Photocatalytic Degradation of Fluoroquinolone Antibiotics in Solution by Au@ZnO-rGO-gC3N4 Composites
Abstract
1. Introduction
2. Results
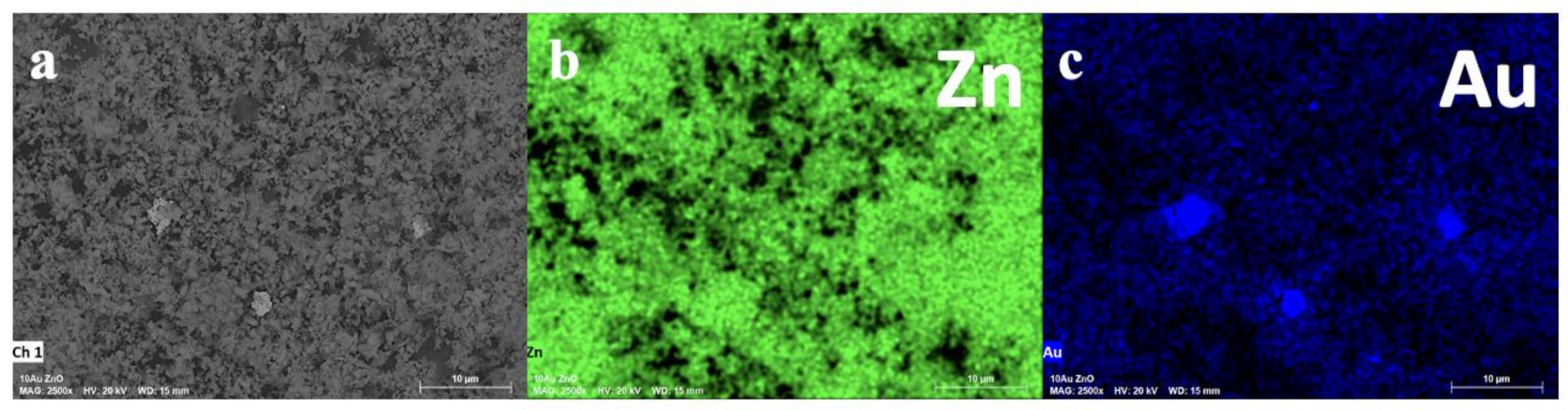
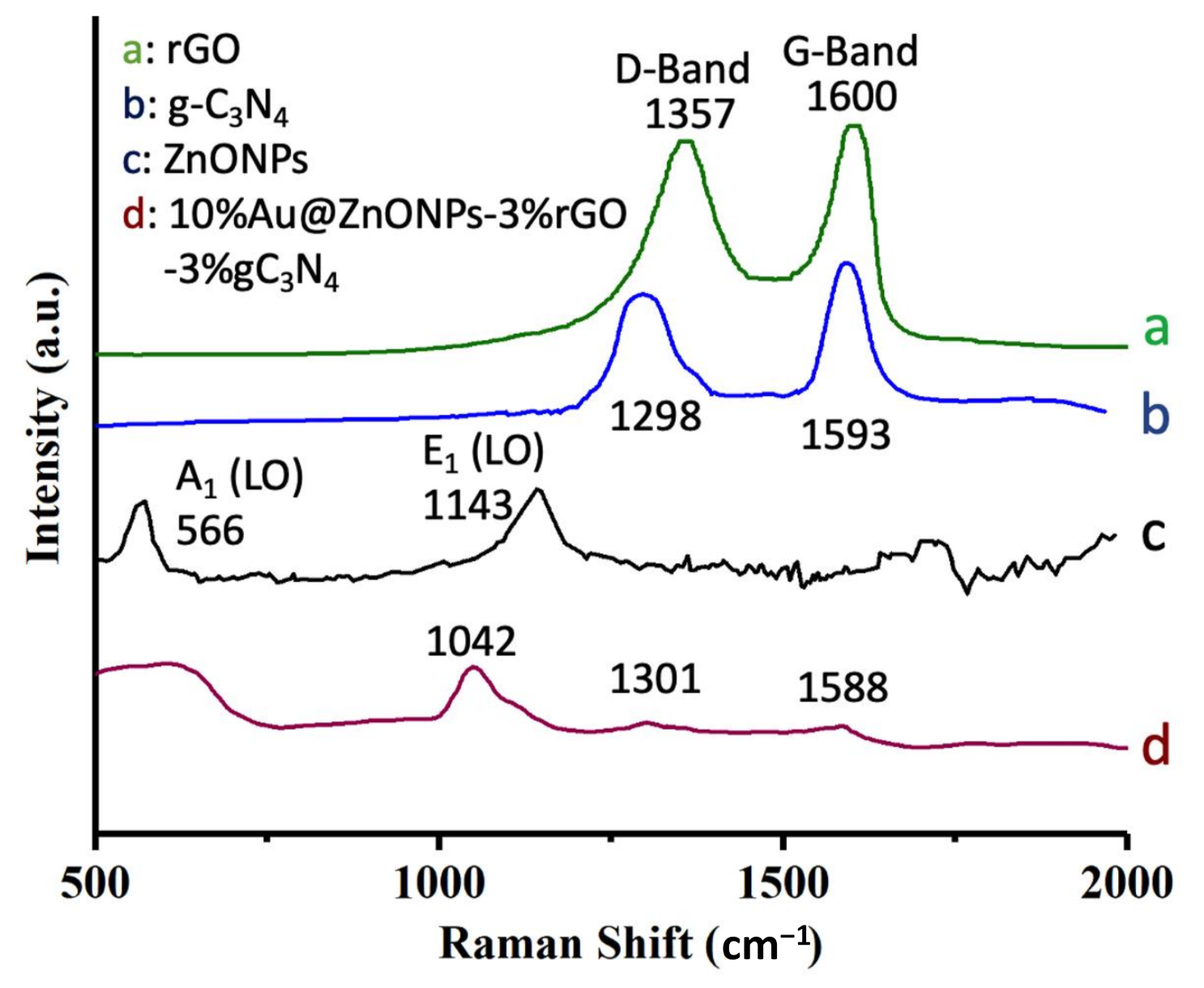
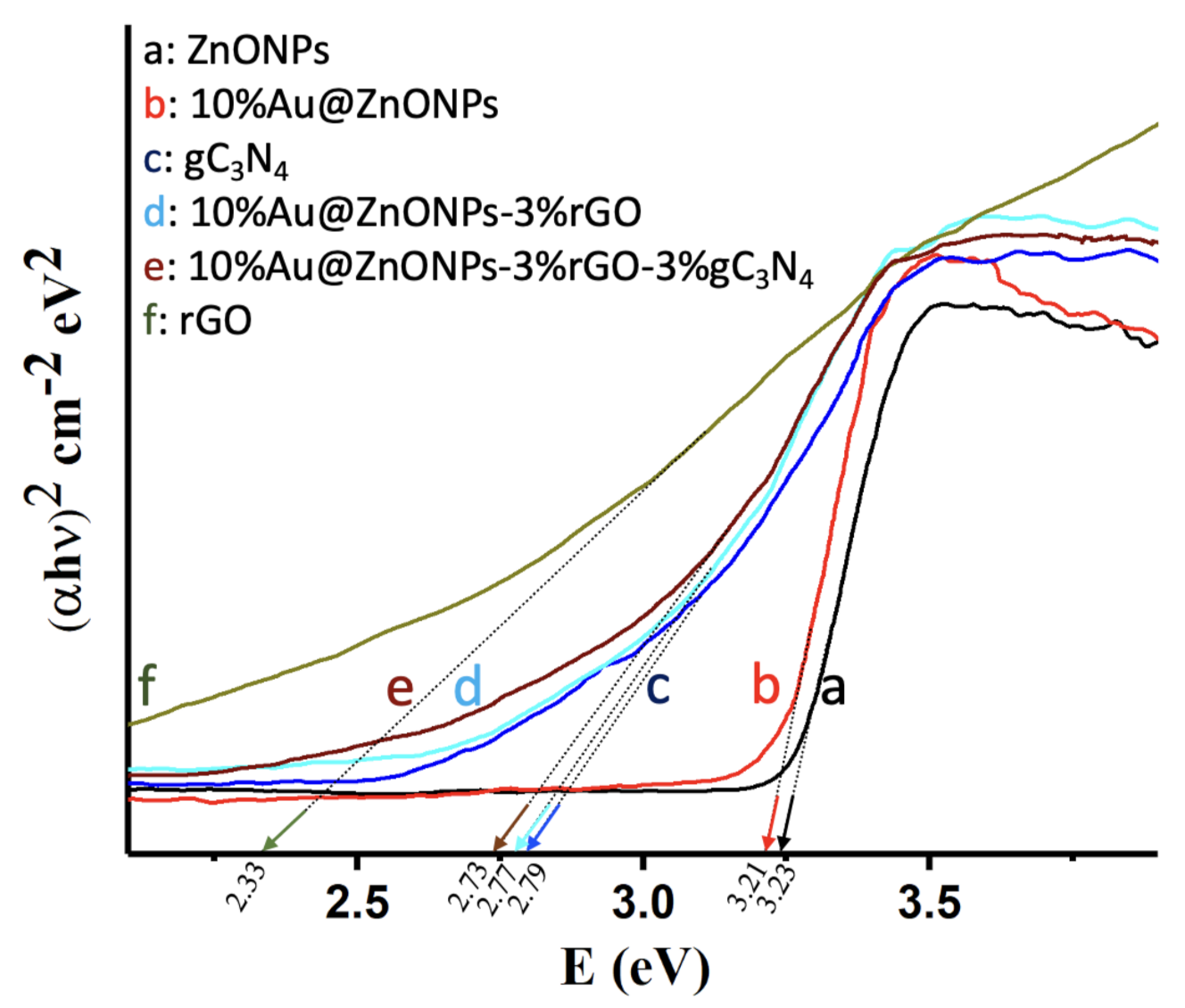
2.1. Characterization of Catalysts
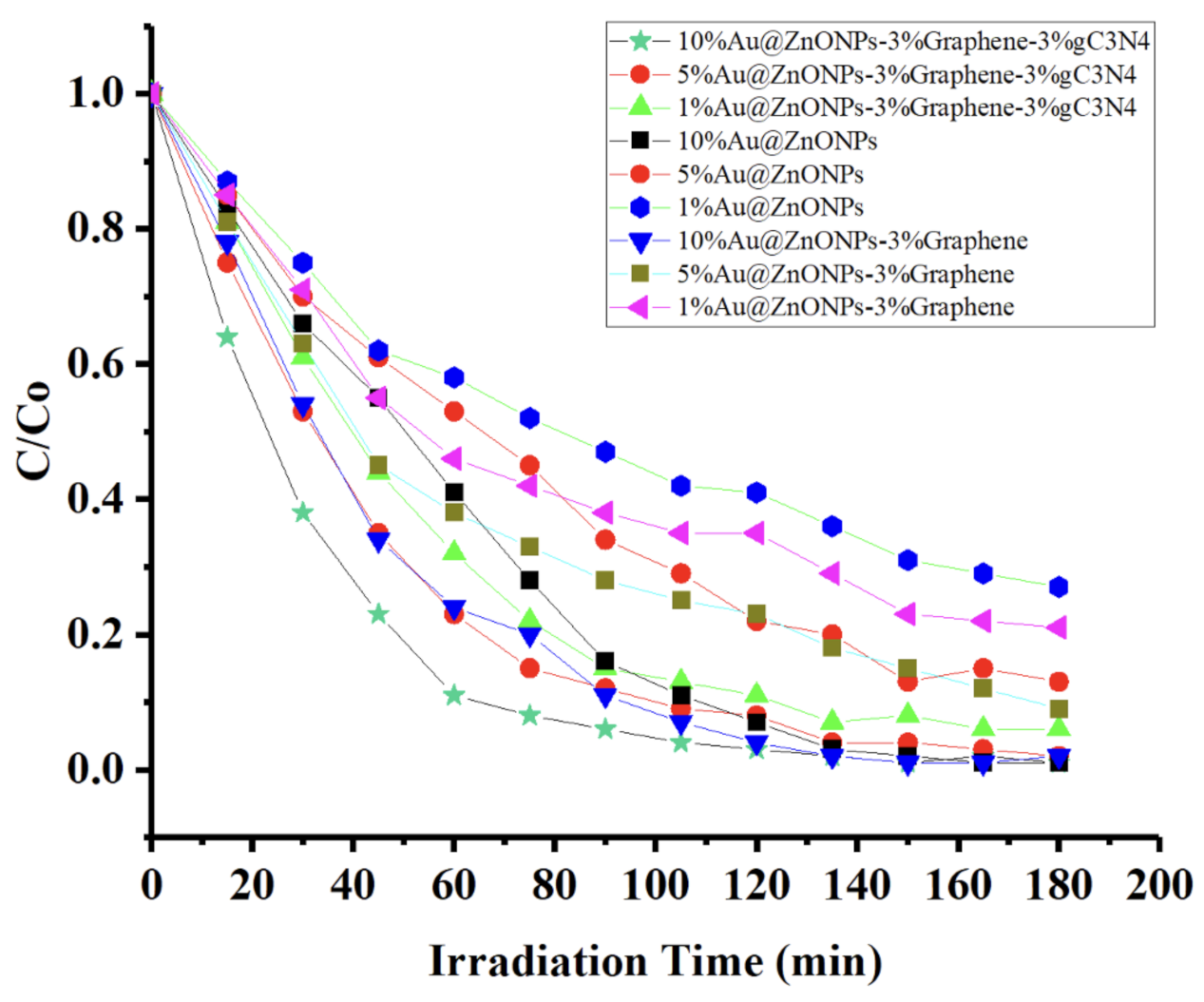
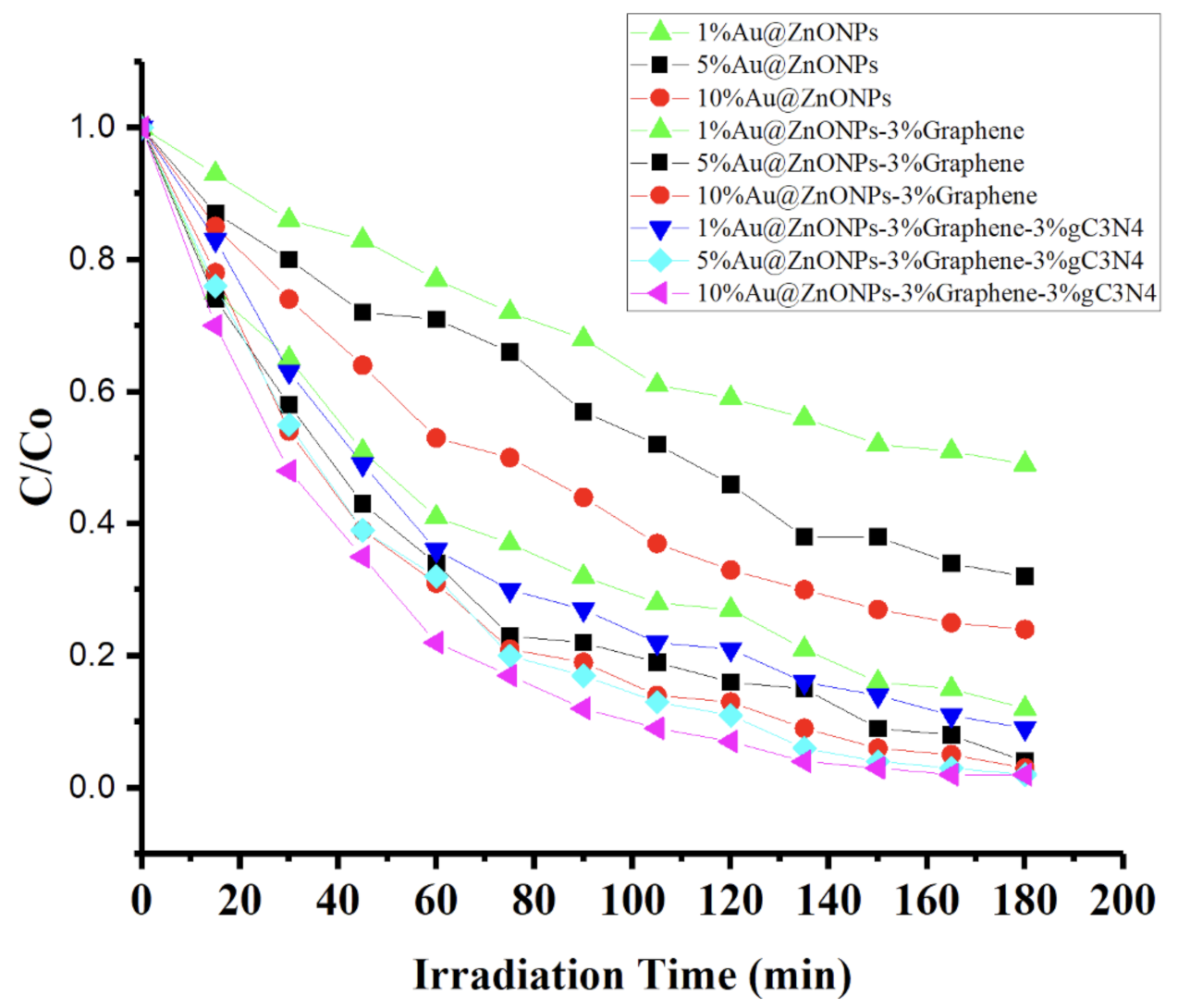
2.2. Photocatalytic Degradation
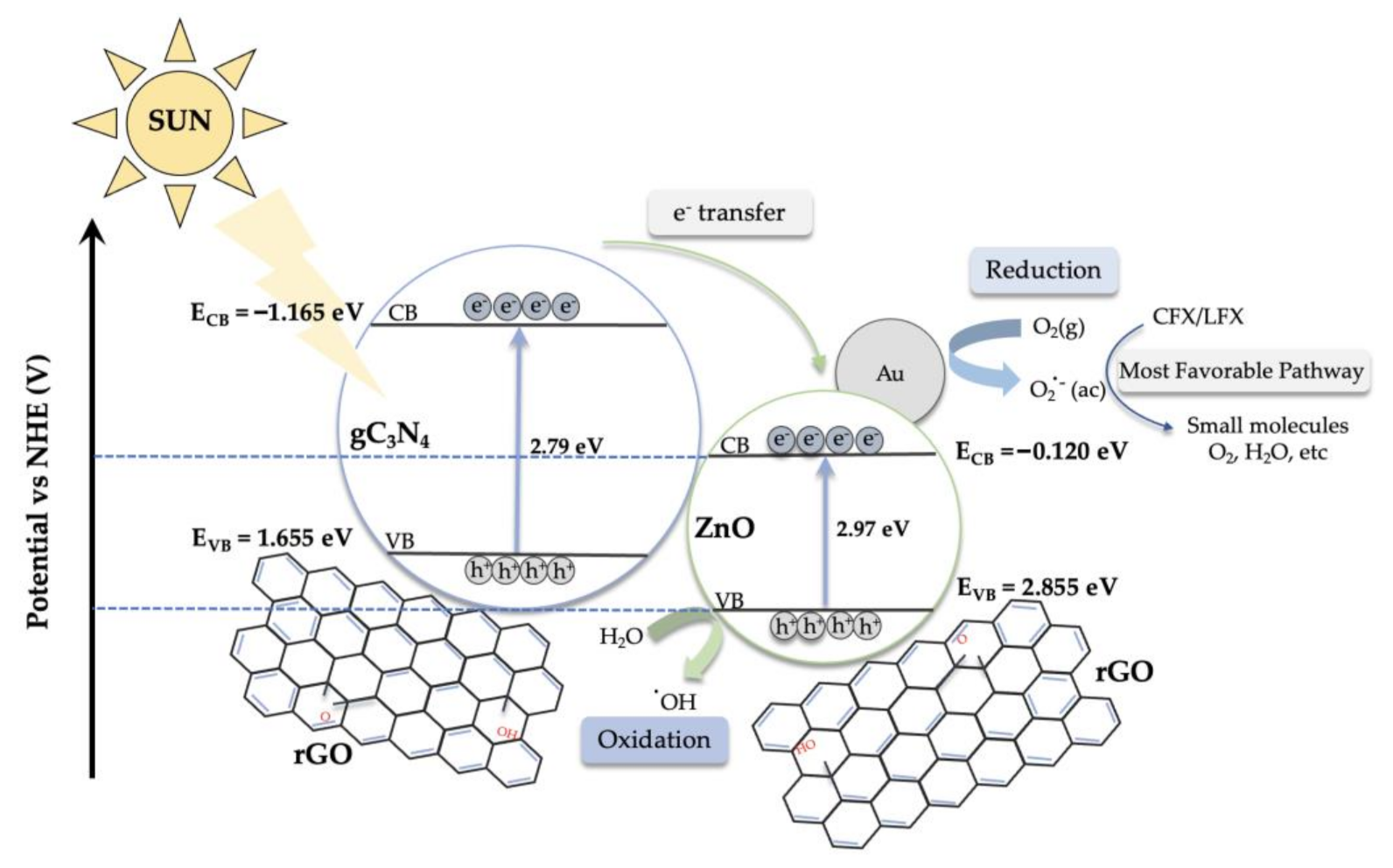
2.3. Proposed Photodegradation Mechanism for CFX and LFX
3. Materials and Methods
3.1. Reagents and Materials
3.2. Synthesis of Nanomaterials
3.3. Characterization of the Catalysts
3.4. Photocatalytic Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.; Svoboda, L.; Němečková, Z.; Sgarzi, M.; Henych, J.; Licciardello, N.; Cuniberti, G. Enhanced Visible-Light Photodegradation of Fluoroquinolone-Based Antibiotics and E. coli Growth Inhibition Using Ag–TiO2 Nanoparticles. RSC Adv. 2021, 11, 13980–13991. [Google Scholar] [CrossRef]
- Rodríguez-López, L.; Cela-Dablanca, R.; Núñez-Delgado, A.; Álvarez-Rodríguez, E.; Fernández-Calviño, D.; Arias-Estévez, M. Photodegradation of Ciprofloxacin, Clarithromycin and Trimethoprim: Influence of PH and Humic Acids. Molecules 2021, 26, 3080. [Google Scholar] [CrossRef] [PubMed]
- El-Maraghy, C.M.; El-Borady, O.M.; El-Naem, O.A. Effective Removal of Levofloxacin from Pharmaceutical Wastewater Using Synthesized Zinc Oxid, Graphen Oxid Nanoparticles Compared with Their Combination. Sci. Rep. 2020, 10, 5914. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.G.J.; de Pedro, C.; Paxeus, N. Effluent from Drug Manufactures Contains Extremely High Levels of Pharmaceuticals. J. Hazard. Mater. 2007, 148, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Rigos, G.; Bitchava, K.; Nengas, I. Antibacterial Drugs in Products Originating from Aquaculture: Assessing the Risks to Public Welfare. Medit. Mar. Sci. 2010, 11, 33. [Google Scholar] [CrossRef][Green Version]
- Hubeny, J.; Harnisz, M.; Korzeniewska, E.; Buta, M.; Zieliński, W.; Rolbiecki, D.; Giebułtowicz, J.; Nałęcz-Jawecki, G.; Płaza, G. Industrialization as a Source of Heavy Metals and Antibiotics Which Can Enhance the Antibiotic Resistance in Wastewater, Sewage Sludge and River Water. PLoS ONE 2021, 16, e0252691. [Google Scholar] [CrossRef]
- Rozman, U.; Duh, D.; Cimerman, M.; Turk, S.Š. Hospital Wastewater Effluent: Hot Spot for Antibiotic Resistant Bacteria. J. Water Sanit. Hyg. Dev. 2020, 10, 171–178. [Google Scholar] [CrossRef]
- Church, N.A.; McKillip, J.L. Antibiotic Resistance Crisis: Challenges and Imperatives. Biologia 2021, 76, 1535–1550. [Google Scholar] [CrossRef]
- Talebi Bezmin Abadi, A.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Sitara, E.; Ehsan, M.F.; Nasir, H.; Iram, S.; Bukhari, S.A.B. Synthesis, Characterization and Photocatalytic Activity of MoS2/ZnSe Heterostructures for the Degradation of Levofloxacin. Catalysts 2020, 10, 1380. [Google Scholar] [CrossRef]
- Dawadi, S.; Thapa, R.; Modi, B.; Bhandari, S.; Timilsina, A.P.; Yadav, R.P.; Aryal, B.; Gautam, S.; Sharma, P.; Thapa, B.B.; et al. Technological Advancements for the Detection of Antibiotics in Food Products. Processes 2021, 9, 1500. [Google Scholar] [CrossRef]
- Hassan, M.; Zhu, G.; Lu, Y.; AL-Falahi, A.H.; Lu, Y.; Huang, S.; Wan, Z. Removal of Antibiotics from Wastewater and Its Problematic Effects on Microbial Communities by Bioelectrochemical Technology: Current Knowledge and Future Perspectives. Environ. Eng. Res. 2020, 26, 16–30. [Google Scholar] [CrossRef]
- Huang, A.; Yan, M.; Lin, J.; Xu, L.; Gong, H.; Gong, H. A Review of Processes for Removing Antibiotics from Breeding Wastewater. Int. J. Environ. Res. Public Health 2021, 18, 4909. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic Residues in Final Effluents of European Wastewater Treatment Plants and Their Impact on the Aquatic Environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Mahdi, M.H.; Mohammed, T.J.; Al-Najar, J.A. Advanced Oxidation Processes (AOPs) for Treatment of Antibiotics in Wastewater: A Review. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012109. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2019, 12, 102. [Google Scholar] [CrossRef]
- Akbari, M.Z.; Xu, Y.; Lu, Z.; Peng, L. Review of Antibiotics Treatment by Advance Oxidation Processes. Environ. Adv. 2021, 5, 100111. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Liu, Y. HZSM-5 Zeolite Supported Boron-Doped TiO2 for Photocatalytic Degradation of Ofloxacin. J. Mater. Res. Technol. 2020, 9, 2557–2567. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic Degradation Mechanisms of Rhodamine B Dye via Radicals Generation by Micro- and Nano-Particles of ZnO. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X.; Tryk, D. TiO2 Photocatalysis and Related Surface Phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Molinari, R.; Lavorato, C.; Argurio, P. Visible-Light Photocatalysts and Their Perspectives for Building Photocatalytic Membrane Reactors for Various Liquid Phase Chemical Conversions. Catalysts 2020, 10, 1334. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, X.; Liu, H.; Liu, C.; Wan, Y.; Long, Y.; Cai, Z. Recent Advances and Applications of Semiconductor Photocatalytic Technology. App. Sci. 2019, 9, 2489. [Google Scholar] [CrossRef]
- Li, H.; Li, Z.; Yu, Y.; Ma, Y.; Yang, W.; Wang, F.; Yin, X.; Wang, X. Surface-Plasmon-Resonance-Enhanced Photoelectrochemical Water Splitting from Au-Nanoparticle-Decorated 3D TiO2 Nanorod Architectures. J. Phys. Chem. C 2017, 121, 12071–12079. [Google Scholar] [CrossRef]
- Kholikov, B.; Hussain, J.; Hayat, S.; Zeng, H. Surface Plasmon Resonance Improved Photocatalytic Activity of Au/TiO2 Nanocomposite under Visible Light for Degradation of Pollutants. J. Chin. Chem. Soc. 2021, 68, 1908–1915. [Google Scholar] [CrossRef]
- Ahmadi, M.; Mistry, H.; Roldan Cuenya, B. Tailoring the Catalytic Properties of Metal Nanoparticles via Support Interactions. J. Phys. Chem. Lett. 2016, 7, 3519–3533. [Google Scholar] [CrossRef]
- Smith, A.T.; LaChance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, Properties, and Applications of Graphene Oxide/Reduced Graphene Oxide and Their Nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Siklitskaya, A.; Gacka, E.; Larowska, D.; Mazurkiewicz-Pawlicka, M.; Malolepszy, A.; Stobiński, L.; Marciniak, B.; Lewandowska-Andrałojć, A.; Kubas, A. Lerf–Klinowski-Type Models of Graphene Oxide and Reduced Graphene Oxide Are Robust in Analyzing Non-Covalent Functionalization with Porphyrins. Sci. Rep. 2021, 11, 7977. [Google Scholar] [CrossRef]
- Fatima, S.; Irfan Ali, S.; Younas, D.; Islam, A.; Akinwande, D.; Rizwan, S. Graphene Nanohybrids for Enhanced Catalytic Activity and Large Surface Area. MRS Commun. 2019, 9, 27–36. [Google Scholar] [CrossRef]
- Yaqoob, A.A.; Mohd Noor, N.H.b.; Serrà, A.; Mohamad Ibrahim, M.N. Advances and Challenges in Developing Efficient Graphene Oxide-Based ZnO Photocatalysts for Dye Photo-Oxidation. Nanomaterials 2020, 10, 932. [Google Scholar] [CrossRef]
- Lu, K.-Q.; Li, Y.-H.; Tang, Z.-R.; Xu, Y.-J. Roles of Graphene Oxide in Heterogeneous Photocatalysis. ACS Mater. Au 2021, 1, 37–54. [Google Scholar] [CrossRef]
- Inagaki, M.; Tsumura, T.; Kinumoto, T.; Toyoda, M. Graphitic Carbon Nitrides (g-C3N4) with Comparative Discussion to Carbon Materials. Carbon 2019, 141, 580–607. [Google Scholar] [CrossRef]
- Vu, M.H.; Nguyen, C.C.; Do, T. Graphitic Carbon Nitride (g-C3N4) Nanosheets as a Multipurpose Material for Detection of Amines and Solar-Driven Hydrogen Production. ChemPhotoChem 2021, 5, 466–475. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y. Mini Review on the Structure and Properties (Photocatalysis), and Preparation Techniques of Graphitic Carbon Nitride Nano-Based Particle, and Its Applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. A Critical Review on Graphitic Carbon Nitride (g-C3N4)-Based Materials: Preparation, Modification and Environmental Application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Mezni, A.; Ben Saber, N.; Ibrahim, M.M.; Shaltout, A.A.; Mersal, G.A.M.; Mostafa, N.Y.; Alharthi, S.; Boukherroub, R.; Altalhi, T. Pt–ZnO/M (M = Fe, Co, Ni or Cu): A New Promising Hybrid-Doped Noble Metal/Semiconductor Photocatalysts. J. Inorg. Organomet. Polym. 2020, 30, 4627–4636. [Google Scholar] [CrossRef]
- Niu, J.; Albero, J.; Atienzar, P.; García, H. Porous Single-Crystal-Based Inorganic Semiconductor Photocatalysts for Energy Production and Environmental Remediation: Preparation, Modification, and Applications. Adv. Funct. Mater. 2020, 30, 1908984. [Google Scholar] [CrossRef]
- Prabavathi, S.L.; Saravanakumar, K.; Park, C.M.; Muthuraj, V. Photocatalytic Degradation of Levofloxacin by a Novel Sm6WO12/g-C3N4 Heterojunction: Performance, Mechanism and Degradation Pathways. Sep. Pur. Technol. 2021, 257, 117985. [Google Scholar] [CrossRef]
- Han, X.-G.; He, H.-Z.; Kuang, Q.; Zhou, X.; Zhang, X.-H.; Xu, T.; Xie, Z.-X.; Zheng, L.-S. Controlling Morphologies and Tuning the Related Properties of Nano/Microstructured ZnO Crystallites. J. Phys. Chem. C 2009, 113, 584–589. [Google Scholar] [CrossRef]
- Ou, H.; Lin, L.; Zheng, Y.; Yang, P.; Fang, Y.; Wang, X. Tri-s-Triazine-Based Crystalline Carbon Nitride Nanosheets for an Improved Hydrogen Evolution. Adv. Mater. 2017, 29, 1700008. [Google Scholar] [CrossRef]
- Xing, W.; Tu, W.; Han, Z.; Hu, Y.; Meng, Q.; Chen, G. Template-Induced High-Crystalline g-C3N4 Nanosheets for Enhanced Photocatalytic H2 Evolution. ACS Energy Lett. 2018, 3, 514–519. [Google Scholar] [CrossRef]
- The International Center for Diffraction Data (ICDD) No. 00-004-0784. Available online: https://www.icdd.com/pdfsearch/ (accessed on 20 December 2021).
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled Graphene Hydrogel via a One-Step Hydrothermal Process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Sumathi, M.; Prakasam, A.; Anbarasan, P.M. High Capable Visible Light Driven Photocatalytic Activity of WO3/g-C3N4 Hetrostructure Catalysts Synthesized by a Novel One Step Microwave Irradiation Route. J. Mater. Sci. Mater. Electron. 2019, 30, 3294–3304. [Google Scholar] [CrossRef]
- Yan, X.; Li, W.; Aberle, A.G.; Venkataraj, S. Investigation of the Thickness Effect on Material and Surface Texturing Properties of Sputtered ZnO:Al Films for Thin-Film Si Solar Cell Applications. Vacuum 2016, 123, 151–159. [Google Scholar] [CrossRef]
- Murmu, P.P.; Kennedy, J.; Ruck, B.J.; Leveneur, J. Structural, Electronic and Magnetic Properties of Er Implanted ZnO Thin Films. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 359, 1–4. [Google Scholar] [CrossRef]
- Naseri, A.; Samadi, M.; Mahmoodi, N.M.; Pourjavadi, A.; Mehdipour, H.; Moshfegh, A.Z. Tuning Composition of Electrospun ZnO/CuO Nanofibers: Toward Controllable and Efficient Solar Photocatalytic Degradation of Organic Pollutants. J. Phys. Chem. C 2017, 121, 3327–3338. [Google Scholar] [CrossRef]
- Qiao, Y.; Li, J.; Li, H.; Fang, H.; Fan, D.; Wang, W. A Label-Free Photoelectrochemical Aptasensor for Bisphenol A Based on Surface Plasmon Resonance of Gold Nanoparticle-Sensitized ZnO Nanopencils. Biosens. Bioelectron. 2016, 86, 315–320. [Google Scholar] [CrossRef]
- Machín, A.; Arango, J.C.; Fontánez, K.; Cotto, M.; Duconge, J.; Soto-Vázquez, L.; Resto, E.; Petrescu, F.I.T.; Morant, C.; Márquez, F. Biomimetic Catalysts Based on Au@ZnO–Graphene Composites for the Generation of Hydrogen by Water Splitting. Biomimetics 2020, 5, 39. [Google Scholar] [CrossRef]
- Briggs, D.; Seah, M. Practical Surface Analysis; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Yue, B.; Li, Q.; Iwai, H.; Kako, T.; Ye, J. Hydrogen Production Using Zinc-Doped Carbon Nitride Catalyst Irradiated with Visible Light. Sci. Technol. Adv. Mater. 2011, 12, 034401. [Google Scholar] [CrossRef]
- Gu, Q.; Gao, Z.; Zhao, H.; Lou, Z.; Liao, Y.; Xue, C. Temperature-Controlled Morphology Evolution of Graphitic Carbon Nitride Nanostructures and Their Photocatalytic Activities under Visible Light. RSC Adv. 2015, 5, 49317–49325. [Google Scholar] [CrossRef]
- Zhu, Y.-P.; Li, M.; Liu, Y.-L.; Ren, T.-Z.; Yuan, Z.-Y. Carbon-Doped ZnO Hybridized Homogeneously with Graphitic Carbon Nitride Nanocomposites for Photocatalysis. J. Phys. Chem. C 2014, 118, 10963–10971. [Google Scholar] [CrossRef]
- Li, N.; Tian, Y.; Zhao, J.; Zhang, J.; Zuo, W.; Kong, L.; Cui, H. Z-Scheme 2D/3D g-C3N4@ZnO with Enhanced Photocatalytic Activity for Cephalexin Oxidation under Solar Light. Chem. Eng. J. 2018, 352, 412–422. [Google Scholar] [CrossRef]
- Zhu, Y.; Xue, J.; Xu, T.; He, G.; Chen, H. Enhanced Photocatalytic Activity of Magnetic Core–Shell Fe3O4@Bi2O3–RGO Heterojunctions for Quinolone Antibiotics Degradation under Visible Light. J. Mater. Sci. Mater. Electron. 2017, 28, 8519–8528. [Google Scholar] [CrossRef]
- Ye, L.; Liu, J.; Gong, C.; Tian, L.; Peng, T.; Zan, L. Two Different Roles of Metallic Ag on Ag/AgX/BiOX (X = Cl, Br) Visible Light Photocatalysts: Surface Plasmon Resonance and Z-Scheme Bridge. ACS Catal. 2012, 2, 1677–1683. [Google Scholar] [CrossRef]
- Jourshabani, M.; Shariatinia, Z.; Badiei, A. Synthesis and Characterization of Novel Sm2O3/S-Doped g-C3N4 Nanocomposites with Enhanced Photocatalytic Activities under Visible Light Irradiation. Appl. Surf. Sci. 2018, 427, 375–387. [Google Scholar] [CrossRef]
- Prabavathi, S.L.; Saravanakumar, K.; Nkambule, T.T.I.; Muthuraj, V.; Mamba, G. Enhanced Photoactivity of Cerium Tungstate-Modified Graphitic Carbon Nitride Heterojunction Photocatalyst for the Photodegradation of Moxifloxacin. J. Mater. Sci. Mater. Electron. 2020, 31, 11434–11447. [Google Scholar] [CrossRef]
- Cao, J.; Li, X.; Lin, H.; Chen, S.; Fu, X. In Situ Preparation of Novel p–n Junction Photocatalyst BiOI/(BiO)2CO3 with Enhanced Visible Light Photocatalytic Activity. J. Hazard. Mater. 2012, 239–240, 316–324. [Google Scholar] [CrossRef]
- Nethercot, A.H. Prediction of Fermi Energies and Photoelectric Thresholds Based on Electronegativity Concepts. Phys. Rev. Lett. 1974, 33, 1088–1091. [Google Scholar] [CrossRef]
- Chen, C.; Bi, W.; Xia, Z.; Yuan, W.; Li, L. Hydrothermal Synthesis of the CuWO4/ZnO Composites with Enhanced Photocatalytic Performance. ACS Omega 2020, 5, 13185–13195. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. The Photocatalytic Degradation of Sodium Diclofenac in Different Water Matrices Using g-C3N4 Nanosheets: A Study of the Intermediate by-Products and Mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]
- Raizada, P.; Sudhaik, A.; Singh, P. Photocatalytic Water Decontamination Using Graphene and ZnO Coupled Photocatalysts: A Review. Mater. Sci. Energy Technol. 2019, 2, 509–525. [Google Scholar] [CrossRef]
- Gomes Silva, C.; Juárez, R.; Marino, T.; Molinari, R.; García, H. Influence of Excitation Wavelength (UV or Visible Light) on the Photocatalytic Activity of Titania Containing Gold Nanoparticles for the Generation of Hydrogen or Oxygen from Water. J. Am. Chem. Soc. 2011, 133, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Cao, W.-R.; Su, Y.; Chen, Z.; Wang, Y. Bismuth Oxyiodide–Graphene Nanocomposites with High Visible Light Photocatalytic Activity. J. Col. Interf. Sci. 2013, 398, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Mo, Z.; She, X.; Li, Y.; Liu, L.; Huang, L.; Chen, Z.; Zhang, Q.; Xu, H.; Li, H. Synthesis of g-C3N4 at Different Temperatures for Superior Visible/UV Photocatalytic Performance and Photoelectrochemical Sensing of MB Solution. RSC Adv. 2015, 5, 101552–101562. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Şimşek, E.B. Doping of boron in TiO2 catalyst: Enhanced photocatalytic degradation of antibiotic under visible light irradiation. J. Boron 2017, 2, 18–27. [Google Scholar]
- Thiruppathi, M.; Kumar, J.V.; Vahini, M.; Ramalingan, C.; Nagarajan, E. A study on divergent functional properties of sphere-like CuWO4 anchored on 2D graphene oxide sheets towards the photocatalysis of ciprofloxacin and electrocatalysis of methanol. J. Mater. Sci. Mater. Electron. 2019, 30, 10172–10182. [Google Scholar] [CrossRef]



| Material | BET Area (m2/g) |
|---|---|
| ZnONPs | 24 |
| 1%Au@ZnONPs | 33 |
| 5%Au@ZnONPs | 49 |
| 10%Au@ZnONPs | 78 |
| 1%Au@ZnONPs-3%rGO | 126 |
| 5%Au@ZnONPs-3%rGO | 161 |
| 10%Au@ZnONPs-3%rGO | 196 |
| 1%Au@ZnONPs-3%rGO-3%gC3N4 | 143 |
| 5%Au@ZnONPs-3%rGO-3%gC3N4 | 177 |
| 10%Au@ZnONPs-3%rGO-3%gC3N4 | 229 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machín, A.; Fontánez, K.; Duconge, J.; Cotto, M.C.; Petrescu, F.I.; Morant, C.; Márquez, F. Photocatalytic Degradation of Fluoroquinolone Antibiotics in Solution by Au@ZnO-rGO-gC3N4 Composites. Catalysts 2022, 12, 166. https://doi.org/10.3390/catal12020166
Machín A, Fontánez K, Duconge J, Cotto MC, Petrescu FI, Morant C, Márquez F. Photocatalytic Degradation of Fluoroquinolone Antibiotics in Solution by Au@ZnO-rGO-gC3N4 Composites. Catalysts. 2022; 12(2):166. https://doi.org/10.3390/catal12020166
Chicago/Turabian StyleMachín, Abniel, Kenneth Fontánez, José Duconge, María C. Cotto, Florian I. Petrescu, Carmen Morant, and Francisco Márquez. 2022. "Photocatalytic Degradation of Fluoroquinolone Antibiotics in Solution by Au@ZnO-rGO-gC3N4 Composites" Catalysts 12, no. 2: 166. https://doi.org/10.3390/catal12020166
APA StyleMachín, A., Fontánez, K., Duconge, J., Cotto, M. C., Petrescu, F. I., Morant, C., & Márquez, F. (2022). Photocatalytic Degradation of Fluoroquinolone Antibiotics in Solution by Au@ZnO-rGO-gC3N4 Composites. Catalysts, 12(2), 166. https://doi.org/10.3390/catal12020166










