Palladium-Catalyzed Synthesis of Novel Quinazolinylphenyl-1,3,4-thiadiazole Conjugates
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis and Characterization
3.2.1. General Procedure for Preparing 4-(N,N-dimethylamino)-2-phenylquinazoline Derivatives (2a–g)
3.2.2. Preparation of 2-(4-Bromophenyl)-5-phenyl-1,3,4-thiadiazole (5a)
3.2.3. Preparation of 2-Phenyl-5-[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1,3,4-thiadiazole (6)
3.2.4. Preparation of Bis(4-bromophenyl)-1,3,4-thiadiazole (5b)
3.2.5. Preparation of Bis[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl]-1,3,4-thiadiazole (7)
3.2.6. General Procedure for the Preparation of Unsymmetrical Quinazolinylphenyl-1,3,4-thiadiazole Derivatives (8a–e) via Suzuki Cross-Coupling from Boronic Acid Pinacol Ester 6
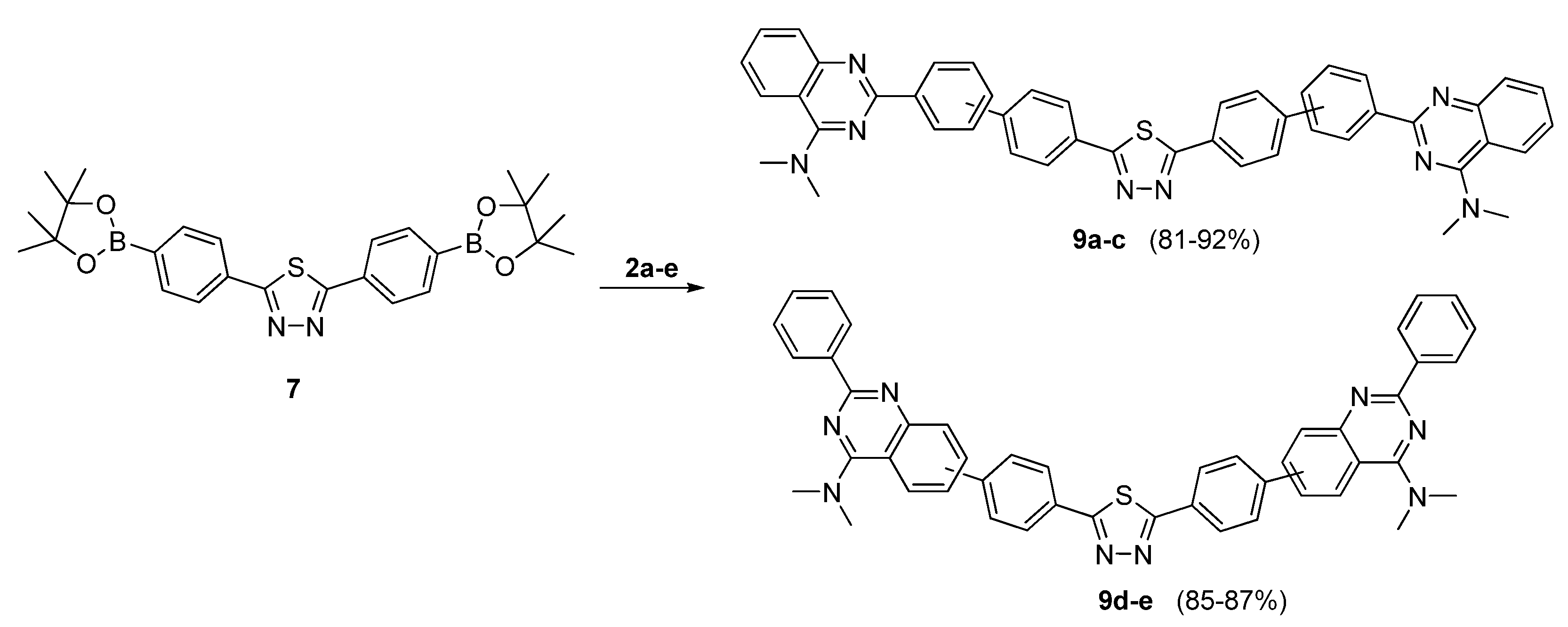
3.2.7. General Procedure for the Preparation of Symmetrical Quinazolinylphenyl-1,3,4-thiadiazole Derivatives (9a–e) via Suzuki Cross-Coupling Using Diboronic Acid Bis(pinacol) Ester 7
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hu, Y.; Li, C.; Wang, X.; Yang, Y.; Zhu, H. 1,3,4-Thiadiazole: Synthesis, Reactions, and Applications in Medicinal, Agricultural, and Materials Chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef]
- Lamani, R.S.; Shetty, N.S.; Kamble, R.R.; Khazi, I.A. Synthesis and antimicrobial studies of novel methylene bridged benzisoxazolyl imidazo[2,1-b][1,3,4]thiadiazole derivatives. Eur. J. Med. Chem. 2009, 44, 2828–2833. [Google Scholar] [CrossRef] [PubMed]
- Brai, A.; Ronzini, S.; Riva, V.; Botta, L.; Zamperini, C.; Borgini, M.; Trivisani, C.I.; Garbelli, A.; Pennisi, C.; Boccuto, A.; et al. Synthesis and Antiviral Activity of Novel 1,3,4-Thiadiazole Inhibitors of DDX3X. Molecules 2019, 24, 3988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janowska, S.; Khylyuk, D.; Bielawska, A.; Szymanowska, A.; Gornowicz, A.; Bielawski, K.; Noworól, J.; Mandziuk, S.; Wujec, M. New 1,3,4-Thiadiazole Derivatives with Anticancer Activity. Molecules 2022, 27, 1814. [Google Scholar] [CrossRef]
- Yusuf, M.; Khan, R.A.; Ahmed, B. Syntheses and anti-depressant activity of 5-amino-1, 3, 4-thiadiazole-2-thiol imines and thiobenzyl derivatives. Bioorg. Med. Chem. 2008, 16, 8029–8034. [Google Scholar] [CrossRef]
- Schenone, S.; Brullo, C.; Bruno, O.; Bondavalli, F.; Ranise, A.; Filippelli, W.; Rinaldi, B.; Capuano, A.; Falcone, G. New 1,3,4-thiadiazole derivatives endowed with analgesic and anti-inflammatory activities. Bioorg. Med. Chem. 2006, 14, 1698–1705. [Google Scholar] [CrossRef]
- Sainy, J.; Mishra, G.P.; Sharma, R.; Chaturvedi, S.C. 2-Amino-5-sulfanyl-1,3,4-thiadiazoles: A novel series of anti-inflammatory and analgesic agents. Pharm. Chem. J. 2009, 43, 19–24. [Google Scholar] [CrossRef]
- Mahmoud, F.I.; Hassan, M.F.M.; Marwa, S.S.; Ali, M.M.M.; Aly, F.A. Design, synthesis and insecticidal activity of new 1,3,4-thiadiazole and 1,3,4-thiadiazolo[3,2-a]pyrimidine derivatives under solvent-free conditions. Synth. Commun. 2021, 51, 2644–2660. [Google Scholar] [CrossRef]
- Chen, M.; Duan, W.-G.; Lin, G.-S.; Fan, Z.-T.; Wang, X. Synthesis, Antifungal Activity, and 3D-QSAR Study of Novel Nopol-Derived 1,3,4-Thiadiazole-Thiourea Compounds. Molecules 2021, 26, 1708. [Google Scholar] [CrossRef]
- Yasuda, T.; Imase, T.; Sasaki, S.; Yamamoto, T. Synthesis, Solid Structure, and Optical Properties of New Thiophene-Based Alternating π-Conjugated Copolymers Containing 4-Alkyl-1,2,4-triazole or 1,3,4-Thiadiazole Unit as the Partner Unit. Macromolecules 2005, 38, 1500–1503. [Google Scholar] [CrossRef]
- Wróblowska, M.; Kudelko, A.; Kuźnik, N.; Łaba, K.; Łapkowski, M. Synthesis of Extended 1,3,4-Oxadiazole and 1,3,4-Thiadiazole Derivatives in the Suzuki Cross-coupling Reactions. J. Heterocycl. Chem. 2016, 54, 1550–1557. [Google Scholar] [CrossRef]
- Kudelko, A.; Olesiejuk, M.; Luczynski, M.; Swiatkowski, M.; Sieranski, T.; Kruszynski, R. 1,3,4-Thiadiazole-Containing Azo Dyes: Synthesis, Spectroscopic Properties and Molecular Structure. Molecules 2020, 25, 2822. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, H.; Tomishima, M.; Kayakiri, N.; Araki, T.; Barrett, D.; Akamatsu, S.; Matsumoto, S.; Uchida, S.; Nakai, T.; Takeda, S.; et al. Synthesis and antifungal activity of ASP9726, a novel echinocandin with potent Aspergillus hyphal growth inhibition. Bioorg. Med. Chem. Lett. 2014, 24, 1172–1175. [Google Scholar] [CrossRef]
- Kudelko, A.; Wróblowska, M.; Łapkowski, M. Efficient Synthesis of Conjugated 1,3,4-Thiadiazole Hybrids through Palladium-Catalyzed Cross-Coupling of 2,5-Bis(4-bromophenyl)-1,3,4-thiadiazole with Boronic Acids. Synlett 2015, 26, 2127–2130. [Google Scholar] [CrossRef]
- Gierczyk, B.; Zalas, M. Synthesis of substituted 1,3,4-thiadiazoles using Lawesson’s reagent. Org. Prep. Proced. Int. 2005, 37, 213–222. [Google Scholar] [CrossRef]
- Ko, I.; Park, S.; Lee, G.; Kim, H. An efficient one-pot synthesis of 2,5-disubstituted-1,3,4-thiadiazoles from aldehydes and hydrazides using Lawesson’s reagent. Arkivoc 2019, 3, 67–78. [Google Scholar] [CrossRef]
- Niu, P.; Kang, J.; Tian, X.; Song, L.; Liu, H.; Wu, J.; Yu, W.; Chang, J. Synthesis of 2-Amino-1,3,4-oxadiazoles and 2-Amino-1,3,4-thiadiazoles via Sequential Condensation and I2-Mediated Oxidative C-O/C-S Bond Formation. J. Org. Chem. 2015, 80, 1018–1024. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Q.; Tang, M.; Zhang, S.; Zhang, Q. Diversity-Oriented Synthesis of 1,2,4-Triazols, 1,3,4-Thiadiazols, and 1,3,4-Selenadiazoles from N-Tosylhydrazones. Org. Lett. 2021, 23, 4420–4425. [Google Scholar] [CrossRef]
- Padmavathi, V.; Nagi Reddy, S.; Dinneswara Reddy, G.; Padmaja, A. Synthesis and bioassay of aminosulfonyl-1,3,4-oxadiazoles and their interconversion to 1,3,4-thiadiazoles. Eur. J. Med. Chem. 2010, 45, 4246–4251. [Google Scholar] [CrossRef]
- Gatadi, S.; Lakshmi, T.V.; Nanduri, S. 4(3H)-Quinazolinone derivatives: Promising antibacterial drug leads. Eur. J. Med. Chem. 2019, 170, 157–172. [Google Scholar] [CrossRef]
- Patel, A. Investigation of the antibacterial activity of new quinazoline derivatives against methicillin and quinolone resistant Staphylococcus aureus. J. Chem. Res. 2020, 44, 315–321. [Google Scholar] [CrossRef]
- Verhaeghe, P.; Azas, N.; Gasquet, M.; Hutter, S.; Ducros, C.; Laget, M.; Rault, S.; Rathelot, P.; Vanelle, P. Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquinazolines. Bioorg. Med. Chem. Lett. 2008, 18, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Haider, K.; Das, S.; Joseph, A.; Yar, M.S. An appraisal of anticancer activity with structure–activity relationship of quinazoline and quinazolinone analogues through EGFR and VEGFR inhibition: A review. Drug Dev. Res. 2022, 83, 859–890. [Google Scholar] [CrossRef]
- Chandrika, P.M.; Yakaiah, T.; Rao, A.R.R.; Narsaiah, B.; Reddy, N.C.; Sridhar, V.; Rao, J.V. Synthesis of novel 4,6-disubstituted quinazoline derivatives, their anti-inflammatory and anti-cancer activity (cytotoxic) against U937 leukemia cell lines. Eur. J. Med. Chem. 2008, 43, 846–852. [Google Scholar] [CrossRef]
- Elshahawi, M.M.; El-Ziaty, A.K.; Morsy, J.M.; Aly, A.F. Synthesis and Insecticidal Efficacy of Novel Bis Quinazolinone Derivatives. J. Heterocyc. Chem. 2015, 53, 1443–1448. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Luo, X.; Chen, Y. Recent research progress and outlook in agricultural chemical discovery based on quinazoline scaffold. Pestic. Biochem. Physiol. 2022, 184, 105122–105130. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, Z.; Su, S.-J.; Guo, F.; Cao, Y.; Zhang, Y. Quinazoline-Based Thermally Activated Delayed Fluorecence for High-Performance OLEDs with External Quantum Efficiencies Exceeding 20%. Adv. Opt. Mater. 2019, 7, 1801496–1801504. [Google Scholar] [CrossRef]
- Wang, Z.; Li, H.; Peng, Z.; Wang, Z.; Wang, Y.; Lu, P. Preparation and photophysical properties of quinazoline-based fluorophores. RSC Adv. 2020, 10, 30297–30303. [Google Scholar] [CrossRef]
- Keruckiene, R.; Vekteryte, S.; Urbonas, E.; Guzauskas, M.; Skuodis, E.; Volyniuk, D.; Grazulevicius, J.V. Synthesis and properties of quinazoline-based versatile exciplex-forming compounds. Beilstein J. Org. Chem. 2020, 16, 1142–1153. [Google Scholar] [CrossRef]
- Jiang, J.B.; Hesson, D.P.; Dusak, B.A.; Dexter, D.L.; Kang, G.L.; Hamel, E. Synthesis and biological evaluation of 2-styrylquinazolin-4(3H)-ones, a new class of antimitotic anticancer agents which inhibit tubulin polymerization. J. Med. Chem. 1990, 33, 1721–1728. [Google Scholar] [CrossRef]
- Bergman, J.; Brynolf, A. Synthesis of Chrysogine, a Metabolite of Penicillium chrysogenum and some related 2-substituted 4-(3H)-Quinazolinones. Tetrahedron Lett. 1990, 46, 1295–1310. [Google Scholar] [CrossRef]
- Witt, A.; Bergman, J. Synthesis and Reactions of some 2-Vinyl-3H-quinazolin-4-ones. Tetrahedron 2000, 56, 7245–7253. [Google Scholar] [CrossRef]
- Rad-Moghadam, K.; Mohseni, M. An Expeditious and Solvent-Free Route to the Synthesis of 2-Substituted Quinazolin-4(3H)-Ones Under Microwave Conditions. J. Chem. Res. 2003, 8, 487–488. [Google Scholar] [CrossRef]
- Connolly, D.J.; Guiry, P.J. A Facile and Versatile Route to 2-Substituted-4(3H)-Quinazolinones and Quinazolines. Synlett 2001, 11, 1707–1710. [Google Scholar] [CrossRef]
- Hess, H.-J.; Cronin, T.H.; Scriabine, A. Antihypertensive 2-amino-4(3H)-quinazolinones. J. Med. Chem. 1968, 11, 130–136. [Google Scholar] [CrossRef]
- Connolly, D.J.; Cusack, D.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202. [Google Scholar] [CrossRef]
- Asif, M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivatives. Int. J. Med. Chem. 2014, 2014, 395637. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, D.; Yu, C.; Wan, C.; Wang, Z. A Simple and Efficient Approach to the Synthesis of 2-Phenylquinazolines via sp3 C−H Functionalization. Org. Lett. 2010, 12, 2841–2843. [Google Scholar] [CrossRef]
- Karnakar, K.; Shangkar, J.; Murthy, S.N.; Ramesch, K.; Nageshwar, Y.V.D. An Efficient Protocol for the Synthesis of 2-Phenylquinazolines Catalyzed by Ceric Ammonium Nitrate (CAN). Synlett 2011, 8, 1089–1096. [Google Scholar] [CrossRef]
- Olesiejuk, M.; Kudelko, A.; Świątkowski, M.; Kruszyński, R. Synthesis of 4-alkyl-4H-1,2,4-triazole derivatives by Suzuki cross-coupling reactions and their luminescence properties. Molecules 2019, 24, 652. [Google Scholar] [CrossRef]
- Wołek, B.; Werłos, M.; Komander, M.; Kudelko, A. Efficient Synthesis of Novel 1,3,4-Oxadiazoles Bearing a 4-N,N-Dimethylaminoquinazoline Scaffold via Palladium-Catalyzed Suzuki Cross-Coupling Reactions. Molecules 2020, 25, 5150. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-coupling reactions via organoboranes. J. Organomett. Chem. 2002, 653, 83–90. [Google Scholar] [CrossRef]
- Miyaura, N. Metal-Catalyzed Cross-Coupling Reactions of Organoboron Compounds with Organic Halides. In Metal-Catalyzed Cross-Coupling Reactions; de Meijere, A., Diederich, F., Eds.; Wiley-VCH: Weinheim, Germany, 2008; pp. 41–123. [Google Scholar]
- D’Alterio, M.C.; Casals-Cruañas, E.; Tzouras, N.V.; Talarico, G.; Nolan, S.P.; Poater, A. Mechanistic Aspects of the Palladium-Catalyzed Suzuki-Miyaura Cross-Coupling Reaction. Chem. Eur. J. 2021, 27, 13481–13493. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Q.; Zhou, Y.; Deng, Z.; Mao, X.; Peng, Y. Synthesis of 4-(Dimethylamino)quinazoline via Direct Amination of Quinazolin-4(3H)-one Using N,N-Dimethylformamide as a Nitrogen Source at Room Temperature. Synthesis 2015, 47, 2055–2062. [Google Scholar] [CrossRef]
- Zieliński, W.; Mazik, M. Synthesis of 4-aminoquinazoline derivatives. Pol. J. Chem. 1994, 68, 487–489. [Google Scholar]
- Zieliński, W.; Kudelko, A. Synthesis and Basicity of 4-Amino-2-phenylquinazolines. Monatsh. Chem. 2000, 131, 895–899. [Google Scholar] [CrossRef]
- Karcz, D.; Starzak, K.; Ciszkowicz, E.; Lecka-Szlachta, K.; Kamiński, D.; Creaven, B.; Jenkins, H.; Radomski, P.; Miłoś, A.; Ślusarczyk, L.; et al. Novel Coumarin-Thiadiazole Hybrids and Their Cu(II) and Zn(II) Complexes as Potential Antimicrobial Agents and Acetylcholinesterase Inhibitors. Int. J. Mol. Sci. 2021, 22, 9709. [Google Scholar] [CrossRef]
- Karcz, D.; Matwijczuk, A.; Kamiński, D.; Creaven, B.; Ciszkowicz, E.; Lecka-Szlachta, K.; Starzak, K. Structural Features of 1,3,4-Thiadiazole-Derived Ligands and Their Zn(II) and Cu(II) Complexes Which Demonstrate Synergistic Antibacterial Effects with Kanamycin. Int. J. Mol. Sci. 2020, 21, 5735. [Google Scholar] [CrossRef]
- Brouwer, A.M. Standards for photoluminescence quantum yield measurements in solution (IUPAC technical report). Pure Appl. Chem. 2011, 83, 2213–2228. [Google Scholar] [CrossRef] [Green Version]
- Melhuish, W.H. Quantum efficiencies of fluorescence of organic substances: Effect of solvent and concentration of the fluorescent solute. J. Phys. Chem. 1961, 65, 229–235. [Google Scholar] [CrossRef]
- Birks, J.B.; Dyson, D.J. The relations between the fluorescence and absorption properties of organic molecules. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1963, 275, 135–148. [Google Scholar] [CrossRef]
- Siegrist, A.E.; Maeder, E.; Duennenberger, M.; Liechti, P. Verfahren zur Herstellung von 1,3,4-Thiadiazolen. Swiss Patent CH426848A, 1967. Available online: https://worldwide.espacenet.com/patent/search/family/025684840/publication/CH426848A?q=CH426848 (accessed on 1 September 2022).






| Entry | Catalyst | Ligand | Base | Solvent | PTC Catalyst | Yield [%] a |
|---|---|---|---|---|---|---|
| 1 | Pd(dppf)Cl2 | - | Na2CO3 | Toluene/water | Bu4NBr | 84 |
| 2 | Pd(dppf)Cl2 | - | Na2CO3 | Toluene/water | - | 82 |
| 3 | PdCl2 | - | Na2CO3 | Toluene/water | Bu4NBr | 61 |
| 4 | Pd(OAc)2 | XPhos | Na2CO3 | Toluene/water | Bu4NBr | 75 |
| 5 | Pd(OAc)2 | cataCXium A | Na2CO3 | Toluene/water | Bu4NBr | 47 |
| 6 | Pd(OAc)2 | Xanthphos | Na2CO3 | Toluene/water | Bu4NBr | 73 |
| 7 | Pd(dppf)Cl2 | - | Na2CO3 | THF/water | - | 77 |
| 8 | Pd(dppf)Cl2 | - | Na2CO3 | dioxane/water | - | 66 |
| 9 | Pd(dppf)Cl2 | - | Na2CO3 | DMF/water | - | 69 |
| 10 | Pd(dppf)Cl2 | - | Na2CO3 | iPrOH/water | - | 82 |
| 11 | Pd(dppf)Cl2 | - | tBuONa | Toluene/water | - | 67 |
| 12 | Pd(dppf)Cl2 | - | K3PO4 | Toluene/water | - | 70 |
| 13 | Pd(dppf)Cl2 | - | NaOH | Toluene/water | - | 43 |
| Entry | Substrate | Product | Yield [%] a | ||
|---|---|---|---|---|---|
| 1. | 2a |  | 8a |  | 84 |
| 2. | 2b |  | 8b |  | 90 |
| 3. | 2c |  | 8c |  | 77 |
| 4. | 2d |  | 8d |  | 82 |
| 5. | 2e |  | 8e |  | 90 |
| Entry | Substrate | Product | Yield [%] a | ||
|---|---|---|---|---|---|
| 1. | 2a |  | 9a |  | 81 |
| 2. | 2b |  | 9b |  | 92 |
| 3. | 2c |  | 9c |  | 88 |
| 4. | 2d |  | 9d |  | 87 |
| 5. | 2e |  | 9e |  | 85 |
| Entry | Compound | λabs (nm) (ε∙10−4 m3/(mol.cm)) | λex(nm) | λem (nm) | Stokes Shift aΔ (nm) | Quantum Yield b Φf |
|---|---|---|---|---|---|---|
| 1 | 5a | 308 (5.6) | 316 | 393 | 85 | 0.06 |
| 2 | 5b | 313 (5.6) | 324 | 400 | 87 | 0.09 |
| 3 | 6 | 306 (4.7) | 316 | 396 | 90 | 0.07 |
| 4 | 7 | 314 (5.5) | 324 | 401 | 87 | 0.11 |
| 5 | 8a | 340 (8.6) | 346 | 434 | 94 | 0.26 |
| 6 | 8b | 325 (5.5) | 338 | 415 | 90 | 0.02 |
| 7 | 8c | 325 (5.3) | 344 | 413 | 88 | 0.05 |
| 8 | 8d | 311 (6.3) | 348 | 415 | 104 | 0.06 |
| 9 | 8e | 342 (5.9) | 346 | 413 | 71 | 0.04 |
| 10 | 9a | 348 (3.9) | 353 | 437 | 89 | >0.98 c |
| 11 | 9b | 334 (8.1) | 342 | 414 | 80 | 0.05 |
| 12 | 9c | 331 (9.7) | 345 | 428 | 97 | 0.02 |
| 13 | 9d | 350 (7.0) | 353 | 435 | 85 | 0.05 |
| 14 | 9e | 359 (9.5) | 360 | 431 | 72 | 0.06 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wołek, B.; Świątkowski, M.; Kudelko, A. Palladium-Catalyzed Synthesis of Novel Quinazolinylphenyl-1,3,4-thiadiazole Conjugates. Catalysts 2022, 12, 1586. https://doi.org/10.3390/catal12121586
Wołek B, Świątkowski M, Kudelko A. Palladium-Catalyzed Synthesis of Novel Quinazolinylphenyl-1,3,4-thiadiazole Conjugates. Catalysts. 2022; 12(12):1586. https://doi.org/10.3390/catal12121586
Chicago/Turabian StyleWołek, Barbara, Marcin Świątkowski, and Agnieszka Kudelko. 2022. "Palladium-Catalyzed Synthesis of Novel Quinazolinylphenyl-1,3,4-thiadiazole Conjugates" Catalysts 12, no. 12: 1586. https://doi.org/10.3390/catal12121586
APA StyleWołek, B., Świątkowski, M., & Kudelko, A. (2022). Palladium-Catalyzed Synthesis of Novel Quinazolinylphenyl-1,3,4-thiadiazole Conjugates. Catalysts, 12(12), 1586. https://doi.org/10.3390/catal12121586








