Preparation of CuS/PbS/ZnO Heterojunction Photocatalyst for Application in Hydrogen Production
Abstract
:1. Introduction
2. Results and Discussion
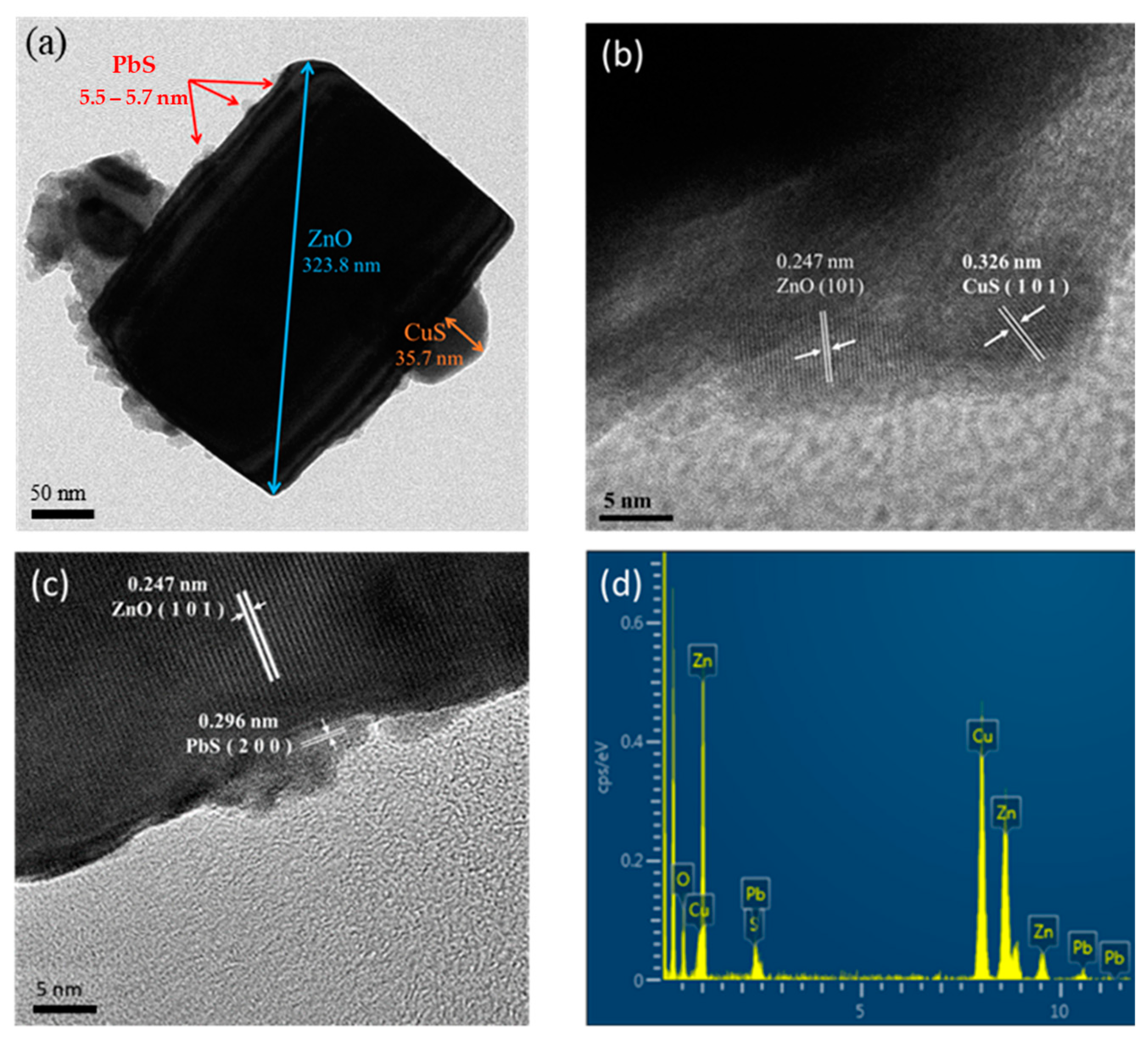
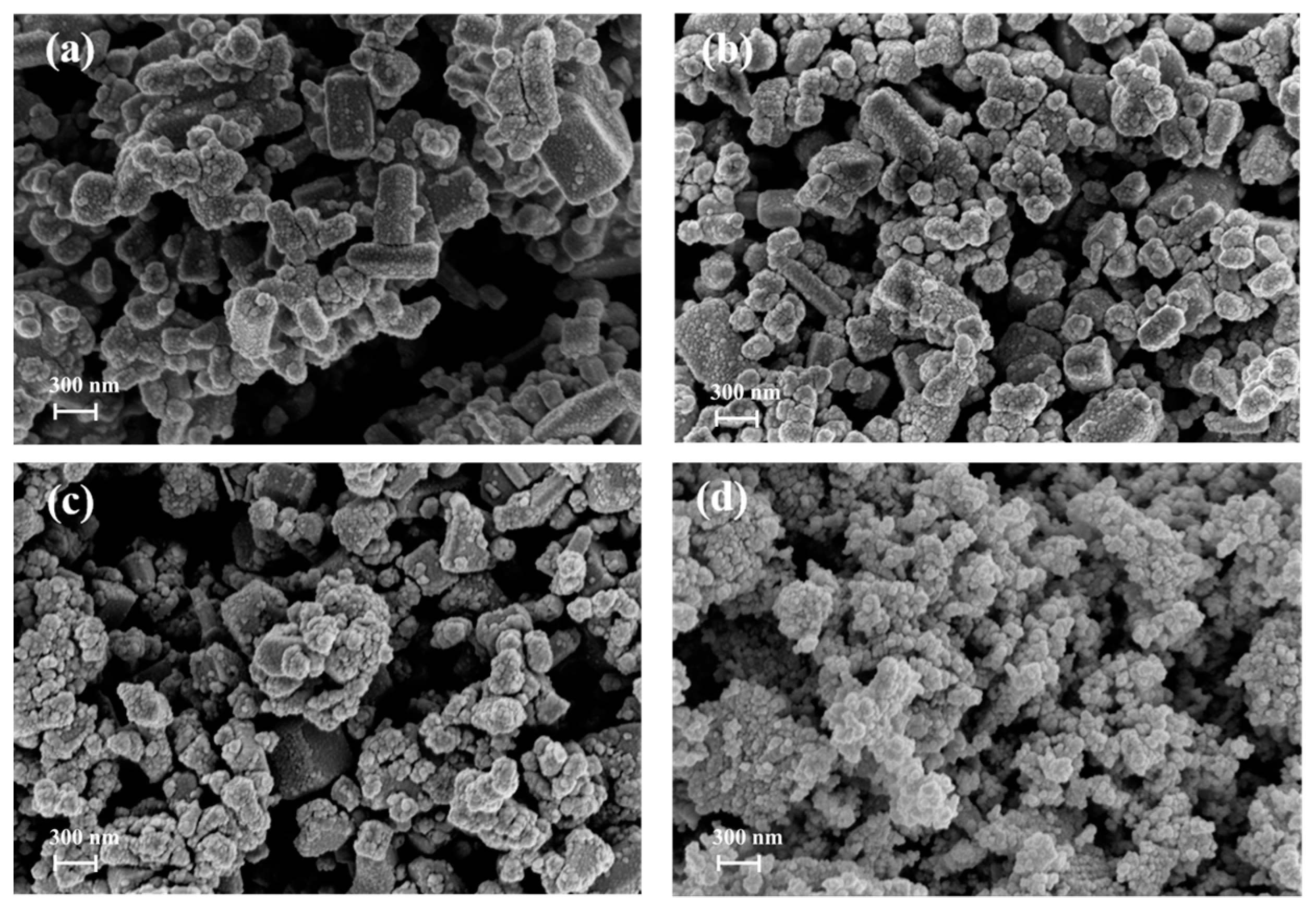
2.1. Analysis of Material Properties
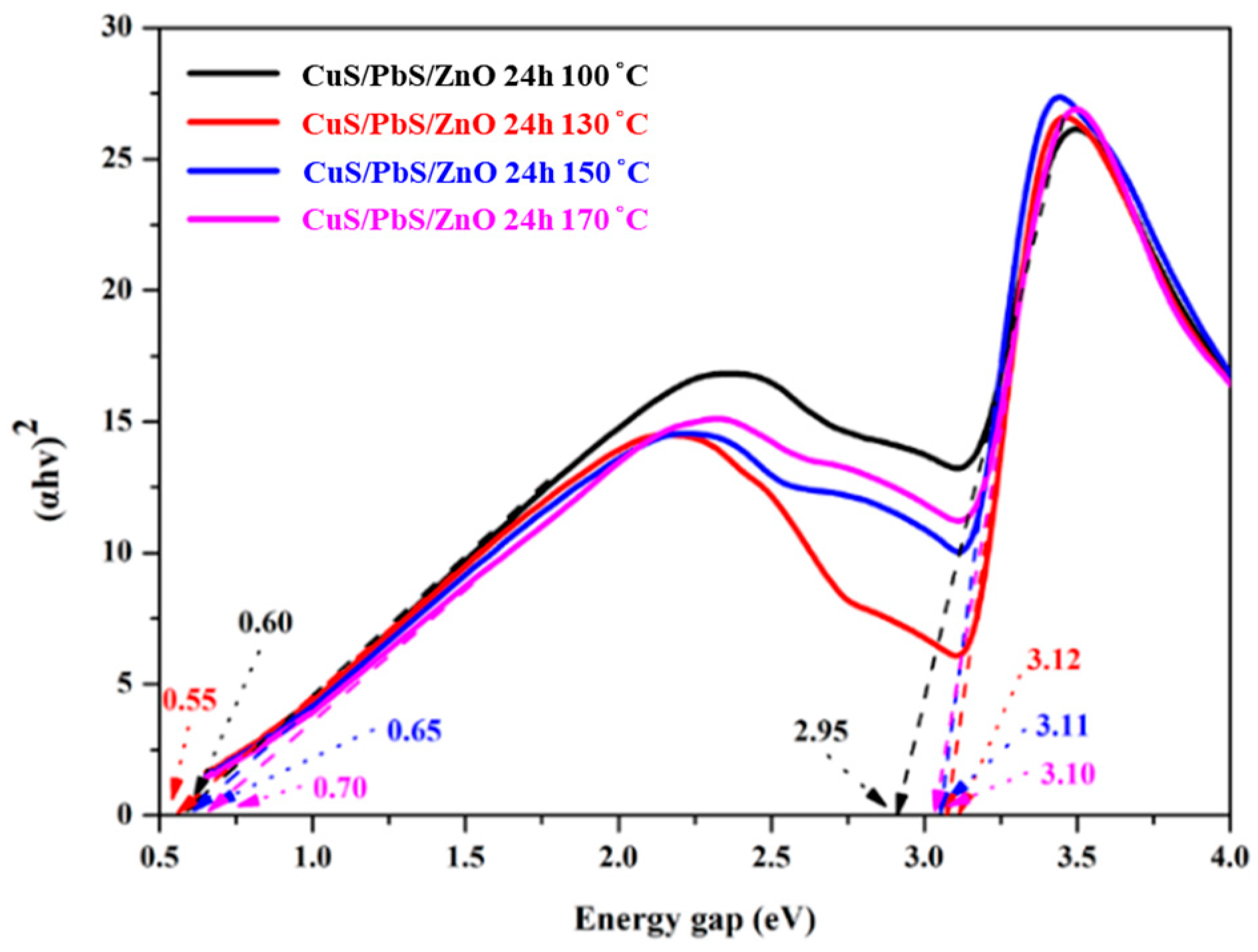
2.2. Spectral Properties of Prepared Heterostructure Photocatalysts
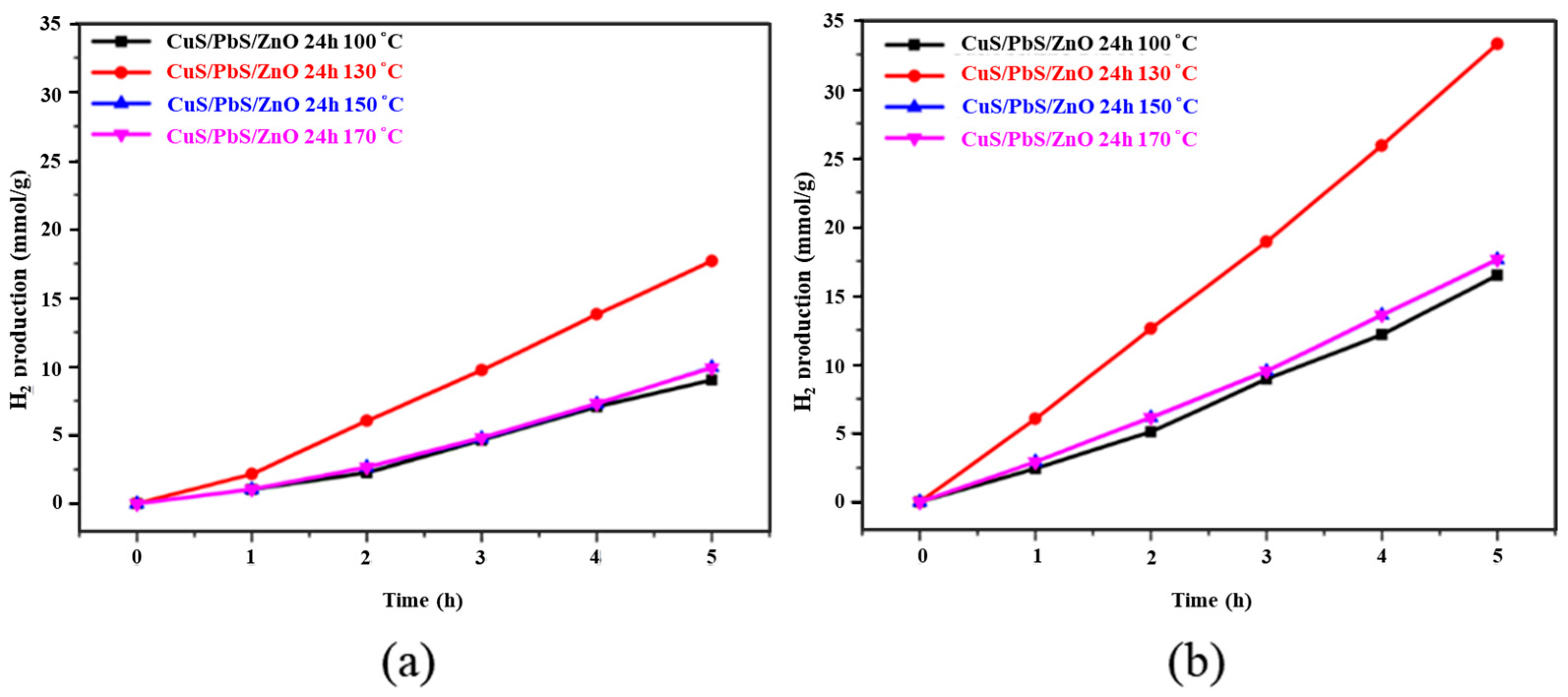
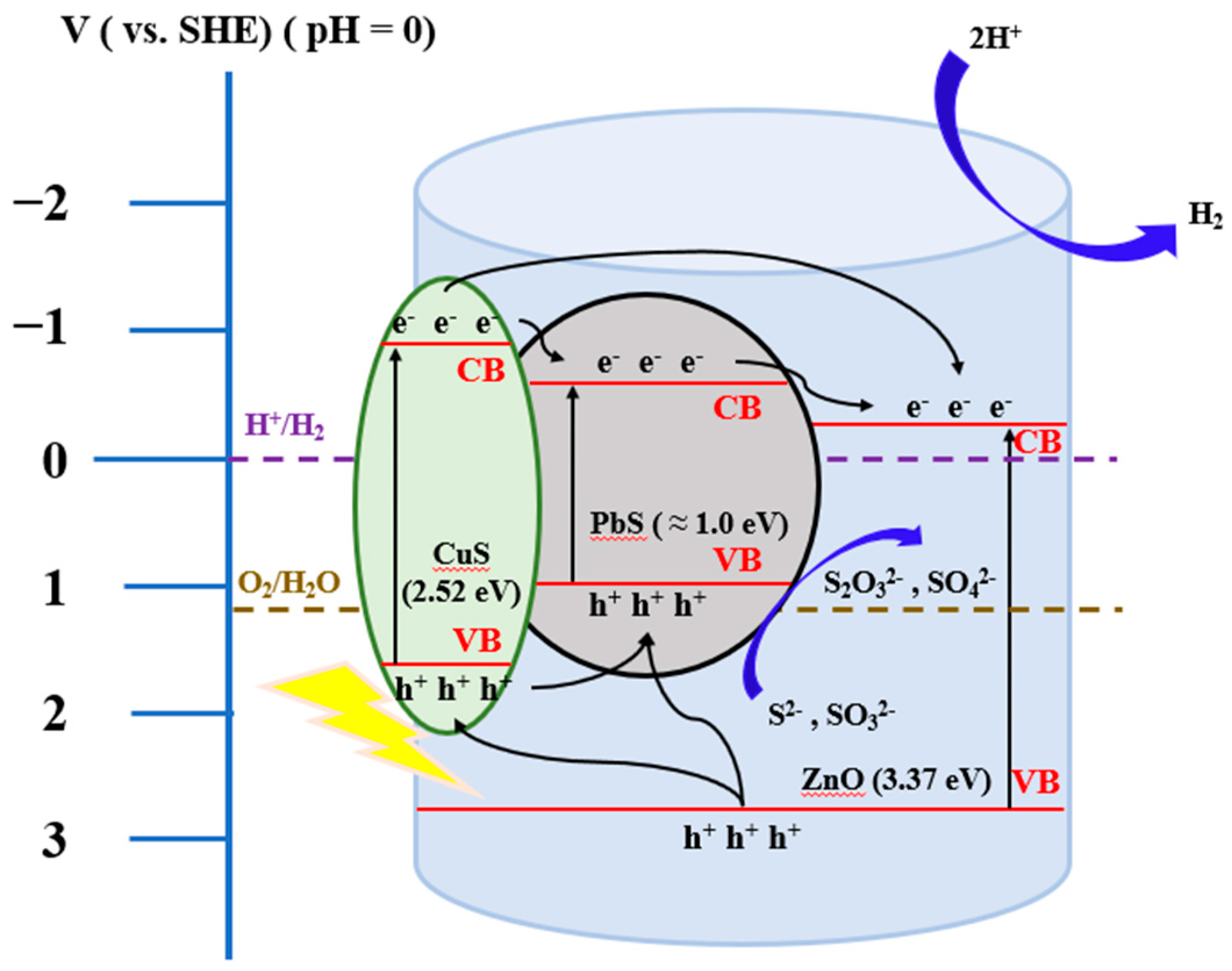
2.3. Hydrogen Production by Water Splitting with Photocatalysts
3. Materials and Methods
3.1. Experimental Reagents
3.2. Synthesis of ZnO Nanomaterials
3.3. Synthesis of CuS/PbS/ZnO Photocatalyst by Hydrothermal Method
3.4. The Degree of Recombination of Photocatalyst
3.5. Photocatalytic Hydrogen Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hermesmann, M.; Müller, T.E. Green, Turquoise, Blue, or Grey? Environmentally Friendly Hydrogen Production in Transforming Energy Systems. Prog. Energy Combust. Sci. 2022, 90, 100996. [Google Scholar] [CrossRef]
- Okonkwo, P.C.; Barhoumi, E.M.; Mansir, I.B.; Emori, W.; Bhowmik, H. Effect of electrode material on the hydrogen production using a low-cost home-made alkaline electrolyzer. Vacuum 2022, 198, 110878. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, Y.G.; Chen, J.K.; Huang, C.Y.; Hsieh, S.C.; Wu, S.Y. Ionic liquid/surfactant-hydrothermal synthesis of dendritic PbS@CuS core-shell photocatalysts with improved photocatalytic performance. Appl. Surf. Sci. 2021, 546, 149106. [Google Scholar] [CrossRef]
- Frank, S.N.; Allen, J. Heterogeneous photocatalytic oxidation of cyanide and sulfite in aqueous solutions at semiconductor powders. J. Phys. Chem. 1977, 15, 1484–1488. [Google Scholar] [CrossRef]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 20, 182–190. [Google Scholar] [CrossRef]
- Fujishima, A.; Honda, K. Electrochemical photolysis of water splitting at a semiconductor electrode. Nature 1972, 5358, 37–38. [Google Scholar] [CrossRef]
- Sun, L.; Koh, Z.Y. PbS quantum dots embedded in a ZnS dielectric matrix for bulk heterojunction solar cell applications. Adv. Mater. 2013, 33, 4598–4604. [Google Scholar] [CrossRef]
- Li, X.; Sun, Z.; Bao, Y.; Xia, X.; Tao, T.; Homewood, K.P.; Li, R.; Gao, Y. Comprehensively improved hydrogen sensing performance via constructing the facets homojunction in rutile TiO2 hierarchical structure. Sens. Actuators B 2022, 350, 130869. [Google Scholar] [CrossRef]
- Xu, Y.; Li, H.; Sun, B.; Qiao, P.; Ren, L.; Guohui, T.; Jiang, B.J.; Pan, K.; Zhou, W. Surface oxygen vacancy defect-promoted electron-hole separation for porous defective ZnO hexagonal plates and enhanced solar-driven photocatalytic performance. Chem. Eng. J. 2020, 379, 122295. [Google Scholar] [CrossRef]
- You, J.; Wang, L.; Bao, W.; Yan, A.; Guo, R. Synthesis and visible-light photocatalytic properties of BiOBr/CdS nanomaterials. J. Mater. Sci. 2021, 56, 6732–6744. [Google Scholar] [CrossRef]
- Abd-Rabboh, H.S.M.; Benaissa, M.; Hamdy, M.S.; Ahmed, M.A.; Glal, M. Synthesis of an efficient, and recyclable mesoporous BiVO4/TiO2 direct Z-scheme heterojunction by sonochemical route for photocatalytic hydrogen production and photodegradation of rhodamine B dye in the visible region. Opt. Mater. 2021, 114, 110761. [Google Scholar] [CrossRef]
- Tentu, R.D.; Basu, S. Photocatalytic water splitting for hydrogen production. Curr. Opin. Electrochem. 2017, 1, 56–62. [Google Scholar] [CrossRef]
- Sclafani, A.; Herrmann, J.M. Influence of metallic silver and of platinum-silver bimetallic deposits on the photocatalytic activity of titania (anatase and rutile) in organic and aqueous media. J. Photochem. Photobiol. A 1998, 113, 181–188. [Google Scholar] [CrossRef]
- Brezova, V.; Blazkova, A.; Karpinsky, L.; Ceppan, M. Phenol decomposition using Mn+/TiO2 photocatalysts supported by the sol-gel technique on glass fibres. J. Photochem. Photobiol. A 1997, 109, 177–183. [Google Scholar] [CrossRef]
- Yang, G.; Yan, W.; Zhang, Q.; Shen, S.; Dingd, S.J. One-dimensional CdS/ZnO core/shell nanofibers via single-spinneret electrospinning: Tunable morphology and efficient photocatalytic hydrogen production. Nanoscale 2013, 24, 12432–12439. [Google Scholar] [CrossRef]
- Goma, P.; Hachisuka, K.; Katsumata, H.; Suzuki, T.; Funasaka, K.; Kaneco, S. Photocatalytic hydrogen production with CuS/ZnO from aqueous Na2S + Na2SO3 solution. Int. J. Hydrogen Energy 2013, 38, 8625–8630. [Google Scholar]
- Zhao, X.; Feng, J.; Liu, J.; Lu, J.; Shi, W.; Yang, G.; Wang, G.; Feng, P.; Cheng, P. Metal-Organic Framework-Derived ZnO/ZnS Heteronanostructures for Efficient Visible-Light-Driven Photocatalytic Hydrogen Production. Adv.Sci. 2018, 4, 1700590. [Google Scholar] [CrossRef]
- Inoue, T.; Watanabe, T.; Fujishma, A.; Honda, K.I. Suppression of Surface Dissolution of CdS Photoanode by Reducing Agents. J. Electrochem. Soc. 1977, 124, 719. [Google Scholar] [CrossRef]
- Alivisatos, A.P. Semiconductor clusters, nanocrystals, and quantum dots. Science 1996, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Nandi, P.; Das, D. ZnO/CdS/CuS heterostructure: A suitable candidate for applications in visible-light photocatalysis. J. Phys. Chem. Solids 2022, 160, 110344. [Google Scholar] [CrossRef]
- Shi, X.F.; Xia, X.Y.; Cui, G.W.; Deng, N.; Zhao, Y.Q.; Zhuo, L.H.; Tang, B. Multiple exciton generation application of PbS quantum dots in ZnO@PbS/graphene oxide for enhanced photocatalytic activity. Appl. Catal. B 2015, 163, 123–128. [Google Scholar] [CrossRef]
- Liu, R.; Feng, S.Y.; Dong, C.D.; Tsai, K.C.; Yang, W.D. Enhancing hydrogen evolution of water splitting under solar spectra using Au/TiO2 heterojunction photocatalysts. Int. J. Hydrogen Energy 2021, 46, 28462–28473. [Google Scholar] [CrossRef]
- Wang, X.W.; Li, Q.C.; Xu, H.P.; Gan, L.; Ji, X.F.; Lui, H.; Zhang, R.G. CuS-modified ZnO rod/reduced graphene oxide/CdS heterostructure for efficient visible-light photocatalytic hydrogen generation. Int. J. Hydrogen Energy 2020, 45, 28394–28403. [Google Scholar] [CrossRef]
- Pahlevanpour, G.; Bashiri, H. Kinetic Monte Carlo simulation of hydrogen production from photocatalytic water splitting in the presence of methanol by 1 wt% Au/TiO2. Int. J. Hydrogen Energy 2022, 26, 12975–12987. [Google Scholar] [CrossRef]
- Patil, A.B.; Jadhav, B.D.; Bhoir, P. Optical band gap modification of Ce/ZnO for visible light photocatalytic H2 production from aqueous methanol solution. Opt. Mater. 2021, 121, 111503. [Google Scholar] [CrossRef]
- Conejeros, S.; Moreira Ide, P.; Alemany, P.; Canadell, E. Nature of Holes, Oxidation States, and Hypervalency in Covellite (CuS). Inorg. Chem. 2014, 53, 12402–12406. [Google Scholar] [CrossRef]
- Saranya, M.; Nirmala Grace, A. Hydrothermal synthesis of CuS nanostructures with different morphology. J. Nano Res. 2012, 18, 43–51. [Google Scholar] [CrossRef]
- Wei, J.; Wei, S.; Chang, N.; Wang, H.; Zhang, J.; Shao, W. Synthesis of PbS quantum dots/ZnO nanorods/Ag nanowires composite photocatalysts with enhanced photocatalytic activity. Mater. Lett. 2021, 285, 129130. [Google Scholar] [CrossRef]
- Yendrapati, T.P.; Gautam, A.; Bojja, S.; Pal, U. Formation of ZnO@CuS nanorods for efficient photocatalytic hydrogen generation. Sol. Energy 2020, 196, 540–548. [Google Scholar] [CrossRef]
- Guo, S.; Chen, W.; Li, M.; Wang, J.; Liu, F.; Cheng, J.P. Effect of reaction temperature on the amorphous-crystalline transition of copper cobalt sulfide for supercapacitors. Electrochim. Acta 2018, 271, 498–506. [Google Scholar] [CrossRef]
- Shalahuddin Al Ja’farawy, M.; Kusumandari; Purwanto, A.; Widiyandari, H. Carbon quantum dots supported zinc oxide (ZnO/CQDs) efficient photocatalyst for organic pollutant degradation-A systematic review. Environ. Nanotechnol. Monit. Manag. 2022, 18, 100681. [Google Scholar] [CrossRef]
- Liang, Y.; Novet, T.; Thorne, J.E.; Parkinson, B.A. Photosensitization of ZnO single crystal electrodes with PbS quantum dots. Phys. Status Solidi A 2014, 9, 1954–1959. [Google Scholar] [CrossRef]
- Guchhait, A.A.; Rath, K.; Pal, A.J. To make polymer: Quantum dot hybrid solar cells NIR-active by increasing diameter of PbS nanoparticles. Sol. Energy Mater. Sol. Cells 2011, 2, 651–656. [Google Scholar] [CrossRef]
- Hong, M.; Zhang, L.; Fang, H.; Feng, X.; Li, Z. Surface engineering of CdS quantum dots modified SiO2@C3N4 nanospheres for effective photocatalytic hydrogen evolution. Mater. Sci. Semicond. Process. 2021, 136, 106134. [Google Scholar] [CrossRef]
- He, B.; Bie, C.; Fei, X.; Cheng, B.; Yu, J.; Ho, W.; Al-Ghamdi, A.A.; Wageh, S. Enhancement in the photocatalytic H2 production activity of CdS NRs by Ag2S and NiS dual cocatalysts. Appl. Catal. B 2021, 288, 119994. [Google Scholar] [CrossRef]
- Chen, W.; Wang, Y.; Liu, S.; Gao, L.; Mao, L.; Fan, Z.; Shangguan, W.; Jiang, Z. Non-noble metal Cu as a cocatalyst on TiO2 nanorod for highly efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2018, 445, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Chang, C.J. Recent Progress on Metal Sulfide Composite Nanomaterials for Photocatalytic Hydrogen Production. Catalysts 2019, 9, 457. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 9, 5155–5164. [Google Scholar] [CrossRef]
- Anuchai, S.; Phanichphant, S.; Tantraviwat, D.; Pluengphon, P.; Bovornratanaraks, T.; Inceesungvorn, B. Low temperature preparation of oxygen-deficient tin dioxide nanocrystals and a role of oxygen vacancy in photocatalytic activity improvement. J. Colloid Interface Sci. 2018, 512, 105–114. [Google Scholar] [CrossRef]
- Yavuz, C.; Erten-Ela, S. Solar light-responsive α-Fe2O3/CdS/g-C3N4 ternary photocatalyst for photocatalytic hydrogen production and photodegradation of methylene blue. J. Alloys Compd. 2022, 908, 164584. [Google Scholar] [CrossRef]
- Shiri, P. An overview on the copper-promoted synthesis of five-membered heterocyclic systems. Appl. Organomet. Chem. 2020, 34, 5. [Google Scholar] [CrossRef]






| Single Wave Length Light (nm) Photocatalyst | 300 | 350 | 400 | 600 | 800 | 1000 | 1400 | 1800 |
| Absorbance of the Photocatalysts (a.u.) | ||||||||
| ZnO | 0.408 | 0.615 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| PbS/ZnO | 0.408 | 0.616 | 0.345 | 0.746 | 0.861 | 0.838 | 0.913 | 0.860 |
| CuS/ZnO | 0.408 | 0.625 | 0.665 | 0.754 | 0.627 | 0.627 | 0.587 | 0.546 |
| CuS/PbS/ZnO | 0.408 | 0.626 | 0.445 | 0.787 | 0.870 | 0.885 | 0.904 | 0.858 |
| Single Wave Length Light (nm) Photocatalyst | 350 | 600 | 800 | 1000 | 1200 |
| Hydrogen Production Rate (μmol min−1) | |||||
| ZnO | 224 | 0 | 0 | 0 | 0 |
| PbS | 0 | 212 | 234 | 188 | 201 |
| CuS | 0 | 197 | 220 | 207 | 194 |
| CuS/PbS/ZnO 24 h 130 °C | 271 | 346 | 375 | 394 | 446 |
| Single Wave Length Light (nm) Photocatalyst | 350 | 600 | 800 | 1000 | 1200 |
| Turnover Number, TON (min−1) | |||||
| ZnO | 4.48 | 0 | 0 | 0 | 0 |
| PbS | 0 | 4.24 | 4.68 | 3.76 | 4.02 |
| CuS | 0 | 3.94 | 4.40 | 4.14 | 3.88 |
| CuS/PbS/ZnO 24 h 130 °C | 5.42 | 6.92 | 7.50 | 7.88 | 8.92 |
| Photocatalyst | BET Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| CuS/PbS/ZnO 100 °C | 28.98 | 0.21 | 29.05 |
| CuS/PbS/ZnO 130 °C | 33.20 | 0.26 | 30.79 |
| CuS/PbS/ZnO 150 °C | 27.57 | 0.23 | 33.13 |
| CuS/PbS/ZnO 170 °C | 23.09 | 0.23 | 39.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiu, M.-H.; Kuo, C.-C.; Huang, C.-W.; Yang, W.-D. Preparation of CuS/PbS/ZnO Heterojunction Photocatalyst for Application in Hydrogen Production. Catalysts 2022, 12, 1677. https://doi.org/10.3390/catal12121677
Chiu M-H, Kuo C-C, Huang C-W, Yang W-D. Preparation of CuS/PbS/ZnO Heterojunction Photocatalyst for Application in Hydrogen Production. Catalysts. 2022; 12(12):1677. https://doi.org/10.3390/catal12121677
Chicago/Turabian StyleChiu, Ming-Huan, Cheng-Ching Kuo, Chao-Wei Huang, and Wein-Duo Yang. 2022. "Preparation of CuS/PbS/ZnO Heterojunction Photocatalyst for Application in Hydrogen Production" Catalysts 12, no. 12: 1677. https://doi.org/10.3390/catal12121677
APA StyleChiu, M.-H., Kuo, C.-C., Huang, C.-W., & Yang, W.-D. (2022). Preparation of CuS/PbS/ZnO Heterojunction Photocatalyst for Application in Hydrogen Production. Catalysts, 12(12), 1677. https://doi.org/10.3390/catal12121677








