Interface Engineering of SRu-mC3N4 Heterostructures for Enhanced Electrochemical Hydrazine Oxidation Reactions
Abstract
:1. Introduction
2. Results and Discussion
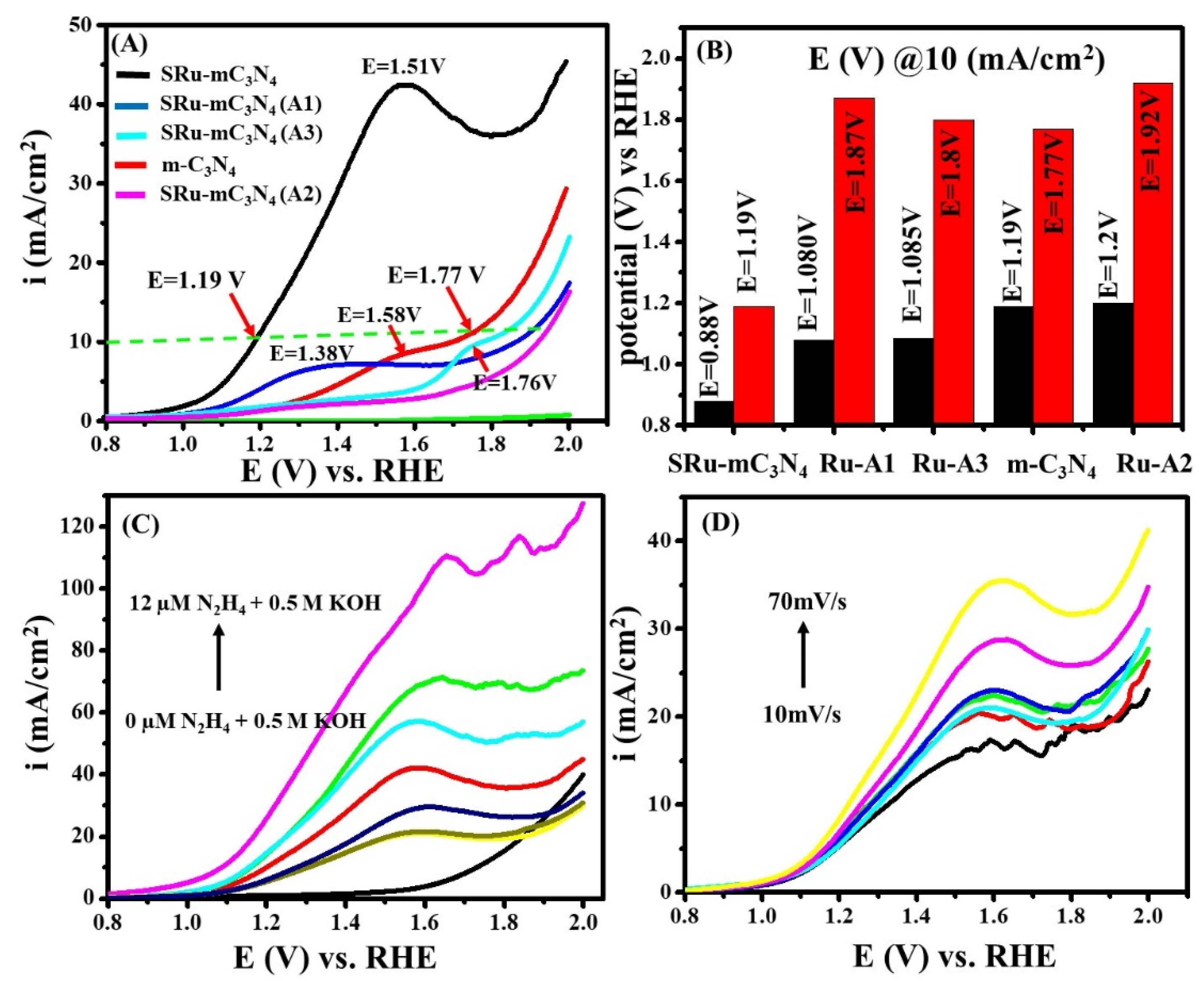
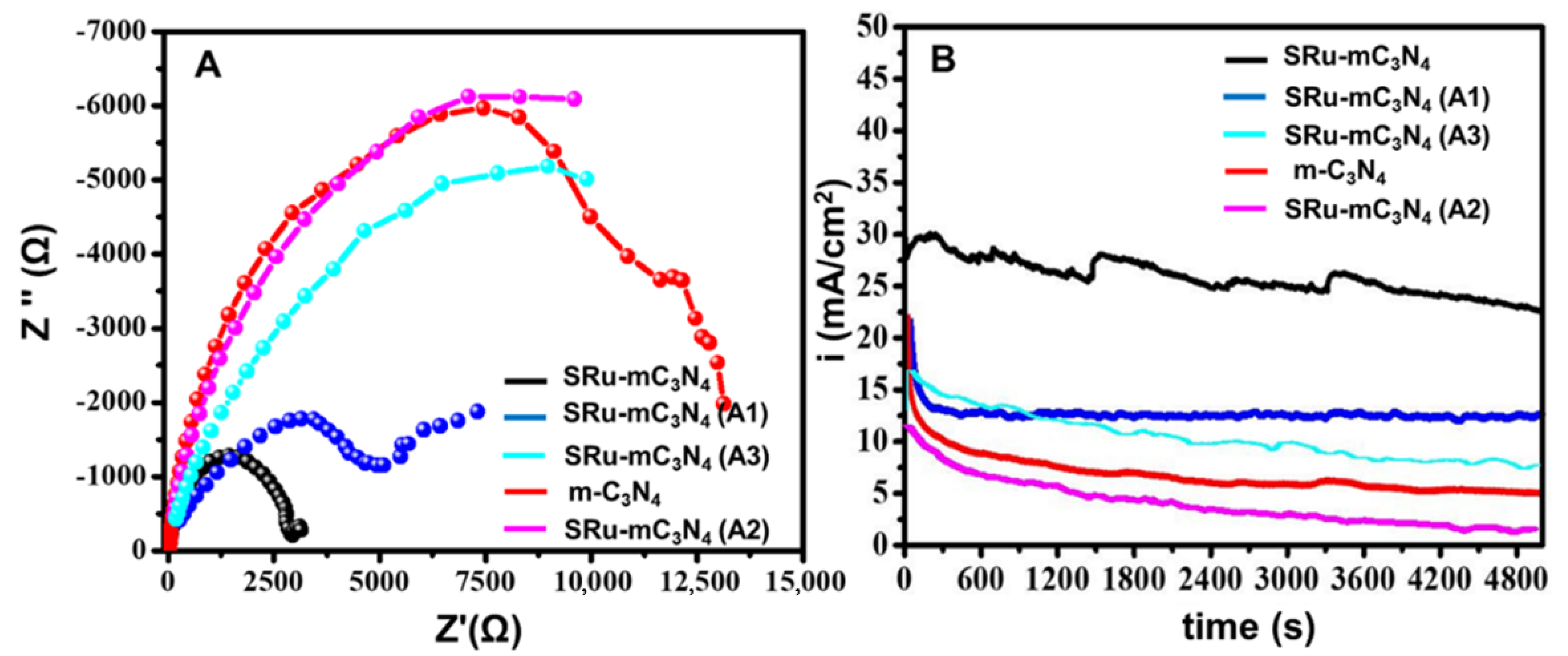
2.1. Electrochemical and Electrocatalytic Studies
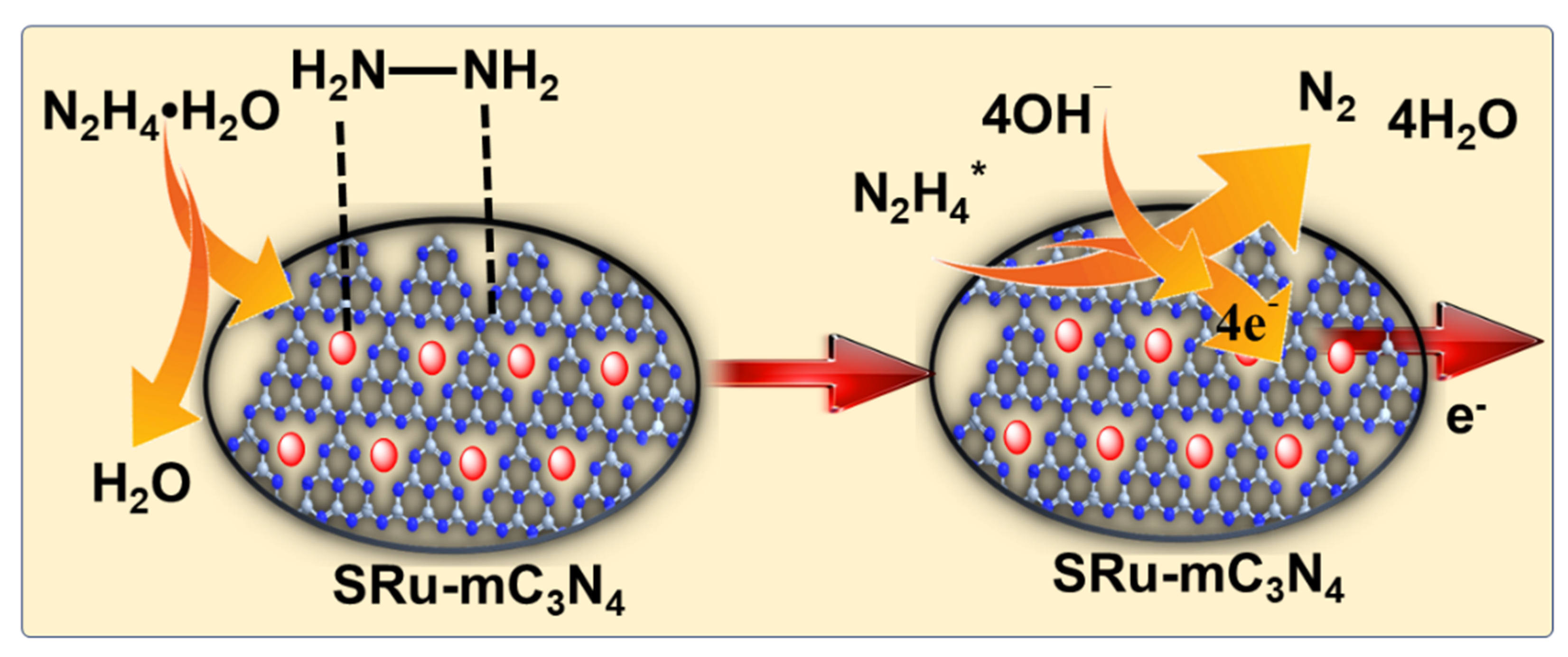
2.2. Mechanistic Pathway for Electrochemical Hydrazine Oxidation on SRu-mC3N4
3. Methods
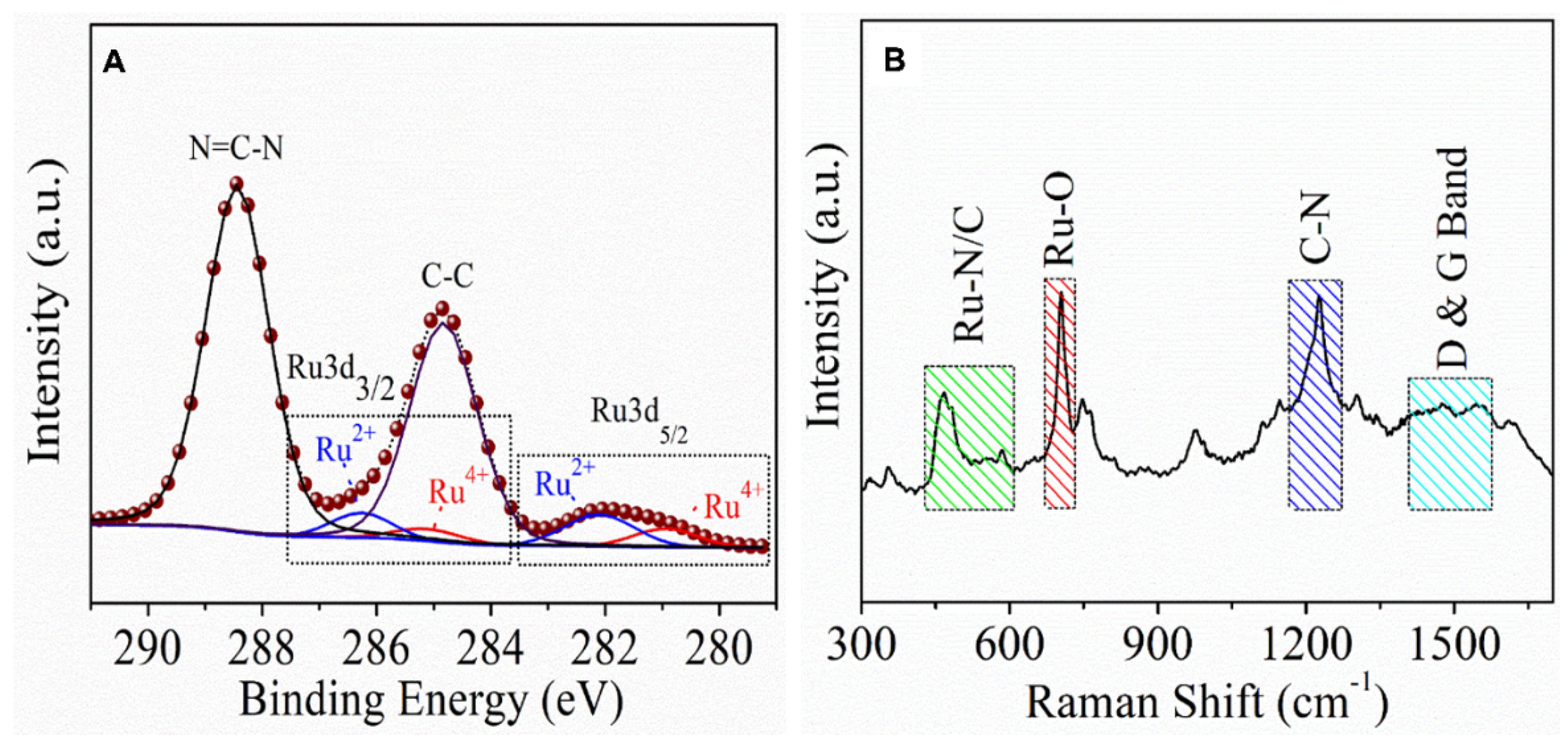
Experimental Details and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cai, W.; Yao, Y.-X.; Zhu, G.-L.; Yan, C.; Jiang, L.-L.; He, C.; Huang, J.-Q.; Zhang, Q. A review on energy chemistry of fast-charging anodes. Chem. Soc. Rev. 2020, 49, 3806–3833. [Google Scholar] [CrossRef] [PubMed]
- Farmahini, A.H.; Krishnamurthy, S.; Friedrich, D.; Brandani, S.; Sarkisov, L. Performance-Based Screening of Porous Materials for Carbon Capture. Chem. Rev. 2021, 121, 10666–10741. [Google Scholar] [CrossRef] [PubMed]
- Bourdrel, T.; Annesi-Maesano, I.; Alahmad, B.; Maesano, C.N.; Bind, M.-A. The impact of outdoor air pollution on COVID-19: A review of evidence from in vitro, animal, and human studies. Eur. Respir. Rev. 2021, 30, 200242. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Han, L.; Zhang, X.; Zhou, W.; Li, W.; Qian, Y. A review of current air quality indexes and improvements under the multi-contaminant air pollution exposure. J. Environ. Manag. 2020, 279, 111681. [Google Scholar] [CrossRef] [PubMed]
- Munde, A.V.; Mulik, B.B.; Chavan, P.P.; Sapner, V.S.; Narwade, S.S.; Mali, S.M.; Sathe, B.R. Electrocatalytic Ethanol Oxidation on Cobalt–Bismuth Nanoparticle-Decorated Reduced Graphene Oxide (Co–Bi@rGO): Reaction Pathway Investigation toward Direct Ethanol Fuel Cells. J. Phys. Chem. C 2021, 125, 2345–2356. [Google Scholar] [CrossRef]
- Peng, L.; Wei, Z. Catalyst Engineering for Electrochemical Energy Conversion from Water to Water: Water Electrolysis and the Hydrogen Fuel Cell. Engineering 2020, 6, 653–679. [Google Scholar] [CrossRef]
- Jiao, F.; Xu, B. Electrochemical Ammonia Synthesis and Ammonia Fuel Cells. Adv. Mater. 2018, 31, e1805173. [Google Scholar] [CrossRef]
- Kowalik, P.; Antoniak-Jurak, K.; Błesznowski, M.; Herrera, C.; Larrubia, M.; Alemany, L.J.; Pieta, I.S. Biofuel steam reforming catalyst for fuel cell application. Catal. Today 2015, 254, 129–134. [Google Scholar] [CrossRef]
- Khalafallah, D.; Zhi, M.; Hong, Z. Development Trends on Nickel-Based Electrocatalysts for Direct Hydrazine Fuel Cells. ChemCatChem 2020, 13, 81–110. [Google Scholar] [CrossRef]
- Rosca, V.; Duca, M.; de Groot, M.T.; Koper, M.T.M. Nitrogen Cycle Electrocatalysis. Chem. Rev. 2009, 109, 2209–2244. [Google Scholar] [CrossRef]
- Munde, A.V.; Mulik, B.B.; Dighole, R.P.; Sathe, B.R. Urea Electro-Oxidation Catalyzed by an Efficient and Highly Stable Ni–Bi Bimetallic Nanoparticles. ACS Appl. Energy Mater. 2021, 4, 13172–13182. [Google Scholar] [CrossRef]
- Kadam, R.G.; Zhang, T.; Zaoralová, D.; Medve, M.; Bakandritsos, A.; Tomanec, O.; Petr, M.; Chen, J.Z.; Miller, J.T.; Otyepka, M.; et al. Single Co-Atoms as Electrocatalysts for Efficient Hydrazine Oxidation Reaction. Small 2021, 17, e2006477. [Google Scholar] [CrossRef]
- Carvalho, L.L.; Colmati, F.; Tanaka, A.A. Nickel–palladium electrocatalysts for methanol, ethanol, and glycerol oxidation reactions. Int. J. Hydrogen Energy 2017, 42, 16118–16126. [Google Scholar] [CrossRef]
- Yang, G.-W.; Gao, G.-Y.; Wang, C.; Xu, C.-L.; Li, H.-L. Controllable deposition of Ag nanoparticles on carbon nanotubes as a catalyst for hydrazine oxidation. Carbon 2008, 46, 747–752. [Google Scholar] [CrossRef]
- Du, J.; Xiang, D.; Zhou, K.; Wang, L.; Yu, J.; Xia, H.; Zhao, L.; Liu, H.; Zhou, W. Electrochemical Hydrogen Production Coupled with Oxygen Evolution, Organic Synthesis, and Waste Reforming. Nano Energy 2022, 104, 107875. [Google Scholar] [CrossRef]
- Sun, H.; Kim, H.; Song, S.; Jung, W. Copper foam-derived electrodes as efficient electrocatalysts for conventional and hybrid water electrolysis. Mater. Rep. Energy 2022, 2, 100092. [Google Scholar] [CrossRef]
- Sun, H.; Xu, X.; Kim, H.; Jung, W.; Zhou, W.; Shao, Z. Electrochemical Water Splitting: Bridging the Gaps between Fundamental Research and Industrial Applications. Energy Environ. Mater. 2022, e12441. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.; Tomanec, O.; Petr, M.; Chen, J.Z.; Miller, J.T.; Varma, R.S.; Gawande, M.B.; Zbořil, R. Carbon Nitride-Based Ruthenium Single Atom Photocatalyst for CO 2 Reduction to Methanol. Small 2021, 17, 2006478. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, N.; Wu, X.-R.; Li, F.-M.; Chen, P.; Jin, P.-J.; Yin, S.; Chen, Y. Rhodium Phosphide Ultrathin Nanosheets for Hydrazine Oxidation Boosted Electrochemical Water Splitting. Appl. Catal. B Environ. 2020, 270, 118880. [Google Scholar] [CrossRef]
- Liu, X.; He, J.; Zhao, S.; Liu, Y.; Zhao, Z.; Luo, J.; Hu, G.; Sun, X.; Ding, Y. Self-Powered H2 Production with Bifunctional Hydrazine as Sole Consumable. Nat. Commun. 2018, 9, 4365. [Google Scholar] [CrossRef]
- Ojha, K.; Farber, E.M.; Burshtein, T.Y.; Eisenberg, D. A Multi-Doped Electrocatalyst for Efficient Hydrazine Oxidation. Angew. Chem. Int. Ed. 2018, 57, 17168–17172. [Google Scholar] [CrossRef]
- Narwade, S.S.; Mali, S.M.; Tanwade, P.D.; Chavan, P.P.; Munde, A.V.; Sathe, B.R. Highly Efficient Metal-Free Ethylenediamine-Functionalized Fullerene (EDA@C 60) Electrocatalytic System for Enhanced Hydrogen Generation from Hydrazine Hydrate. New J. Chem. 2022, 46, 14004–14009. [Google Scholar] [CrossRef]
- Muthukumar, P.; John, S.A. Synergistic effect of gold nanoparticles and amine functionalized cobalt porphyrin on electrochemical oxidation of hydrazine. New J. Chem. 2014, 38, 3473–3479. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Kannan, P.; Ji, S. Cobalt nanoparticles intercalated nitrogen-doped mesoporous carbon nanosheet network as potential catalyst for electro-oxidation of hydrazine. Int. J. Hydrogen Energy 2020, 45, 19344–19356. [Google Scholar] [CrossRef]
- Ding, J.; Kannan, P.; Wang, P.; Ji, S.; Wang, H.; Liu, Q.; Gai, H.; Liu, F.; Wang, R. Synthesis of nitrogen-doped MnO/carbon network as an advanced catalyst for direct hydrazine fuel cells. J. Power Sources 2018, 413, 209–215. [Google Scholar] [CrossRef]
- Gawande, M.B.; Fornasiero, P.; Zbořil, R. Carbon-Based Single-Atom Catalysts for Advanced Applications. ACS Catal. 2020, 10, 2231–2259. [Google Scholar] [CrossRef]
- Pieta, I.S.; Kadam, R.G.; Pieta, P.; Mrdenovic, D.; Nowakowski, R.; Bakandritsos, A.; Tomanec, O.; Petr, M.; Otyepka, M.; Kostecki, R.; et al. The Hallmarks of Copper Single Atom Catalysts in Direct Alcohol Fuel Cells and Electrochemical CO2 Fixation. Adv. Mater. Interfaces 2021, 8, 2001822. [Google Scholar] [CrossRef]
- Niu, W.; Yang, Y. Graphitic Carbon Nitride for Electrochemical Energy Conversion and Storage. ACS Energy Lett. 2018, 3, 2796–2815. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Jin, Z.; Xing, W.; Zhuang, Z.; Ye, J.; Wei, X.; Cao, R.; Gu, L.; Sun, S.; et al. Single-Atom Rh/N-Doped Carbon Electrocatalyst for Formic Acid Oxidation. Nat. Nanotechnol. 2020, 15, 390–397. [Google Scholar] [CrossRef]
- Mulik, B.B.; Munde, A.V.; Bankar, B.D.; Biradar, A.V.; Sathe, B.R. Highly Efficient Manganese Oxide Decorated Graphitic Carbon Nitrite Electrocatalyst for Reduction of CO2 to Formate. Catal. Today 2020, 370, 104–113. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. Applied Surface Science A Review on G-C3N4 -Based Photocatalysts. New J. Chem. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Mulik, B.B.; Bankar, B.D.; Munde, A.V.; Chavan, P.P.; Biradar, A.V.; Sathe, B.R. Electrocatalytic and Catalytic CO2 Hydrogenation on ZnO/g-C3N4 Hybrid Nanoelectrodes. Appl. Surf. Sci. 2021, 538, 148120. [Google Scholar] [CrossRef]
- Kumar, S.; Gawande, M.B.; Kopp, J.; Kment, S.; Varma, R.S.; Zbořil, R. P-and F-co-doped Carbon Nitride Nanocatalysts for Photocatalytic CO2 Reduction and Thermocatalytic Furanics Synthesis from Sugars. ChemSusChem 2020, 13, 5231–5238. [Google Scholar] [CrossRef] [PubMed]
- Aghajani, H.; Tabrizi, A.T.; Ghorbani, R.; Behrangi, S.; Stupavska, M.; Abdian, N. Evaluation of Electrochemical Hydrogen Storage Capability of Three-Dimensional Nano-Structured Nitrogen-Doped Graphene. J. Alloy. Compd. 2022, 906, 164284. [Google Scholar] [CrossRef]
- Yousefi Bonab, S.S.; Kouzehgar, H.; Taghizadeh Tabrizi, A.; Aghajani, H. Assessment of the Effect of Electrophoretic Deposition Parameters on Hydrogen Storage Performance of Graphene Oxide Layer Applied on Nickel Foam. Int. J. Hydrogen Energy 2022, 47, 2491–2499. [Google Scholar] [CrossRef]
- Ghorbani, R.; Behrangi, S.; Aghajani, H.; Taghizadeh Tabrizi, A.; Abdian, N. Application of Synthesized Porous 3D Graphene Structure for Electrochemical Hydrogen Storage. Mater. Sci. Eng. B 2021, 268, 115139. [Google Scholar] [CrossRef]
- Munde, A.V.; Mulik, B.B.; Dighole, R.P.; Sathe, B.R. Cobalt Oxide Nanoparticle-Decorated Reduced Graphene Oxide (Co3O4–RGO): Active and Sustainable Nanoelectrodes for Water Oxidation Reaction. New J. Chem. 2020, 44, 15776–15784. [Google Scholar] [CrossRef]
- Munde, A.V.; Mulik, B.B.; Chavan, P.P.; Sathe, B.R. Enhanced Electrocatalytic Activity towards Urea Oxidation on Ni Nanoparticle Decorated Graphene Oxide Nanocomposite. Electrochim. Acta 2020, 349, 136386. [Google Scholar] [CrossRef]
- Miao, R.; Compton, R.G. Mechanism of Hydrazine Oxidation at Palladium Electrodes: Long-Lived Radical Di-Cation Formation. Electrochim. Acta 2021, 388, 138655. [Google Scholar] [CrossRef]
- Narwade, S.S.; Mulik, B.B.; Mali, S.M.; Sathe, B.R. Silver Nanoparticles Sensitized C60(Ag@C60) as Efficient Electrocatalysts for Hydrazine Oxidation: Implication for Hydrogen Generation Reaction. Appl. Surf. Sci. 2017, 396, 939–944. [Google Scholar] [CrossRef]
- Roy, N.; Bhunia, K.; Terashima, C.; Fujishima, A.; Pradhan, D. Citrate-Capped Hybrid Au-TiO2 Nanomaterial for Facile and Enhanced Electrochemical Hydrazine Oxidation. ACS Omega 2017, 2, 1215–1221. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Shi, S.; Yin, H. CuPd Alloy Oxide Nanobelts as Electrocatalyst Towards Hydrazine Oxidation. ChemElectroChem 2019, 6, 1514–1519. [Google Scholar] [CrossRef]
- Du, M.; Sun, H.; Li, J.; Ye, X.; Yue, F.; Yang, J.; Liu, Y.; Guo, F. Integrative Ni@Pd-Ni Alloy Nanowire Array Electrocatalysts Boost Hydrazine Oxidation Kinetics. ChemElectroChem 2019, 6, 5581–5587. [Google Scholar] [CrossRef]
- Deng, J.; Li, X.; Imhanria, S.; Chen, K.; Deng, X.; Wang, W. Molybdenum Carbide-Nitrogen Doped Carbon Composites as Effective Non-Precious Electrocatalyst for Direct Hydrazine Fuel Cell. Electrochim. Acta 2021, 384, 138417. [Google Scholar] [CrossRef]
- Er, O.F.; Cavak, A.; Aldemir, A.; Kivrak, H. Hydrazine Electrooxidation Activities of Novel Carbon Nanotube Supported Tin Modified Palladium Nanocatalysts. Surf. Interfaces 2022, 28, 101680. [Google Scholar] [CrossRef]
- Liu, F.; Jiang, X.; Wang, H.-H.; Chen, C.; Yang, Y.-H.; Sheng, T.; Wei, Y.-S.; Zhao, X.-S.; Wei, L. Boosting Electrocatalytic Hydrazine Oxidation Reaction on High-Index Faceted Au Concave Trioctahedral Nanocrystals. ACS Sustain. Chem. Eng. 2022, 10, 696–702. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.; Shen, T.; Zheng, X.; Zhao, Y.; Song, Y.-F. Atomically Dispersed Rh-Doped NiFe Layered Double Hydroxides: Precise Location of Rh and Promoting Hydrazine Electrooxidation Properties. Nanoscale 2021, 13, 1869–1874. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, S.-M.; Xu, Y.; Tan, L.; Wang, X.; Zhao, Y.; Duan, H.; Song, Y.-F. Single Ru Atoms with Precise Coordination on a Monolayer Layered Double Hydroxide for Efficient Electrooxidation Catalysis. Chem. Sci. 2019, 10, 378–384. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Xue, Y.; He, F.; Li, Y. Controlled Growth of a High Selectivity Interface for Seawater Electrolysis. Proc. Natl. Acad. Sci. USA 2022, 119, e2206946119. [Google Scholar] [CrossRef]
- Zheng, X.; Xue, Y.; Zhang, C.; Li, Y. Controlled Growth of Multidimensional Interface for High-Selectivity Ammonia Production. CCS Chem. 2022, 1–10. [Google Scholar] [CrossRef]
- Gao, Y.; Xue, Y.; Qi, L.; Xing, C.; Zheng, X.; He, F.; Li, Y. Rhodium Nanocrystals on Porous Graphdiyne for Electrocatalytic Hydrogen Evolution from Saline Water. Nat. Commun. 2022, 13, 5227. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Huang, B.; Yi, Y.; Guo, Y.; Zuo, Z.; Li, Y.; Jia, Z.; Liu, H.; Li, Y. Anchoring Zero Valence Single Atoms of Nickel and Iron on Graphdiyne for Hydrogen Evolution. Nat. Commun. 2018, 9, 1460. [Google Scholar] [CrossRef] [PubMed]

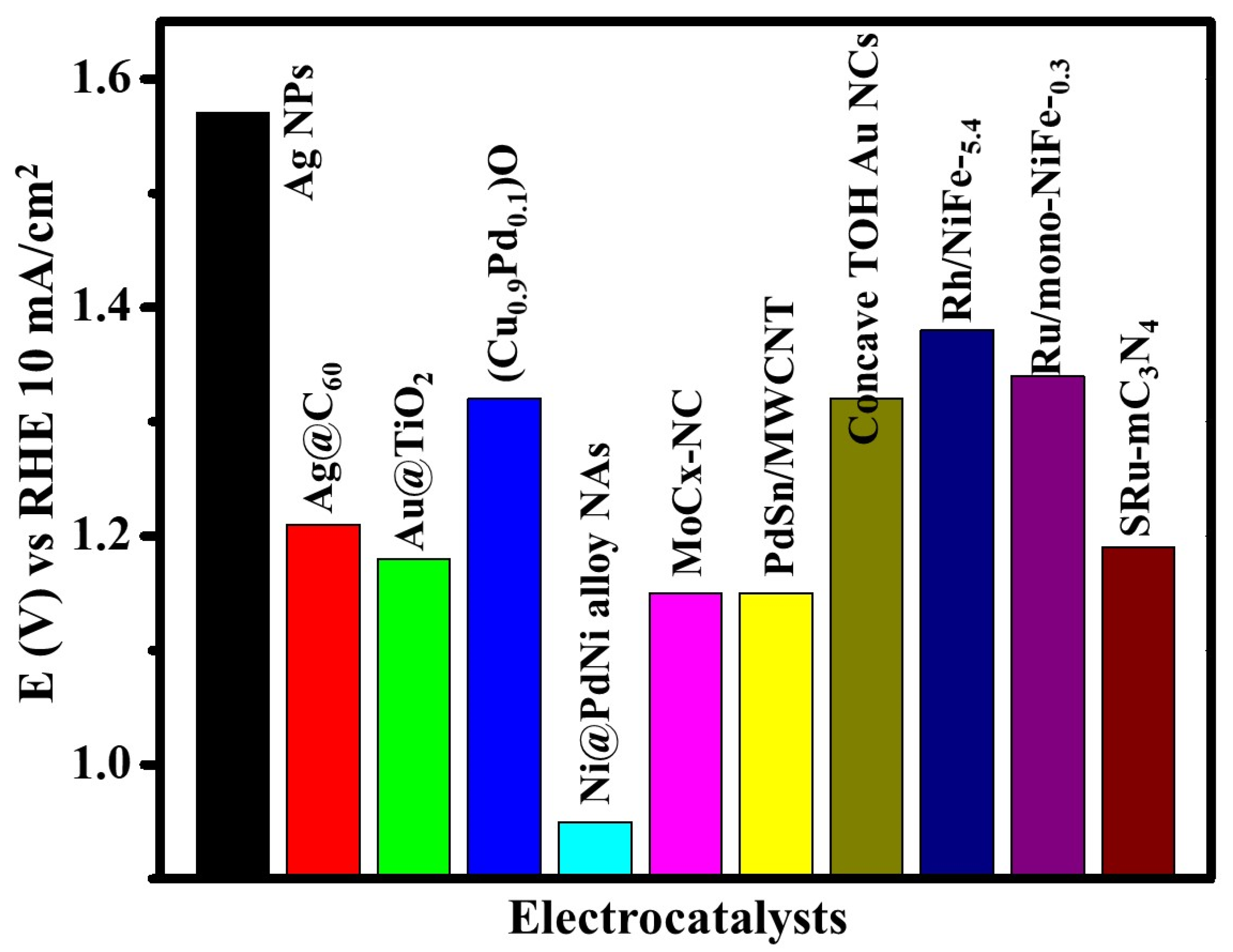
| Sr. No. | Electrocatalyst | @Potential (V) vs. RHE | Current Density (mA/cm2) | Enhancement Factor (ƞ) |
|---|---|---|---|---|
| (1) | bare GCE | 1.3 | 0.85 | - |
| (2) | m-C3N4 | 1.3 | 3.2 | 376 |
| (3) | SRu-mC3N4 (A1) | 1.3 | 5.25 | 617 |
| (4) | SRu-mC3N4 (A3) | 1.3 | 3.28 | 386 |
| (5) | SRu-mC3N4 (A2) | 1.3 | 3.1 | 364 |
| (6) | SRu-mC3N4 | 1.3 | 19.2 | 2258 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munde, A.; Sharma, P.; Dhawale, S.; Kadam, R.G.; Kumar, S.; Kale, H.B.; Filip, J.; Zboril, R.; Sathe, B.R.; Gawande, M.B. Interface Engineering of SRu-mC3N4 Heterostructures for Enhanced Electrochemical Hydrazine Oxidation Reactions. Catalysts 2022, 12, 1560. https://doi.org/10.3390/catal12121560
Munde A, Sharma P, Dhawale S, Kadam RG, Kumar S, Kale HB, Filip J, Zboril R, Sathe BR, Gawande MB. Interface Engineering of SRu-mC3N4 Heterostructures for Enhanced Electrochemical Hydrazine Oxidation Reactions. Catalysts. 2022; 12(12):1560. https://doi.org/10.3390/catal12121560
Chicago/Turabian StyleMunde, Ajay, Priti Sharma, Somnath Dhawale, Ravishankar G. Kadam, Subodh Kumar, Hanumant B. Kale, Jan Filip, Radek Zboril, Bhaskar R. Sathe, and Manoj B. Gawande. 2022. "Interface Engineering of SRu-mC3N4 Heterostructures for Enhanced Electrochemical Hydrazine Oxidation Reactions" Catalysts 12, no. 12: 1560. https://doi.org/10.3390/catal12121560
APA StyleMunde, A., Sharma, P., Dhawale, S., Kadam, R. G., Kumar, S., Kale, H. B., Filip, J., Zboril, R., Sathe, B. R., & Gawande, M. B. (2022). Interface Engineering of SRu-mC3N4 Heterostructures for Enhanced Electrochemical Hydrazine Oxidation Reactions. Catalysts, 12(12), 1560. https://doi.org/10.3390/catal12121560