Advances in Catalytic C–F Bond Activation and Transformation of Aromatic Fluorides
Abstract
1. Introduction
2. Transition Metal-Promoted Catalytic C–F Activation of Aromatic Hydrocarbons
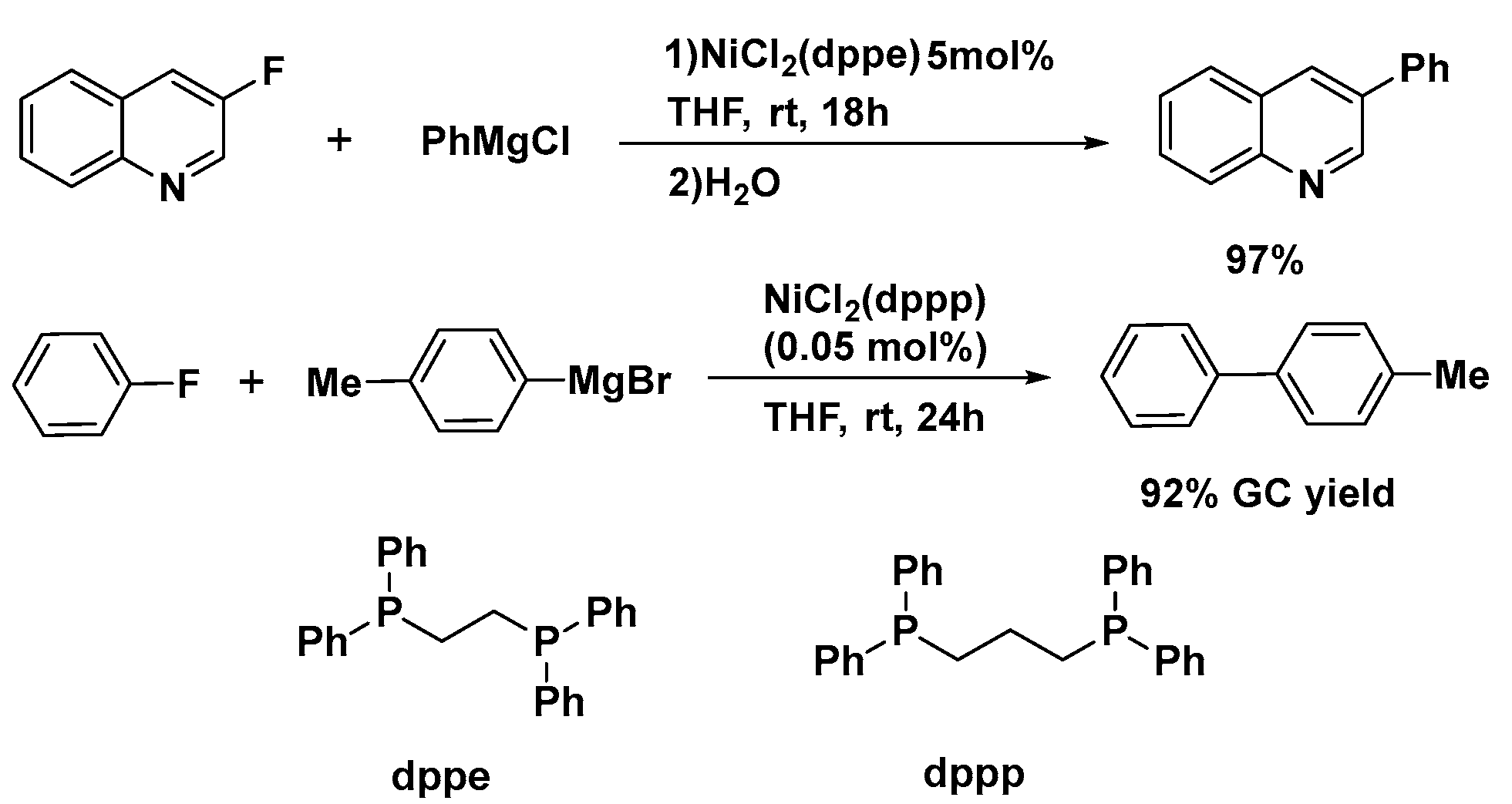
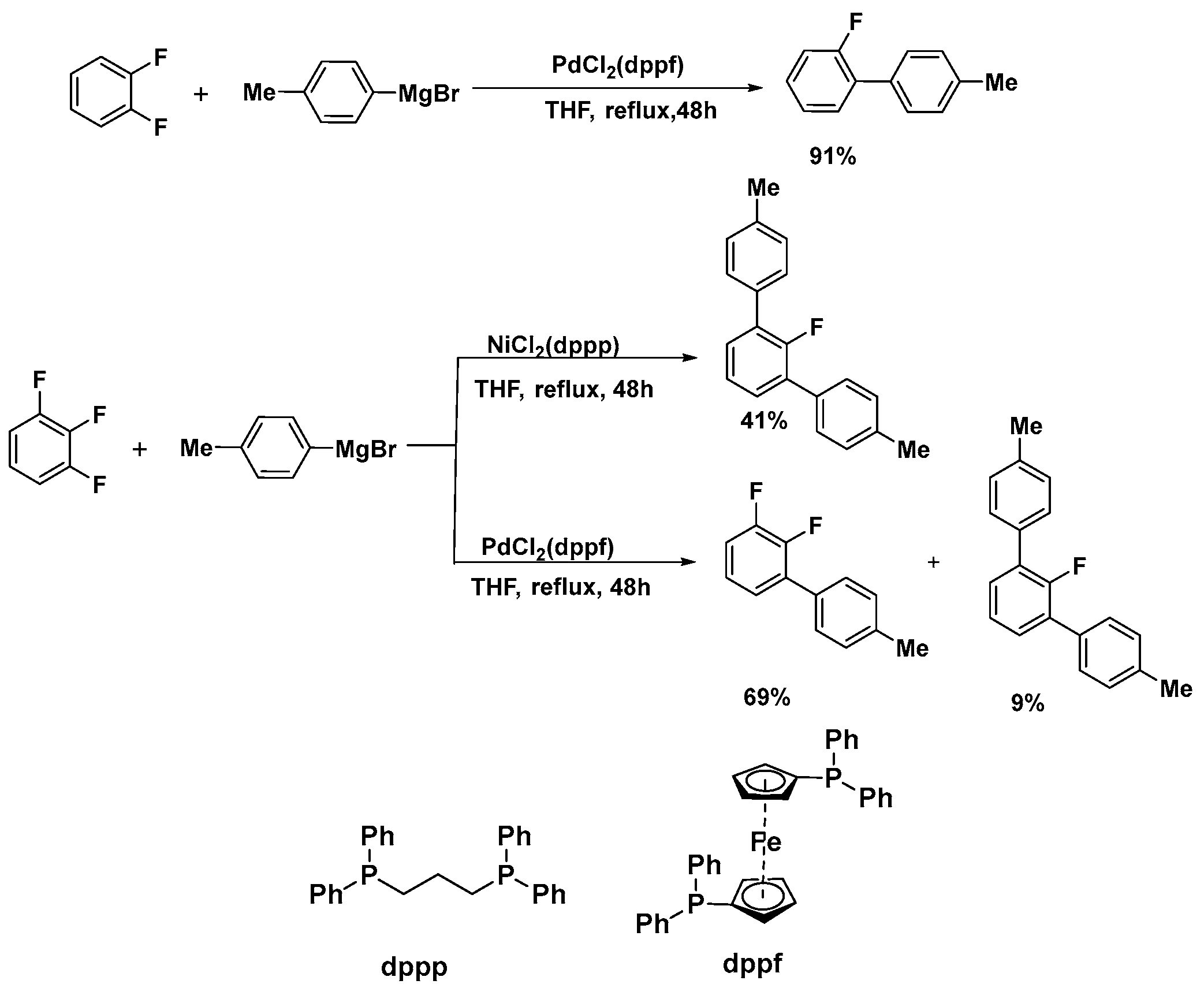
2.1. Ni-Catalyzed Activation of C–F Bonds in Fluoro-Aromatics
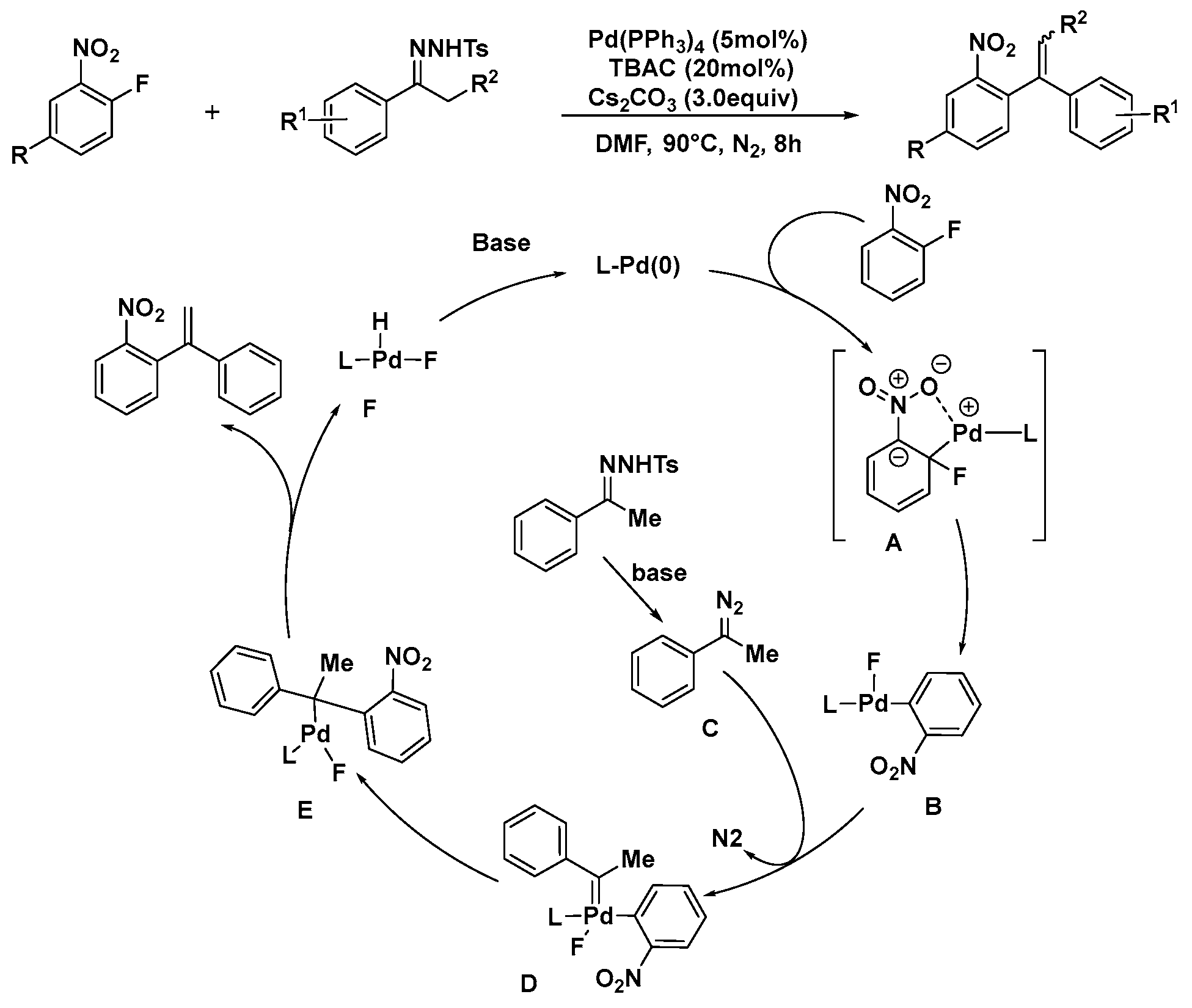
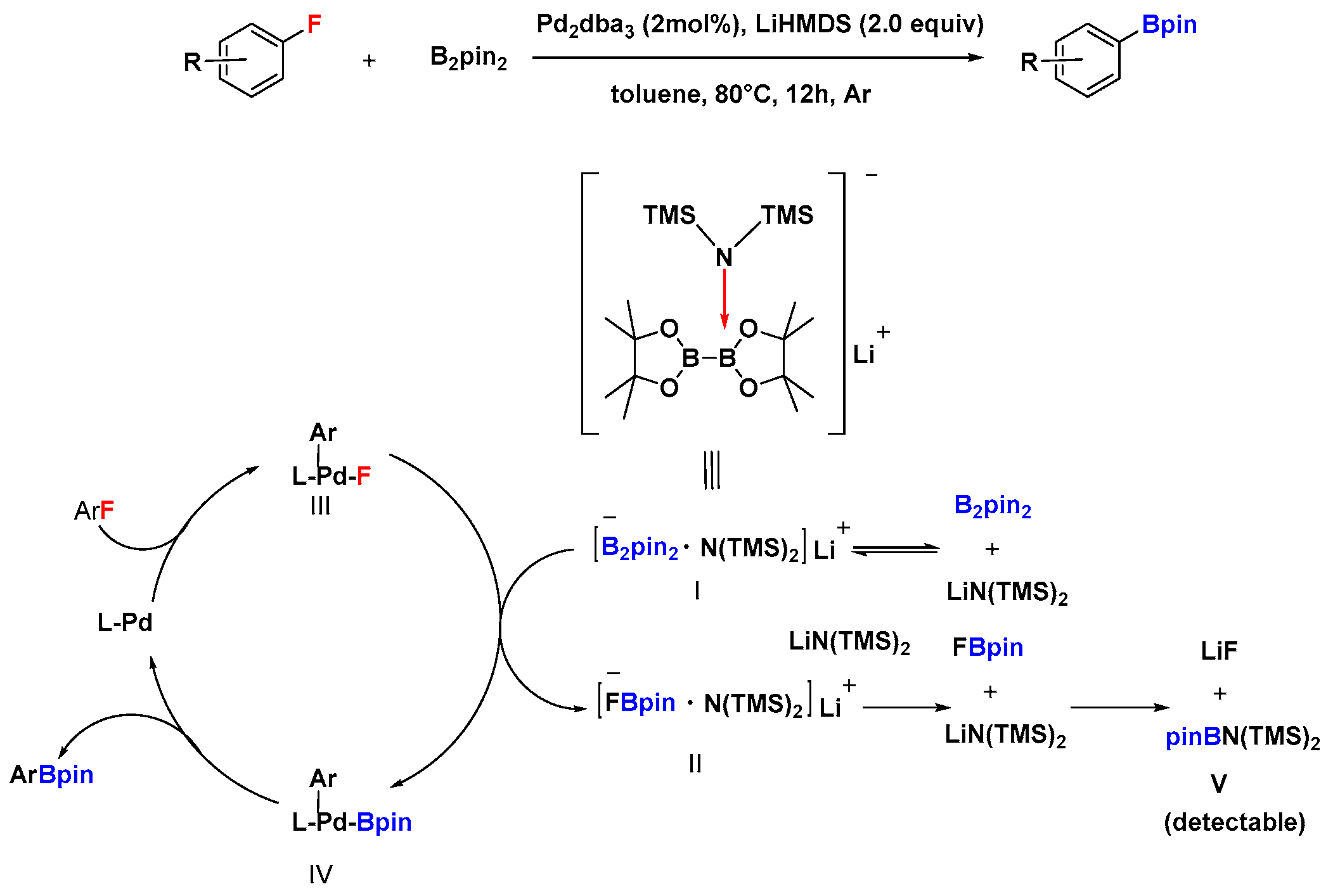
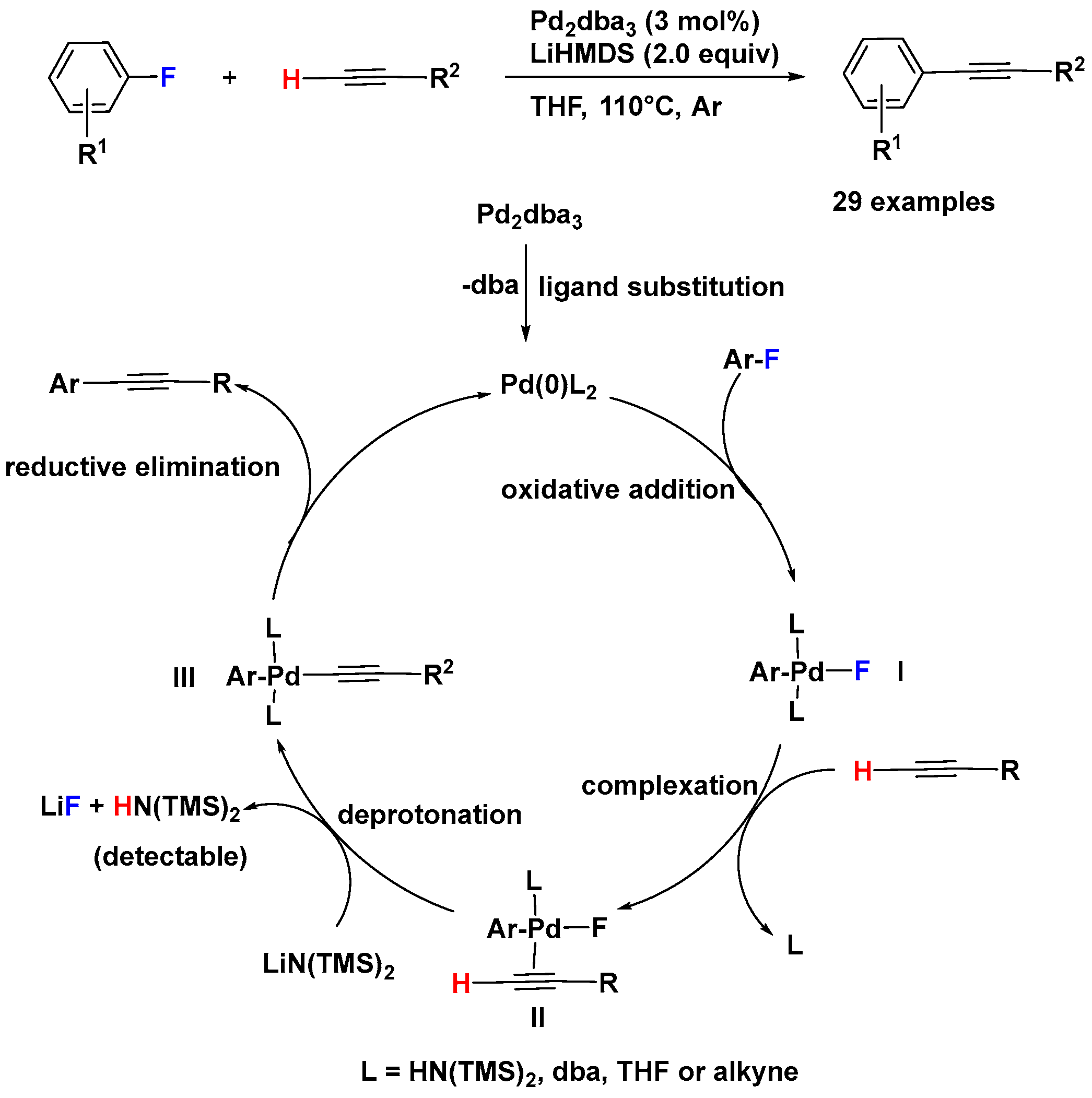
2.2. Pd-Catalyzed Activation of C–F Bonds in Fluoro-Aromatics
2.3. Other Transition Metal Complexes Catalyzed C–F Activation of Fluoro-Aromatics
3. Activation of C–F Bond in Fluoro-Aromatics Promoted by Transition Metal-Free Processes
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Politanskaya, L.V.; Selivanova, G.A.; Panteleeva, E.V.; Tretyakov, E.V.; Platonov, V.E.; Nikul’shin, P.V.; Vinogradov, A.S.; Zonov, Y.V.; Karpov, V.M.; Mezhenkova, T.V. Organofluorine chemistry: Promising growth areas and challenges. Russ. Chem. Rev. 2019, 88, 425–569. [Google Scholar] [CrossRef]
- O’Hagan, D. Understanding organofluorine chemistry. An introduction to the C–F bond. Chem. Soc. Rev. 2008, 37, 308–319. [Google Scholar] [CrossRef] [PubMed]
- Tsui, G.C.; Hu, J. Organofluorine chemistry. Asian J. Org. Chem. 2019, 8, 566–567. [Google Scholar] [CrossRef]
- Uneyama, K. Organofluorine Chemistry; 9600 Garsington Road; Blackwell Publishing Ltd.: Oxford, UK, 2008; pp. 1–90. [Google Scholar]
- Zhou, Y.; Wang, J.; Gu, Z.; Wang, S.; Zhu, W.; Aceña, J.L.; Soloshonok, V.A.; Izawa, K.; Liu, H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem. Rev. 2016, 116, 422–518. [Google Scholar] [CrossRef]
- Reichel, M.; Karaghiosoff, K. Reagents for selective fluoromethylation: A challenge in organofluorine chemistry. Angew. Chem. Int. Ed. 2020, 59, 12268–12281. [Google Scholar] [CrossRef]
- Meera, G.; Rohit, K.; Treesa, G.S.; Anilkumar, G. Advances and Prospects in Gold-Catalyzed C–H Activation. Asian. J. Org. Chem. 2020, 9, 144–161. [Google Scholar] [CrossRef]
- Bumberger, A.E.; Gordon, C.P.; Trummer, D.; Copéret, C. C–H Activation and Olefin Insertion in d8 and d0 Complexes: Same Elementary Steps, Different Electronics. Helv. Chim. Acta 2020, 103, e1900278. [Google Scholar] [CrossRef]
- Jin, L.; Yao, Q.-J.; Xie, P.-P.; Li, Y.; Zhan, B.-B.; Han, Y.-Q.; Hong, X.; Shi, B.-F. Atroposelective synthesis of axially chiral styrenes via an asymmetric C–H functionalization strategy. Chem 2020, 6, 497–511. [Google Scholar] [CrossRef]
- Liu, M.; Niu, J.-L.; Yang, D.; Song, M.-P. Development of a traceless directing group: Cp*-free cobalt-catalyzed C–H activation/annulations to access isoquinolinones. J. Org. Chem. 2020, 85, 4067–4078. [Google Scholar] [CrossRef]
- Palumbo, C.T.; Scopelliti, R.; Zivkovic, I.; Mazzanti, M. C–H bond activation by an isolated dinuclear U (III)/U (IV) nitride. J. Am. Chem. Soc. 2020, 142, 3149–3157. [Google Scholar] [CrossRef]
- Shi, Y.; Xing, H.; Huang, T.; Liu, X.; Chen, J.; Guo, X.; Li, G.-B.; Wu, Y. Divergent C–H activation synthesis of chalcones, quinolones and indoles. Chem. Commun. 2020, 56, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Noor, A.; Qayyum, S.; Schwarz, S.; Dietel, T.; Kempe, R. Formation of a dimeric tungsten (i) complex via C–H activation. Dalton Trans. 2020, 49, 1992–1996. [Google Scholar] [CrossRef] [PubMed]
- Bhaskararao, B.; Singh, S.; Anand, M.; Verma, P.; Prakash, P.; Athira, C.; Malakar, S.; Schaefer, H.F.; Sunoj, R.B. Is silver a mere terminal oxidant in palladium catalyzed C–H bond activation reactions? Chem. Sci. 2020, 11, 208–216. [Google Scholar] [CrossRef]
- Hadlington, T.J.; Kostenko, A.; Driess, M. Cycloaddition Chemistry of a Silylene-Nickel Complex toward Organic π-Systems: From Reversibility to C–H Activation. Chem.-Eur. J. 2020, 26, 1958–1962. [Google Scholar] [CrossRef] [PubMed]
- Fricke, C.; Dahiya, A.; Reid, W.B.; Schoenebeck, F. Gold-catalyzed C–H functionalization with aryl germanes. ACS Catal. 2019, 9, 9231–9236. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.-N.; Wang, G.; Poelman, D.; Van Der Voort, P. Metal organic frameworks based materials for heterogeneous photocatalysis. Molecules 2018, 23, 2947. [Google Scholar] [CrossRef]
- Li, G.H.; Dong, D.Q.; Yang, Y.; Yu, X.Y.; Wang, Z.L. Direct Carbamoylation of Quinoline N-oxides with Hydrazinecarboxamides via C–H Bond Activation Catalyzed by Copper Catalyst. Adv. Synth. Catal. 2019, 361, 832–835. [Google Scholar]
- Ren, W.; Jin, M.; Zuo, Q.-M.; Yang, S.-D. Allylation of β-amino phosphonic acid precursor via palladium-NHC catalyzed allylic C–H activation. Org. Chem. Front. 2020, 7, 298–302. [Google Scholar] [CrossRef]
- Deng, L.; Dong, G. Carbon–carbon bond activation of ketones. Trends Chem. 2020, 2, 183–198. [Google Scholar] [CrossRef]
- Zhong, J.; Long, Y.; Yan, X.; He, S.; Ye, R.; Xiang, H.; Zhou, X. Rhodium-catalyzed pyridine N-oxide assisted Suzuki–Miyaura coupling reaction via C (O)–C bond activation. Org. Lett. 2019, 21, 9790–9794. [Google Scholar] [CrossRef]
- Nanda, T.; Ravikumar, P. A Palladium-Catalyzed Cascade C–C Activation of Cyclopropenone and Carbonylative Amination: Easy Access to Highly Functionalized Maleimide Derivatives. Org. Lett. 2020, 22, 1368–1374. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Lin, Z. Theoretical studies on Rh-catalyzed cycloisomerization of homopropargylallene-alkynes through C (sp3)–C (sp) bond activation. ACS Catal. 2020, 10, 1828–1837. [Google Scholar] [CrossRef]
- Long, Y.; Su, Z.; Zheng, Y.; He, S.; Zhong, J.; Xiang, H.; Zhou, X. Rhodium-catalyzed transarylation of benzamides: C–C bond vs C–N bond activation. ACS Catal. 2020, 10, 3398–3403. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, P.-h.; Lu, G.; Liu, P.; Dong, G. Ruthenium-catalyzed reductive cleavage of unstrained aryl–aryl bonds: Reaction development and mechanistic study. J. Am. Chem. Soc. 2019, 141, 18630–18640. [Google Scholar] [CrossRef] [PubMed]
- Ambler, B.R.; Turnbull, B.W.; Suravarapu, S.R.; Uteuliyev, M.M.; Huynh, N.O.; Krische, M.J. Enantioselective Ruthenium-Catalyzed Benzocyclobutenone–Ketol Cycloaddition: Merging C–C Bond Activation and Transfer Hydrogenative Coupling for Type II Polyketide Construction. J. Am. Chem. Soc. 2018, 140, 9091–9094. [Google Scholar] [CrossRef] [PubMed]
- Avullala, T.; Asgari, P.; Hua, Y.; Bokka, A.; Ridlen, S.G.; Yum, K.; Dias, H.R.; Jeon, J. Umpolung α-Silylation of Cyclopropyl Acetates via Low-Temperature Catalytic C–C Activation. ACS Catal. 2018, 9, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Lübbesmeyer, M.; Mackay, E.G.; Raycroft, M.A.; Elfert, J.; Pratt, D.A.; Studer, A. Base-Promoted C–C Bond Activation Enables Radical Allylation with Homoallylic Alcohols. J. Am. Chem. Soc. 2020, 142, 2609–2616. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Wang, F.; Qian, P. Density functional calculations for Rh (I)-catalyzed C–C bond activation of siloxyvinylcyclopropanes and diazoesters. Appl. Organomet. Chem. 2019, 33, e4869. [Google Scholar] [CrossRef]
- Xia, Y.; Wang, J.; Dong, G. Suzuki–Miyaura coupling of simple ketones via activation of unstrained carbon–carbon bonds. J. Am. Chem. Soc. 2018, 140, 5347–5351. [Google Scholar] [CrossRef]
- Yu, C.-G.; Matsuo, Y. Nickel-catalyzed deaminative acylation of activated aliphatic amines with aromatic amides via C–N bond activation. Org. Lett. 2020, 22, 950–955. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Yang, B. Chemical transformations of quaternary ammonium salts via C–N bond cleavage. Org. Biomol. Chem. 2020, 18, 1057–1072. [Google Scholar] [CrossRef] [PubMed]
- Kadam, A.A.; Metz, T.L.; Qian, Y.; Stanley, L.M. Ni-catalyzed three-component alkene carboacylation initiated by amide C–N bond activation. ACS Catal. 2019, 9, 5651–5656. [Google Scholar] [CrossRef]
- Li, C.-L.; Jiang, X.; Lu, L.-Q.; Xiao, W.-J.; Wu, X.-F. Cobalt (II)-catalyzed alkoxycarbonylation of aliphatic amines via C–N bond activation. Org. Lett. 2019, 21, 6919–6923. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.; Chauhan, S.; Verma, P.; Singh, S.; Srivastava, V. TBHP-Initiated Transamidation of Secondary Amides via C− N Bond Activation: A Metal-Free Approach. Asian. J. Org. Chem. 2019, 8, 853–857. [Google Scholar] [CrossRef]
- Wang, G.; Shi, Q.; Hu, W.; Chen, T.; Guo, Y.; Hu, Z.; Gong, M.; Guo, J.; Wei, D.; Fu, Z. Organocatalytic asymmetric N-sulfonyl amide CN bond activation to access axially chiral biaryl amino acids. Nat. Commun. 2020, 11, 946. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, M.M.; Xiong, W.; Lu, L.Q.; Xiao, W.J. Deaminative (Carbonylative) Alkyl-Heck-type Reactions Enabled by Photocatalytic C− N Bond Activation. Angew. Chem. Int. Ed. 2019, 58, 2402–2406. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, B.; Gao, B.; Zhang, T.; Huang, H. Triple-bond insertion triggers highly regioselective 1, 4-aminomethylamination of 1, 3-enynes with aminals enabled by Pd-catalyzed C–N bond activation. Org. Lett. 2019, 21, 535–539. [Google Scholar] [CrossRef]
- Zhou, T.; Ji, C.-L.; Hong, X.; Szostak, M. Palladium-catalyzed decarbonylative Suzuki–Miyaura cross-coupling of amides by carbon–nitrogen bond activation. Chem. Sci. 2019, 10, 9865–9871. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, S.-Q.; Hong, X. Computational studies on Ni-catalyzed amide C–N bond activation. Chem. Commun. 2019, 55, 11330–11341. [Google Scholar] [CrossRef]
- Lang, S.M.; Bernhardt, T.M.; Bakker, J.M.; Yoon, B.; Landman, U. Methanol C–O Bond Activation by Free Gold Clusters Probed via Infrared Photodissociation Spectroscopy. Z. Phys. Chem. 2019, 233, 865–880. [Google Scholar] [CrossRef]
- Goulas, K.A.; Mironenko, A.V.; Jenness, G.R.; Mazal, T.; Vlachos, D.G. Fundamentals of C–O bond activation on metal oxide catalysts. Nat. Catal. 2019, 2, 269–276. [Google Scholar] [CrossRef]
- Khakyzadeh, V.; Rostami, A.; Veisi, H.; Shaghasemi, B.S.; Reimhult, E.; Luque, R.; Xia, Y.; Darvishi, S. Direct C–S bond formation via C–O bond activation of phenols in a crossover Pd/Cu dual-metal catalysis system. Org. Biomol. Chem. 2019, 17, 4491–4497. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Bai, C.; Bao, Y.-S. Heterogeneous Suzuki–Miyaura coupling of heteroaryl ester via chemoselective C (acyl)–O bond activation. RSC Adv. 2019, 9, 17266–17272. [Google Scholar] [CrossRef] [PubMed]
- Tobisu, M.; Chatani, N. Nickel-catalyzed cross-coupling reactions of unreactive phenolic electrophiles via C–O bond activation. In Ni-and Fe-Based Cross-Coupling Reactions; Springer: Berlin/Heidelberg, Germany, 2017; pp. 129–156. [Google Scholar]
- Bisz, E.; Szostak, M. Iron-Catalyzed C− O Bond Activation: Opportunity for Sustainable Catalysis. ChemSusChem 2017, 10, 3964–3981. [Google Scholar] [CrossRef] [PubMed]
- Hoang, G.T.; Walsh, D.J.; McGarry, K.A.; Anderson, C.B.; Douglas, C.J. Development and mechanistic study of quinoline-directed acyl C–O bond activation and alkene oxyacylation reactions. J. Org. Chem. 2017, 82, 2972–2983. [Google Scholar] [CrossRef] [PubMed]
- Mondal, M.; Begum, T.; Bora, U. Chemoselective acyl C–O bond activation in esters for Suzuki–Miyaura coupling. Org. Chem. Front. 2017, 4, 1430–1434. [Google Scholar] [CrossRef]
- Malapit, C.A.; Caldwell, D.R.; Sassu, N.; Milbin, S.; Howell, A.R. Pd-catalyzed acyl C–O bond activation for selective ring-opening of α-methylene-β-lactones with amines. Org. Lett. 2017, 19, 1966–1969. [Google Scholar] [CrossRef]
- Purohit, P.; Seth, K.; Kumar, A.; Chakraborti, A.K. C–O Bond Activation by Nickel–Palladium Hetero-Bimetallic Nanoparticles for Suzuki–Miyaura Reaction of Bioactive Heterocycle-Tethered Sterically Hindered Aryl Carbonates. ACS Catal. 2017, 7, 2452–2457. [Google Scholar] [CrossRef]
- Wiensch, E.M.; Montgomery, J. Nickel-Catalyzed Amination of Silyloxyarenes through C–O Bond Activation. Angew. Chem. Int. Ed. 2018, 57, 11045–11049. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Gao, F.; Shen, L. Advances in Transition Metal-Catalyzed Selective Functionalization of Inert C–O Bonds Assisted by Directing Groups. Adv. Synth. Catal. 2019, 361, 3915–3924. [Google Scholar] [CrossRef]
- Iyori, Y.; Takahashi, K.; Yamazaki, K.; Ano, Y.; Chatani, N. Nickel-catalyzed reductive defunctionalization of esters in the absence of an external reductant: Activation of C–O bonds. Chem. Commun. 2019, 55, 13610–13613. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.A. Recent advances in the selective formation of the carbon-fluorine bond. Chem. Rev. 1992, 92, 505–519. [Google Scholar] [CrossRef]
- Furuya, T.; Kuttruff, C.A.; Ritter, T. Carbon-fluorine bond formation. Curr. Opin. Drug Discov. Devel. 2008, 11, 803–819. [Google Scholar] [PubMed]
- Furuya, T.; Klein, J.E.; Ritter, T. Carbon-fluorine bond formation for the synthesis of aryl fluorides. Synthesis 2010, 2010, 1804–1821. [Google Scholar] [PubMed]
- Barthazy, P.; Hintermann, L.; Stoop, R.M.; Wörle, M.; Mezzetti, A.; Togni, A. Carbon–Fluorine Bond Formation via a Five-Coordinate Fluoro Complex of Ruthenium (II), Preliminary Communication. Helv. Chim. Acta 1999, 82, 2448–2453. [Google Scholar] [CrossRef]
- Campbell, M.G.; Ritter, T. Late-Stage Formation of Carbon–Fluorine Bonds. Chem. Rec. 2014, 14, 482–491. [Google Scholar] [CrossRef]
- Neumann, C.N.; Ritter, T. Facile C–F bond formation through a concerted nucleophilic aromatic substitution mediated by the phenofluor reagent. Acc. Chem. Res. 2017, 50, 2822–2833. [Google Scholar] [CrossRef]
- Pfeifer, L. New Methods and Reagents for Carbon-Fluorine Bond Formation; University of Oxford: Oxford, UK, 2016. [Google Scholar]
- Ye, Y.; Schimler, S.D.; Hanley, P.S.; Sanford, M.S. Cu (OTf) 2-mediated fluorination of aryltrifluoroborates with potassium fluoride. J. Am. Chem. Soc. 2013, 135, 16292–16295. [Google Scholar] [CrossRef]
- Park, N.H.; Senter, T.J.; Buchwald, S.L. Rapid synthesis of aryl fluorides in continuous flow through the Balz–Schiemann reaction. Angew. Chem. Int. Ed. 2016, 128, 12086–12090. [Google Scholar] [CrossRef]
- Thomas, H.P.; Wang, Y.-M.; Lorenzini, F.; Coles, S.J.; Horton, P.N.; Marr, A.C.; Saunders, G.C. Cyclometalation via carbon–fluorine bond activation induced by silver particles. Organometallics 2017, 36, 960–963. [Google Scholar] [CrossRef]
- Fujita, T.; Fuchibe, K.; Ichikawa, J. Transition-Metal-Mediated and-Catalyzed C–F Bond Activation by Fluorine Elimination. Angew. Chem. Int. Ed. 2019, 58, 390–402. [Google Scholar] [CrossRef]
- Wei, J.; Liu, K.-M.; Duan, X.-F. Cobalt-catalyzed biaryl couplings via C–F bond activation in the absence of phosphine or NHC ligands. J. Org. Chem. 2017, 82, 1291–1300. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.-Q.; Wang, R.; Song, H.; Liu, Y.; Wang, Q. Visible-light-induced deoxygenation/defluorination protocol for synthesis of γ, γ-difluoroallylic ketones. Org. Lett. 2020, 22, 709–713. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.; Wang, S.; Norton, J.; Hammond, M. Catalyzing the hydrodefluorination of CF3-substituted alkenes by PhSiH3. H• transfer from a nickel hydride. J. Am. Chem. Soc. 2020, 142, 4793–4799. [Google Scholar] [CrossRef]
- Briceno-Strocchia, A.I.; Johnstone, T.C.; Stephan, D.W. Using frustrated Lewis pairs to explore C–F bond activation. Dalton Trans. 2020, 49, 1319–1324. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.-A.M.; Suliman, A.M.; Gong, T.-J.; Fu, Y. Palladium-catalyzed stereoselective defluorination arylation/alkenylation/alkylation of gem-difluorinated cyclopropanes. Org. Lett. 2019, 21, 5645–5649. [Google Scholar] [CrossRef]
- Coates, G.; Tan, H.Y.; Kalff, C.; White, A.J.; Crimmin, M.R. Defluorosilylation of industrially relevant fluoroolefins using nucleophilic silicon reagents. Angew. Chem. Int. Ed. 2019, 58, 12514–12518. [Google Scholar] [CrossRef]
- Ding, D.; Lan, Y.; Lin, Z.; Wang, C. Synthesis of gem-difluoroalkenes by merging Ni-catalyzed C–F and C–C bond activation in cross-electrophile coupling. Org. Lett. 2019, 21, 2723–2730. [Google Scholar] [CrossRef]
- He, Y.; Anand, D.; Sun, Z.; Zhou, L. Visible-light-promoted redox neutral γ, γ-difluoroallylation of cycloketone oxime ethers with trifluoromethyl alkenes via C–C and C–F bond cleavage. Org. Lett. 2019, 21, 3769–3773. [Google Scholar] [CrossRef]
- Jiang, L.-F.; Ren, B.-T.; Li, B.; Zhang, G.-Y.; Peng, Y.; Guan, Z.-Y.; Deng, Q.-H. Nucleophilic substitution of gem-difluoroalkenes with TMSNu promoted by catalytic amounts of Cs2CO3. J. Org. Chem. 2019, 84, 6557–6564. [Google Scholar] [CrossRef]
- Kondoh, A.; Koda, K.; Terada, M. Organocatalytic Nucleophilic Substitution Reaction of gem-Difluoroalkenes with Ketene Silyl Acetals. Org. Lett. 2019, 21, 2277–2280. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhu, C.; Jiang, H. Single C (sp3)–F Bond Activation in a CF3 Group: Ipso-Defluorooxylation of (Trifluoromethyl) alkenes with Oximes. Org. Lett. 2019, 21, 1130–1133. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.-S.; Wu, D.-S.; Ye, J.-H.; Gong, L.; Zeng, X.; Ran, C.-K.; Gui, Y.-Y.; Li, J.; Yu, D.-G. Copper-Catalyzed Carboxylation of C–F bonds with CO2. ACS Catal. 2019, 9, 6987–6992. [Google Scholar] [CrossRef]
- Ma, T.; Chen, Y.; Li, Y.; Ping, Y.; Kong, W. Nickel-catalyzed enantioselective reductive aryl fluoroalkenylation of alkenes. ACS Catal. 2019, 9, 9127–9133. [Google Scholar] [CrossRef]
- Kiplinger, J.L.; Richmond, T.G.; Osterberg, C.E. Activation of carbon-fluorine bonds by metal complexes. Chem. Rev. 1994, 94, 373–431. [Google Scholar] [CrossRef]
- Burdeniuc, J.; Jedicka, B.; Crabtree, R.H. Recent advances in C–F bond activation. Chem. Ber. 1997, 130, 145–154. [Google Scholar] [CrossRef]
- Torrens, H. Carbonfluorine bond activation by platinum group metal complexes. Coord. Chem. Rev. 2005, 249, 1957–1985. [Google Scholar] [CrossRef]
- Wilklow-Marnell, M.; Brennessel, W.W.; Jones, W.D. C (sp 2)–F Oxidative Addition of Fluorinated Aryl Ketones by iPrPCPIr. Organometallics 2017, 36, 3125–3134. [Google Scholar] [CrossRef]
- Mongin, F.; Mojovic, L.; Guillamet, B.; Trécourt, F.; Quéguiner, G. Cross-coupling reactions of phenylmagnesium halides with fluoroazines and fluorodiazines. J. Org. Chem. 2002, 67, 8991–8994. [Google Scholar] [CrossRef]
- Saeki, T.; Takashima, Y.; Tamao, K. Nickel-and palladium-catalyzed cross-coupling reaction of polyfluorinated arenes and alkenes with Grignard reagents. Synlett 2005, 2005, 1771–1774. [Google Scholar] [CrossRef]
- Dankwardt, J.W. Transition metal catalyzed cross-coupling of aryl Grignard reagents with aryl fluorides via Pd-or Ni-activation of the C–F bond: An efficient synthesis of unsymmetrical biaryls–application of microwave technology in ligand and catalyst screening. J. Organomet. Chem. 2005, 690, 932–938. [Google Scholar] [CrossRef]
- Xiao, S.-H.; Xiong, Y.; Zhang, X.-X.; Cao, S. Nickel-catalyzed N-heterocycle-directed cross-coupling of fluorinated arenes with organozinc reagents. Tetrahedron 2014, 70, 4405–4411. [Google Scholar] [CrossRef]
- He, Y.; Chen, Z.; He, C.Y.; Zhang, X. Nickel-Catalyzed ortho-Selective Hydrodefluorination of N-Containing Heterocycle-Polyfluoroarenes. Chin. J. Chem. 2013, 31, 873–877. [Google Scholar] [CrossRef]
- Cui, B.; Jia, S.; Tokunaga, E.; Shibata, N. Defluorosilylation of fluoroarenes and fluoroalkanes. Nat. Commun. 2018, 9, 4393. [Google Scholar] [CrossRef] [PubMed]
- Harada, T.; Ueda, Y.; Iwai, T.; Sawamura, M. Nickel-catalyzed amination of aryl fluorides with primary amines. Chem. Commun. 2018, 54, 1718–1721. [Google Scholar] [CrossRef]
- Yin, Y.; Yue, X.; Zhong, Q.; Jiang, H.; Bai, R.; Lan, Y.; Zhang, H. Ni-Catalyzed C–F Bond Functionalization of Unactivated Aryl Fluorides and Corresponding Coupling with Oxazoles. Adv. Synth. Catal. 2018, 360, 1639–1643. [Google Scholar] [CrossRef]
- Kim, Y.M.; Yu, S. Palladium (0)-catalyzed amination, Stille coupling, and Suzuki coupling of electron-deficient aryl fluorides. J. Am. Chem. Soc. 2003, 125, 1696–1697. [Google Scholar] [CrossRef]
- Albéniz, A.C.; Casares, J.A. Palladium-mediated organofluorine chemistry. Adv. Organomet. Chem. 2014, 62, 1–110. [Google Scholar]
- Dehury, N.; Maity, N.; Tripathy, S.K.; Basset, J.-M.; Patra, S. Dinuclear tetrapyrazolyl palladium complexes exhibiting facile tandem transfer hydrogenation/Suzuki coupling reaction of fluoroarylketone. ACS Catal. 2016, 6, 5535–5540. [Google Scholar] [CrossRef]
- Widdowson, D.A.; Wilhelm, R. Palladium catalysed Suzuki reactions of fluoroarenes. Chem. Commun. 2003, 578–579. [Google Scholar] [CrossRef]
- Luo, H.; Wu, G.; Xu, S.; Wang, K.; Wu, C.; Zhang, Y.; Wang, J. Palladium-catalyzed cross-coupling of aryl fluorides with N-tosylhydrazones via C–F bond activation. Chem. Commun. 2015, 51, 13321–13323. [Google Scholar] [CrossRef] [PubMed]
- Manabe, K.; Ishikawa, S. Ortho-selective cross-coupling of fluorobenzenes with Grignard reagents: Acceleration by electron-donating ortho-directing groups. Synthesis 2008, 2008, 2645–2649. [Google Scholar] [CrossRef]
- Zhao, X.; Wu, M.; Liu, Y.; Cao, S. LiHMDS-promoted palladium or iron-catalyzed ipso-defluoroborylation of aryl fluorides. Org. Lett. 2018, 20, 5564–5568. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Yang, K.; Zhao, J.; Cao, S. LiHMDS-promoted palladium-catalyzed Sonogashira cross-coupling of aryl fluorides with terminal alkynes. Org. Lett. 2019, 21, 9714–9718. [Google Scholar] [CrossRef]
- Chen, Z.; He, C.Y.; Yin, Z.; Chen, L.; He, Y.; Zhang, X. Palladium-Catalyzed Ortho-Selective C-F Activation of Polyfluoroarenes with Triethylsilane: A Facile Access to Partially Fluorinated Aromatics. Angew. Chem. Int. Ed. 2013, 52, 5813–5817. [Google Scholar] [CrossRef]
- Luo, Z.-J.; Zhao, H.-Y.; Zhang, X. Highly selective Pd-catalyzed direct C–F bond arylation of polyfluoroarenes. Org. Lett. 2018, 20, 2543–2546. [Google Scholar] [CrossRef]
- Gair, J.J.; Grey, R.L.; Giroux, S.; Brodney, M.A. Palladium catalyzed hydrodefluorination of fluoro-(hetero) arenes. Org. Lett. 2019, 21, 2482–2487. [Google Scholar] [CrossRef]
- Matsunami, A.; Kayaki, Y.; Kuwata, S.; Ikariya, T. Nucleophilic Aromatic Substitution in Hydrodefluorination Exemplified by Hydridoiridium (III) Complexes with Fluorinated Phenylsulfonyl-1, 2-diphenylethylenediamine Ligands. Organometallics 2018, 37, 1958–1969. [Google Scholar] [CrossRef]
- Lv, H.; Cai, Y.B.; Zhang, J.L. Copper-Catalyzed Hydrodefluorination of Fluoroarenes by Copper Hydride Intermediates. Angew. Chem. Int. Ed. 2013, 52, 3203–3207. [Google Scholar] [CrossRef]
- Ekkert, O.; Strudley, S.D.; Rozenfeld, A.; White, A.J.; Crimmin, M.R. Rhodium Catalyzed, Carbon–Hydrogen Bond Directed Hydrodefluorination of Fluoroarenes. Organometallics 2014, 33, 7027–7030. [Google Scholar] [CrossRef]
- Chen, J.; Huang, D.; Ding, Y. Rhodium-Catalyzed ortho-Selective C-F Activation and Hydrodefluorination of Heterocycle-Substituted Polyfluoroarenes: Dominated by Phosphine Ligands. Chem. Sel. 2017, 2, 1219–1224. [Google Scholar]
- Iwasaki, T.; Yamashita, K.; Kuniyasu, H.; Kambe, N. Co-catalyzed cross-coupling reaction of alkyl fluorides with alkyl Grignard reagents. Org. Lett. 2017, 19, 3691–3694. [Google Scholar] [CrossRef]
- Lim, S.; Song, D.; Jeon, S.; Kim, Y.; Kim, H.; Lee, S.; Cho, H.; Lee, B.C.; Kim, S.E.; Kim, K. Cobalt-Catalyzed C–F Bond Borylation of Aryl Fluorides. Org. Lett. 2018, 20, 7249–7252. [Google Scholar] [CrossRef] [PubMed]
- Aoki, Y.; O’Brien, H.M.; Kawasaki, H.; Takaya, H.; Nakamura, M. Ligand-Free Iron-Catalyzed C–F Amination of Diarylamines: A One-Pot Regioselective Synthesis of Diaryl Dihydrophenazines. Org. Lett. 2019, 21, 461–464. [Google Scholar] [CrossRef]
- Kuehnel, M.F.; Lentz, D.; Braun, T. Synthesis of fluorinated building blocks by transition-metal-mediated hydrodefluorination reactions. Angew. Chem. Int. Ed. 2013, 52, 3328–3348. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Zhao, L.; Luo, M.; Zeng, X. Chromium-Catalyzed Selective Cross-Electrophile Coupling between Unactivated C (aryl)–F and C (aryl)–O Bonds. Organometallics 2022, 41, 561–568. [Google Scholar] [CrossRef]
- Matsunami, A.; Kuwata, S.; Kayaki, Y. Regioselective Transfer Hydrogenative Defluorination of Polyfluoroarenes Catalyzed by Bifunctional Azairidacycle. Organics 2022, 3, 150–160. [Google Scholar] [CrossRef]
- Ahrens, T.; Kohlmann, J.; Ahrens, M.; Braun, T. Functionalization of fluorinated molecules by transition-metal-mediated C–F bond activation to access fluorinated building blocks. Chem. Rev. 2015, 115, 931–972. [Google Scholar] [CrossRef]
- Chen, J.; Huang, D.; Ding, Y. C–N Bond Coupling of Fluorobenzenes: N-Heterocycle-Assisted Selective C–F Bond Cleavage through an Li/F Interaction. Eur. J. Org. Chem. 2017, 2017, 4300–4304. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, H.; Jia, J.; Du, A.; Li, X. Transition-Metal-Free Synthesis of Fluorinated Arenes from Perfluorinated Arenes Coupled with Grignard Reagents. Organometallics 2014, 33, 1079–1081. [Google Scholar] [CrossRef]
- Lu, F.; Sun, H.; Du, A.; Feng, L.; Li, X. Selective alkylation and arylation of C–F bond with Grignard reagents. Org. Lett. 2014, 16, 772–775. [Google Scholar] [CrossRef]
- Xiong, Y.; Wu, J.; Xiao, S.; Xiao, J.; Cao, S. Noncatalytic pyridyl-directed alkylation and arylation carbon–fluorine bond of polyfluoroarenes with Grignard reagents. J. Org. Chem. 2013, 78, 4599–4603. [Google Scholar] [CrossRef]
- Ji, X.; Li, J.; Wu, M.; Cao, S. Facile Synthesis of 3-Arylindenes by HMPA-Promoted Direct Arylation of Indenes with Aryl Fluorides. ACS Omega 2018, 3, 10099–10106. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, D.; Ding, Y. Transition-metal-free site-selective C–F bond activation for synthesis of 8-aminoquinolines. Tetrahedron Lett. 2017, 58, 4240–4242. [Google Scholar] [CrossRef]
- Platonov, V.E.; Haas, A.; Schelvis, M.; Lieb, M.; Dvornikova, K.V.; Osina, O.I.; Gatilov, Y.V. Polyfluorinated arylnitramines. J. Fluor. Chem. 2001, 109, 131–139. [Google Scholar] [CrossRef]
- Tokárová, Z.; Balogh, R.; Tisovský, P.; Hrnčariková, K.; Vegh, D. Direct nucleophilic substitution of polyfluorobenzenes with pyrrole and 2,5-dimethylpyrrole. J. Fluor. Chem. 2017, 204, 59–64. [Google Scholar] [CrossRef]
- Phillips, N.A.; O’Hanlon, J.; Hooper, T.N.; White, A.J.; Crimmin, M.R. Dihydridoboranes: Selective Reagents for Hydroboration and Hydrodefluorination. Org. Lett. 2019, 21, 7289–7293. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Jiang, H.; Leng, J.; Ong, H.-W.; Wu, J. Visible-Light-Induced Selective Defluoroborylation of Polyfluoroarenes, gem-Difluoroalkenes, and Trifluoromethylalkenes. Angew. Chem. Int. Ed. 2020, 132, 4038–4045. [Google Scholar] [CrossRef]
- Lu, J.; Khetrapal, N.S.; Johnson, J.A.; Zeng, X.C.; Zhang, J. “π-Hole– π” Interaction Promoted Photocatalytic Hydrodefluorination via Inner-Sphere Electron Transfer. J. Am. Chem. Soc. 2016, 138, 15805–15808. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.-J.; Ye, Z.-P.; Hu, Y.-Z.; Xiao, J.-A.; Chen, K.; Xiang, H.-Y.; Chen, X.-Q.; Yang, H. Photocatalytic C–F Bond Borylation of Polyfluoroarenes with NHC-boranes. Org. Lett. 2020, 22, 1742–1747. [Google Scholar] [CrossRef]
- Mallick, S.; Xu, P.; Würthwein, E.U.; Studer, A. Silyldefluorination of Fluoroarenes by Concerted Nucleophilic Aromatic Substitution. Angew. Chem. Int. Ed. 2019, 131, 289–293. [Google Scholar] [CrossRef]
- Su, J.; Chen, Q.; Lu, L.; Ma, Y.; Auyoung, G.H.L.; Hua, R. Base-promoted nucleophilic fluoroarenes substitution of C-F bonds. Tetrahedron 2018, 74, 303–307. [Google Scholar] [CrossRef]
- Hough, S.E.; Hargrove, W.R., Jr.; Deck, P.A. Regioselective, nucleophilic activation of C-F bonds in o-fluoroanilines. J. Fluorine Chem. 2019, 224, 121–126. [Google Scholar] [CrossRef]
- Liu, H.; Yuan, H.; Shi, X. Synthesis of nickel and palladium complexes with diarylamido-based unsymmetrical pincer ligands and application for norbornene polymerization. Dalton Trans. 2019, 48, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Slootweg, J.C.; Jupp, A.R. (Eds.) Frustrated Lewis Pairs. In Molecular Catalysis; Springer: Cham, Switzerland, 2021; Volume 2. [Google Scholar]
- Stephan, D.W. Frustrated Lewis Pairs: From Concept to Catalysis. Acc. Chem. Res. 2015, 48, 306–316. [Google Scholar] [CrossRef] [PubMed]
- Stephan, D.W.; Erker, G. Frustrated Lewis Pairs: Metal-free Hydrogen Activation and More. Angew. Chem. Int. Ed. 2010, 49, 46–76. [Google Scholar] [CrossRef] [PubMed]

































Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, R.; Hu, H.; Li, X.; Mao, G.; Song, Y.; Xin, S. Advances in Catalytic C–F Bond Activation and Transformation of Aromatic Fluorides. Catalysts 2022, 12, 1665. https://doi.org/10.3390/catal12121665
Ma R, Hu H, Li X, Mao G, Song Y, Xin S. Advances in Catalytic C–F Bond Activation and Transformation of Aromatic Fluorides. Catalysts. 2022; 12(12):1665. https://doi.org/10.3390/catal12121665
Chicago/Turabian StyleMa, Rongqing, Hongfan Hu, Xinle Li, Guoliang Mao, Yuming Song, and Shixuan Xin. 2022. "Advances in Catalytic C–F Bond Activation and Transformation of Aromatic Fluorides" Catalysts 12, no. 12: 1665. https://doi.org/10.3390/catal12121665
APA StyleMa, R., Hu, H., Li, X., Mao, G., Song, Y., & Xin, S. (2022). Advances in Catalytic C–F Bond Activation and Transformation of Aromatic Fluorides. Catalysts, 12(12), 1665. https://doi.org/10.3390/catal12121665







