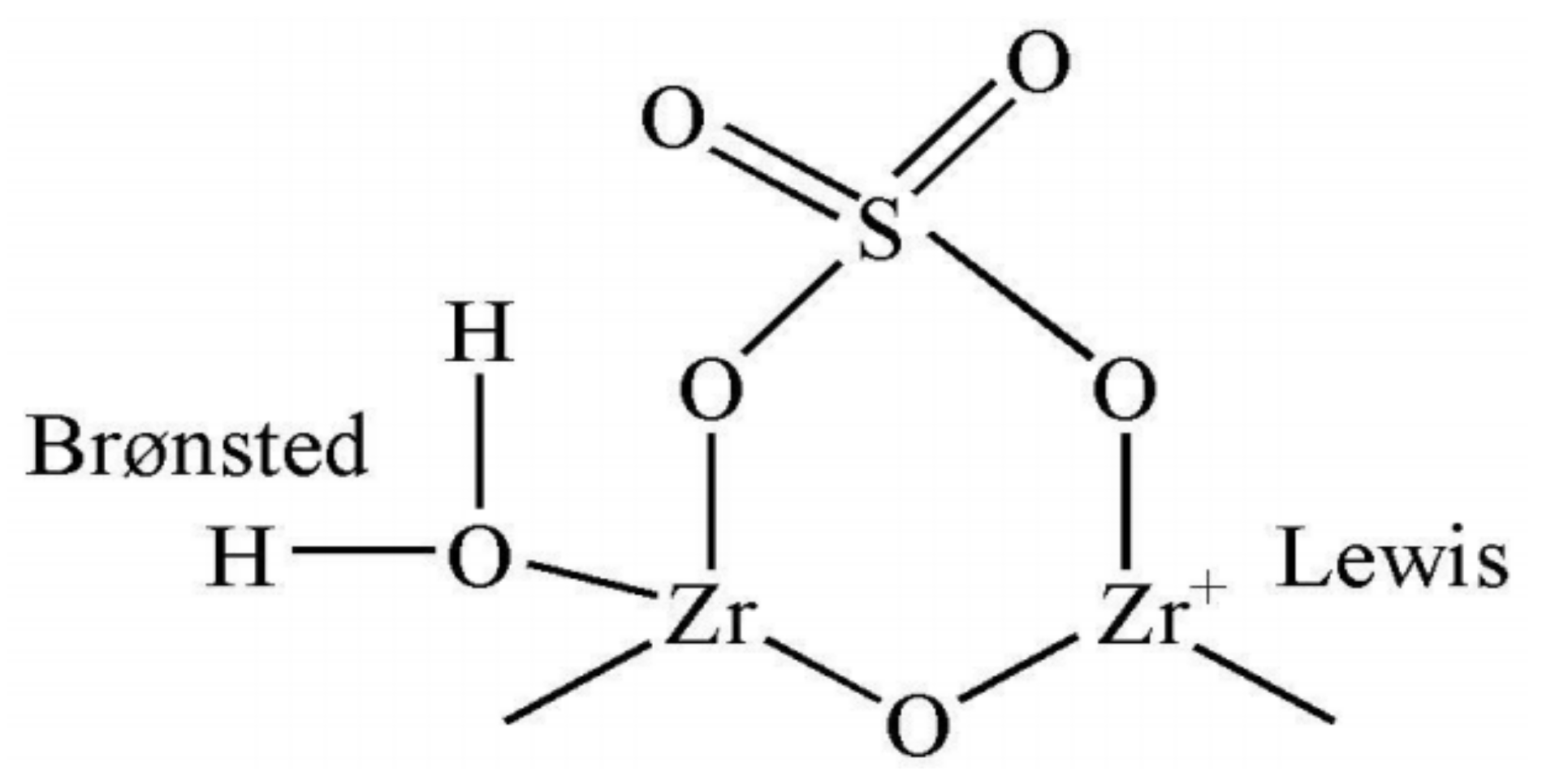
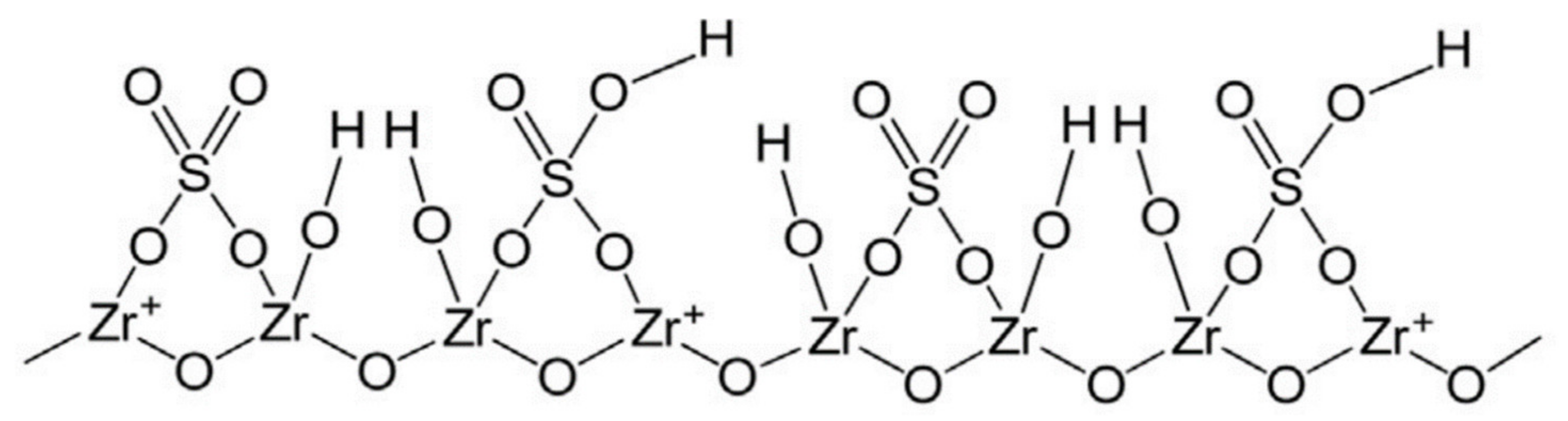
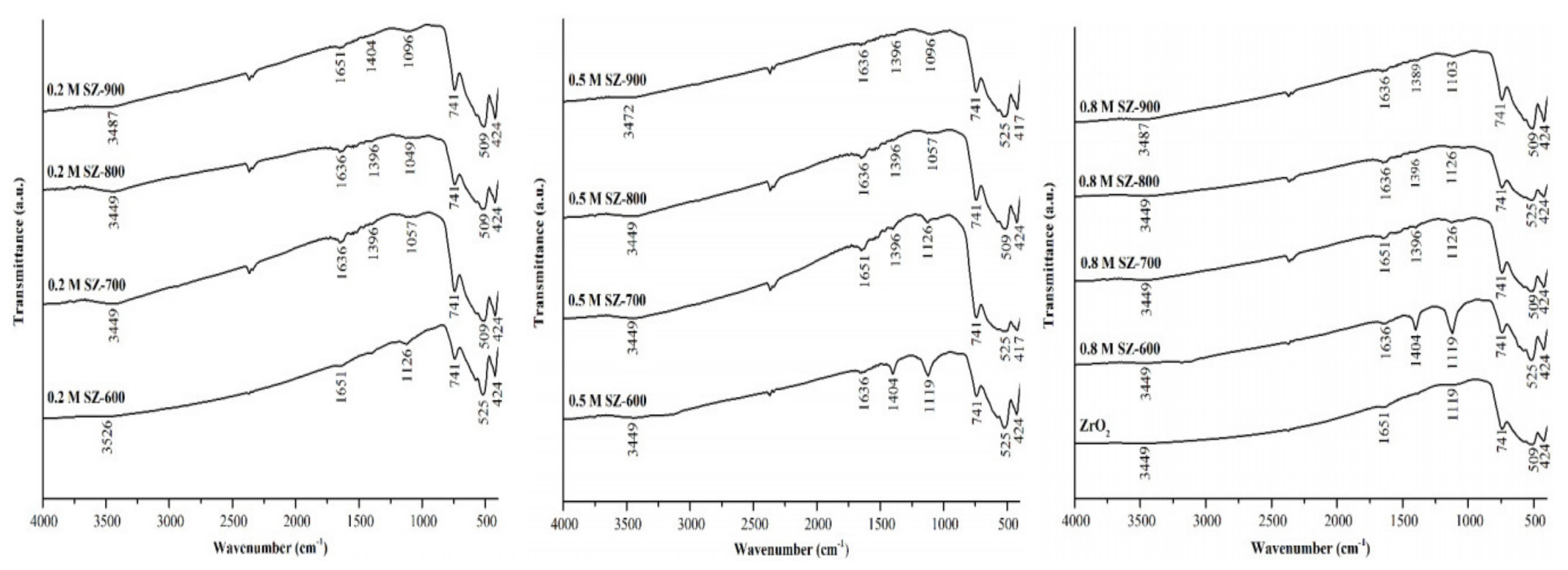
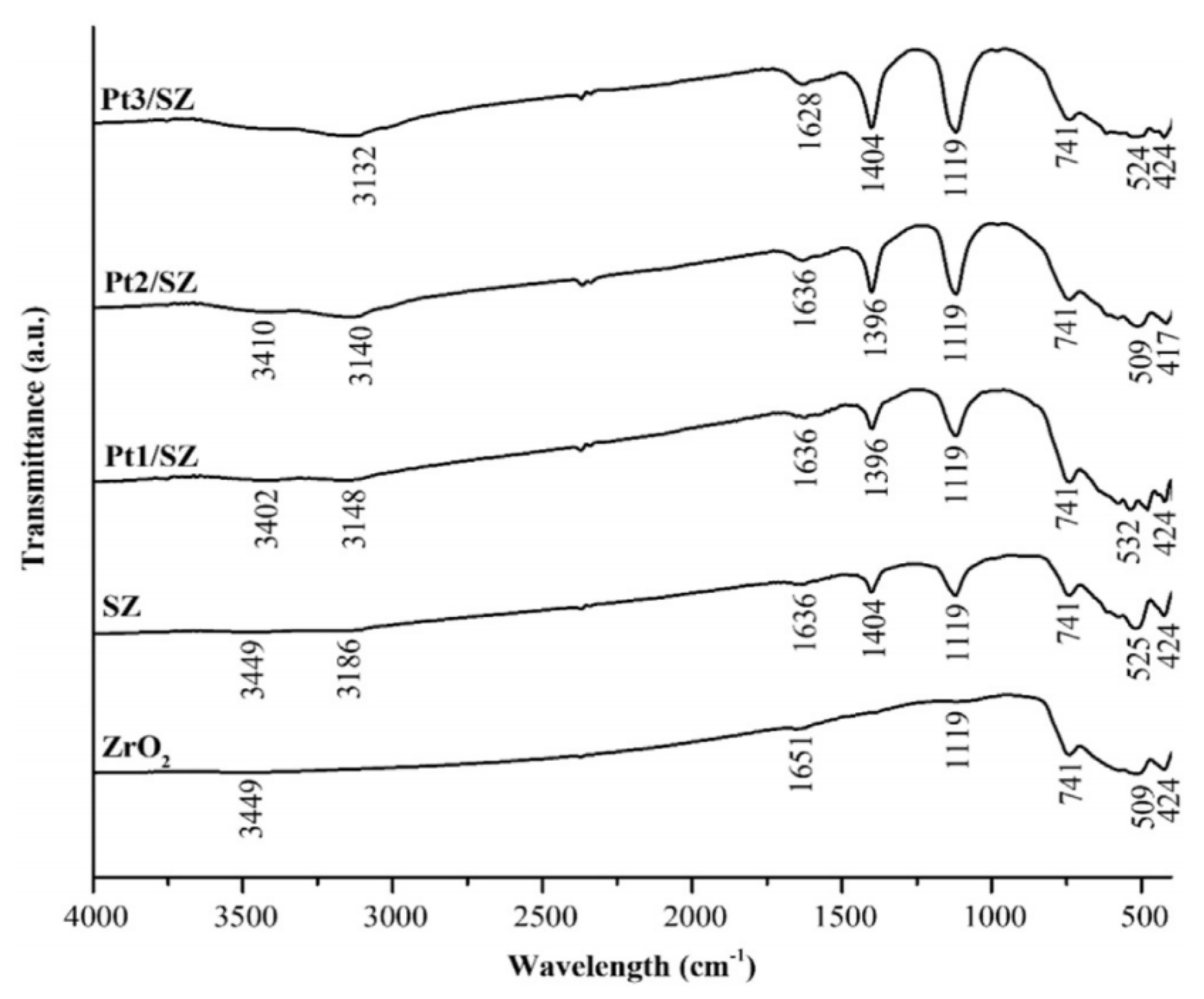
3.1. Functional Group Characterization for ZrO2-SO4
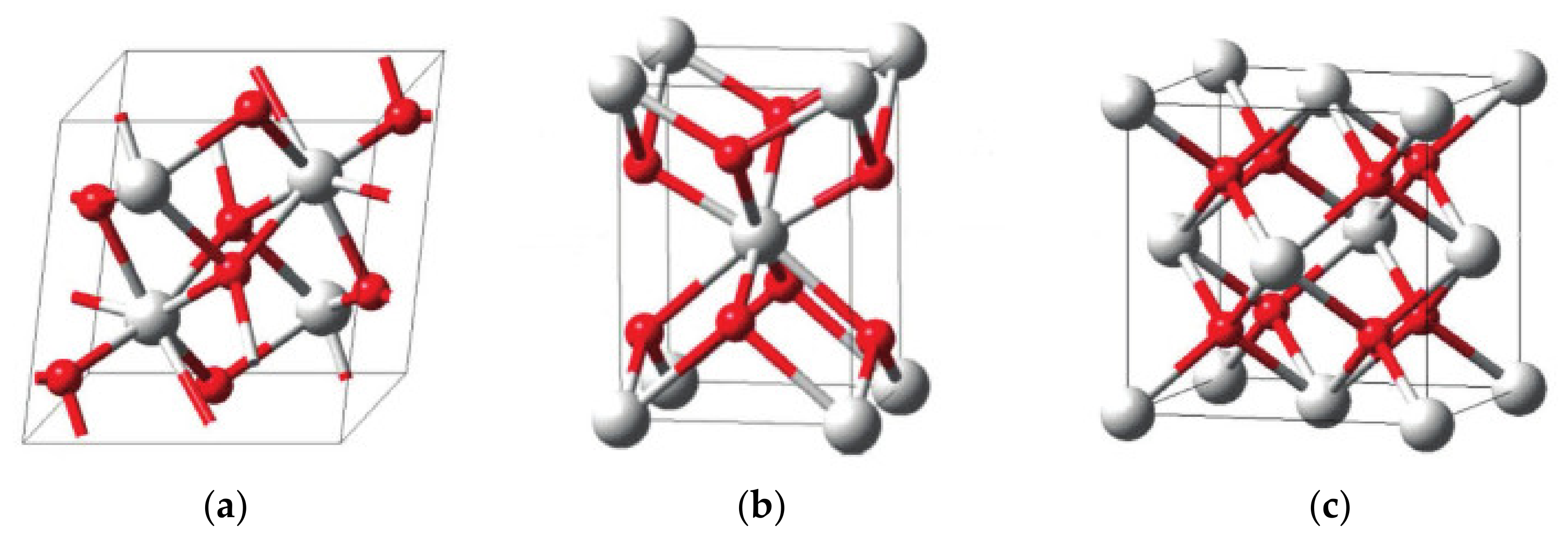
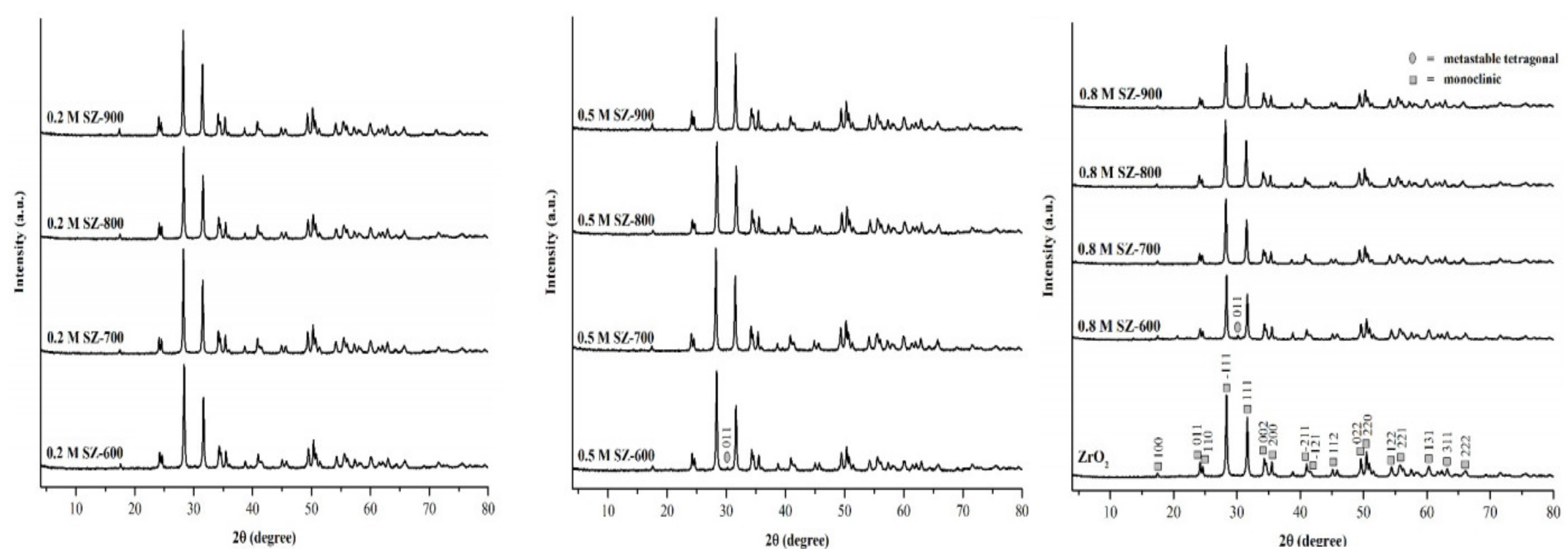
3.2. ZrO2-SO4 Crystal Structure Characterization
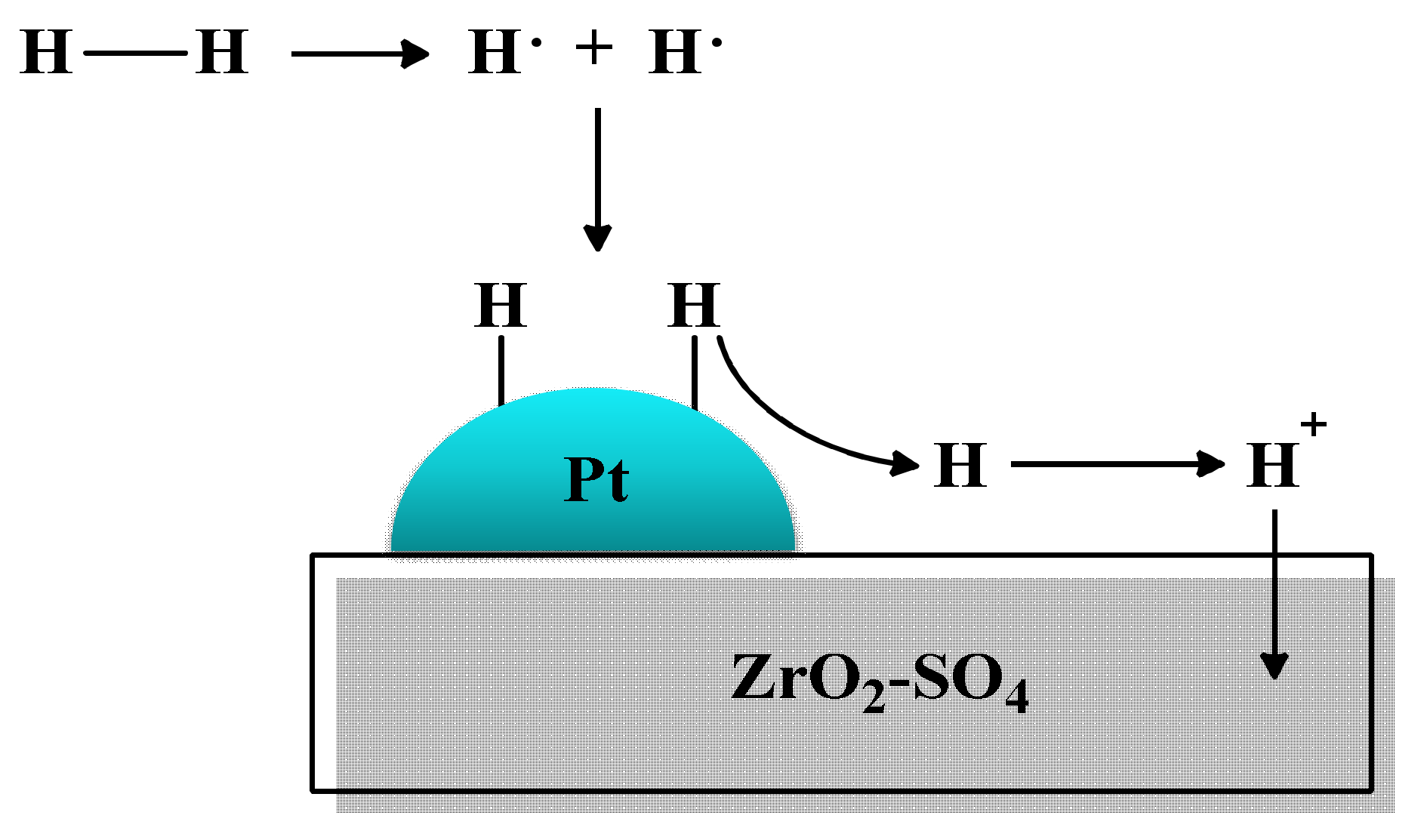
4. Platinum/Sulfated Zirconia (Pt/ZrO2-SO4) Catalyst
4.1. FTIR and Acidity Characterization of Pt/ZrO2-SO4 Catalyst
4.2. XRD and GSA Characterizations of Pt/ZrO2-SO4 Catalyst
4.3. Elemental Composition Characterization Using EDXRF
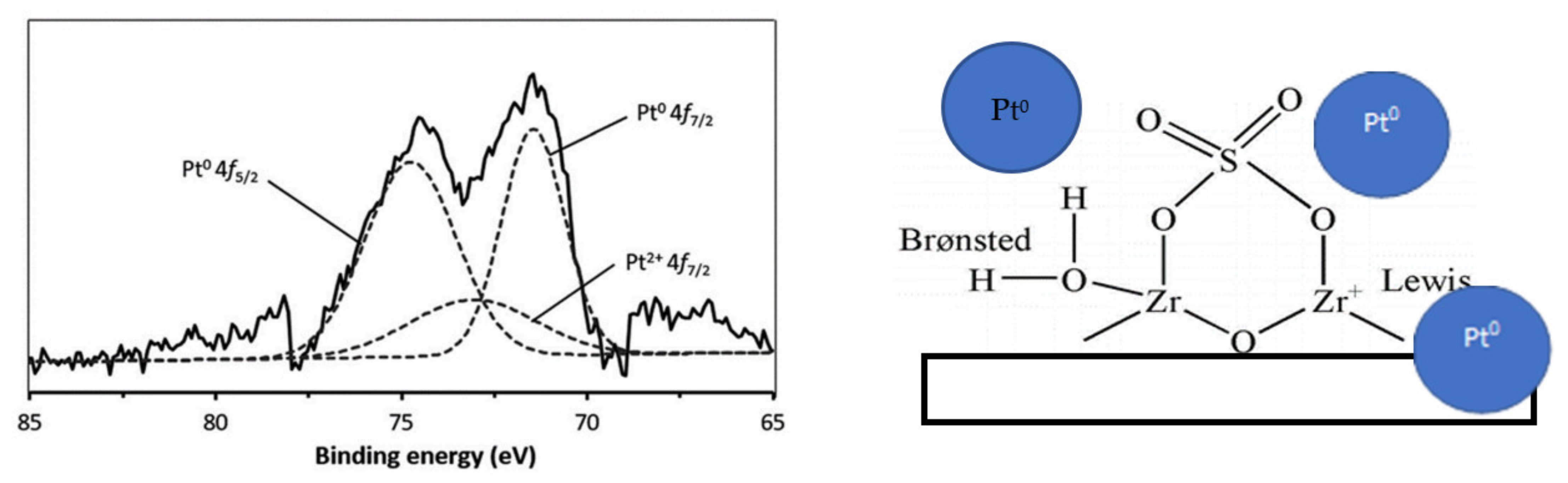
4.4. Pt metal Composition Identification Using XPS
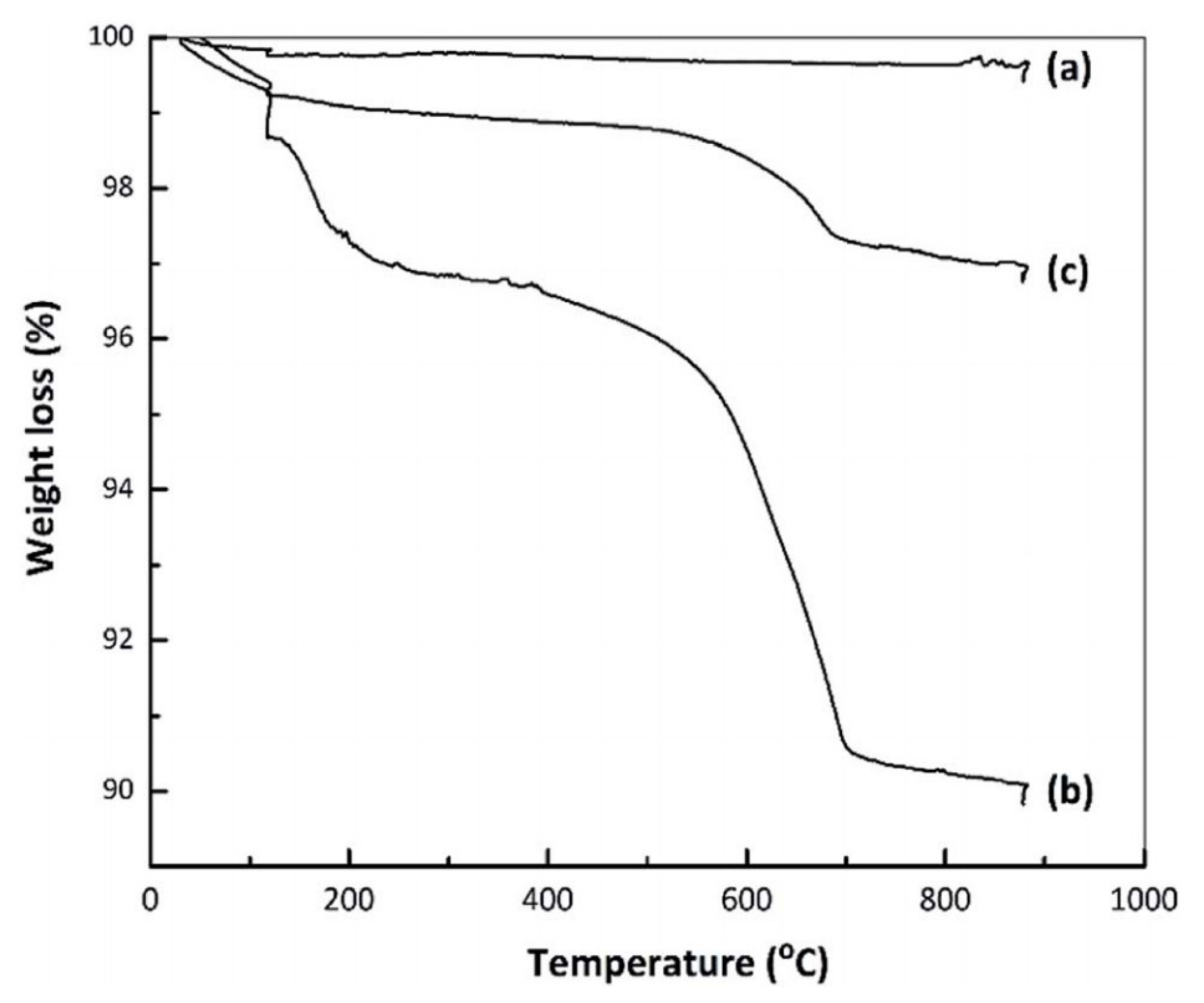
4.5. Thermal Stability Characterization with TG/DTA
4.6. Activity and Selectivity of Pt/ZrO2-SO4 Catalyst in LPDE Hydrocracking Application
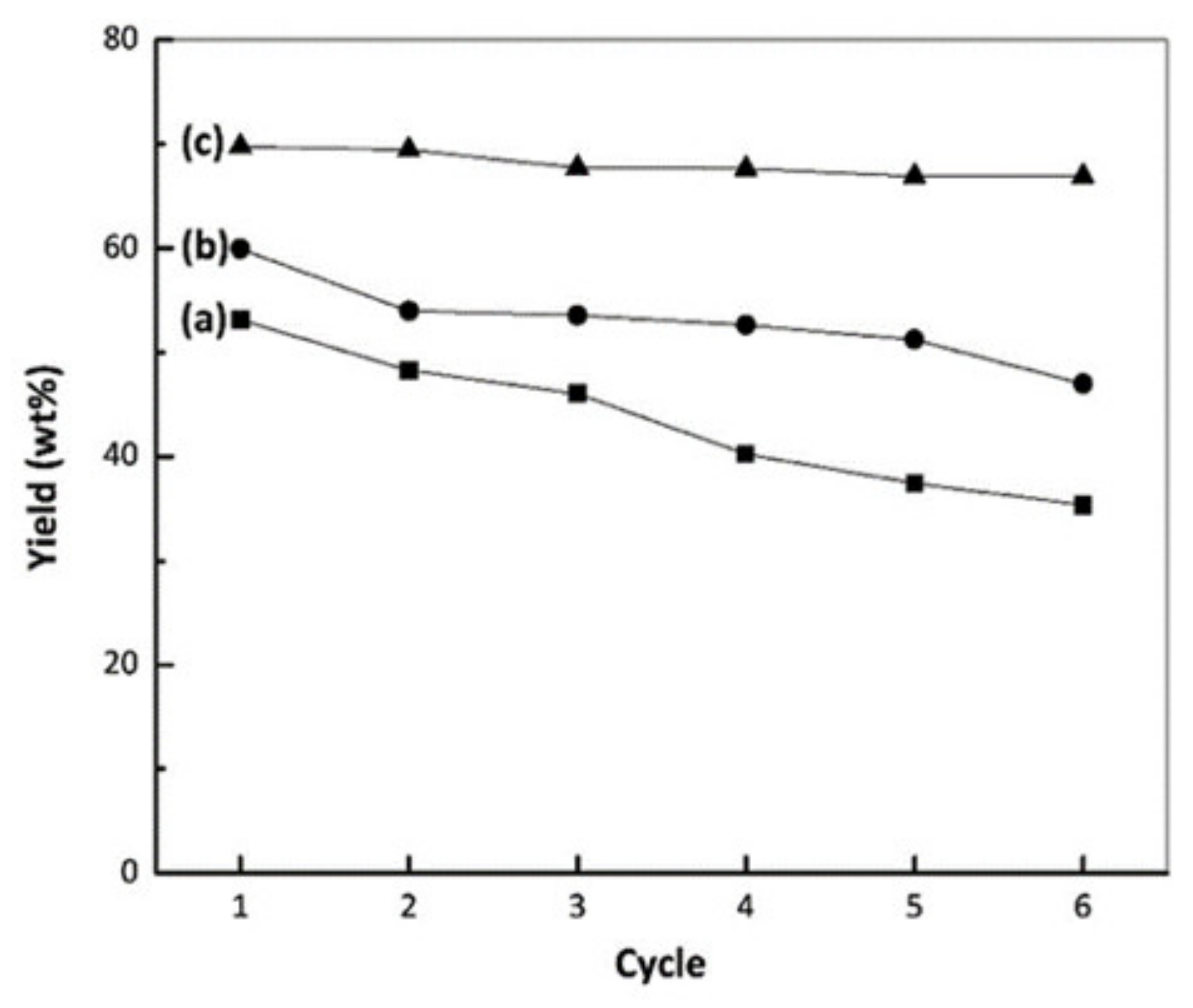
4.7. Stability Test of Pt/ZrO2-SO4
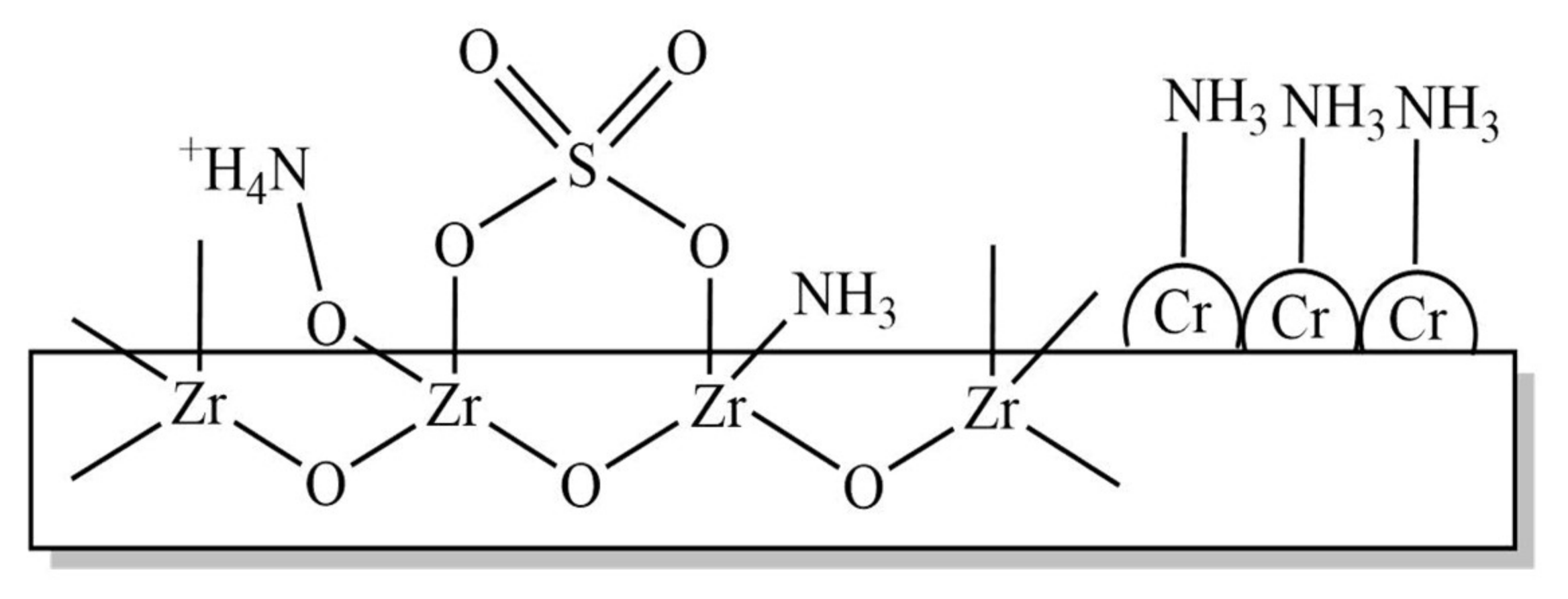
5. Cr/ZrO2-SO4 Catalyst
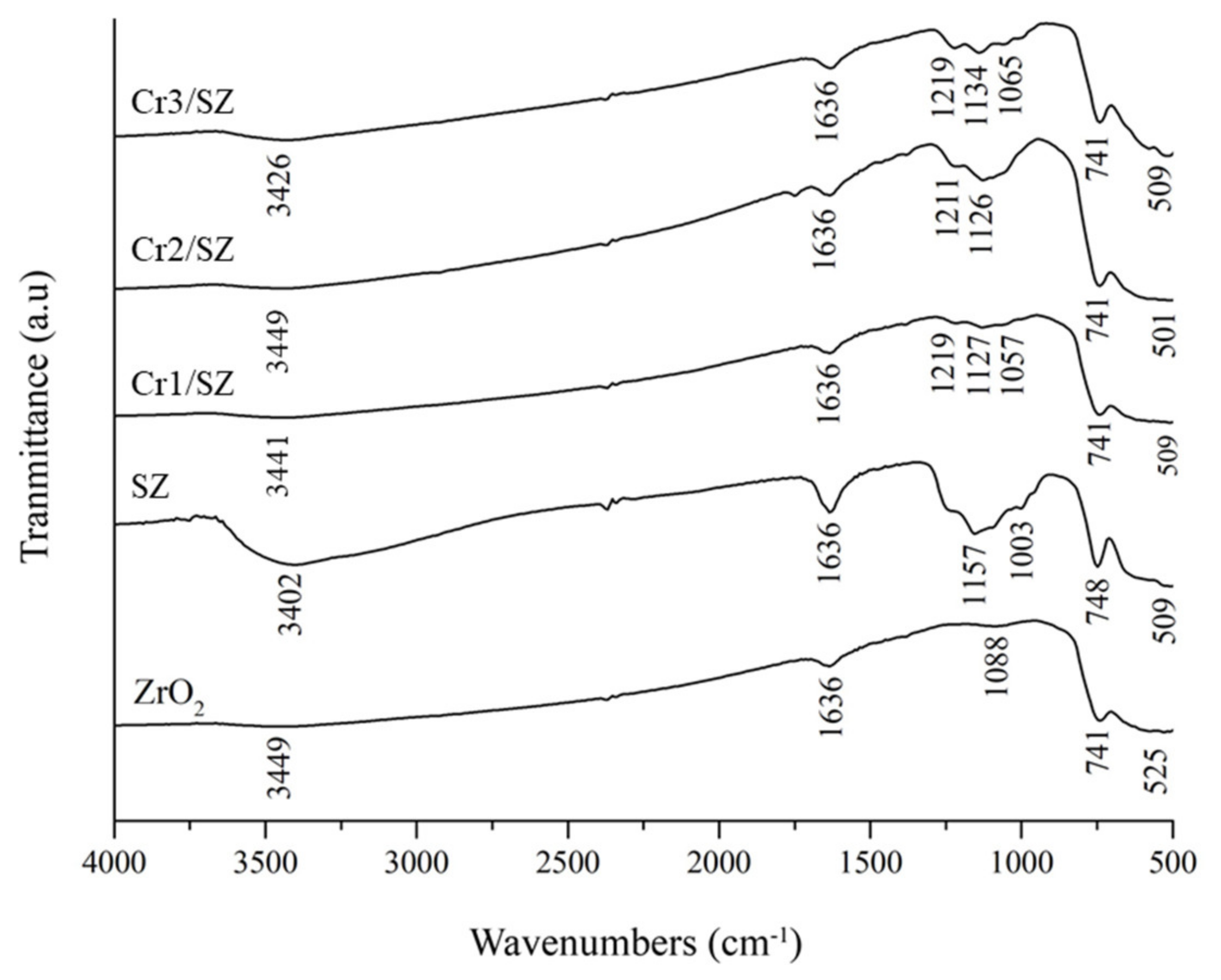
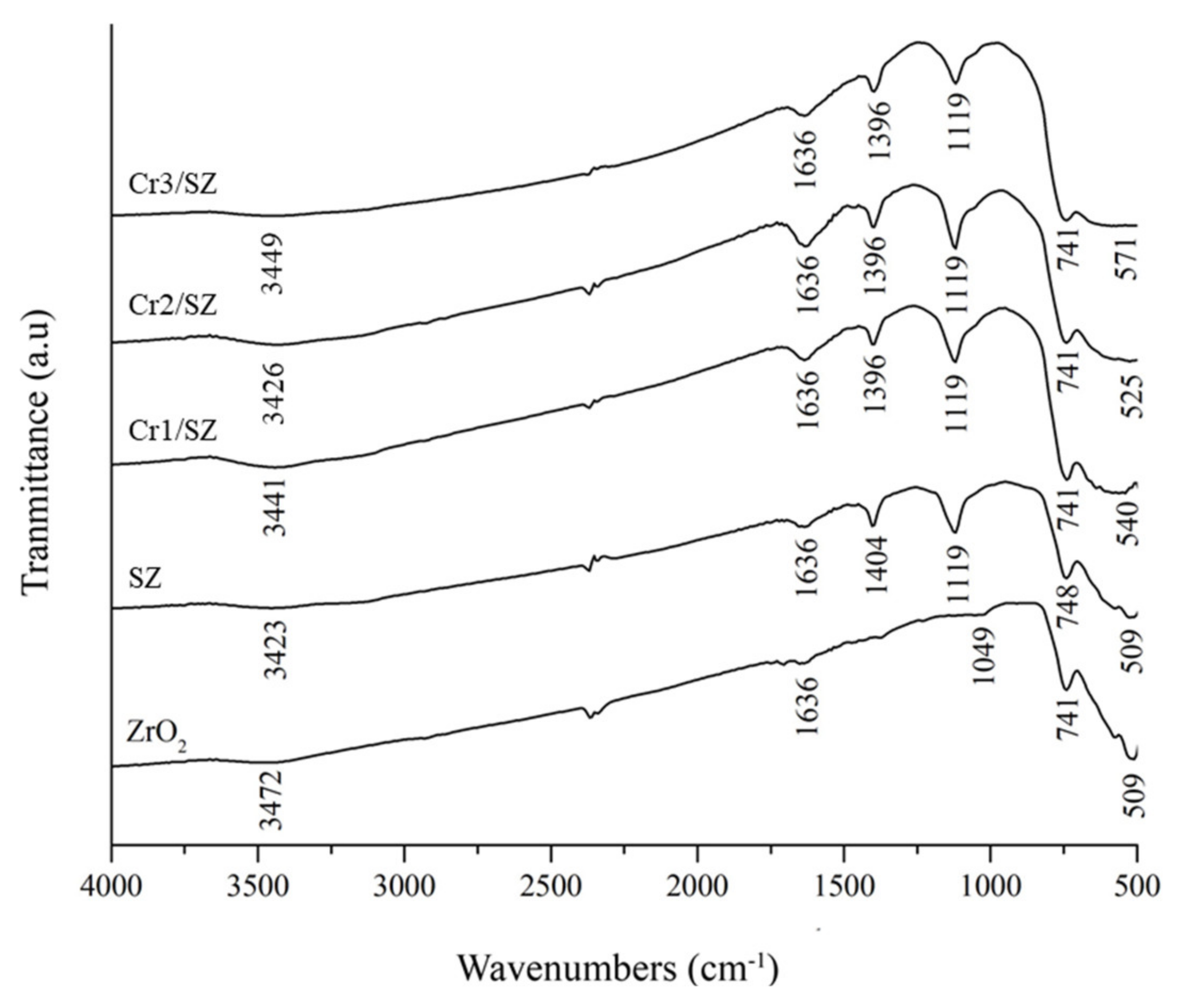
5.1. FITR and Acidity Characterizations of Cr/ZrO2-SO4 Catalyst
5.2. XRD Characterization of Cr/ZrO2-SO4 Catalyst
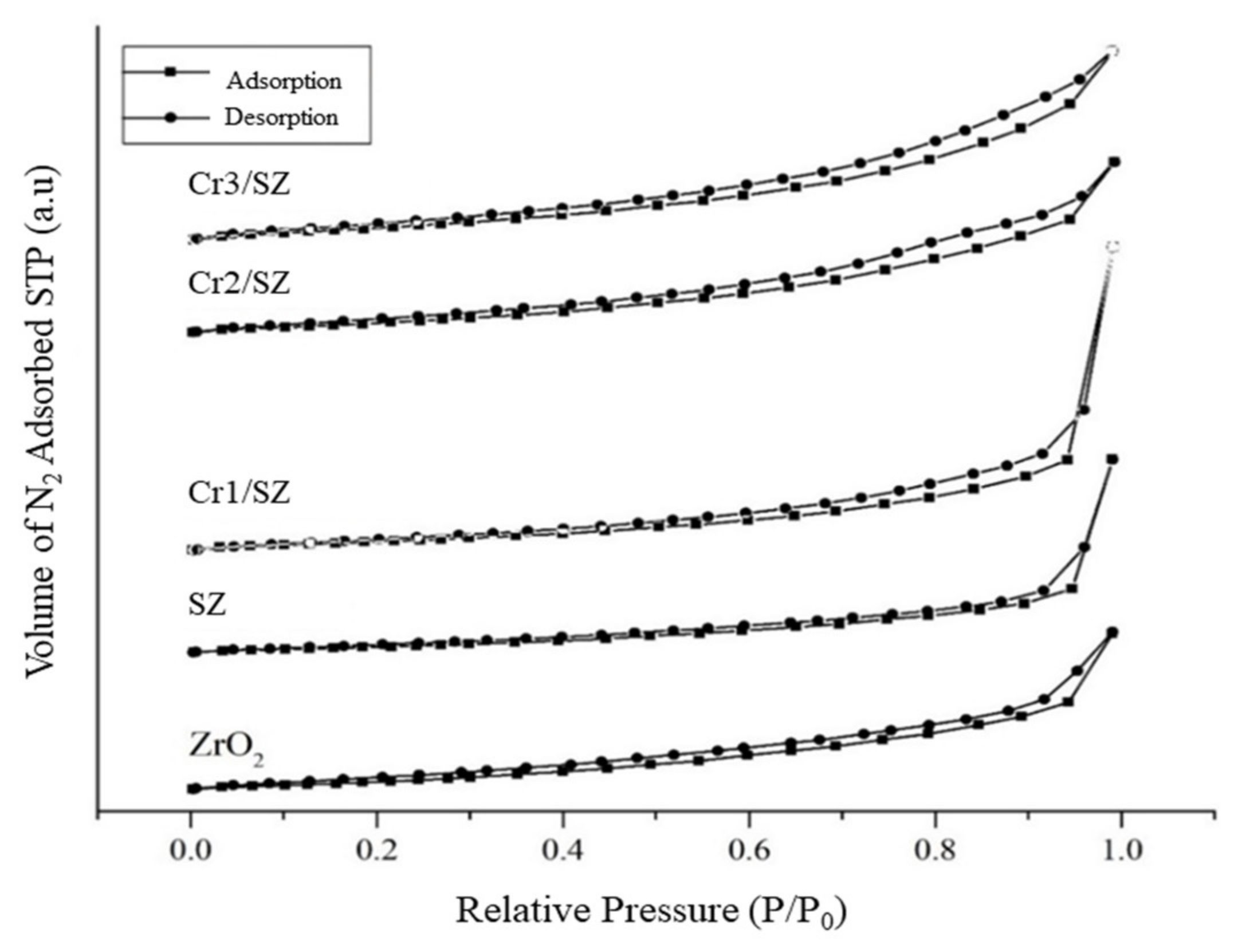
5.3. SAA Characterization of Cr/ZrO2-SO4 Catalyst
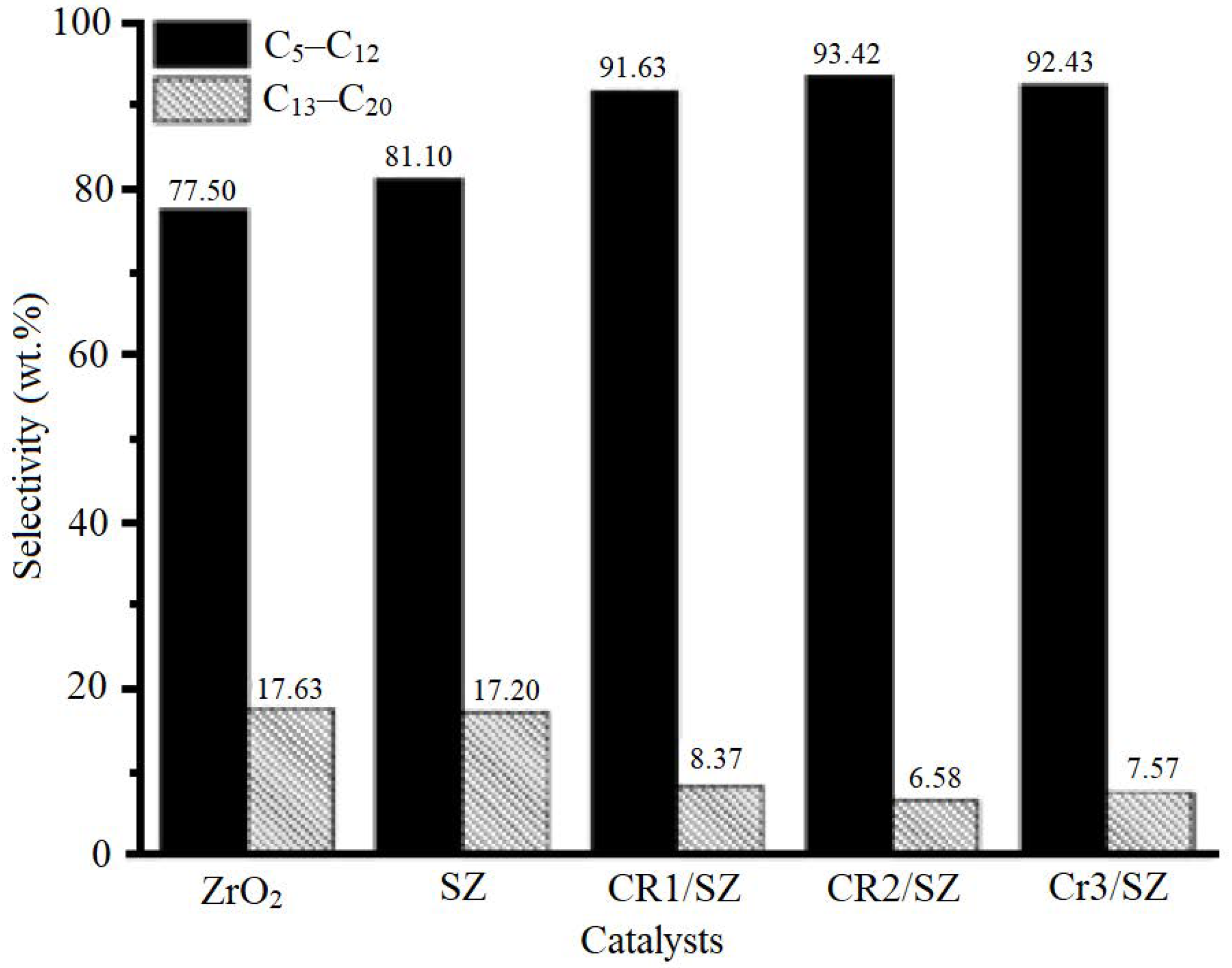
5.4. Activity and Selectivity Tests of Cr/ZrO2-SO4 Catalyst
6. Ni/ZrO2-SO4 Catalyst
6.1. FITR Characterization of Ni/ZrO2-SO4 Catalyst
6.2. Acidity and SAA Analysis of Ni/ZrO2-SO4 Catalyst
7. Conclusions
8. Future Suggestion
Author Contributions
Funding
Conflicts of Interest
References
- Li, D.; Feng, W.; Chen, C.; Chen, S.; Fan, G.; Liao, S.; Wu, G.; Wang, Z. Transesterification of Litsea cubeba kernel oil to biodiesel over zinc supported on zirconia heterogeneous catalysts. Renew. Energy 2021, 177, 13–22. [Google Scholar] [CrossRef]
- Hanafi, M.F.; Sapawe, N. Reusability study of zirconia catalyst toward photocatalytic degradation of Remazol brilliant blue dye. Mater. Today Proc. 2020, 31, 266–2688. [Google Scholar] [CrossRef]
- Amin, A.K.; Trisunaryanti, W.; Wijaya, K. Effect of promoters and calcination temperature on surface and acidity of modified zirconia. J. Nano Res. 2018, 57, 31–39. [Google Scholar] [CrossRef]
- Hauli, L.; Wijaya, K.; Armunanto, R. Preparation and characterization of sulfated zirconia from a commercial zirconia nanopowder. Orient. J. Chem. 2018, 34, 1559–1564. [Google Scholar] [CrossRef]
- Yan, C.; Zheng, S.; Chen, N.; Yuan, S.; Chen, Y.; Li, B.; Zhang, Y. Sulfated zirconia catalysts supported on mesoporous Mg-SBA-15 with different morphologies for highly efficient conversion of fructose to 5-hydroxymethylfurfural. Micropor. Mesopor. Mater. 2021, 328, 111507. [Google Scholar] [CrossRef]
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Pt-promoted Sulfated Zirconia as Catalyst for Hydrocracking of LDPE Plastic Waste into Liquid Fuels. Mater. Chem. Phys. 2018, 213, 548–555. [Google Scholar] [CrossRef]
- Yan, G.X.; Wang, A.; Wachs, I.E.; Baltrusaitis, J. Critical review on the active site structure of sulfated zirconia catalysts and prospects in fuel production. Appl. Catal. A. Gen. 2019, 572, 210–225. [Google Scholar] [CrossRef]
- Wijaya, K.; Kurniawan, M.A.; Saputri, W.D.; Trisunaryanti, W.; Mirzan, M.; Hariani, P.L.; Tikoalu, A.D. Synthesis of nickel catalyst supported on ZrO2/SO4 pillared bentonite and its application for conversion of coconut oil into gasoline via hydrocracking process. J. Environ. Chem. Eng. 2021, 9, 105399. [Google Scholar] [CrossRef]
- Utami, M.; Trisunaryanti, W.; Shida, K.; Tsushida, M.; Kawakita, H.; Ohto, K.; Wijaya, K.; Tominaga, M. Hydrothermal preparation of a platinum-loaded sulphated nanozirconia catalyst for the effective conversion of waste low density polyethylene into gasoline-range hydrocarbons. RSC Adv. 2019, 9, 41392–41401. [Google Scholar] [CrossRef] [Green Version]
- Hauli, L.; Wijaya, K.; Syoufian, A. Fuel production from LDPE-based plastic waste over chromium supported on sulfated zirconia. Indones. J. Chem. 2019, 20, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Amin, A.K.; Trisunaryanti, W.; Wijaya, K. The Catalytic Performance of ZrO2-SO4 and Ni/ZrO2-SO4 Prepared from Commercial ZrO2 in Hydrocracking of LDPE Plastic Waste into Liquid Fuels. Orient. J. Chem. 2018, 34, 3070–3078. [Google Scholar] [CrossRef] [Green Version]
- Treccani, L.; Klein, T.Y.; Meder, F.; Pardun, K.; Rezwan, K. Functionalized ceramics for biomedical, biotechnological and environmental applications. Acta Biomater. 2013, 9, 7115–7150. [Google Scholar] [CrossRef] [PubMed]
- Skovgaard, M.; Gudik-Sorensen, M.; Almdal, K.; Ahniyas, A. Nanoporous zirconia microspheres prepared by salt-assisted spray drying. SN Appl. Sci. 2020, 2, 784. [Google Scholar] [CrossRef] [Green Version]
- Utami, M. Platinum/Sulfated Nanozirconia: Preparation, Characterization, and Their Application in Conversion of LDPE Plastic Waste into Liquid Fuels. Ph.D. Thesis, Department of Chemistry, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2019. [Google Scholar]
- Le Ore, M.S.; Wijaya, K.; Trisunaryanti, W.; Saputri, W.D.; Heraldy, E.; Yuwana, M.W.; Hariani, P.L.; Budiman, A.; Sudiono, S. The Synthesis of SO4/ZrO2 and Zr/CaO catalysts via hydrothermal treatment and their application for conversion of low-grade coconut oil into biodiesel. J. Environ. Chem. Eng. 2020, 8, 104205. [Google Scholar] [CrossRef]
- Burger, W.; Kiefer, G. Alumina, zirconia and their composite ceramics with properties tailored for medical applications. J. Compos. Sci. 2021, 5, 306. [Google Scholar] [CrossRef]
- Siddiqui, M.R.H.; Al-Wassil, A.I.; Alotaibi, A.M.; Mahfouz, R. Effects of precursor on the morphology and size of ZrO2 nanoparticles, synthesized by sol-gel method in non-aqueous medium. Mater. Res. 2012, 15, 986–989. [Google Scholar] [CrossRef] [Green Version]
- Halter, W.; Eisele, R.; Rothenstein, D.; Bill, J.; Allgower, F. Moment dynamics of zirconia particle formation foe optimizing particle size distribution. Nanomaterials 2019, 9, 333. [Google Scholar] [CrossRef] [Green Version]
- Maleki, F.; Pacchiono, G. Characterization of acid and basic sites on zirconia surfaces and nanoparticles by adsorbed probe molecules: A theoretical study. Top. Catal. 2020, 63, 1717–1730. [Google Scholar] [CrossRef]
- Glorius, M.; Markovits, M.A.C.; Breitkopf, C. Design of specific acid-base-properties in CeO2-ZrO2-mixed oxides via templating and Au modification. Catalysts 2018, 8, 358. [Google Scholar] [CrossRef] [Green Version]
- De Souza, E.F.; Appel, L.G. Oxygen vacancy formation and their role in the CO2 activation on Ca doped ZrO2 surface: An an-initio DFT study. App. Surf. Sci. 2021, 553, 149589. [Google Scholar] [CrossRef]
- Song, L.; Cao, X.; Li, L. Engineering stable surface oxygen vacancies on ZrO2 by hydrogen-etching technology: An efficient support of gold catalysts for water-gas shift reaction. ACS Appl. Mater. Interfaces 2018, 109, 31249–31259. [Google Scholar] [CrossRef] [PubMed]
- Gautam, C.; Joyner, J.; Gautam, A.; Rao, J.; Vajtai, R. Zirconia Based Dental Ceramics: Structure, Mechanical Properties, Biocompatibility and Applications. Dalton Trans. 2016, 45, 19194–19215. [Google Scholar] [CrossRef] [PubMed]
- Hauli, L.; Wijaya, K.; Syoufian, A. Hydrocracking of LDPE plastic waste into liquid fuel over sulfated zirconia from a commercial zirconia nanopowder. Orient. J. Chem. 2019, 35, 128–133. [Google Scholar] [CrossRef]
- El-Desouki, D.S.; Ibrahim, A.H.; Abdelazim, S.M.; Aboul-Gheit, N.A.K.; Abdel-Hafizar, D.R. The optimum condition for methanol conversion to dimethyl ether over modified sulfated zirconia catalysts prepared by different methods. J. Fuel Chem. Technol. 2021, 49, 63–71. [Google Scholar] [CrossRef]
- Yu, S.; Wu, S.; Li, L.; Ge, X. Upgrading bio-oil from waste cooking oil by esterification using SO42−/ZrO2 as catalyst. Fuel 2020, 276, 118019. [Google Scholar] [CrossRef]
- Wang, P.; Yue, Y.; Wang, T.; Bao, X. Alkane isomerization over sulfated zirconia solid acid system. Int. J. Energ. Res. 2020, 44, 3270–3294. [Google Scholar] [CrossRef]
- Rabee, A.I.M.; Mekhemer, G.A.H.; Osatiashtiani, A.; Isaacs, M.A.; Lee, A.F.; Wilson, K.; Zaki, M.I. Acidity-reactivity relationships in catalytic esterification over ammonium sulfate-derived sulfated zirconia. Catalysts 2017, 7, 204. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Zhang, J.; Han, C.; Yang, C.; Li, C. Effect of Modification Methods on the Surface Properties and n-Butane Isomerization Performance of La/Ni-Promoted SO42−/ZrO2-Al2O3. Appl. Surf. Sci. 2016, 378, 489–495. [Google Scholar] [CrossRef]
- Saravanan, K.; Tyagi, B.; Bajaj, H.C. Esterifcation of caprylic acid with alcohol over nano-crystalline sulfated zirconia. J. Sol-Gel Sci. Technol. 2012, 62, 13–17. [Google Scholar] [CrossRef]
- Utami, M.; Wijaya, K.; Trisunaryanti, W. Effect of Sulfuric Acid Treatment and Calcination on Commercial Zirconia Nanopowder. Key Eng. Mater. 2017, 757, 131–137. [Google Scholar] [CrossRef]
- Yang, X.; Ma, X.; Yu, X.; Ge, M. Exploration of strong metal-support interaction in zirconia supported catalysts for toluene oxidation. App. Catal. B Environ. 2020, 263, 118355. [Google Scholar] [CrossRef]
- Mirzan, M.; Syoufian, A.; Wijaya, K. Physico-chemical properties of nano ZrO2-pillared bentonite with nickel as supporting metal. Nano Hybrids Compos. 2020, 30, 9–17. [Google Scholar] [CrossRef]
- Loveless, B.T.; Gyanani, A.; Muggli, D.S. Discrepancy between TPD- and FTIR- based measurements of Brønsted and Lewis acidity for sulfated zirconia. Appl. Catal. B Environ. 2008, 84, 591–597. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Li, H.; Yu, S.; Liu, S.; Yu, H.; Ge, X. SO42−/ZrO2 as catalyst for upgrading of pyrolysis oil by esterification. Fuel 2018, 226, 190–194. [Google Scholar] [CrossRef]
- Pratika, R.A.; Wijaya, K.; Trisunaryanti, W. Hydrothermal treatment of SO4/TiO2 and TiO2/CaO as heterogeneous catalysts for the conversion of Jatropha oil into biodiesel. J. Environ. Chem. Eng. 2021, 9, 106547. [Google Scholar] [CrossRef]
- Utami, M.; Safitri, R.; Pradipta, M.F.; Wijaya, K.; Chang, S.W.; Ravindran, B.; Ovi, D.; Rajabathar, J.R.; Poudineh, N.; Gengan, R.M. Enhanced catalytic conversion of palm oil into biofuels by Cr-incorporated sulphated zirconia. Mater. Lett. 2022, 309, 131472. [Google Scholar] [CrossRef]
- Hanifah, A.; Nadia, A.; Saputri, W.D.; Syoufian, A.; Wijaya, K. Performance of Ni-Mo sulfated nanozirconia catalyst for conversion of waste cooking oil into biofuel via hydrocracking process. Mater. Sci. Forum 2021, 1045, 79–89. [Google Scholar] [CrossRef]
- Sari, E.P.; Wijaya, K.; Trisunaryanti, W.; Syoufian, A.; Hasanudin, H.; Saputri, W.D. The effective combination of zirconia superacid and zirconia-imregnated CaO in biodiesel manufacturing: Utilization of used coconut cooking oil (UCCO). Int. J. Energ. Environ. Eng. 2021, 1–12. [Google Scholar] [CrossRef]
- Wijaya, K.; Nadia, A.; Dinana, A.; Pratiwi, A.F.; Tikoalu, A.D.; Wibowo, A.C. Catalytic hydrocracking of fresh and waste frying oil over Ni- and Mo-based catalysts supported on sulfated silica for biogasoline production. Catalysts 2021, 11, 1150. [Google Scholar] [CrossRef]
- Fernandez-Morales, J.M.; Castillejos, E.; Asedegbega-Nieto, E.; Dongil, A.B.; Rodrigues-Ramos, I.; Guerrero-Ruiz, A. Comparative study of different acidic surface structures in solid catalysts applied for the isobutene dimerization reaction. Nanomaterial 2020, 10, 1235. [Google Scholar] [CrossRef]
- Purba, S.E.; Wijaya, K.; Trisunaryanti, W.; Pratika, R.A. Dealuminated and desilicated natural zeolite as a catalyst for hydrocracking of used cooking oil into biogasoline. Mediterr. J. Chem. 2020, 11, 74–83. [Google Scholar] [CrossRef]
- Wijaya, K.; Malau, M.L.L.; Utami, M.; Mulijani, S.; Patah, A.; Wibowo, A.C.; Chandrasekaran, M.; Rajabathar, J.R.; Al-Lohedan, H. Synthesis, characterizations and catalysis of sulfated silica and nickel modified silica catalysts for diethyl ether (DEE) production from ethanol towards renewable energy applications. Catalysts 2021, 11, 1511. [Google Scholar] [CrossRef]
- Serrano, D.P.; Garcia, R.A.; Linares, M.; Gil, B. Influence of the calcination treatment on the catalytic properties of hierarchical ZSM-5. Catal. Today 2012, 179, 91–101. [Google Scholar] [CrossRef]
- Swamidoss, C.M.A.; Sheraz, M.; Anus, A.; Jeong, S.; Park, Y.-K.; Kim, Y.-M.; Kim, S. Effect of Mg/Al2O3 and calcination temperature on the catalytic decomposition of HFC-134a. Catalysts 2019, 9, 270. [Google Scholar] [CrossRef] [Green Version]
- Abedin, M.A.; Kanitkar, S.; Bhattar, S.; Spivey, J.J. Methane dehydroaromatization using Mo supported on sulfated zirconia catalyst: Effect of promoters. Catal. Today 2021, 365, 71–79. [Google Scholar] [CrossRef]
- Susi, E.P.; Wijaya, K.; Wangsa; Pratika, R.A.; Hariani, P.L. Effect of nickel concentration in natural zeolite as catalyst in hydrocracking process of used cooking oil. Asian J. Chem. 2020, 32, 2773–2777. [Google Scholar] [CrossRef]
- Yin, P.; Hu, S.; Qian, K.; Wei, Z.; Zhang, L.-L.; Lin, Y.; Huang, W.; Xiong, H.; Li, W.X.; Liang, H.-W. Quantification of critical particle distance for mitigating catalyst sintering. Nat. Commun. 2021, 12, 4865. [Google Scholar] [CrossRef]
- Prašnikar, A.; Pavlišič, A.; Ruiz-Zepeda, F.; Kovač, J.; Likozar, B. Mechanism of Copper-based catalyst deactivation during CO2 reduction to methanol. Ind. Eng. Chem. Res. 2019, 58, 13021–13029. [Google Scholar] [CrossRef] [Green Version]
- Rahmati, M.; Safdari, M.S.; Fletcher, T.H.; Argyle, M.D.; Bartholomew, C.H. Chemical and thermal sintering of supported metals with emphasis on cobalt catalysts during Fischer–Tropsch synthesis. Chem. Rev. 2020, 120, 4455–4533. [Google Scholar] [CrossRef]
- Zhou, S.; Song, Y.; Zhao, J.; Zhou, X.; Chen, L. Study on the Mechanism of Water Poisoning Pt-Promoted Sulfated Zirconia Alumina in n-Hexane Isomerization. Energy Fuels 2021, 35, 14860–14867. [Google Scholar] [CrossRef]
- Peng, S.; Li, M.; Yang, X.; Li, P.; Liu, H.; Xiong, W.; Peng, X. Atomic layer deposition of Pt nanoparticles on ZrO2 based metal-organic frameworks for increased photocatalytic activity. Ceram. Int. 2019, 45, 18128–18134. [Google Scholar] [CrossRef]
- Bikmetova, L.I.; Kazantsev, K.V.; Zatolokina, L.V.; Smolikov, M.D.; Belyi, A.S. A study on the synthesis steps of alumina-supported Pt/SO4/ZrO2 catalysts for isomerization of n-hexane. AIP Conf. Proc. 2020, 2285, 020003. [Google Scholar] [CrossRef]
- Souza, I.C.A.; Manfro, R.L.; Souza, M.M.V.M. Hydrogen production from steam reforming of acetic acid over Pt-Ni bimetallic catalysts supported on ZrO2. Biomass Bioenergy 2022, 156, 106317. [Google Scholar] [CrossRef]
- Ullah, I.; Taha, T.A.; Alenad, A.M.; Uddin, I.; Hayat, A.; Hayat, A.; Sohail, M.; Irfan, A.; Khan, J.; Palamanit, A. Platinum-alumina modified SO42−ZrO2/Al2O3 based bifunctional catalyst for significantly improved n-butane isomerization performance. Surf. Interfaces 2021, 25, 101227. [Google Scholar] [CrossRef]
- Tamizhdurai, P.; Lavanya, M.; Meenakshisundaram, A.; Shanti, K.; Sivasanker, S. Isomerization of alaknes over Pt-sulphated zirconia supported on SBA-15. Adv. Porous Mater. 2017, 5, 169–174. [Google Scholar] [CrossRef]
- Cho, H.J.; Kim, D.; Li, S.; Su, D.; Ma, D.; Xu, B. Molecular-level proximity of metal and acid sites in zeolite-encapsulated Pt nanoparticles for selective multistep tandem catalysis. ACS Catal. 2020, 10, 3340–3348. [Google Scholar] [CrossRef]
- Gan, T.; Yang, J.; Morris, D.; Chu, X.; Zhang, P.; Zhang, W.; Zuo, Y.; Yan, W.; Wei, S.-H.; Liu, G. Electron donation of non-oxide supports boosts O2 activation on nano-platinum catalysts. Nat. Commun. 2021, 12, 2741. [Google Scholar] [CrossRef] [PubMed]
- Aboul-Gheit, A.K.; Gad, F.K.; Abdel-Aleem, G.M.; El-Desouki, D.S.; Abdel Hamid, S.M.; Ghoneim, S.S.; Ibrahim, A.H. Pt, Re and Pt-Re incorporation in sulfated zirconia as catalysts for n-pentane isomerization. Egypt. J. Pet. 2014, 23, 303–314. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.-P.; Chen, J.; Yu, H.-B.; Cen, B.-H.; Wang, W.-Y.; Luo, M.-F.; Lu, J.-Q. Insight into propane combustion over MoO3 promoted Pt/ZrO2 catalysts: The generation of Pt-MoO3 interface and its promotional role on catalytic activity. J. Catal. 2020, 391, 80–90. [Google Scholar] [CrossRef]
- Kondratowicz, T.; Drozdek, M.; Michalik, M.; Gac, W.; Gajewska, M.; Kustroski, P. Catalytic activity of Pt species variously dispersed on hollow ZrO2 spheres in combustion of volatile organic compounds. App. Surf. Sci. 2020, 513, 145788. [Google Scholar] [CrossRef]
- Verga, L.G.; Rusell, A.E.; Skylaris, C.K. Ethanol, O, and CO adsorption on Pt nanoparticles: Effect of nanoparticle size and graphene support. Phys. Chem. Chem. Phys. 2018, 20, 25918–25930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Pu, J.; Wu, J.; Liu, H.; Xu, H.; Li, X.; Wang, H. SO42−/ZrO2 as a solid acid for the esterification of palmitic acid with methanol: Effects of the calcination time and recycle method. ACS Omega 2020, 5, 30139–30147. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Kong, D.; Meng, X.; Wang, X.; Shi, L.; Liu, N. The effect of ammonium sulfate and sulfamic acid on the surface acidity of sulfated zirconia. J. Saudi Chem. Soc. 2019, 23, 198–204. [Google Scholar] [CrossRef]
- Vance, B.C.; Kots, P.A.; Wang, C.; Hinton, Z.R.; Quinn, C.M.; Epps, T.H., III; Korley, L.T.J.; Vlachos, D.G. Single pot catalyst strategy to branched products via adhesive isomerization and hydrocracking of polyethylene over platinum tungstated zirconia. App. Catal. B. Environ. 2021, 299, 120483. [Google Scholar] [CrossRef]
- Liu, S.; Lots, P.; Vance, B.C.; Danielson, A.; Vlachos, D.G. Plastic waste to fuels by hydrocracking at mild conditions. Sci. Adv. 2021, 7, eabf8283. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, Y.; Chen, X.; Dai, S.; Bao, Z.; Yang, Q.; Ren, Q.; Zhang, Z. Cooperative interplay of Bronsted acid and Lewis acid sites in MIL-101 (Cr) for cross-dehydrogenative coupling of C–H. Bonds. ACS Appl. Mater. Interfaces 2021, 13, 10845–10854. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.-P.; Wang, Z.; Yuan, Z.-Y. Cr/Al2O3 catalysts with strong metal-support interactions for stable catalytic dehydrogenation of propane to propylene. Mol. Catal. 2020, 493, 111052. [Google Scholar] [CrossRef]
- Annuar, N.H.R.; Triwahyono, S.; Jalil, A.A.; Basar, N.; Abdullah, T.A.T.; Ahmad, A. Effect of Cr2O3 loading on the properties and cracking activity of Pt/Cr2O3-ZrO2. App. Catal. A Gen. 2017, 541, 77–86. [Google Scholar] [CrossRef]
- Wang, H.; Lin, N.; Xu, R.; Yu, Y.; Zhao, X. First principles studies of electronic, mechanical and optical properties of Cr-doped cubic ZrO2. Chem. Phys. 2020, 539, 110972. [Google Scholar] [CrossRef]
- Hauli, L. Chromium/Sulfated Nanozirconia: Preparation, Characterization, and Their Application in Conversion of LDPE Plastic Waste into Liquid Fuels. Ph.D. Thesis, Department of Chemistry, Universitas Gadjah Mada, Yogyakarta, Indonesia, 2019. [Google Scholar]
- Hauli, L.; Wijaya, K.; Armunanto, R. Preparation of Cr metal supported on sulfated zirconia catalyst. Mater. Sci. Forum 2019, 948, 221–227. [Google Scholar] [CrossRef]
- Aziz, I.T.A.; Saputri, W.D.; Trisunaryanti, W.; Sudiono, S.; Syoufian, A.; Budiman, A.; Wijaya, K. Synthesis of Nickel-loaded sulfated zirconia catalyst and its application for converting used palm cooking oil to gasoline via hydrocracking process. Period. Polytech. Chem. Eng. 2021, 66, 101–113. [Google Scholar] [CrossRef]
- Tao, Y.; Zhu, Y.; Liu, C.; Yue, H.; Ji, J.; Yuan, S.; Jiang, W.; Liang, B. A highly selective Cr/ZrO2 catalyst for the reverse water-gas shift reaction prepared from simulated Cr-containing wastewater by a photocatalytic deposition process with ZrO2. J. Environ. Chem. Eng. 2018, 6, 6761–6770. [Google Scholar] [CrossRef]
- Serrano, D.; Escola, J.; Briones, L.; Arroyo, M. Hydroprocessing of the LDPE thermal cracking oil into transportation fuels over Pd supported on hierarchical ZSM-5 catalyst. Fuel 2017, 206, 190–198. [Google Scholar] [CrossRef]
- Wong, S.L.; Ngadi, N.; Abdullah, T.A.T.; Inuwa, I.M. Conversion of low-density polyethylene (LDPE) over ZSM-5 zeolite to liquid fuel. Fuel 2017, 192, 71–82. [Google Scholar] [CrossRef]
- Lyu, Y.; Xu, R.; Williams, O.; Wang, Z.; Sievers, C. Reaction paths of methane activation and oxidation of surface intermediates over NiO on Ceria-Zirconia catalysts studied by In-situ FTIR spectroscopy. J. Catal. 2021, 404, 334–347. [Google Scholar] [CrossRef]
- Dahdah, E.; Estephane, J.; Gennequin, C.; Aboukais, A.; Abi-Aad, E.; Aouad, S. Zirconia supported nickel catalysts for glycerol steam reforming: Effect of zirconia structure on the catalytic performance. Int. J. Hydrogen Energy 2020, 45, 4457–4467. [Google Scholar] [CrossRef]
- Kou, J.; Yi, L.; Li, G.; Cheng, K.; Wang, R.; Zhnag, D.; Jin, H.; Guo, L. Structural effect of ZrO2 on supported Ni-based catalysts for supercritical water gasification of oil-containing water. Int. J. Hydrogen Energ. 2021, 46, 12874–12885. [Google Scholar] [CrossRef]
- Li, X.; Shao, Y.; Zhang, S.; Wang, Y.; Xiang, J.; Hu, S.; Xu, L.; Hu, X. Pore diameters of Ni/ZrO2 catalysts affect properties of the coke in steam reforming of acetic acid. Int. J. Hydrogen Energy 2021, 46, 23642–23657. [Google Scholar] [CrossRef]
- Kou, J.; Feng, H.; Wei, W.; Wang, G.; Sun, J.; Jin, H.; Guo, L. Study on the detailed reaction pathways and catalytic mechanism of a Ni/ZrO2 catalyst for supercritical water gasification of diesel oil. Fuel 2022, 312, 122849. [Google Scholar] [CrossRef]
- Papageridis, K.N.; Charisiou, N.D.; Douvartzides, S.L.; Sebastian, V.; Hinder, S.J.; Baker, M.A.; Alkhoori, S.; Polychronopoulou, K.; Goula, M.A. Effect of operating parameters on the selective catalytic deoxygenation of palm oil to produce renewable diesel over Ni supported on Al2O3, ZrO2, and SiO2 catalysts. Fuel Process. Technol. 2020, 209, 106547. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Nizami, A.-S.; Sultana, S.; Zafar, M.; Rehan, M.G.; Srinivasan, G.R.; Ali, A.M.; et al. Biodeisel production from novel non-edible caper (Capparis spinosa L.) seeds oil employing Cu–Ni doped ZrO2 catalyst. Renew. Sustain. Energy Rev. 2021, 138, 110558. [Google Scholar] [CrossRef]









| Catalyst | Acidity (mmol/g) | |||
|---|---|---|---|---|
| 600 °C | 700 °C | 800 °C | 900 °C | |
| ZrO2 | 0.18 | - | - | - |
| SZ-0.2 | 0.39 | 0.32 | 0.29 | 0.26 |
| SZ-0.5 | 0.83 | 0.33 | 0.30 | 0.28 |
| SZ-0.8 | 1.06 | 0.56 | 0.53 | 0.33 |
| Sample | Acidity (mmol/g) |
|---|---|
| ZrO2 | 0.18 |
| SZ | 1.06 |
| Pt1/SZ | 10.75 |
| Pt2/SZ | 11.05 |
| Pt3/SZ | 11.14 |
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| ZrO2 | 22.05 | 0.08 | 3.49 |
| SZ | 12.49 | 0.05 | 3.82 |
| Pt1/SZ | 13.49 | 0.05 | 3.83 |
| Pt2/SZ | 20.23 | 0.06 | 3.84 |
| Pt3/SZ | 29.48 | 0.08 | 3.86 |
| Sample | Crystal Size (nm) |
|---|---|
| ZrO2 | 31.54 |
| SZ | 34.06 |
| Pt1/SZ | 32.80 |
| Pt2/SZ | 31.54 |
| Pt3/SZ | 31.53 |
| Sample | Elemental Compositions (% w/w) | |||
|---|---|---|---|---|
| Zr | Pt | O | S | |
| ZrO2 | 64.08 | - | 35.46 | 0.46 |
| SZ | 60.51 | - | 37.42 | 2.07 |
| Pt1/SZ | 75.24 | 0.35 | 24.02 | 0.74 |
| Pt2/SZ | 74.37 | 0.90 | 25.02 | 0.61 |
| Pt3/SZ | 75.34 | 1.19 | 24.40 | 0.26 |
| Pt 4f Peak | Peak Position (eV) | Relative Area (%) |
|---|---|---|
| Pt0 4f7/2 | 71.45 | 36.36 |
| Pt0 4f5/2 | 74.75 | 45.46 |
| Pt2+4f7/2 | 73.08 | 18.18 |
| Sample | Hydrocracking Product (% w/w) | |||
|---|---|---|---|---|
| Liquid | Solid | Gas | ||
| C5–C12 | C13–C20 | |||
| SZ | 42.95 | 14.97 | 0.35 | 41.73 |
| Pt1/SZ | 48.76 | 21.82 | 0.15 | 29.27 |
| Pt2/SZ | 64.22 | 6.93 | 0.16 | 28.69 |
| Pt3/SZ | 67.51 | 7.09 | 0.15 | 25.25 |
| Sample | Acidity (mmol/g) |
|---|---|
| SZ | 3.81 |
| Cr1/SZ | 6.24 |
| Cr2/SZ | 8.22 |
| Cr3/SZ | 6.75 |
| Sample | Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| ZrO2 | 12.27 | 3.70 | 0.07 |
| SZ | 7.79 | 3.68 | 0.08 |
| Cr1/SZ | 12.62 | 3.70 | 0.12 |
| Cr2/SZ | 14.56 | 3.70 | 0.08 |
| Cr3/SZ | 11.91 | 9.04 | 0.04 |
| Catalyst | Product Conversion (% b/b) | ||
|---|---|---|---|
| Liquid | Coke | Gas | |
| ZrO2 | 17.39 | 0.36 | 32.23 |
| SZ | 28.72 | 0.34 | 29.51 |
| Cr1/SZ | 33.48 | 0.01 | 25.78 |
| Cr2/SZ | 40.99 | 0.01 | 24.84 |
| Cr3/SZ | 37.51 | 0.01 | 26.77 |
| Sample | Acidity | Surface Area (m2/g) | Pore Diameter (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|
| ZrO2 | 0.355 | 5.63 | 30.72 | 0.04 |
| SZ | 2.004 | 4.04 | 35.84 | 0.04 |
| Ni-SZ 1 | 3.905 | 7.09 | 3.82 | 0.06 |
| Ni-SZ 2 | 4.061 | 8.75 | 3.82 | 0.05 |
| Ni-SZ 3 | 4.235 | 11.68 | 3.82 | 0.05 |
| Catalyst | Selectivity (wt%) | |||
|---|---|---|---|---|
| (C1–C4) | (C5–C12) | (C13–C20) | Non-Hydrocarbon | |
| ZrO2 | 1.86 | 77.31 | 19.26 | 1.57 |
| SZ | 1.93 | 67.83 | 28.15 | 2.54 |
| Ni-SZ 1 | 0.00 | 51.59 | 15.16 | 33.25 |
| Ni-SZ 2 | 0.00 | 87.01 | 12.99 | 0.00 |
| Ni-SZ 3 | 0.00 | 100.00 | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekewael, S.J.; Pratika, R.A.; Hauli, L.; Amin, A.K.; Utami, M.; Wijaya, K. Recent Progress on Sulfated Nanozirconia as a Solid Acid Catalyst in the Hydrocracking Reaction. Catalysts 2022, 12, 191. https://doi.org/10.3390/catal12020191
Sekewael SJ, Pratika RA, Hauli L, Amin AK, Utami M, Wijaya K. Recent Progress on Sulfated Nanozirconia as a Solid Acid Catalyst in the Hydrocracking Reaction. Catalysts. 2022; 12(2):191. https://doi.org/10.3390/catal12020191
Chicago/Turabian StyleSekewael, Serly Jolanda, Remi Ayu Pratika, Latifah Hauli, Amalia Kurnia Amin, Maisari Utami, and Karna Wijaya. 2022. "Recent Progress on Sulfated Nanozirconia as a Solid Acid Catalyst in the Hydrocracking Reaction" Catalysts 12, no. 2: 191. https://doi.org/10.3390/catal12020191
APA StyleSekewael, S. J., Pratika, R. A., Hauli, L., Amin, A. K., Utami, M., & Wijaya, K. (2022). Recent Progress on Sulfated Nanozirconia as a Solid Acid Catalyst in the Hydrocracking Reaction. Catalysts, 12(2), 191. https://doi.org/10.3390/catal12020191






