Catalytic Enantioselective Diels Alder Reaction: Application in the Synthesis of Antiviral Agents †
Abstract
1. Introduction
2. Discussion
2.1. Asymmetric DAR
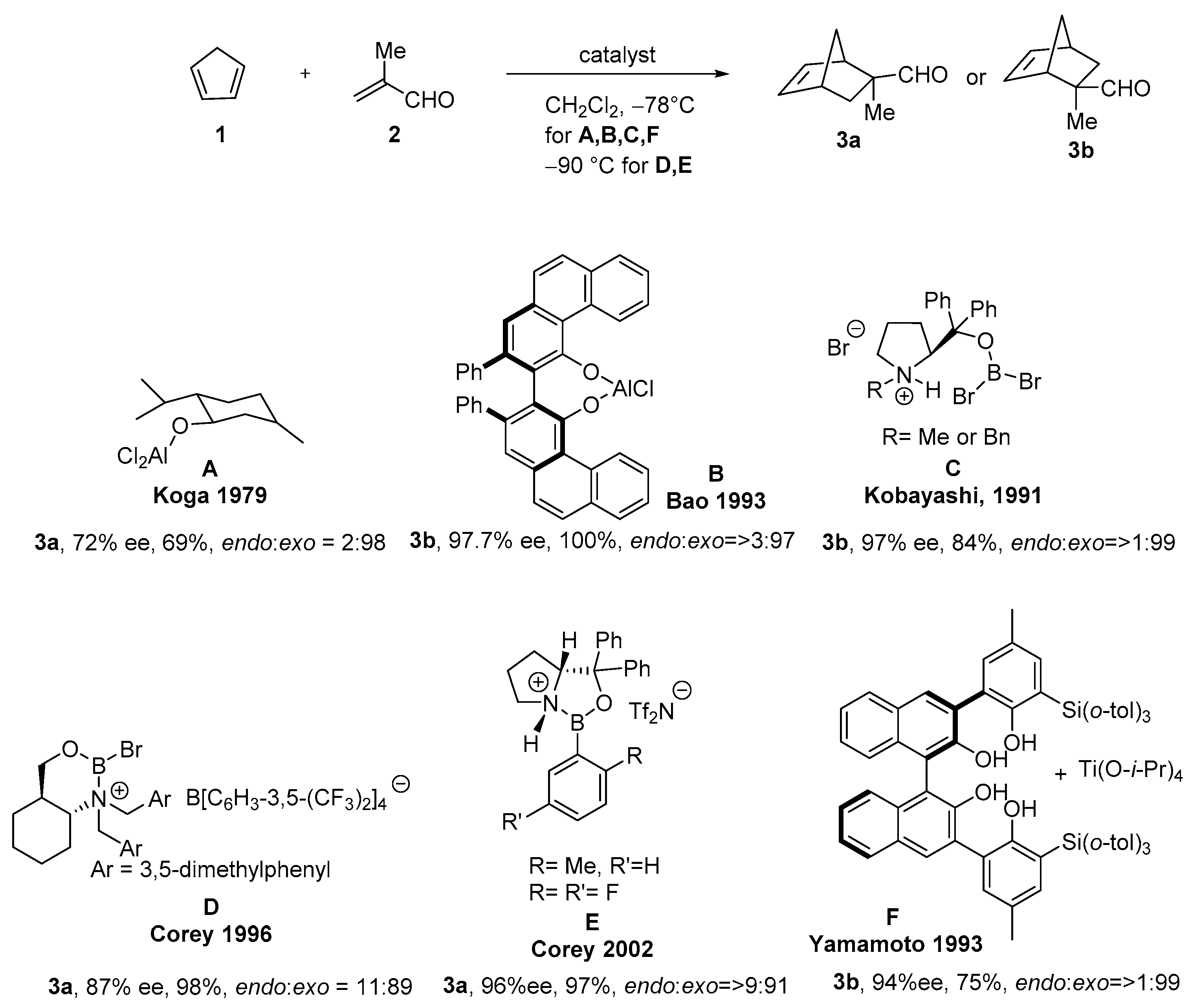
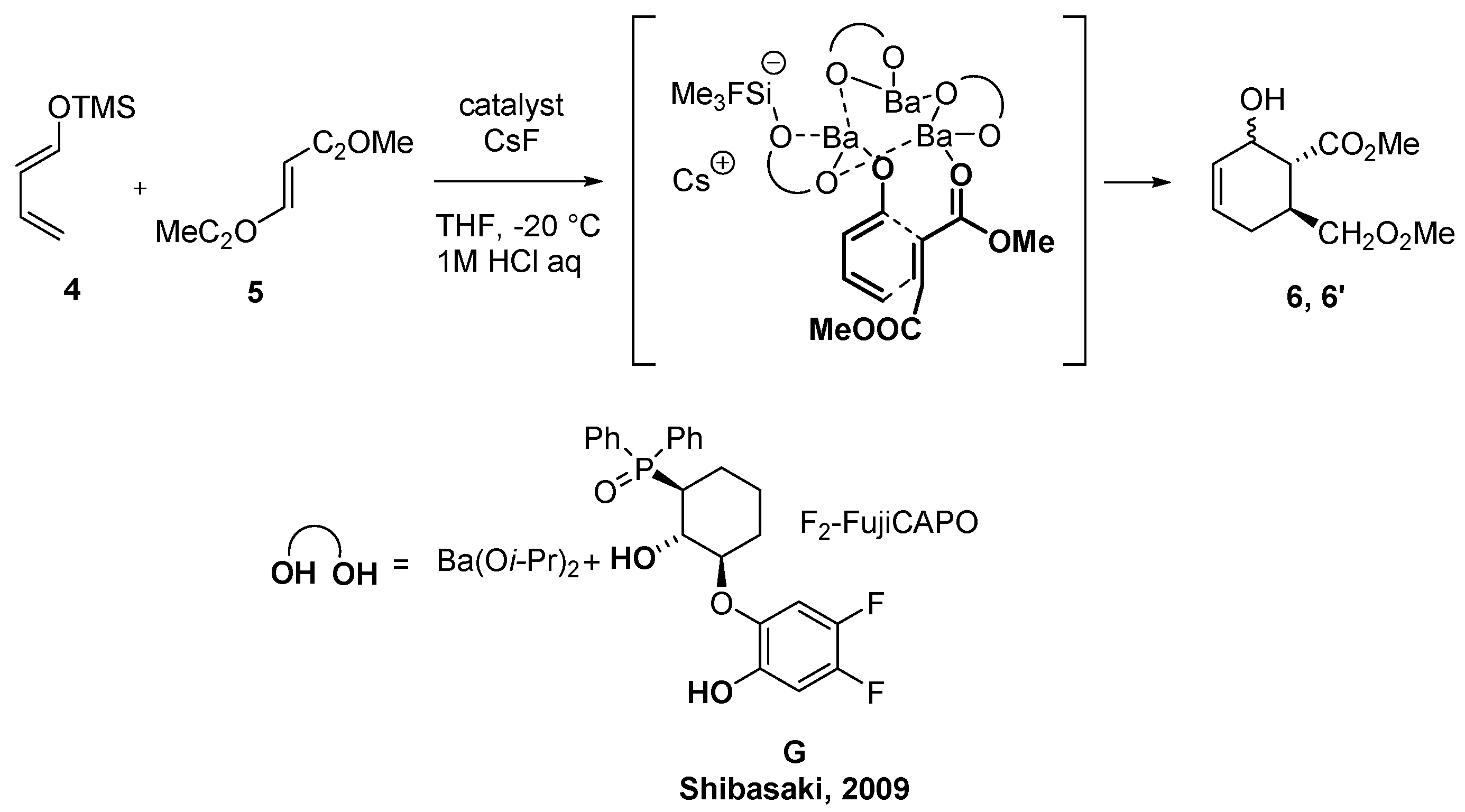
2.2. Asymmetric DAR (Unsaturated Aldehydes or Ester as Dienophiles)
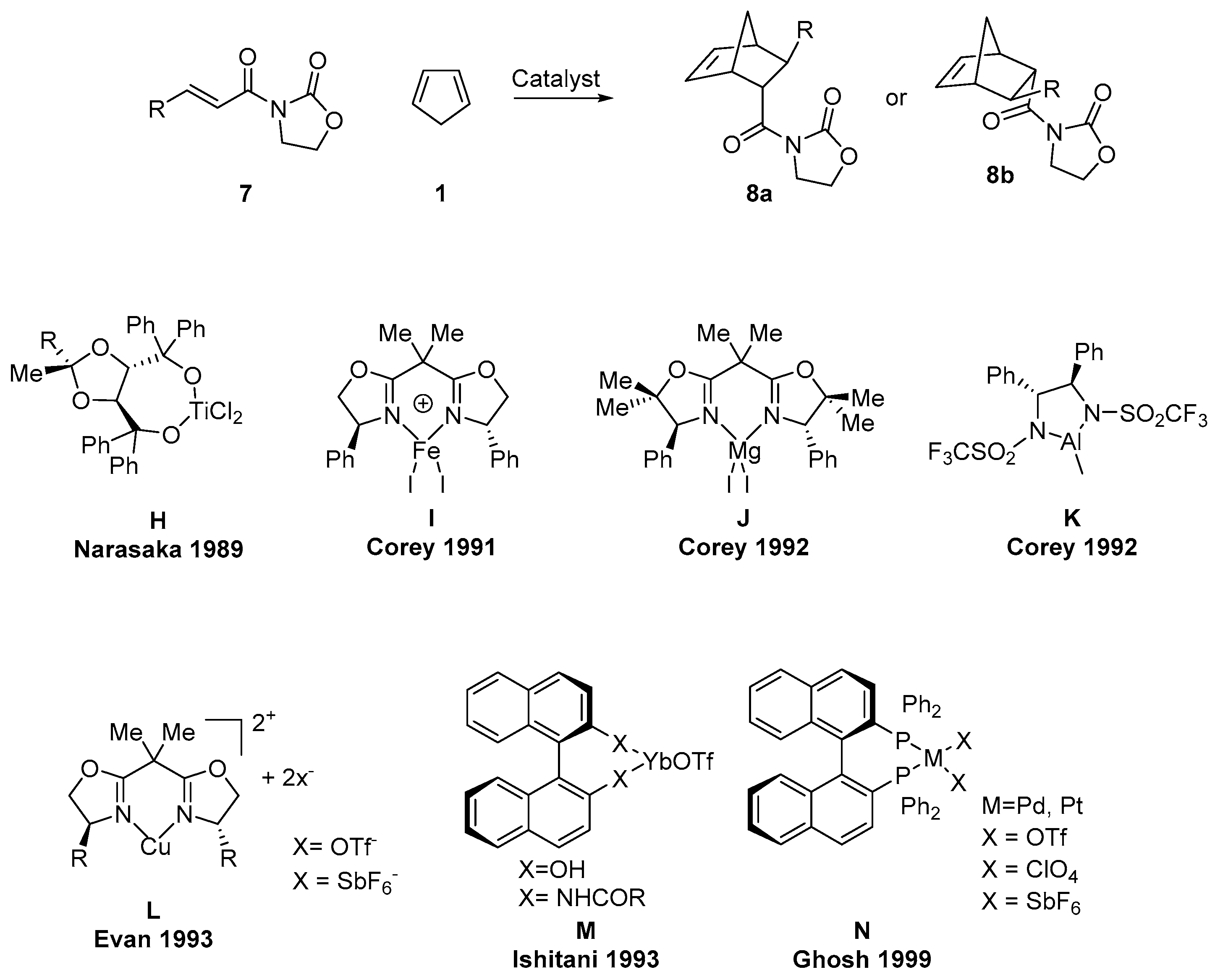
2.3. Asymmetric DAR (3-Alkenoyl-1,3-Oxazolidin-2-Ones as Diene)
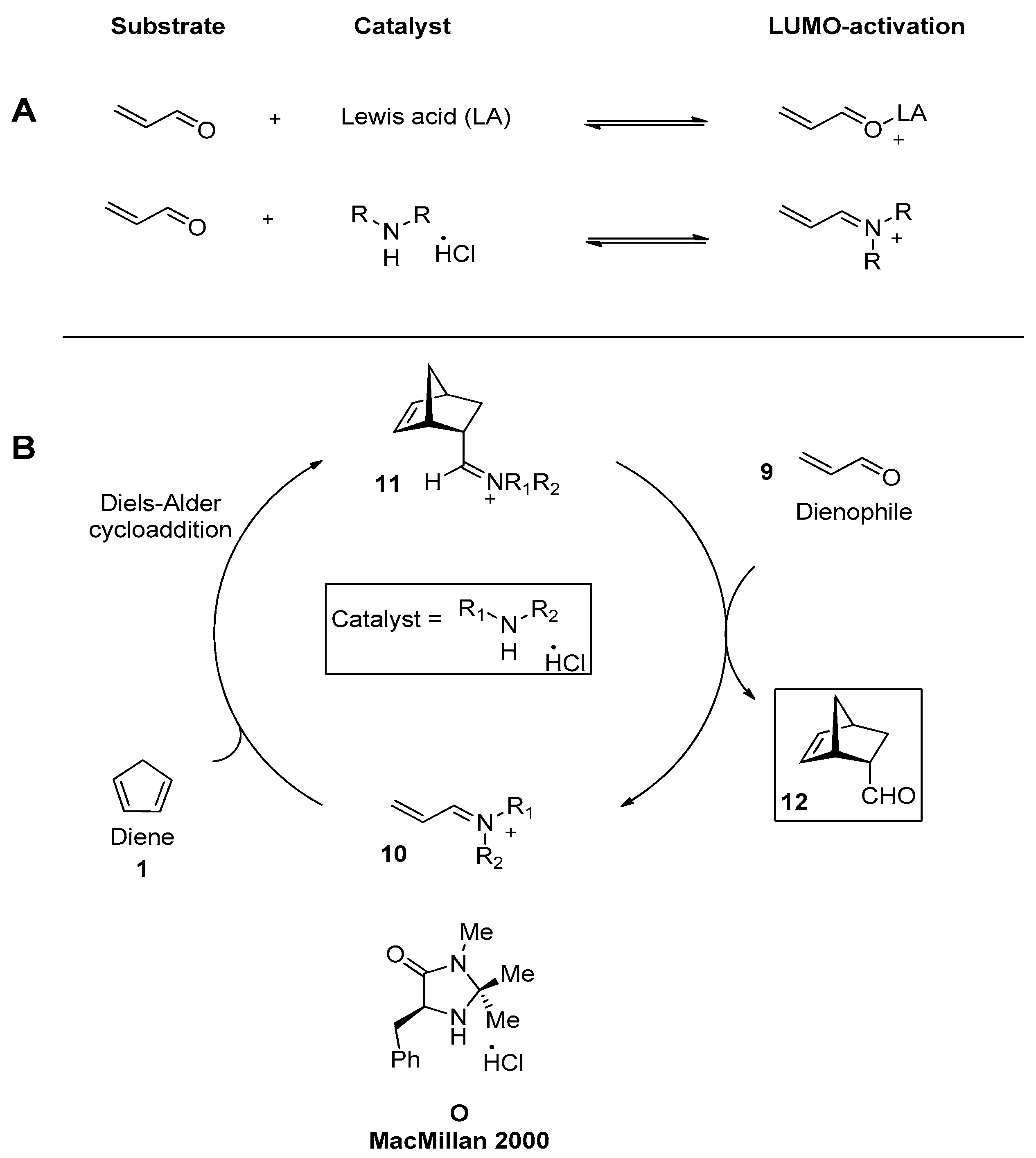
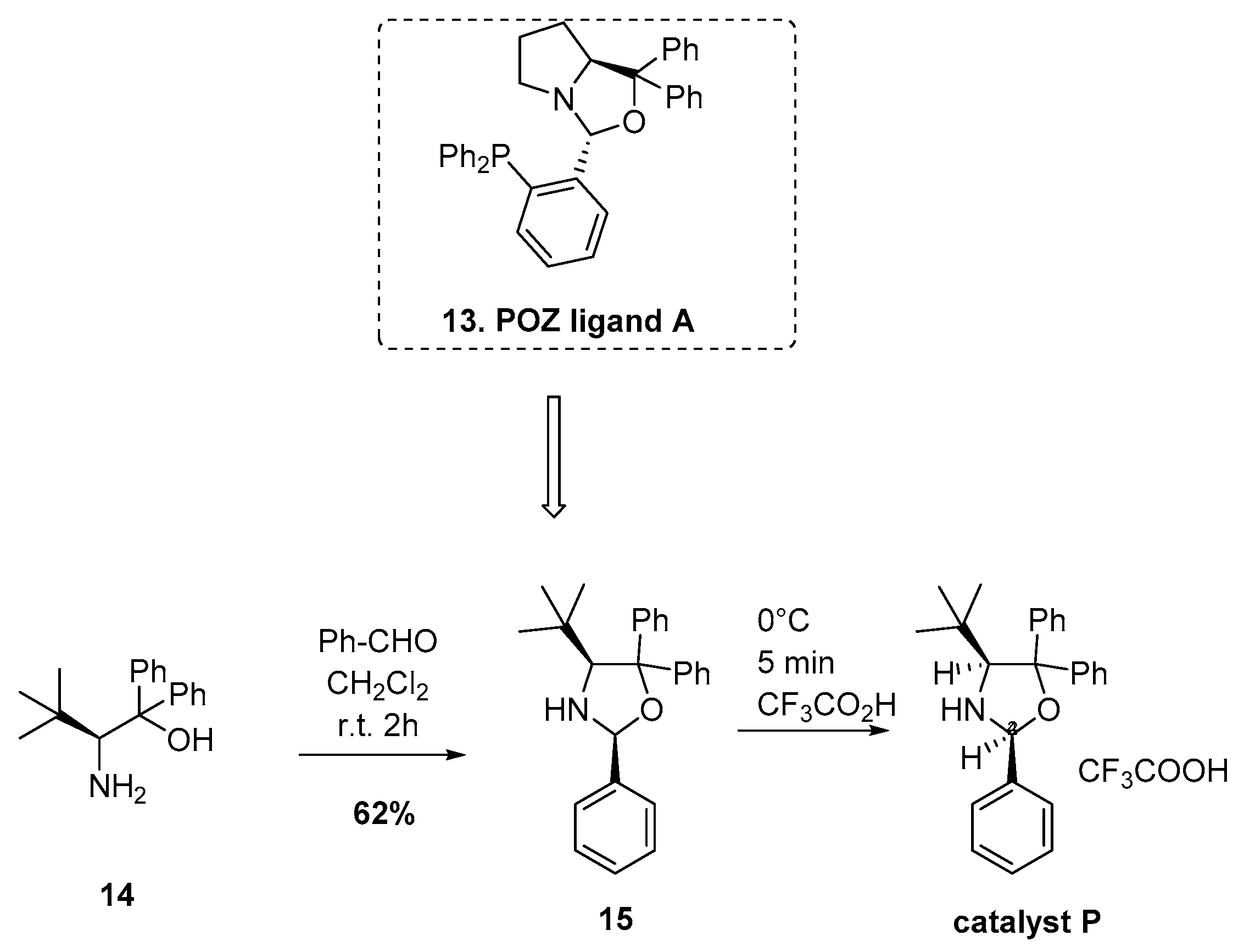
2.4. Base Catalyzed Asymmetric DAR (Organocatalysis)
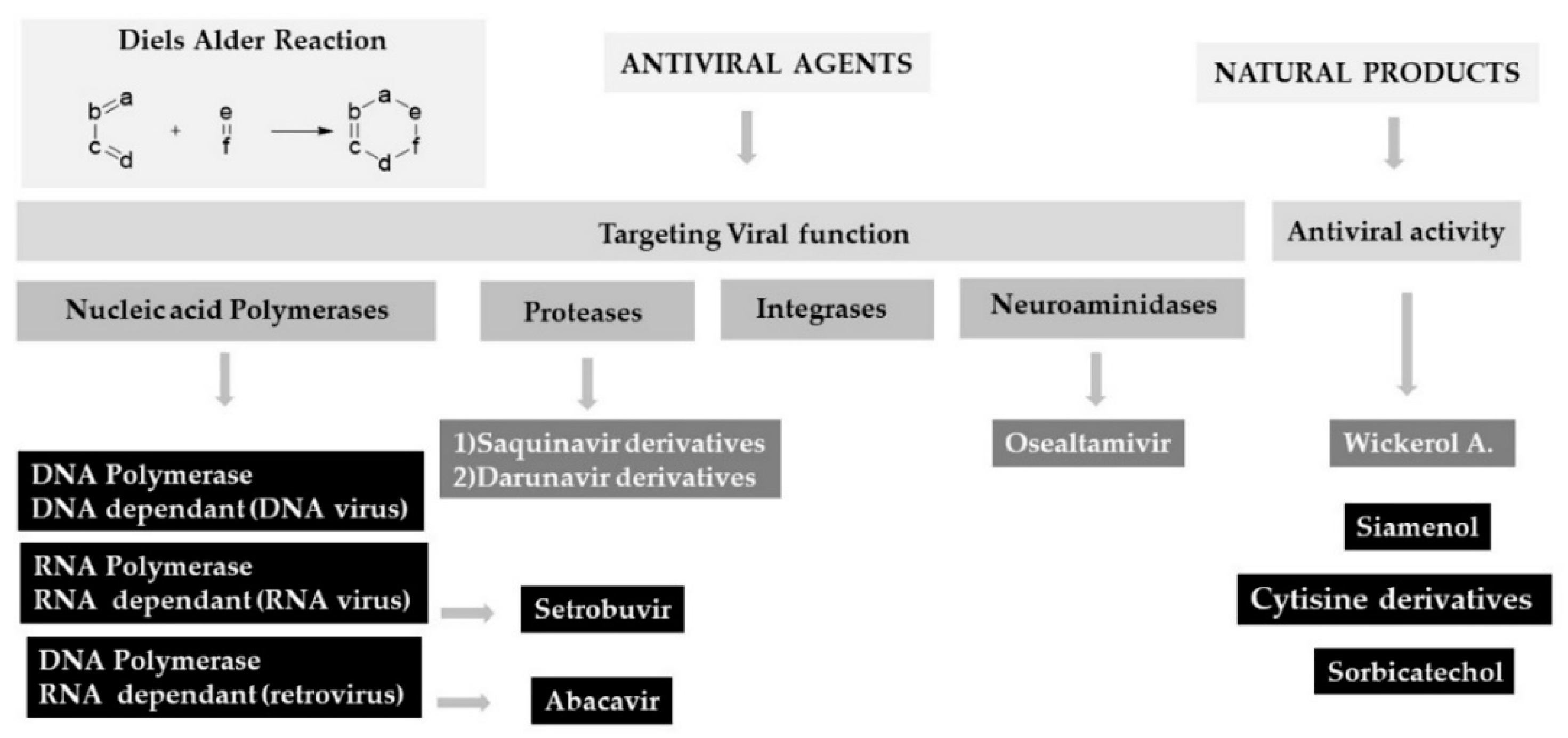
2.5. Chiral DAR Applied to the Antiviral Agents
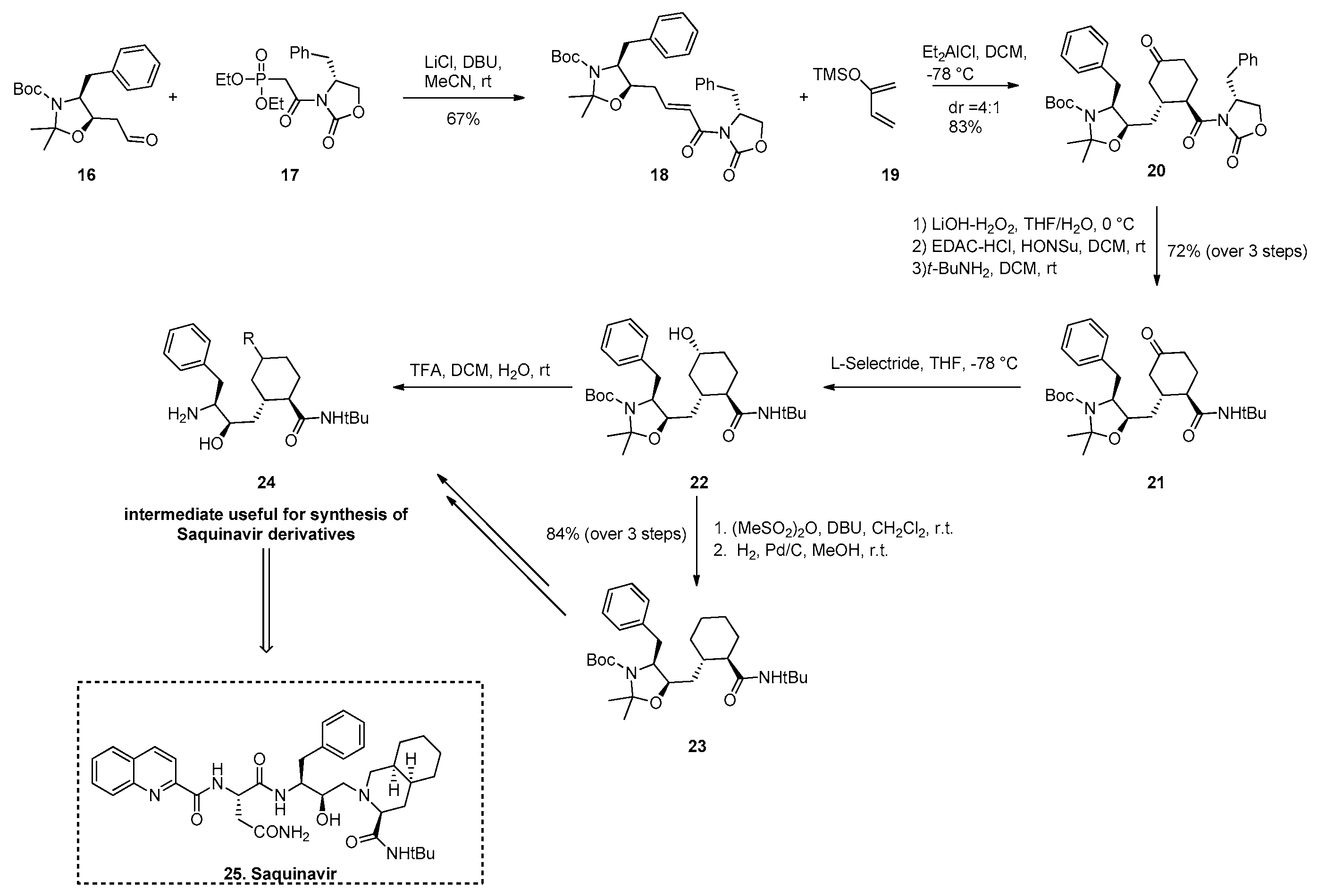
2.5.1. Synthesis of Saquinavir Derivatives
2.5.2. Darunavir Derivatives
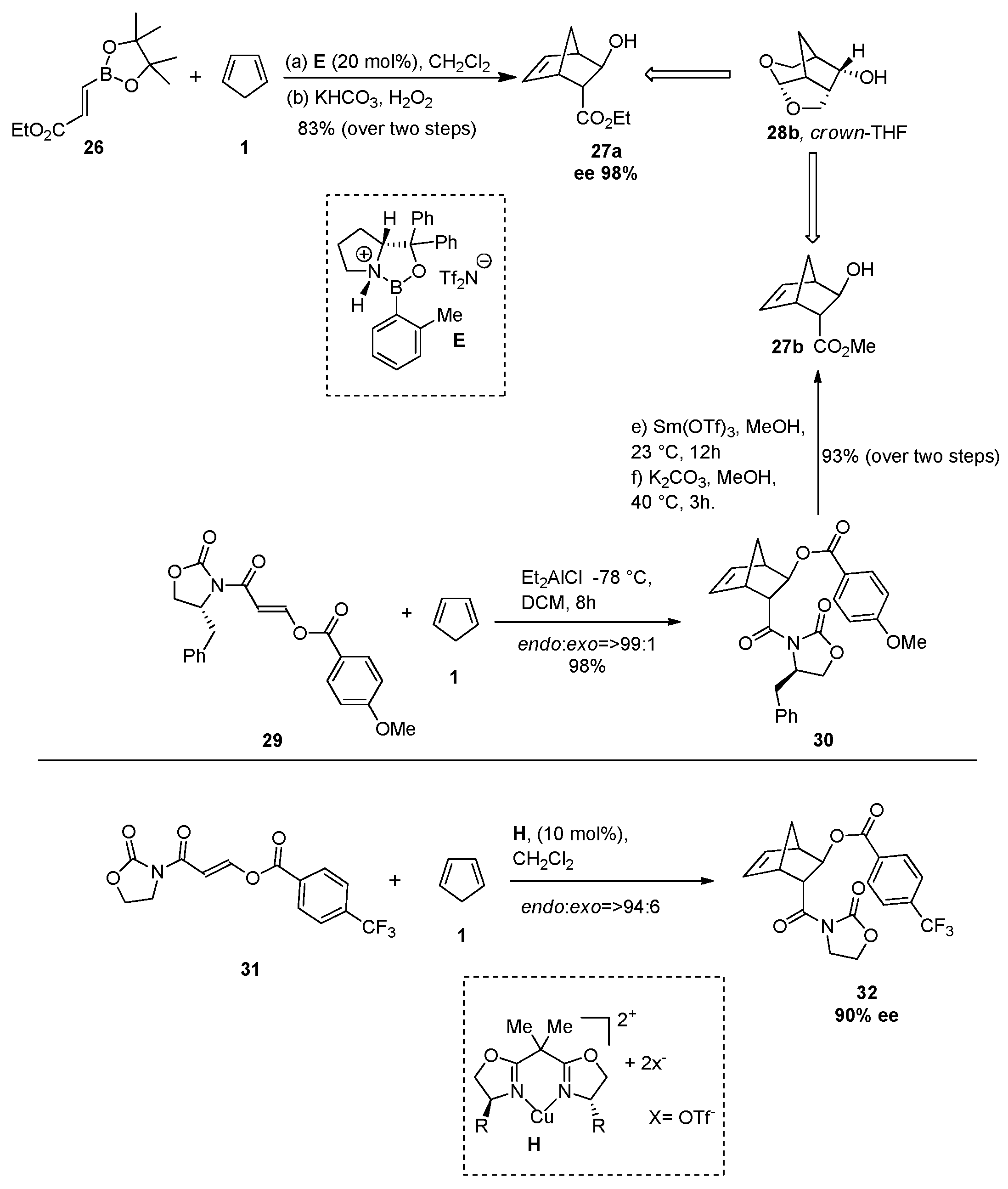
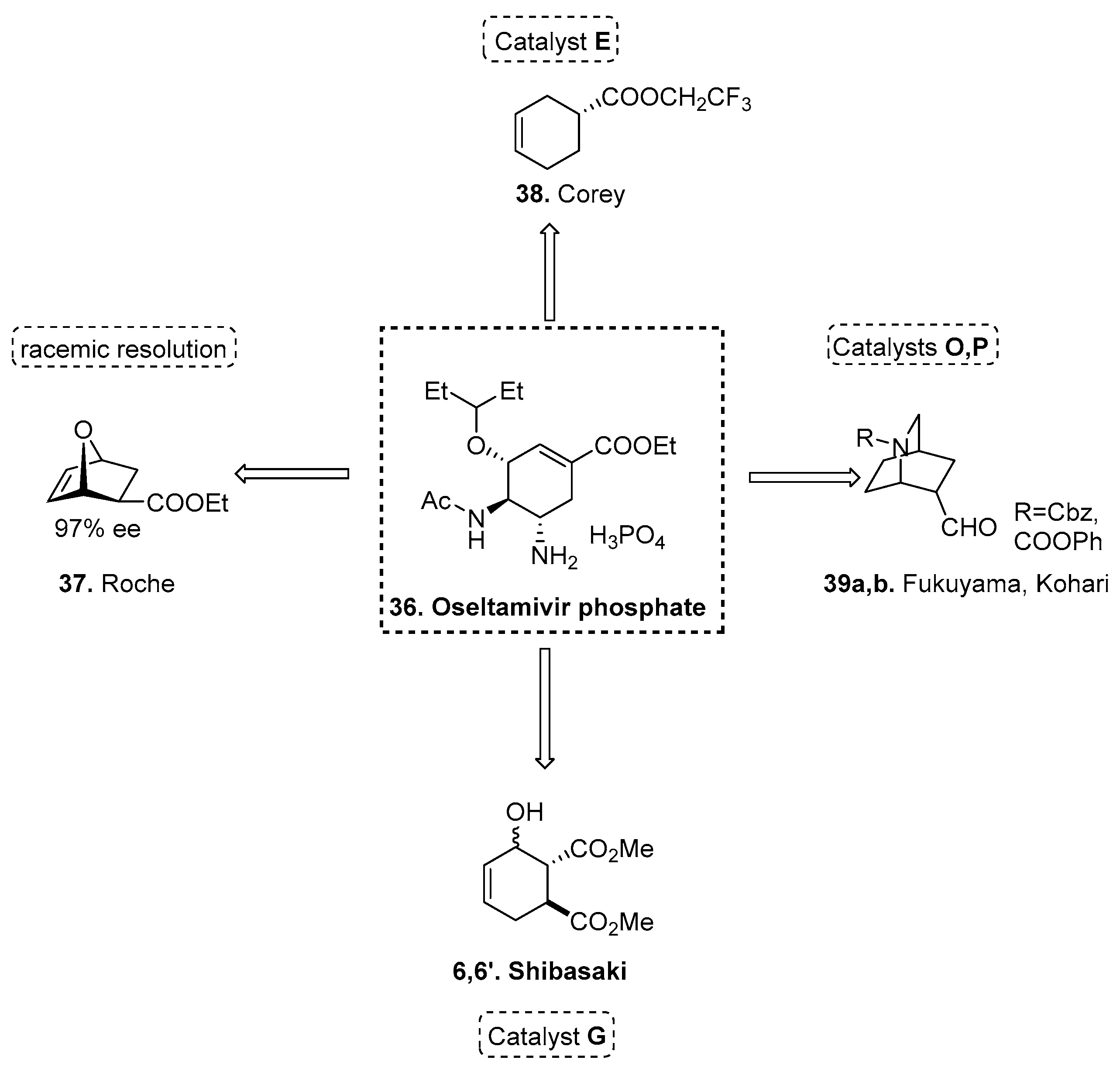
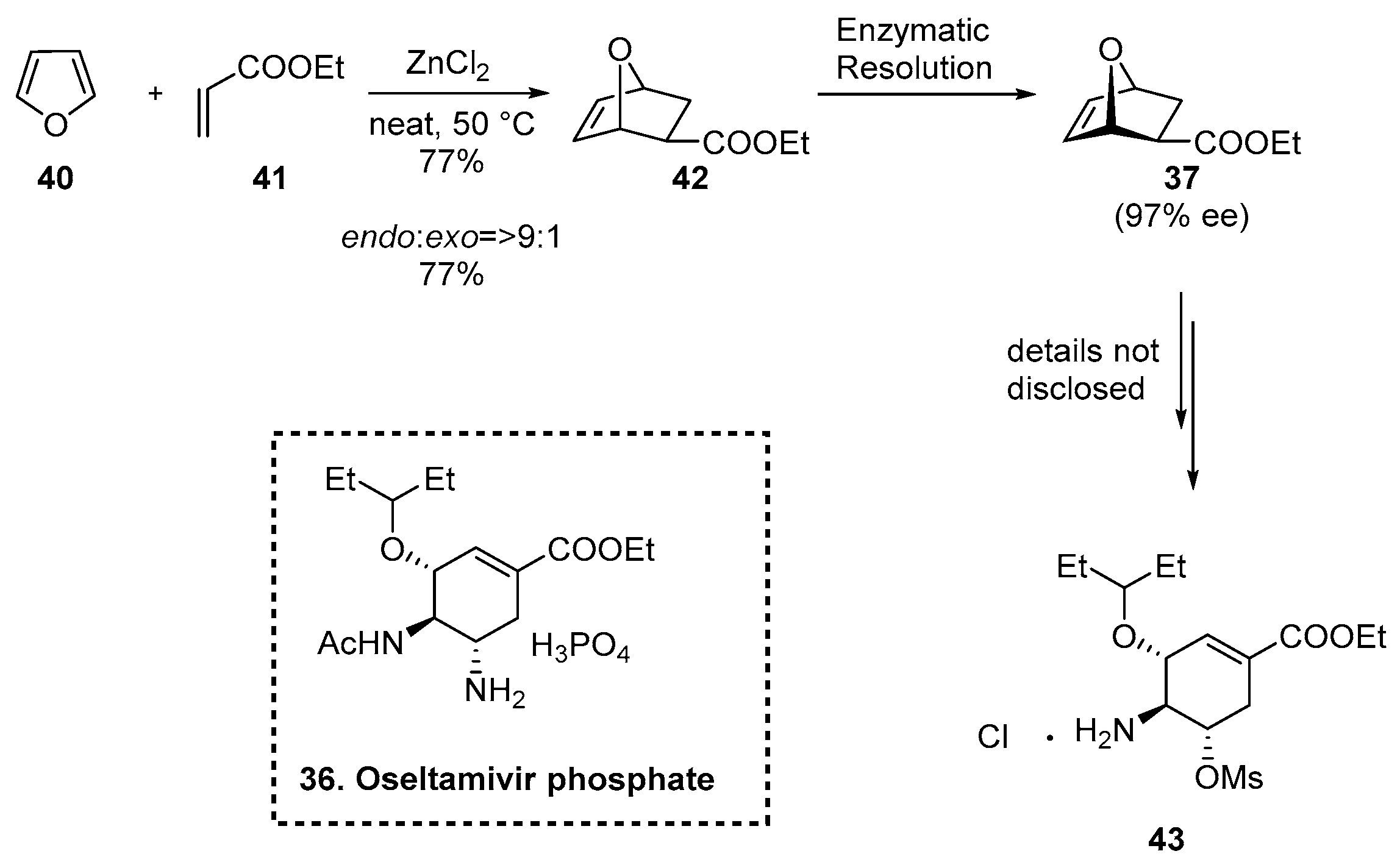
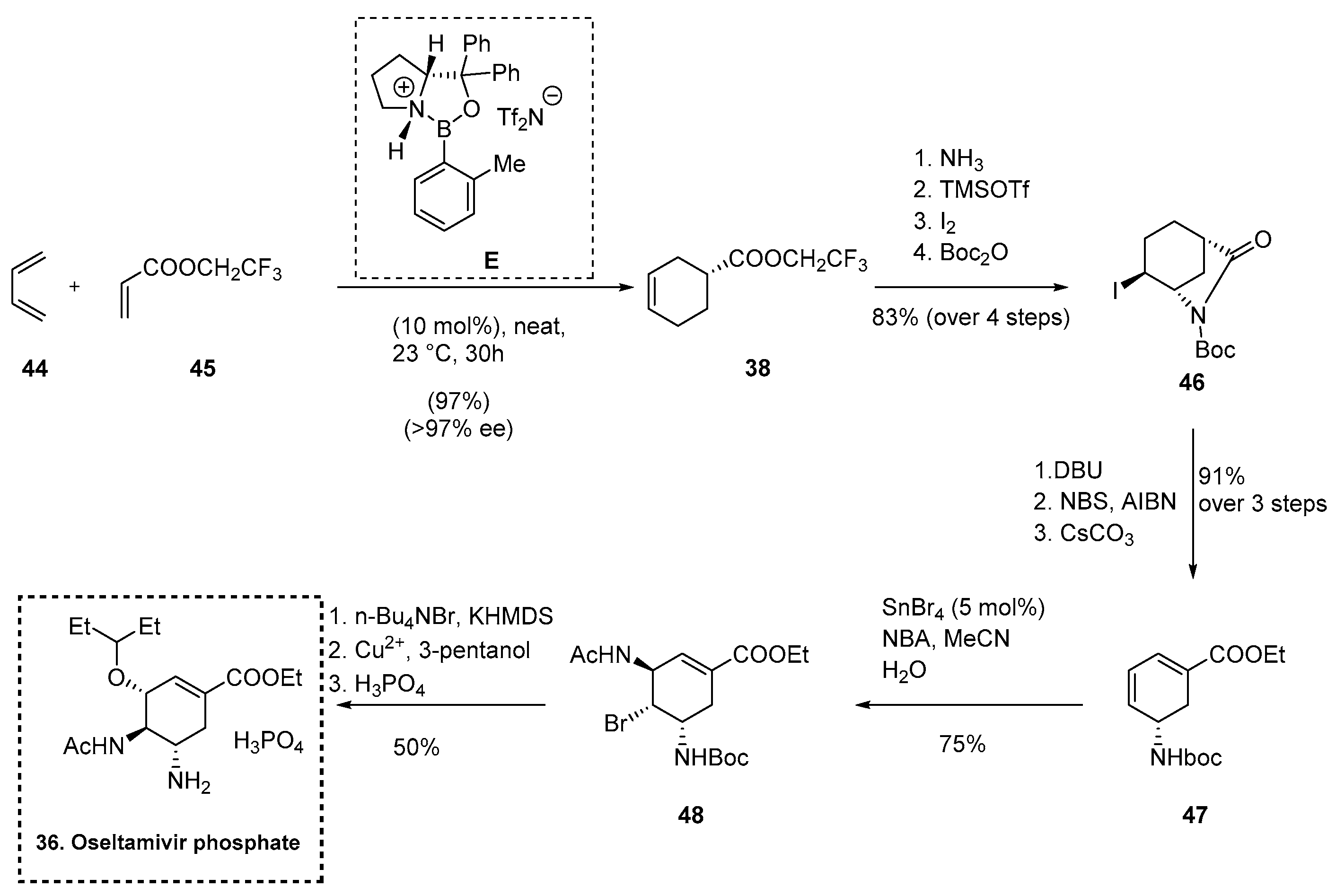
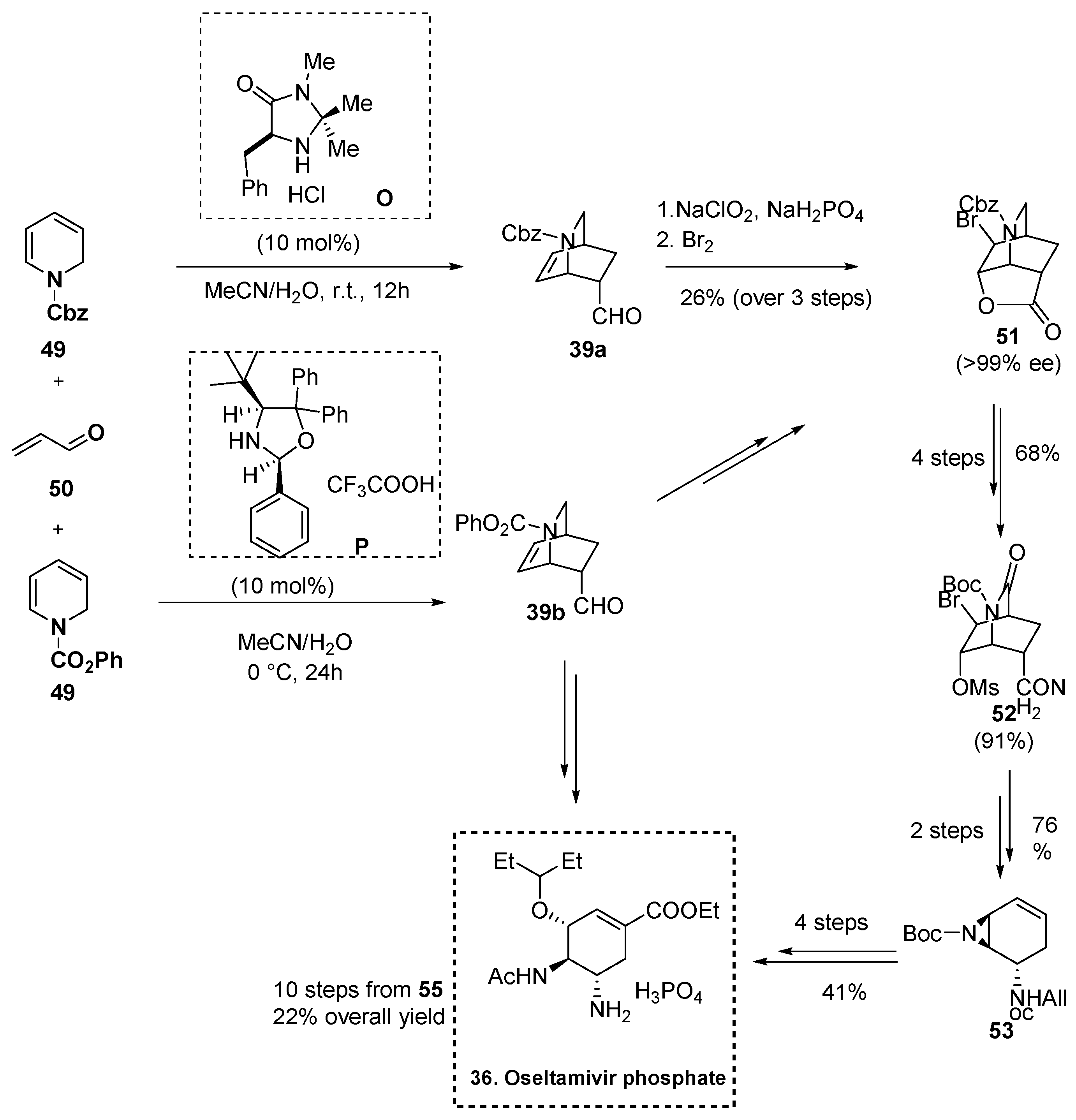
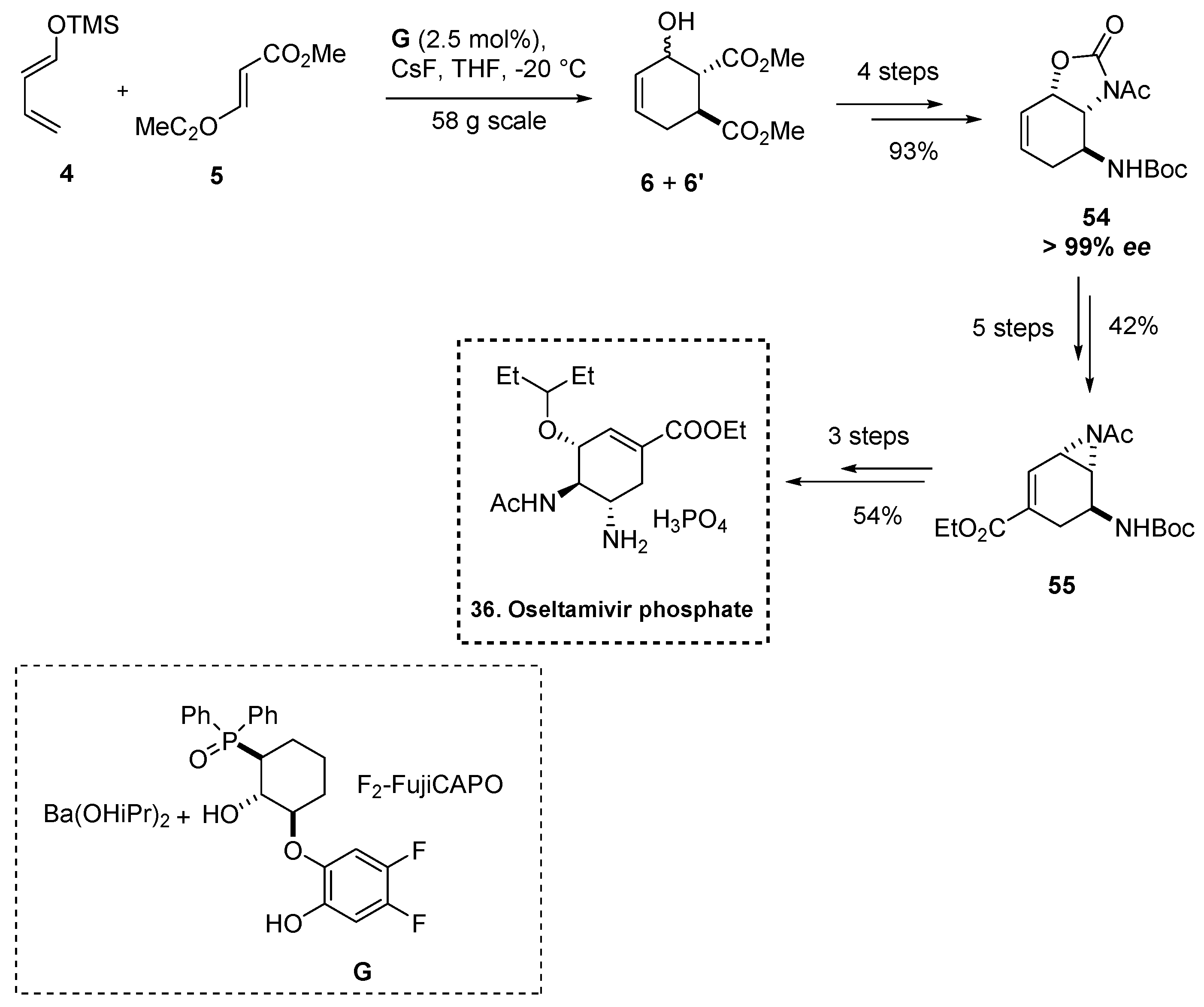
2.5.3. Synthesis of Oseltamivir
2.6. DA Applied for the Synthesis of Natural Compound Endowing Antiviral Activity
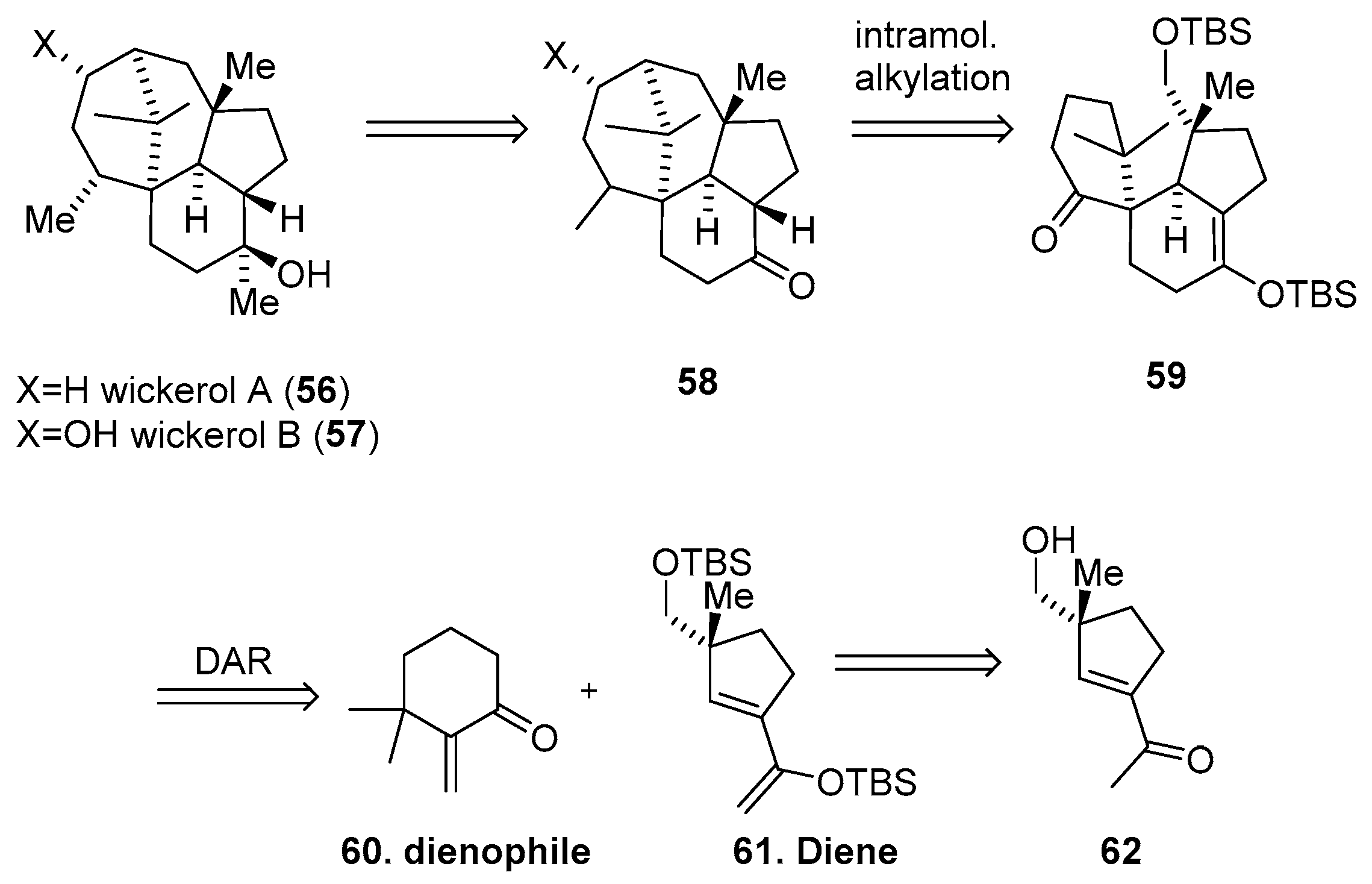
Synthesis of Wickerols
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Nino, A.; Maiuolo, L.; Merino, P.; Nardi, M.; Procopio, A.; Roca-López, D.; Russo, B.; Algieri, V. Efficient Organocatalyst Supported on a Simple Ionic Liquid as a Recoverable System for the Asymmetric Diels-Alder Reaction in the Presence of Water. ChemCatChem 2015, 7, 830–835. [Google Scholar] [CrossRef]
- Carruthers, W. Cycloaddition Reactions in Organic Synthesis; Pergamon: Oxford, UK, 1990; 359p. [Google Scholar]
- Constantino, A.F.; Francisco, C.S.; Cubides-Roman, D.C.; Lacerda, V. Hetero-Diels-Alder Reactions in the Synthesis of Biologically Active Nitrogen Compounds: A Review. Curr. Org. Synth. 2017, 15, 84–104. [Google Scholar] [CrossRef]
- Gregoritza, M.; Brandl, F.P. The Diels-Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials. Eur. J. Pharm. Biopharm. 2015, 97, 438–453. [Google Scholar] [CrossRef]
- Arumugam, S.; Popik, V.V. Light-induced hetero-diels-alder cycloaddition: A facile and selective photoclick reaction. J. Am. Chem. Soc. 2011, 133, 5573–5579. [Google Scholar] [CrossRef]
- Pałasz, A. Recent Advances in Inverse-Electron-Demand Hetero-Diels–Alder Reactions of 1-Oxa-1,3-Butadienes. Top. Curr. Chem. 2016, 374, 24–61. [Google Scholar] [CrossRef][Green Version]
- Quadrelli, P.; Moiola, M. Cycloaddition Reactions for Antiviral Compounds, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2019; 83p. [Google Scholar]
- Slagman, S.; Fessner, W.D. Biocatalytic routes to anti-viral agents and their synthetic intermediates. Chem. Soc. Rev. 2021, 50, 1968–2009. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Fanelli, A.; Botta, L.; De Angelis, M.; Palamara, A.T.; Nencioni, L.; Saladino, R. Aminomalononitrile inspired prebiotic chemistry as a novel multicomponent tool for the synthesis of imidazole and purine derivatives with anti-influenza A virus activity. RSC Adv. 2021, 11, 30020–30029. [Google Scholar] [CrossRef]
- Zippilli, C.; Botta, L.; Bizzarri, B.M.; Nencioni, L.; De Angelis, M.; Protto, V.; Giorgi, G.; Baratto, M.C.; Pogni, R.; Saladino, R. Laccase-Catalyzed 1,4-Dioxane-Mediated Synthesis of Belladine N-Oxides with Anti-Influenza A Virus Activity. Int. J. Mol. Sci. 2021, 22, 1337. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Fanelli, A.; Piccinino, D.; De Angelis, M.; Dolfa, C.; Palamara, A.T.; Nencioni, L.; Zippilli, C.; Crucianelli, M.; Saladino, R. Synthesis of Stilbene and Chalcone Inhibitors of Influenza A Virus by SBA-15 Supported Hoveyda-Grubbs Metathesis. Catalysts 2019, 9, 983. [Google Scholar] [CrossRef]
- Bizzarri, B.M.; Botta, L.; Capecchi, E.; Celestino, I.; Checconi, P.; Palamara, A.T.; Nencioni, L.; Saladino, R. Regioselective IBX-Mediated Synthesis of Coumarin Derivatives with Antioxidant and Anti-influenza Activities. J. Nat. Prod. 2017, 80, 3247–3254. [Google Scholar] [CrossRef]
- Botta, G.; Bizzarri, B.M.; Garozzo, A.; Timpanaro, R.; Bisignano, B.; Amatore, D.; Palamara, A.T.; Nencioni, L.; Saladino, R. Carbon nanotubes supported tyrosinase in the synthesis of lipophilic hydroxytyrosol and dihydrocaffeoyl catechols with antiviral activity against DNA and RNA viruses. Bioorg. Med. Chem. 2015, 23, 5345–5351. [Google Scholar] [CrossRef] [PubMed]
- Sirous, H.; Fassihi, A.; Brogi, S.; Campiani, G.; Christ, F.; Debyser, Z.; Gemma, S.; Butini, S.; Chemi, G.; Grillo, A.; et al. Synthesis, Molecular Modelling and Biological Studies of 3-hydroxy-pyrane-4-one and 3-hydroxy-pyridine-4-one Derivatives as HIV-1 Integrase Inhibitors. Med. Chem. 2019, 15, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Grillo, A.; Raghavaiah, J.; Kovela, S.; Johnson, M.E.; Kneller, D.W.; Wang, Y.F.; Hattori, S.-I.; Higashi-Kuwata, N.; Weber, I.T.; et al. Design, Synthesis, and X-ray Studies of Potent HIV-1 Protease Inhibitors with P2-Carboxamide Functionalities. ACS Med. Chem. Lett. 2020, 11, 1965–1972. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, R.; Donnici, E.; Guidotti, L.; Iannacone, M.; Di Fabio, R.; Summa, V.; Prandi, A.; Randazzo, P.; Ivanova Bencheva, L.; De Mtteo, M.; et al. Oxalamido-Substituted Triciclic Inhibitors of Hepatitis B Virus. WO Patent WO2020234483A1, 26 November 2020. [Google Scholar]
- De Francesco, R.; Donnici, L.; Guidotti, L.; Iannaccone, M.; Di Fabio, R.; Summa, V.; Prandi, A.; Randazzo, P.; Gornati, D.; Grillo, A.; et al. Tricyclic Inhibitors of Hepatitis B Virus. WO Patent WO2020030781A1, 13 February 2020. [Google Scholar]
- Ghosh, A.K.; Grillo, A.; Kovela, S.; Brindisi, M. Asymmetric Diels-Alder reaction of 3-(acyloxy)acryloyl oxazolidinones: Optically active synthesis of a high-affinity ligand for potent HIV-1 protease inhibitors. RSC Adv. 2019, 9, 41755–41763. [Google Scholar] [CrossRef]
- Evans, D.A.; Chapman, K.T.; Bisaha, J. New asymmetric Diels-Alder cycloaddition reactions. Chiral alpha, beta-Unsaturated Carboximides as practical chiral acrylate and crotonate dienophile synthons. J. Am. Chem. Soc. 1984, 106, 4261–4263. [Google Scholar] [CrossRef]
- Du, H.; Ding, K. Asymmetric Catalysis of Diels–Alder Reaction. ChemInform 2010, 41, 1–58. [Google Scholar] [CrossRef]
- Hashimoto, S.; Komeshima, N.; Koga, K. Asymmetric Diels–Alder reaction catalysed by chiral alkoxyaluminium dichloride. J. Chem. Soc. Chem. Commun. 1979, 10, 437–438. [Google Scholar] [CrossRef]
- Bao, J.; Wulff, W.D.; Rheingold, A.L. Vaulted Biaryls as Chiral Ligands for Asymmetric Catalytic Diels-Alder Reactions. J. Am. Chem. Soc. 1993, 115, 3814–3815. [Google Scholar] [CrossRef]
- Bao, J.; Wulff, W.D.; Dominy, J.B.; Fumo, M.J.; Grant, E.B.; Rob, A.C.; Whitcomb, M.C.; Yeung, S.M.; Ostrander, R.L.; Rheingold, A.L. Synthesis, resolution, and determination of absolute configuration of a vaulted 2,2′-binaphthol and a vaulted 3,3′-biphenanthrol (VAPOL). J. Am. Chem. Soc. 1996, 118, 3392–3405. [Google Scholar] [CrossRef]
- Kobayashi, S.; Murakami, M.; Harada, T.; Mukaiyama, T. The Asymmetric Diels–Alder Reaction of α,β-Unsaturated Aldehydes with Dienes using a chiral boron reagent as a catalyst. Chem. Lett. 1991, 20, 1341–1344. [Google Scholar] [CrossRef]
- Hayashi, Y.; Rohde, J.J.; Corey, E.J.; March, R.V. A Novel Chiral Super-Lewis Acidic Catalyst for Enantioselective Synthesis. J. Am. Chem. Soc. 1996, 118, 5502–5503. [Google Scholar] [CrossRef]
- Corey, E.J.; Shibata, T.; Lee, T.W. Asymmetric Diels−Alder Reactions Catalyzed by a Triflic Acid Activated Chiral Oxazaborolidine. J. Am. Chem. Soc. 2002, 124, 3808–3809. [Google Scholar] [CrossRef]
- Mukherjee, S.; Corey, E.J. [4 + 2] Cycloaddition Reactions Catalyzed by a Chiral Oxazaborolidinium Cation. Reaction Rates and Diastereo-, Regio-, and Enantioselectivity Depend on Whether Both Bonds Are Formed Simultaneously. Org. Lett. 2010, 12, 1024–1027. [Google Scholar] [CrossRef]
- Mahender Reddy, K.; Bhimireddy, E.; Thirupathi, B.; Breitler, S.; Yu, S.; Corey, E.J. Cationic Chiral Fluorinated Oxazaborolidines. More Potent, Second-Generation Catalysts for Highly Enantioselective Cycloaddition Reactions. J. Am. Chem. Soc. 2016, 138, 2443–2453. [Google Scholar] [CrossRef]
- Maruoka, K.; Murase, N.; Yamamoto, H. Chiral helical Lewis acids for asymmetric Diels-Alder catalysts. J. Org. Chem. 1993, 58, 2938–2939. [Google Scholar] [CrossRef]
- Yamatsugu, K.; Yin, L.; Kamijo, S.; Kimura, Y.; Kanai, M.; Shibasaki, M. A Synthesis of Tamiflu by using a Barium-Catalyzed Asymmetric Diels–Alder-Type reaction. Angew. Chem. Int. Ed. Engl. 2009, 48, 1070–1076. [Google Scholar] [CrossRef]
- Corey, E.J.; Kurti, L. Enantioselective Chemical Synthesis: Methods, Logic, and Practice; Elsevier: Amsterdam, The Netherlands, 2013; 151p. [Google Scholar]
- Carreira, E.M.; Kvaerno, L. Classics in Stereoselective Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2009; 158p. [Google Scholar]
- Walsh, P.J.; Kozlowski, M.C. Fundamentals of Asymmetric Catalysis; University Science Books: Sausalito, CA, USA, 2009; 549p. [Google Scholar]
- Narasaka, K.; Inoue, M.; Okada, N. Asymmetric Diels-Alder reaction promoted by a chiral titanium reagent. Chem. Lett. 1986, 15, 1109–1112. [Google Scholar] [CrossRef]
- Corey, E.J.; Imai, N.; Zhang, H.Y. Designed Catalyst for Enantioselective Diels-Alder addition from a C2-symmetric chiral bis(oxazoline)-Fe(III) complex. J. Am. Chem. Soc. 1991, 113, 728–729. [Google Scholar] [CrossRef]
- Corey, E.J.; Ishihara, K. Highly enantioselective catalytic Diels-Alder addition promoted by a chiral bis(oxazoline)-magnesium complex. Tetrahedron Lett. 1992, 33, 6807–6810. [Google Scholar] [CrossRef]
- Corey, E.J.; Imwinkelried, R.; Pikul, S.; Xiang, Y.B. Practical enantioselective Diels-Alder and aldol reactions using a new chiral controller system. J. Am. Chem. Soc. 1989, 111, 5493–5495. [Google Scholar] [CrossRef]
- Evans, D.A.; Barnes, D.M.; Johnson, J.S.; Lectka, T.; Matt, P.V.; Miller, S.J.; Murry, J.A.; Norcross, R.D.; Shaughnessy, E.A.; Campos, K.R. Bis(oxazoline) and Bis(oxazolinyl)pyridine Copper Complexes as Enantioselective Diels−Alder Catalysts: Reaction Scope and Synthetic Applications. J. Am. Chem. Soc. 1999, 121, 7582–7594. [Google Scholar] [CrossRef]
- Evans, D.A.; Murry, J.A.; Von Matt, P.; Norcross, R.D.; Miller, S.J. C2-Symmetric Cationic Copper(II) Complexes as Chiral Lewis Acids: Counterion Effects in the Enantioselective Diels–Alder Reaction. Angew. Chem. Int. Ed. Engl. 1995, 34, 798–800. [Google Scholar] [CrossRef]
- Kobayashi, S.; Ishitani, H. Lanthanide(III)-Catalyzed Enantioselective Diels-Alder Reactions. Stereoselective Synthesis of Both Enantiomers by Using a Single Chiral Source and a Choice of Achiral Ligands. J. Am. Chem. Soc. 1994, 116, 4083–4084. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Matsuda, H. Counterions of BINAP-Pt(II) and -Pd(II)Complexes: Novel Catalysts for Highly Enantioselective Diels-Alder Reaction. Org. Lett. 1999, 1, 2157–2159. [Google Scholar] [CrossRef]
- Ahrendt, K.A.; Borths, C.J.; MacMillan, D.W.C. New strategies for organic catalysis: The first highly enantioselective organocatalytic Diels—Alder reaction. J. Am. Chem. Soc. 2000, 122, 4243–4244. [Google Scholar] [CrossRef]
- Riant, O.; Kagan, H.B. Asymmetric Diels-Alder reaction catalyzed by chiral bases. Tetrahedron Lett. 1989, 30, 7403–7406. [Google Scholar] [CrossRef]
- Suttibut, C.; Kohari, Y.; Igarashi, K.; Nakano, H.; Hirama, M.; Seki, C.; Matsuyama, H.; Uwai, K.; Takano, N.; Okuyama, Y.; et al. A highly enantioselective Diels–Alder reaction of 1,2-dihydropyridine using a simple b-amino alcohol organocatalyst for a practical synthetic methodology of oseltamivir intermediate. Tetrahedron Lett. 2011, 52, 4745–4748. [Google Scholar] [CrossRef]
- Nakano, H.; Tsugawa, N.; Fujita, R. The highly enantioselective Diels–Alder reaction of 1,2-dihydropyridine using chiral cationic palladium–phosphinooxazolidine catalyst for the synthesis of chiral isoquinuclidines. Tetrahedron Lett. 2005, 46, 5677–5681. [Google Scholar] [CrossRef]
- Nakano, H.; Tsugawa, N.; Takahashi, K.; Okuyama, Y.; Fujita, R. An efficient synthetic methodology of chiral isoquinuclidines by the enantioselective Diels–Alder reaction of 1,2-dihydropyridines using chiral cationic palladium–phosphinooxazolidine catalyst. Tetrahedron 2006, 62, 10879–10887. [Google Scholar] [CrossRef]
- Corey, E. Catalytic Enantioselective Diels-Alder Reactions: Methods, Mechanistic Fundamentals, Pathways, and Applications. Angew. Chem. Int. Ed. Engl. 2002, 41, 1650–1667. [Google Scholar] [CrossRef]
- Brocksom, T.J.; Nakamura, J.; Maria, L.; Brocksom, U. The Diels-Alder Reaction: An Update. J. Braz. Chem. Soc. 2001, 12, 597–622. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder reaction in total synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Funel, J.-A.; Abele, S. Industrial Applications of the Diels-Alder Reaction. Angew. Chem. Int. Ed. Engl. 2013, 52, 2–44. [Google Scholar] [CrossRef]
- Daluge, S.; Vince, R. Synthesis of Carbocyclic Aminonucleosides. J. Org. Chem. 1978, 43, 2311–2320. [Google Scholar] [CrossRef]
- Griffiths, G.J.; Previdoli, F.E. Diels-Alder reaction of methanesulfonyl cyanide with cyclopentadiene. Industrial synthesis of 2-azabicyclo[2.2.1]hept-5-en-3-one. J. Org. Chem. 1993, 58, 6129–6131. [Google Scholar] [CrossRef]
- Ruebsam, F.; Murphy, D.E.; Tran, C.V.; Li, L.S.; Zhao, J.; Dragovich, P.S.; McGuire, H.M.; Xiang, A.X.; Sun, Z.; Ayida, B.K.; et al. Discovery of tricyclic 5,6-dihydro-1H-pyridin-2-ones as novel, potent, and orally bioavailable inhibitors of HCV NS5B polymerase. Bioorg. Med. Chem. Lett. 2009, 19, 6404–6412. [Google Scholar] [CrossRef]
- Tran, C.V.; Ruebsam, F.; Murphy, D.E.; Dragovich, P.; Zhou, Y.; Chen, L.; Kucera, D.; Blatter, F.; Viertelhaus, M. 5,6-Dihydro-1H-Pyridin-2-One Compounds. WO Patent WO2008124450A1, 16 October 2008. [Google Scholar]
- Ghosh, A.K.; Bilcer, G.; Schiltz, G. Syntheses of FDA approved HIV protease inhibitors. Synthesis 2001, 43, 2203–2229. [Google Scholar] [CrossRef]
- Crackett, P.; Demont, E.; Eatherton, A.; Frampton, C.S.; Gilbert, J.; Kahn, I.; Redshaw, S.; Watson, W. HIV protease inhibitor part 1: Use of Evans’ oxazolidinone in intermolecular Diels-Alder reaction en route to 3,4-substituted cyclohexanones. Synlett 2004, 4, 679–683. [Google Scholar]
- Evans, D.; Weber, A.E. Asymmetric Glycine Enolate Aldol Reaction. J. Am. Chem. Soc. 1986, 108, 6757–6761. [Google Scholar] [CrossRef]
- Blanchette, M.A.; Choy, W.; Davis, J.T.; Essenfeld, A.P.; Masamune, S.; Roush, W.R.; Sakai, T. Horner-wadsworth-emmons reaction: Use of lithium chloride and an amine for base-sensitive compounds. Tetrahedron Lett. 1984, 25, 2183–2186. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Chapsal, B.D.; Weber, I.T.; Mitsuya, H. Design of HIV Protease Inhibitors Targeting Protein Backbone: An Effective Strategy for Combating Drug Resistance. Acc. Chem. Res. 2008, 41, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Sridhar, P.R.; Leshchenko, S.; Hussain, A.K.; Li, J.; Kovalevsky, A.Y.; Walters, D.E.; Wedekind, J.; Grum-Tokars, V.; Das, D.; et al. Structure-Based Design of Novel HIV-1 Protease Inhibitors to Combat Drug Resistance. J. Med. Chem. 2006, 49, 5252–5261. [Google Scholar] [CrossRef] [PubMed]
- Sterrantino, G.; Zaccarelli, M.; Colao, G.; Baldanti, F.; Di Giambenedetto, S.; Carli, T.; Maggiolo, F.; Zazzi, M. Genotypic resistance profiles associated with virological failure to darunavir-containing regimens: A cross-sectional analysis. Infection 2012, 40, 311–318. [Google Scholar] [CrossRef] [PubMed]
- De Meyer, S.; Lathouwers, E.; Dierynck, I.; De Paepe, E.; Van Baelen, B.; Vangeneugden, T.; Spinosa-Guzman, S.; Lefebvre, E.; Picchio, G.; De Bethune, M.P. Characterization of virologic failure patients on darunavir/ritonavir in treatment-experienced patients. AIDS 2009, 23, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Kincaid, J.F.; Cho, W.; Walters, D.E.; Krishnan, K.; Hussain, K.A.; Koo, Y.; Cho, H.; Rudall, C.; Holland, L.; et al. Potent HIV Protease Inhibitors Incorporating High-Affinity P2-Ligands and (R)-(Hydroxyethylamino)sulfonamide Isostere. Bioorg. Med. Chem. Lett. 1998, 8, 687–690. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Dawson, Z.L.; Mitsuya, H. Darunavir, a conceptually new HIV-1 protease inhibitor for the treatment of drug-resistant HIV. Bioorg. Med. Chem. 2007, 15, 7576–7580. [Google Scholar] [CrossRef]
- Sibi, M.P.; Matsunaga, H. Enantioselective Diels–Alder reactions of 3-(acyloxy)acrylates. Tetrahedron Lett. 2004, 45, 5925–5929. [Google Scholar] [CrossRef]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: Design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- Ishikawa, H.; Suzuki, T.; Hayashi, Y. High-Yielding Synthesis of the Anti-Influenza Neuramidase Inhibitor (−)-Oseltamivir by Three “One-Pot” Operations. Angew. Chem. 2009, 121, 1330–1333. [Google Scholar] [CrossRef]
- Farina, V.; Brown, J.D. Tamiflu: The supply problem. Angew. Chem. Int. Ed. 2006, 45, 7330–7334. [Google Scholar] [CrossRef]
- Hřebabecký, H.; Dračínský, M.; De Palma, A.M.; Neyts, J.; Holý, A. Synthesis of novel carbocyclic nucleoside analogues derived from 7-oxabicyclo[2.2.1]heptane-2-methanol. Collect. Czech. Chem. Commun. 2009, 74, 487–502. [Google Scholar] [CrossRef]
- Gong, J.; Xu, W. Different Synthetic Strategies of Oseltamivir Phosphate: A Potent Influenza Neuraminidase Inhibitor. Curr. Med. Chem. 2008, 15, 3145–3159. [Google Scholar] [CrossRef]
- Sagandira, C.R.; Mathe, F.M.; Guyo, U.; Watts, P. The evolution of Tamiflu synthesis, 20 years on: Advent of enabling technologies the last piece of the puzzle? Tetrahedron 2020, 76, 131440. [Google Scholar] [CrossRef]
- Abrecht, S.; Harrington, P.; Iding, H.; Karpf, M.; Trussardi, R.; Wirz, B.; Zutter, U. The Synthetic Development of the Anti-Influenza Neuraminidase Inhibitor Oseltamivir Phosphate (Tamiflu®): A Challenge for Synthesis & Process Research. CHIMIA Int. J. Chem. 2004, 58, 621–629. [Google Scholar]
- Shaim, H.; McCaffrey, P.; Trieu, J.A.; DeAnda, A.; Yates, S.G. Evaluating the effects of oseltamivir phosphate on platelet counts: A retrospective review. Platelets 2020, 31, 1080–1084. [Google Scholar] [CrossRef]
- Yeung, Y.Y.; Hong, S.; Corey, E.J. A short enantioselective pathway for the synthesis of the anti-influenza neuramidase inhibitor oseltamivir from 1,3-butadiene and acrylic acid. J. Am. Chem. Soc. 2006, 128, 6310–6311. [Google Scholar] [CrossRef]
- Satoh, N.; Akiba, T.; Yokoshima, S.; Fukuyama, T. Practical Synthesis of (−)-Oseltamivir. Angew. Chem. Int. Ed. 2007, 46, 5734–5736. [Google Scholar] [CrossRef]
- Nakano, H.; Osone, K.; Takeshita, M.; Kwon, E.; Seki, C.; Matsuyama, H.; Takano, N.; Kohari, Y. A novel chiral oxazolidine organocatalyst for the synthesis of an oseltamivir intermediate using a highly enantioselective Diels-Alder reaction of 1,2-dihydropyridine. Chem. Commun. 2010, 46, 4827–4829. [Google Scholar] [CrossRef]
- Tsypysheva, I.; Koval’skaya, A.; Petrova, P.; Lobov, A.; Borisevich, S.; Tsypyshev, D.; Fedo-rova, V.; Gorbunova, E.; Galochkina, A.; Zarubaev, V. Diels-Alder adducts of 3-N-substituted derivatives of (−)-Cytisine as influenza A/H1N1 virus inhibitors; ste-reodifferentiation of antiviral properties and preliminary assessment of action mechanism. Tetrahedron 2019, 75, 2933–2943. [Google Scholar] [CrossRef]
- Sib, A.; Milzarek, T.M.; Herrmann, A.; Oubraham, L.; Meller, J.; Pichlmair, A.; Brack-Werner, R.; Gulder, T.A. Chemoenzymatic Total Synthesis of Sorbicatechol Structural Analogues and Evaluation of Their Antiviral Potential. ChemBioChem 2020, 21, 492–495. [Google Scholar] [CrossRef]
- Naffziger, M.R.; Ashburn, B.O.; Perkins, J.R.; Carter, R.G. Diels-Alder Approach for the Construction of Halogenated, o-Nitro Biaryl Templates and Application to the Total Synthesis of the Anti-HIV Agent Siamenol. J. Org. Chem. 2007, 72, 9857–9865. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Izumi, N.; Ui, H.; Sueki, A.; Masuma, R.; Nonaka, K.; Hirose, T.; Sunazuka, T.; Nagai, T.; Yamada, H.; et al. Wickerols A and B: Novel anti-influenza virus diterpenes produced by Trichoderma atroviride FKI-3849. Tetrahedron 2012, 68, 9267–9271. [Google Scholar] [CrossRef]
- Kwong Jia Ye, D.; Richard, J.-A. Synthesis of the 6-6-6 tricyclic skeleton of the anti-influenza A diterpene wickerol A. Tetrahedron Lett. 2014, 55, 2183–2186. [Google Scholar] [CrossRef]
- Liu, S.A.; Trauner, D. Asymmetric Synthesis of the Antiviral Diterpene Wickerol A. J. Am. Chem. Soc. 2017, 139, 9491–9494. [Google Scholar] [CrossRef]
- Deng, J.; Ning, Y.; Tian, H.; Gui, J. Divergent Synthesis of Antiviral Diterpenes Wickerols A and B. J. Am. Chem. Soc. 2020, 142, 4690–4695. [Google Scholar] [CrossRef]
- Adams, J.; Lepine-frenette, C.; Spero, D.M. Intramolecular Cyclopropanation-Ring Fragmentation Leading to Spirocyclic Ring Construction: A Stereoselective Synthesis. J. Org. Chem. 1991, 56, 4494–4498. [Google Scholar] [CrossRef]
- Inoue, M.; Carson, M.W.; Frontier, A.J.; Danishefsky, S.J. Total Synthesis and Determination of the Absolute Configuration of Frondosin B. J. Am. Chem. Soc. 2001, 123, 1878–1889. [Google Scholar] [CrossRef]
- Maruoka, K.; Ooi, T.; Nagahara, S.; Yamamoto, H. Organoaluminum-catalyzed rearrangement of epoxides a facile route to the synthesis of optically active β-siloxy aldehydes. Tetrahedron 1991, 47, 6983–6998. [Google Scholar] [CrossRef]
- Yang, Z.; Liao, H.; Sheng, K.; Chen, Y.; Yao, Z. Enantioselective Total Synthesis of Marine Diterpenoid Clavulactone. Angew. Chem. 2012, 124, 6590–6593. [Google Scholar] [CrossRef]
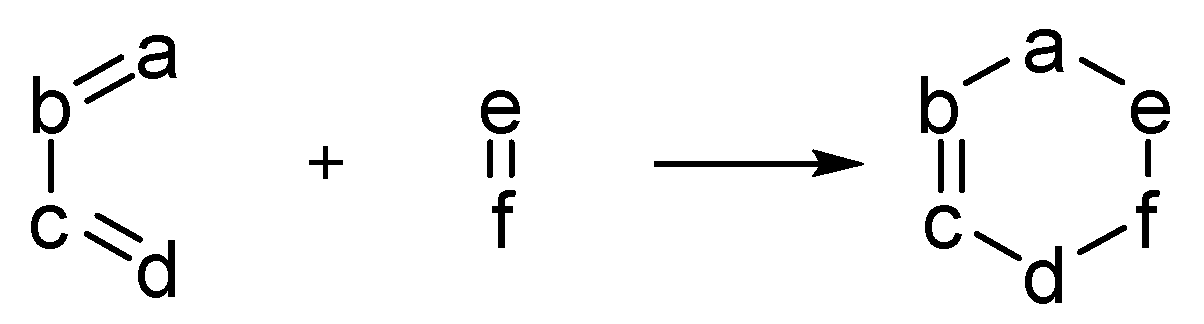

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grillo, A.; Bizzarri, B.M. Catalytic Enantioselective Diels Alder Reaction: Application in the Synthesis of Antiviral Agents. Catalysts 2022, 12, 150. https://doi.org/10.3390/catal12020150
Grillo A, Bizzarri BM. Catalytic Enantioselective Diels Alder Reaction: Application in the Synthesis of Antiviral Agents. Catalysts. 2022; 12(2):150. https://doi.org/10.3390/catal12020150
Chicago/Turabian StyleGrillo, Alessandro, and Bruno Mattia Bizzarri. 2022. "Catalytic Enantioselective Diels Alder Reaction: Application in the Synthesis of Antiviral Agents" Catalysts 12, no. 2: 150. https://doi.org/10.3390/catal12020150
APA StyleGrillo, A., & Bizzarri, B. M. (2022). Catalytic Enantioselective Diels Alder Reaction: Application in the Synthesis of Antiviral Agents. Catalysts, 12(2), 150. https://doi.org/10.3390/catal12020150






