Aerial Oxidation of Phenol/Catechol in the Presence of Catalytic Amounts of [(Cl)2Mn(RCOOET)], RCOOET= Ethyl-5-Methyl-1-(((6-Methyl-3-Nitropyridin-2-yl)Amino)Methyl)-1H-Pyrazole-3-Carboxylate
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structure of C14H17N5O4 (RCOOET)
2.2. IR Analysis
2.3. Thermal Analysis
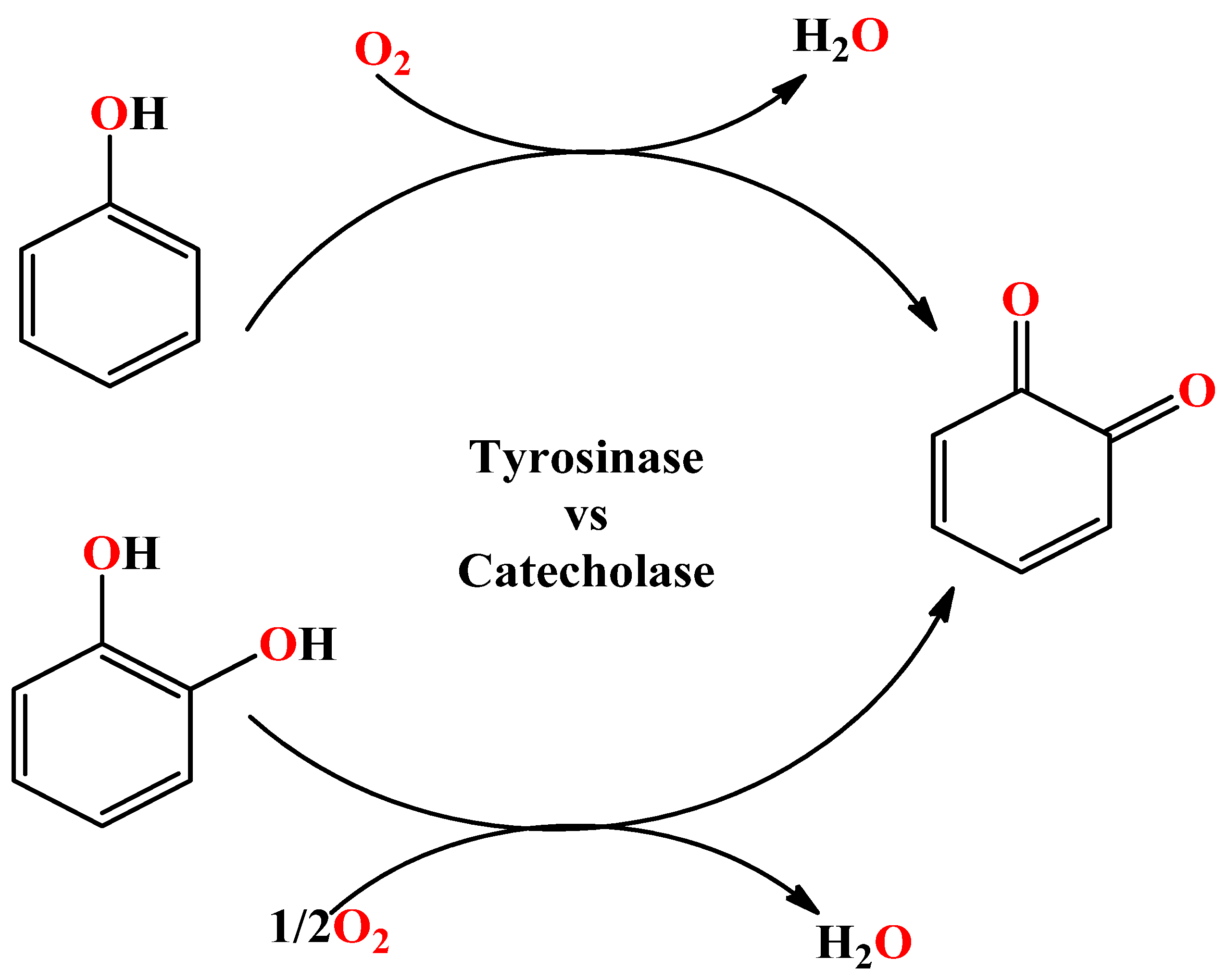
2.4. Catecholase Mimicking Activity of [(Cl)2Mn(RCOOET)]
2.5. Kinetic Study
2.6. Tyrosinase Mimicking Activity of [(Cl)2Mn(RCOOET)]
2.7. Proposed Mechanism of Action of Catechol Oxidase
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Ethyl-5-Methyl-1-(((6-Methyl-3-Nitropyridin-2-yl)Amino)Methyl)-1H-Pyrazole Carboxylate (RCOOET)
3.2.2. X-ray Single Crystal Study of RCOOET
3.2.3. Synthesis of [(Cl)2Mn(RCOOET)]
3.2.4. Catecholase and Tyrosinase Activity of [(Cl)2Mn(RCOOET)]
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gligorich, K.M.; Sigman, M.S. Recent advancements and challenges of palladium II-catalyzed oxidation reactions with molecular oxygen as the sole oxidant. Chem. Commun. 2009, 3854–3867. [Google Scholar] [CrossRef] [PubMed]
- Piera, J.; Backvall, J.E. Catalytic oxidation of organic substrates by molecular oxygen and hydrogen peroxide by multistep electron transfer—A biomimetic approach. Angew. Chem. Int. Ed. 2008, 47, 3506–3523. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S. Palladium-Catalyzed Oxidation of Organic Chemicals with O2. Science 2005, 309, 1824–1826. [Google Scholar] [CrossRef] [PubMed]
- Punniyamurthy, T.; Velusamy, S.J. Recent advances in transition metal catalyzed oxidation of organic substrates with molecular oxygen. Iqbal Chem. Rev. 2005, 105, 2329–2363. [Google Scholar] [CrossRef] [PubMed]
- Stahl, S.S. Palladium oxidase catalysis: Selective oxidation of organic chemicals by direct dioxygen-coupled turnover. Angew. Chem. Int. Ed. 2004, 43, 3400–3420. [Google Scholar] [CrossRef] [PubMed]
- Gerdemann, C.; Eicken, C.; Krebs, B. The crystal structure of catechol oxidase: New insight into the function of type-3 copper proteins. Acc. Chem. Res. 2002, 35, 183–191. [Google Scholar] [CrossRef]
- Palm-Espling, M.E.; Niemiec, M.S.; Wittung-Stafshede, P. Role of metal in folding and stability of copper proteins in vitro. Biochim. Biophys. Acta-Mol. Cell Res. 2012, 1823, 1594–1603. [Google Scholar] [CrossRef]
- Huffman, D.L.; O’Halloran, T.V. Function, structure, and mechanism of intracellular copper trafficking proteins. Annu. Rev. Biochem. 2001, 70, 677–701. [Google Scholar] [CrossRef]
- Puig, S.; Thiele, D.J. Molecular mechanisms of copper uptake and distribution. Curr. Opin. Chem. Biol. 2002, 6, 171–180. [Google Scholar] [CrossRef]
- Harris, E.D. Basic and clinical aspects of copper. Crit. Rev. Clin. Lab. Sci. 2003, 40, 547–586. [Google Scholar] [CrossRef]
- Malmström, B.G. Enzymology of oxygen. Annu. Rev. Biochem. 1982, 51, 21–59. [Google Scholar] [CrossRef]
- Messerschmidt, A.; Rossi, A.; Ladenstein, R.; Huber, R.; Bolognesi, M.; Gatti, G.; Marchesini, A.; Petruzzelli, R.; Finazziagro, A.J. X-ray crystal structure of the blue oxidase ascorbate oxidase from zucchini: Analysis of the polypeptide fold and a model of the copper sites and ligands. Mol. Biol. 1989, 206, 513–529. [Google Scholar] [CrossRef]
- Spira-Solomon, D.J.; Allendorf, M.D.; Solomon, E.I. Low-temperature magnetic circular dichroism studies of native laccase: Confirmation of a trinuclear copper active site. J. Am. Chem. Soc. 1986, 108, 5318–5328. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper oxidases and oxygenases. Chem. Rev. 1996, 96, 2563–2605. [Google Scholar] [CrossRef]
- Gerdemann, C.; Eicken, C.; Galla, H.-J.; Krebs, B.J. Comparative modeling of the latent form of a plant catechol oxidase using a molluskan hemocyanin structure. Inorg. Biochem. 2002, 89, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.; Giri, G.C.; Sen, T.K.; Carrella, L.; Mandal, S.K.; Bera, M. Bis (μ-alkoxo) bridged dinuclear CuII2 and ZnII2 complexes of an isoindol functionality based new ligand: Synthesis, structure, spectral characterization, magnetic properties and catechol oxidase activity. Polyhedron 2014, 67, 495–504. [Google Scholar] [CrossRef]
- Mandal, S.; Mukherjee, J.; Lloret, F.; Mukherjee, F. Modeling Tyrosinase and Catecholase Activity Using New m-Xylyl-Based Ligands with Bidentate Alkylamine Terminal Coordination. Inorg. Chem. 2012, 51, 13148–13161. [Google Scholar] [CrossRef]
- Biswas, A.; Das, L.K.; Drew, M.G.B.; Diaz, C.; Ghosh, A. Insertion of a hydroxido bridge into a diphenoxido dinuclear copper (II) complex: Drastic change of the magnetic property from strong antiferromagnetic to ferromagnetic and enhancement in the catecholase activity. Inorg. Chem. 2012, 51, 10111–10121. [Google Scholar] [CrossRef]
- Agotegaray, M.A.; Dennehy, M.; Boeris, M.A.; Grela, M.A.; Burrow, R.A.; Quin-zani, O.V. Therapeutic properties, SOD and catecholase mimetic activities of novel ternary copper (II) complexes of the anti-inflammatory drug Fenoprofen with imidazole and caffeine. Polyhedron 2012, 34, 74–83. [Google Scholar] [CrossRef]
- Sadhukhan, D.; Ray, A.; Butcher, R.J.; Gomez Garcia, C.J.; Dede, B.; Mitra, S. Magnetic and catalytic properties of a new copper (II)–Schiff base 2D coordination polymer formed by connected helical chains. Inorg. Chim. Acta 2011, 376, 245–254. [Google Scholar] [CrossRef]
- Panda, M.K.; Shaikh, M.M.; Butcher, R.J.; Ghosh, P. Functional mimics of catechol oxidase by mononuclear copper complexes of sterically demanding [NNO] ligands. Inorg. Chim. Acta 2011, 372, 145–151. [Google Scholar] [CrossRef]
- Majumder, S.; Sarkar, S.; Sasmal, S.; Sanudo, E.C.; Mohanta, S. Heterobridged dinuclear, tetranuclear, dinuclear-based 1-D, and heptanuclear-based 1-D complexes of copper (II) derived from a dinucleating ligand: Syntheses, structures, magnetochemistry, spectroscopy, and catecholase activity. Inorg. Chem. 2011, 50, 7540–7554. [Google Scholar] [CrossRef] [PubMed]
- Bakshi, R.; Rossi, M.; Caruso, F.; Mathur, P. Copper (II) complexes of a new N-picolylated bis benzimidazolyl diamide ligand: Synthesis, crystal structure and catechol oxidase studies. Inorg. Chim. Acta 2011, 376, 175–188. [Google Scholar] [CrossRef]
- El Boutaybi, M.; Bouroumane, N.; Azzouzi, M.; Bacroume, S.; Touzani, R.; Bahari, Z. Catecholase, phenoxazinone synthase and copper (CuII) complex based on pyrazolic ligand: Preparation and characterization. Mater. Today Proc. 2022. [CrossRef]
- Kaddouri, Y.; Haddari, H.; Titi, A.; Yousfi, E.B.; Chetouani, A.; El Kodadi, M.; Touzani, R. Catecholase catalytic properties of copper (II) complexes prepared in-situ with heterocyclic ligands: Experimental and DFT study. Moroc. J. Chem. 2020, 8, 184–196. [Google Scholar] [CrossRef]
- Titi, A.; Al-Noaimi, M.; Kaddouri, Y.; El Ati, R.; Yousfi, E.B.; El Kodadi, M.; Touzani, R. Study of the catecholase catalytic properties of copper (II) complexes prepared in-situ with monodentate ligands. Mater. Today Proc. 2019, 13, 1134–1142. [Google Scholar] [CrossRef]
- El Ati, R.; Takfaoui, A.; El Kodadi, M.; Touzani, R.; Yousfi, E.B.; Almalki, F.A.; Ben Hadda, T. Catechol oxidase and Copper(I/II) Complexes Derived from Bipyrazol Ligand: Synthesis, molecular structure investigation of new biomimetic functional model and mechanistic study. Mater. Today Proc. 2019, 13, 1229–1237. [Google Scholar] [CrossRef]
- Boyaala, R.; El Ati, R.; Khoutoul, M.; El Kodadi, M.; Touzani, R.; Hammouti, B. Biomimetic oxidation of catechol employing complexes formed in situ with heterocyclic ligands and different copper(II) salts. J. Iran. Chem. Soc 2018, 15, 85–92. [Google Scholar] [CrossRef]
- Bouroumane, N.; El Kodadi, M.; Touzani, R.; El Boutaybi, M.; Oussaid, A.; Hammouti, B.; Nandiyanto, A.B.D. New pyrazole-based ligands: Synthesis, characterization, and catalytic activity of their copper complexes. Arab. J. Sci. Eng 2021, 47, 269–279. [Google Scholar] [CrossRef]
- Indira, S.; Vinoth, G.; Bharathi, M.; Bharathi, S.; Rahiman, A.K.; Bharathi, K.S. Catechol oxidase and phenoxazinone synthase mimicking activities of mononuclear Fe(III) and Co(III) complexes of aminobis(phenolate)-based mixed ligands: Synthesis, spectral and electrochemical studies. Inorg. Chim. Acta 2019, 495, 118988. [Google Scholar] [CrossRef]
- Lo, S.-I.; Lu, J.-W.; Chen, W.-J.; Wang, S.-R.; Wei, H.-H.; Katada, M. Functional model for catecholase-like activity: Synthesis, structure, spectra, and catalytic activity of iron (III) complexes with substituted-salicylaldimine ligands. Inorg. Chim. Acta 2009, 362, 4699–4705. [Google Scholar] [CrossRef]
- Megyes, T.; May, Z.; Schubert, G.; Grosz, T.; Simandi, L.I.; Radnai, T. Synthesis and structure study of some catecholase-mimetic iron complexes. Inorg. Chim. Acta 2006, 359, 2329–2336. [Google Scholar] [CrossRef]
- Simandi, L.I.; Simandi, T.M.; May, Z.; Besenyei, G. Catalytic activation of dioxygen by oximatocobalt (II) and oximatoiron (II) complexes for catecholase-mimetic oxidations of o-substituted phenols. Coord. Chem. Rev. 2003, 245, 85–93. [Google Scholar] [CrossRef]
- Majumder, S.; Mondal, S.; Lemoine, P.; Mohanta, S. Dinuclear mixed-valence Co III Co II complexes derived from a macrocyclic ligand: Unique example of a Co III Co II complex showing catecholase activity. Dalton Trans. 2013, 42, 4561–4569. [Google Scholar] [CrossRef]
- Banerjee, A.; Guha, A.; Adhikary, J.; Khan, A.; Manna, K.; Dey, S.; Zangrando, E.; Das, D. Dinuclear cobalt (II) complexes of Schiff-base compartmental ligands: Syntheses, crystal structure and bio-relevant catalytic activities. Polyhedron 2013, 60, 102–109. [Google Scholar] [CrossRef]
- Zhang, Y.; Meng, X.G.; Liao, Z.R.; Li, D.F.; Liu, C.L. Synthesis, structures and polyphenol oxidase activities of dicopper and dicobalt complexes. J. Coord. Chem. 2009, 62, 876–885. [Google Scholar] [CrossRef]
- Titi, A.; Warad, I.; Tillard, M.; Touzani, R.; Messali, M.; El Kodadi, M.; Eddike, D.; Zarrouk, A. Inermolecular interaction in [C6H10N3] 2 [CoCl4] complex: Synthesis, XRD/HSA relation, spectral and catecholase catalytic analysis. J. Mol. Struct. 2020, 1217, 128422. [Google Scholar] [CrossRef]
- Titi, A.; Shiga, T.; Oshio, H.; Touzani, R.; Mouslim, M.; Warad, I. Synthesis of novel Cl2Co4L6 clusterusing 1-hydroxymethyl-3,5-dimethylpyrazole (LH) ligand: Crystal structure, spectral, thermal, Hirschfeld surface analysis and catalytic oxidation evaluation. J. Mol. Struct. 2020, 1199, 126995. [Google Scholar] [CrossRef]
- Maji, A.K.; Ghosh, B.K.; Kaur, G.; Roy Choudhury, A.; Lin, C.-H.; Ribas, J.; Ghosh, R.; Mitra, M. Synthesis, crystallographic characterization and catecholase activity of a monocopper (II) and a dimanganese (III) complex with an anionic Schiff base ligand. Polyhedron 2013, 61, 15–19. [Google Scholar]
- Jana, A.; Aliaga-Alcalde, N.; Ruiz, E.; Mohanta, S. Structures, magnetochemistry, spectroscopy, theoretical study, and catechol oxidase activity of dinuclear and dimer-of-dinuclear mixed-valence MnIIIMnII complexes derived from a macrocyclic ligand. Inorg. Chem. 2013, 52, 7732–7746. [Google Scholar] [CrossRef]
- Seth, P.; Das, L.K.; Drew, M.G.B.; Ghosh, A. Synthesis, Crystal Structure, and Catecholase Activity of Three Trinuclear Heterometallic Ni2II–MnII Complexes Derived from a Salen-Type Schiff Base Ligand. Eur. J. Inorg. Chem. 2012, 2012, 2232–2242. [Google Scholar] [CrossRef]
- Guha, A.; Banu, K.S.; Banerjee, A.; Ghosh, T.; Bhattacharya, S.; Zangrando, E.; Das, D. Bio-relevant manganese (II) compartmental ligand complexes: Syntheses, crystal structures and studies of catalytic activities. J. Mol. Catal. A Chem. 2011, 338, 51–57. [Google Scholar] [CrossRef]
- Banu, K.S.; Chattopadhyay, T.; Banerjee, A.; Mukherjee, M.; Bhattacharya, S.; Patra, G.K.; Zangrando, E.; Das, D. Mono-and dinuclear manganese (III) complexes showing efficient catechol oxidase activity: Syntheses, characterization and spectroscopic studies. Dalton Trans. 2009, 8755–8764. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, J.; Barath, G.; Csonka, R.; Speier, G.; Korecz, L.; Rockenbauer, A.; Parkanyi, L. Catechol oxidase and phenoxazinone synthase activity of a manganese (II) isoindoline complex. J. Inorg. Biochem. 2008, 102, 773–780. [Google Scholar] [CrossRef]
- Kaizer, J.; Csonka, R.; Barath, G.; Speier, G. Synthesis, properties, and catecholase-like activity of the [1, 4-di (6′-methyl-2′-pyridyl) aminophthalazine] dimanganese (II) complex, Mn2 (6′ Me2PAP) 2Cl4. Transit. Met. Chem. 2007, 32, 1047–1050. [Google Scholar] [CrossRef]
- Majumder, A.; Goswami, S.; Batten, S.R.; El Fallah, M.S.; Ribas, J.; Mitra, S. Catalytic oxidation of 3, 5-di-tert-butylcatechol by a manganese (III) 18-azametallacrown-6 compound: Synthesis, crystal structure, fluorescence, magnetic and kinetic investigation. Inorg. Chim. Acta 2006, 359, 2375–2382. [Google Scholar] [CrossRef]
- Blay, G.; Fernandez, I.; Pedro, J.R.; Ruiz-Garcia, R.; Temporal-Sanchez, T.; Pardo, E.; Lloret, F.; Munoz, M.C. Chemistry and reactivity of dinuclear manganese oxamate complexes: Aerobic catechol oxidation catalyzed by high-valent bis (oxo)-bridged dimanganese (IV) complexes with a homologous series of binucleating 4, 5-disubstituted-o-phenylenedioxamate ligands. J. Mol. Catal. A Chem. 2006, 250, 20–26. [Google Scholar] [CrossRef]
- Hitomi, Y.; Ando, A.; Matsui, H.; Ito, T.; Tanaka, T.; Ogo, S.; Funabiki, T. Aerobic catechol oxidation catalyzed by a Bis (μ-oxo) dimanganese (III, III) complex via a manganese (II)− semiquinonate complex. Inorg. Chem. 2005, 44, 3473–3478. [Google Scholar] [CrossRef]
- Mukherjee, S.; Weyhermueller, T.; Bothe, E.; Wieghardt, K.; Chaudhuri, P. Dinuclear and mononuclear manganese (IV)-radical complexes and their catalytic catecholase activity. Dalton Trans. 2004, 3842–3853. [Google Scholar] [CrossRef]
- Triller, M.U.; Pursche, D.; Hsieh, W.-Y.; Pecoraro, V.L.; Rompel, A.; Krebs, B. Catalytic Oxidation of 3,5-Di-tert-butylcatechol by a Series of Mononuclear Manganese Complexes: Synthesis, Structure, and Kinetic Investigation. Inorg. Chem. 2003, 42, 6274–6283. [Google Scholar] [CrossRef]
- Guha, A.; Chattopadhyay, T.; Paul, N.D.; Mukherjee, M.; Goswami, S.; Mondal, T.K.; Zangrando, E.; Das, D. Radical pathway in catecholase activity with zinc-based model complexes of compartmental ligands. Inorg. Chem. 2012, 51, 8750–8759. [Google Scholar] [CrossRef]
- Olivares, C.; Solano, F. New insights into the active site structure and catalytic mechanism of tyrosinase and its related proteins. Pigment Cell Melanoma Res. 2009, 22, 750–760. [Google Scholar] [CrossRef]
- Nikkhahi, M.; Souri, E.; Sarkhail, P.; Baeeri, M.; Mohammadhosseini, N. Evaluation of anti-tyrosinase activity of Allium ursinum extracts and their metal complexes. Acta Sci. Pol. Technol. Aliment. 2018, 17, 219–226. [Google Scholar]
- Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 1 November 2022).
- Titi, A.; Messali, M.; Alqurashy, B.A.; Touzani, R.; Shiga, T.; Oshio, H.; Fettouhi, M.; Rajabi, M.; Almalki, F.A.; Ben Hadda, T. Synthesis, characterization, X-ray crystal study and bioctivities of pyrazole derivatives: Identification of antitumor, antifungal and antibacterial pharmacophore sites. J. Mol. Struct. 2020, 1205, 127625. [Google Scholar] [CrossRef]
- Choi, S.; Kim, S.; Lee, H.-J.; Lee, H. Cadmium (II) complexes containing N’-substituted N,N-bispyrazolyl ligands: The formation of 4- and 5-coordinated monomers versus 6-coordinated dimer. Inorg. Chem. Commun. 2014, 44, 164. [Google Scholar] [CrossRef]
- APEX3; Version 2017.3-0; Bruker AXS, Inc.: Madison, WI, USA, 2017.
- Sheldrick, G.M. Acta Crystallogr. Sect. A Fundam. Crystallogr. 2015, 71, 3. [Google Scholar]












| Formula | C14H17N5O4 |
|---|---|
| CCDC | 1912583 |
| Space group, | P |
| Z, Formula weight | 2, 318.33 |
| Temperature (K) | 298 (2) |
| Lattice (Å, °) | a = 6.8641(3), b = 11.2751(6), c = 11.6535(6) |
| α = 112.886(2), β = 104.104(2), γ = 99.536(2) | |
| Volume (Å3) | 770.87(7) |
| Crystal (mm) | 0.14 × 0.14 × 0.30 |
| Recorded/unique reflections | 21,566/3384 [Rint = 0.0354] |
| Goodness-of-fit on F2 | 1.017 |
| Final R1, wR2 indices [I > 2σ(I)] | 0.059, 0.1489 |
| Δρ Fourier residuals (e.Å−3) | 0.25/−0.26 |
| Compound | Temperature (°C) | Mass Loss (%) | Proposed Lost Species |
|---|---|---|---|
| Cl2MnRCOOET | 0–82 | 5.35 | Solvent |
| 82–172 | 4.24 | Chlorine | |
| 172–278 | 16.18 | C5H5N2 | |
| 278–467 | 19.77 | C3H5O2 | |
| 467–635 | 23.43 | C5H6N3 |
| Complex | MeOH | THF | |
|---|---|---|---|
| Solvent | |||
| The synthesized complex | 0.16 | 3.74 | |
| the prepared complex in situ | 0.2 | 1.51 | |
| Complex | Vmax (μmol. L−1. min−1) | Km (mol. L−1) |
|---|---|---|
| [(Cl)2Mn(RCOOET)] | 0.307 | 0.083 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutaybi, M.E.; Titi, A.; Alzahrani, A.Y.A.; Bahari, Z.; Tillard, M.; Hammouti, B.; Touzani, R. Aerial Oxidation of Phenol/Catechol in the Presence of Catalytic Amounts of [(Cl)2Mn(RCOOET)], RCOOET= Ethyl-5-Methyl-1-(((6-Methyl-3-Nitropyridin-2-yl)Amino)Methyl)-1H-Pyrazole-3-Carboxylate. Catalysts 2022, 12, 1642. https://doi.org/10.3390/catal12121642
Boutaybi ME, Titi A, Alzahrani AYA, Bahari Z, Tillard M, Hammouti B, Touzani R. Aerial Oxidation of Phenol/Catechol in the Presence of Catalytic Amounts of [(Cl)2Mn(RCOOET)], RCOOET= Ethyl-5-Methyl-1-(((6-Methyl-3-Nitropyridin-2-yl)Amino)Methyl)-1H-Pyrazole-3-Carboxylate. Catalysts. 2022; 12(12):1642. https://doi.org/10.3390/catal12121642
Chicago/Turabian StyleBoutaybi, Mohamed El, Abderrahim Titi, Abdullah Y. A. Alzahrani, Zahra Bahari, Monique Tillard, Belkheir Hammouti, and Rachid Touzani. 2022. "Aerial Oxidation of Phenol/Catechol in the Presence of Catalytic Amounts of [(Cl)2Mn(RCOOET)], RCOOET= Ethyl-5-Methyl-1-(((6-Methyl-3-Nitropyridin-2-yl)Amino)Methyl)-1H-Pyrazole-3-Carboxylate" Catalysts 12, no. 12: 1642. https://doi.org/10.3390/catal12121642
APA StyleBoutaybi, M. E., Titi, A., Alzahrani, A. Y. A., Bahari, Z., Tillard, M., Hammouti, B., & Touzani, R. (2022). Aerial Oxidation of Phenol/Catechol in the Presence of Catalytic Amounts of [(Cl)2Mn(RCOOET)], RCOOET= Ethyl-5-Methyl-1-(((6-Methyl-3-Nitropyridin-2-yl)Amino)Methyl)-1H-Pyrazole-3-Carboxylate. Catalysts, 12(12), 1642. https://doi.org/10.3390/catal12121642








