TiO2-Based Heterostructure Containing g-C3N4 for an Effective Photocatalytic Treatment of a Textile Dye
Abstract
:1. Introduction
2. Results and Discussion
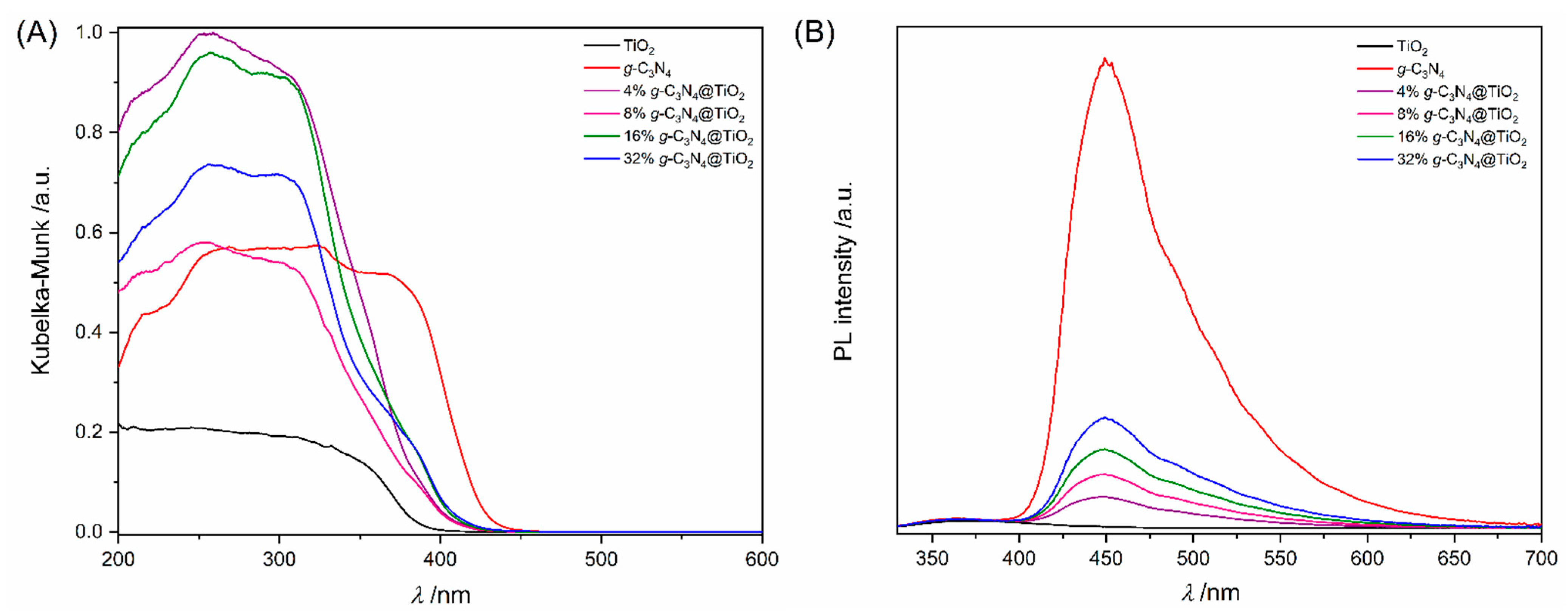
2.1. Characterization of the Prepared Materials
2.2. Evaluation of Photocatalytic Activity
3. Materials and Methods
3.1. Chemicals
3.2. Synthesis of TiO2, g-C3N4, and g-C3N4@TiO2 Nanocomposites
3.3. Physico-Chemical Characterization
3.4. Photolytic, Adsorption and Photocatalytic Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khalid, S.; Shahid, M.; Natasha; Bibi, I.; Sarwar, T.; Shah, A.H.; Niazi, N.K. A Review of Environmental Contamination and Health Risk Assessment of Wastewater Use for Crop Irrigation with a Focus on Low and High-Income Countries. Int. J. Environ. Res. Public Health 2018, 15, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Smith, D.W. Advanced technologies in water and wastewater treatment. Can. J. Civ. Eng. 2001, 28, 49–66. [Google Scholar] [CrossRef]
- Zhang, K.; Zhou, M.; Yang, K.; Yu, C.; Mu, P.; Yu, Z.; Lu, K.; Huang, W.; Dai, W. Photocatalytic H2O2 production and removal of Cr (VI) via a novel Lu3NbO7: Yb, Ho/CQDs/AgInS2/In2S3 heterostructure with broad spectral response. J. Hazard. Mater. 2022, 423, 127172. [Google Scholar] [CrossRef] [PubMed]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key Points of Advanced Oxidation Processes (AOPs) for Wastewater, Organic Pollutants and Pharmaceutical Waste Treatment: A Mini Review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Liu, X.; Huang, W.Y.; Zhou, Q.; Chen, X.R.; Yang, K.; Li, D.; Dionysiou, D.D. Ag-decorated 3D flower-like Bi2MoO6/rGO with boosted photocatalytic performance for removal of organic pollutants. Rare Met. 2021, 40, 1086–1098. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, K.; Yu, C.; Lu, K.; Huang, W.; Xu, L.; Zou, L.; Wang, S.; Chen, Z.; Hu, J.; et al. Steering Unit Cell Dipole and Internal Electric Field by Highly Dispersed Er atoms Embedded into NiO for Efficient CO2 Photoreduction. Adv. Funct. Mater. 2022, 32, 2111999. [Google Scholar] [CrossRef]
- Darkwah, W.K.; Ao, Y. Mini Review on the Structure and Properties (Photocatalysis), and Preparation Techniques of Graphitic Carbon Nitride Nano-Based Particle, and Its Applications. Nanoscale Res. Lett. 2018, 13, 388. [Google Scholar] [CrossRef]
- Ibhadon, A.O.; Fitzpatrick, P. Heterogeneous Photocatalysis: Recent Advances and Applications. Catalysts 2013, 3, 189–218. [Google Scholar] [CrossRef] [Green Version]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, N.; Tang, Z.-R.; Xu, Y.-J. Improving the photocatalytic performance of graphene–TiO2 nanocomposites via a combined strategy of decreasing defects of graphene and increasing interfacial contact. Phys. Chem. Chem. Phys. 2012, 14, 9167–9175. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Liao, J.; Dong, F.; Li, H.; Liu, H. Growth of g-C3N4 Layer on Commercial TiO2 for Enhanced Visible Light Photocatalytic Activity. J. Nanomater. 2014, 2014, 869094. [Google Scholar] [CrossRef] [Green Version]
- Mohini, R.; Lakshminarasimhan, N. Coupled semiconductor nanocomposite g-C3N4/TiO2 with enhanced visible light photocatalytic activity. Mater. Res. Bull. 2016, 76, 370–375. [Google Scholar] [CrossRef]
- Li, C.; Zhu, D.; Cheng, S.; Zuo, Y.; Wang, Y.; Ma, C.; Dong, H. Recent research progress of bimetallic phosphides-based nanomaterials as cocatalyst for photocatalytic hydrogen evolution. Chin. Chem. Lett. 2022, 33, 1141–1153. [Google Scholar] [CrossRef]
- Miranda, C.; Mansilla, H.; Yáñez, J.; Obregón, S.; Colón, G. Improved photocatalytic activity of g-C3N4/TiO2 composites prepared by a simple impregnation method. J. Photochem. Photobiol. A Chem. 2013, 253, 16–21. [Google Scholar] [CrossRef]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef]
- Yu, T.; Liu, L.; Yang, F. Heterojunction between anodic TiO2/g-C3N4 and cathodic WO3/W nano-catalysts for coupled pollutant removal in a self-biased system. Chinese J. Catal. 2017, 38, 270–277. [Google Scholar] [CrossRef]
- Tian, Q.; Shen, X.; Wang, Z.; Zhu, B.; Osotsi, M.I.; Xie, X.; Jin, Y.; Chen, Z.; Zhang, L. Growth of Cu2O Spherical Superstructures on g-C3N4 as Efficient Visible-Light-Driven p-n Heterojunction Photocatalysts for Degrading Various Organic Pollutants. J. Nanosci. Nanotechnol. 2018, 18, 7355–7363. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.-G.; He, H.-W.; Wang, X.-X.; Zhang, J.; Zhang, Q.-Q.; Tong, Y.-F.; Liu, H.-L.; Ramakrishna, S.; Yan, S.-Y.; et al. One-Step Synthesis Heterostructured g-C3N4/TiO2 Composite for Rapid Degradation of Pollutants in Utilizing Visible Light. Nanomaterials 2018, 8, 842. [Google Scholar] [CrossRef] [Green Version]
- Xia, Y.; Xu, L.; Peng, J.; Han, J.; Guo, S.; Zhang, L.; Han, Z.; Komarneni, S. TiO2@g-C3N4 core/shell spheres with uniform mesoporous structures for high performance visible-light photocatalytic application. Ceram. Int. 2019, 45, 18844–18851. [Google Scholar] [CrossRef]
- Tang, Q.; Meng, X.; Wang, Z.; Zhou, J.; Tang, H. One-step electrospinning synthesis of TiO2/g-C3N4 nanofibers with enhanced photocatalytic properties. Appl. Surf. Sci. 2018, 430, 253–262. [Google Scholar] [CrossRef]
- Razali, M.H.; Md Fauzi, M.A.F.; Mohd Azam, B.; Yusoff, M. g-C3N4/TiO2 nanocomposite photocatalyst for methylene blue photodegradation under visible light. Appl. Nanosci. 2022, 12, 3197–3206. [Google Scholar] [CrossRef]
- Song, G.; Chu, Z.; Jin, W.; Sun, H. Enhanced performance of g-C3N4/TiO2 photocatalysts for degradation of organic pollutants under visible light. Chinese J. Chem. Eng. 2015, 23, 1326–1334. [Google Scholar] [CrossRef]
- Ma, L.; Wang, G.; Jiang, C.; Bao, H.; Xu, Q. Synthesis of core-shell TiO2@g-C3N4 hollow microspheres for efficient photocatalytic degradation of rhodamine B under visible light. Appl. Surf. Sci. 2018, 430, 263–272. [Google Scholar] [CrossRef]
- Li, K.; Gao, S.; Wang, Q.; Xu, H.; Wang, Z.; Huang, B.; Dai, Y.; Lu, J. In-Situ-Reduced Synthesis of Ti3+ Self-Doped TiO2/g-C3N4 Heterojunctions with High Photocatalytic Performance under LED Light Irradiation. ACS Appl. Mater. Interfaces 2015, 7, 9023–9030. [Google Scholar] [CrossRef]
- Barzegar, M.H.; Sabzehmeidani, M.M.; Ghaedi, M.; Avargani, V.M.; Moradi, Z.; Roy, V.A.L.; Heidari, H. S-scheme heterojunction g-C3N4/TiO2 with enhanced photocatalytic activity for degradation of a binary mixture of cationic dyes using solar parabolic trough reactor. Chem. Eng. Res. Des. 2021, 174, 307–318. [Google Scholar] [CrossRef]
- Adeleye, A.T.; John, K.I.; Adeleye, P.G.; Akande, A.A.; Banjoko, O.O. One-dimensional titanate nanotube materials: Heterogeneous solid catalysts for sustainable synthesis of biofuel precursors/value-added chemicals—A review. J. Mater. Sci. 2021, 56, 18391–18416. [Google Scholar] [CrossRef]
- Dong, G.; Zhao, K.; Zhang, L. Carbon self-doping induced high electronic conductivity and photoreactivity of g-C3N4. Chem. Commun. 2012, 48, 6178–6180. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Li, Y.; Ho, W.; Yu, J. Fabrication and photocatalytic activity enhanced mechanism of direct Z-scheme g-C3N4/Ag2WO4 photocatalyst. Appl. Surf. Sci. 2017, 391, 175–183. [Google Scholar] [CrossRef]
- Thomas, A.; Fischer, A.; Goettmann, F.; Antonietti, M.; Müller, J.O.; Schlögl, R.; Carlsson, J.M. Graphitic carbon nitride materials: Variation of structure and morphology and their use as metal-free catalysts. J. Mater. Chem. 2008, 18, 4893–4908. [Google Scholar] [CrossRef]
- Aleksandrzak, M.; Baranowska, D.; Kukulka, W.; Onyszko, M.; Zielinska, B.; Mijowska, E. Bifunctional polymeric carbon nitride via tuning fabrication conditions for photocatalysis. Catalysts 2021, 11, 651. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, N.; Xia, J.; Meng, Q.; Ding, J.; Lu, J. Synthesis and characterization of TiO2/graphene oxide nanocomposites for photoreduction of heavy metal ions in reverse osmosis concentrate. RSC Adv. 2018, 8, 34241–34251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.F.; Lee, C.Y.; Yeng, M.Y.; Chiu, H.T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Amorin, L.H.; Suzuki, V.Y.; De Paula, N.H.; Duarte, J.L.; Da Silva, M.A.T.; Taft, C.A.; De Almeida La Porta, F. Electronic, structural, optical, and photocatalytic properties of graphitic carbon nitride. New J. Chem. 2019, 43, 13647–13653. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, H.; Chen, C. Fabrication of g-C3N4/TiO2 composite photocatalyst with extended absorption wavelength range and enhanced photocatalytic performance. J. Photochem. Photobiol. A Chem. 2016, 317, 151–160. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhu, C.; Wan, W.; Qian, K.; Xie, J. Constructing graphite-like carbon nitride modified hierarchical yolk-shell TiO2 spheres for water pollution treatment and hydrogen production. J. Mater. Chem. A 2016, 4, 1806–1818. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV-Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [Green Version]
- Pham, M.T.; Luu, H.Q.; Nguyen, T.M.T.; Tran, H.H.; You, S.J.; Wang, Y.F. Rapid and Scalable Fabrication of TiO2@g-C3N4 Heterojunction for Highly Efficient Photocatalytic NO Removal under Visible Light. Aerosol Air Qual. Res. 2021, 21, 210276. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Radošević, T.; Podlogar, M. Enhanced Photocatalytic Activity of Hybrid rGO@TiO2/CN Nanocomposite for Organic Pollutant Degradation under Solar Light Irradiation. Catalysts 2021, 11, 1023. [Google Scholar] [CrossRef]
- Alfaifi, M.Q.; Bagabas, A.A. Preparation, characterization, and application in water purification of g-C3N4/I-TiO2 composite photocatalysts. Adv. Mater. Sci. 2019, 4, 1–10. [Google Scholar] [CrossRef]
- Parvathiraja, C.; Katheria, S.; Siddiqui, M.R.R.; Wabaidur, S.M.M.; Islam, M.A.A.; Lai, W.-C. Activated Carbon-Loaded Titanium Dioxide Nanoparticles and Their Photocatalytic and Antibacterial Investigations. Catalysts 2022, 12, 834. [Google Scholar] [CrossRef]
- Zhang, Y.; Pan, Q.; Chai, G.; Liang, M.; Dong, G.; Zhang, Q.; Qiu, J. Synthesis and luminescence mechanism of multicolor-emitting g-C3N4 nanopowders by low temperature thermal condensation of melamine. Sci. Rep. 2013, 3, 1943. [Google Scholar] [CrossRef] [Green Version]
- Bian, J.; Li, J.; Kalytchuk, S.; Wang, Y.; Li, Q.; Lau, T.C.; Niehaus, T.A.; Rogach, A.L.; Zhang, R.Q. Efficient emission facilitated by multiple energy level transitions in uniform graphitic carbon nitride films deposited by thermal vapor condensation. ChemPhysChem 2015, 16, 954–959. [Google Scholar] [CrossRef]
- Bayan, S.; Midya, A.; Gogurla, N.; Singha, A.; Ray, S.K. Origin of Modified Luminescence Response in Reduced Graphitic Carbon Nitride Nanosheets. J. Phys. Chem. C 2017, 121, 19383–19391. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, L.; Xing, J.; Utama, M.I.B.; Lu, X.; Du, K.; Li, Y.; Hu, X.; Wang, S.; Genç, A.; et al. High-yield synthesis and optical properties of g-C3N4. Nanoscale 2015, 7, 12343–12350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.; Shinde, S.L.; Nanda, K.K. Temperature-Dependent Photoluminescence of g-C3N4: Implication for Temperature Sensing. ACS Appl. Mater. Interfaces 2016, 8, 2181–2186. [Google Scholar] [CrossRef] [PubMed]
- Chubenko, E.B.; Baglov, A.V.; Leonenya, M.S.; Yablonskii, G.P.; Borisenko, V.E. Structure of Photoluminescence Spectra of Oxygen-Doped Graphitic Carbon Nitride. J. Appl. Spectrosc. 2020, 87, 9–14. [Google Scholar] [CrossRef]
- Zhang, X.; Jiang, S.P.; Yang, P. Bright and tunable photoluminescence from the assembly of red g-C3N4 nanosheets. J. Lumin. 2021, 235, 118055. [Google Scholar] [CrossRef]
- Choudhury, B.; Paul, K.K.; Sanyal, D.; Hazarika, A.; Giri, P.K. Evolution of Nitrogen-Related Defects in Graphitic Carbon Nitride Nanosheets Probed by Positron Annihilation and Photoluminescence Spectroscopy. J. Phys. Chem. C 2018, 122, 9209–9219. [Google Scholar] [CrossRef]
- Tang, W.; Tian, Y.; Chen, B.; Xu, Y.; Li, B.; Jing, X.; Zhang, J.; Xu, S. Supramolecular Copolymerization Strategy for Realizing the Broadband White Light Luminescence Based on N-Deficient Porous Graphitic Carbon Nitride (g-C3N4). ACS Appl. Mater. Interfaces 2020, 12, 6396–6406. [Google Scholar] [CrossRef]
- Samanta, S.; Martha, S.; Parida, K. Facile synthesis of Au/g-C3N4 nanocomposites: An inorganic/organic hybrid plasmonic photocatalyst with enhanced hydrogen gas evolution under visible-light irradiation. ChemCatChem 2014, 6, 1453–1462. [Google Scholar] [CrossRef]
- Hu, S.W.; Yang, L.W.; Tian, Y.; Wei, X.L.; Ding, J.W.; Zhong, J.X.; Chu, P.K. Non-covalent doping of graphitic carbon nitride with ultrathin graphene oxide and molybdenum disulfide nanosheets: An effective binary heterojunction photocatalyst under visible light irradiation. J. Colloid Interface Sci. 2014, 431, 42–49. [Google Scholar] [CrossRef]
- Horti, N.C.; Kamatagi, M.D.; Patil, N.R.; Nataraj, S.K.; Sannaikar, M.S.; Inamdar, S.R. Synthesis and photoluminescence properties of titanium oxide (TiO2) nanoparticles: Effect of calcination temperature. Optik 2019, 194, 163070. [Google Scholar] [CrossRef]
- Abazović, N.D.; Čomor, M.I.; Dramićanin, M.D.; Jovanović, D.J.; Ahrenkiel, S.P.; Nedeljković, J.M. Photoluminescence of anatase and rutile TiO2 particles. J. Phys. Chem. B 2006, 110, 25366–25370. [Google Scholar] [CrossRef]
- Pallotti, D.K.; Passoni, L.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Photoluminescence Mechanisms in Anatase and Rutile TiO2. J. Phys. Chem. C 2017, 121, 9011–9021. [Google Scholar] [CrossRef]
- Jiménez-Salcedo, M.; Monge, M.; Tena, M.T. The photocatalytic degradation of sodium diclofenac in different water matrices using g-C3N4 nanosheets: A study of the intermediate by-products and mechanism. J. Environ. Chem. Eng. 2021, 9, 105827. [Google Scholar] [CrossRef]
- Iovino, P.; Chianese, S.; Canzano, S.; Prisciandaro, M.; Musmarra, D. Ibuprofen photodegradation in aqueous solutions. Environ. Sci. Pollut. Res. 2016, 23, 22993–23004. [Google Scholar] [CrossRef]
- Shehzad, N.; Tahir, M.; Johari, K.; Murugesan, T.; Hussain, M. A critical review on TiO2 based photocatalytic CO2 reduction system: Strategies to improve efficiency. J. CO2 Util. 2018, 26, 98–122. [Google Scholar] [CrossRef]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef]
- Petala, A.; Mantzavinos, D.; Frontistis, Z. Impact of water matrix on the photocatalytic removal of pharmaceuticals by visible light active materials. Curr. Opin. Green Sustain. Chem. 2021, 28, 100445. [Google Scholar] [CrossRef]
- Dharwadkar, S.; Yu, L.; Achari, G. Photocatalytic degradation of sulfolane using a led-based photocatalytic treatment system. Catalysts 2021, 11, 624. [Google Scholar] [CrossRef]
- Cruz, M.; Gomez, C.; Duran-Valle, C.J.; Pastrana-Martínez, L.M.; Faria, J.L.; Silva, A.M.T.; Faraldos, M.; Bahamonde, A. Bare TiO2 and graphene oxide TiO2 photocatalysts on the degradation of selected pesticides and influence of the water matrix. Appl. Surf. Sci. 2017, 416, 1013–1021. [Google Scholar] [CrossRef]
- Faisal, M.; Alsaiari, M.; Rashed, M.A.; Harraz, F.A. Biomass-derived active Carbon@ZnO/SnO2 novel visible-light photocatalyst for rapid degradation of linezolid antibiotic and imidacloprid insecticide. J. Taiwan Inst. Chem. Eng. 2021, 120, 313–324. [Google Scholar] [CrossRef]
- Vavilapalli, D.S.; Peri, R.G.; Sharma, R.K.; Goutam, U.K.; Muthuraaman, B.; Ramachandra Rao, M.S.; Singh, S. g-C3N4/Ca2Fe2O5 heterostructures for enhanced photocatalytic degradation of organic effluents under sunlight. Sci. Rep. 2021, 11, 19639. [Google Scholar] [CrossRef]
- Gogoi, D.; Makkar, P.; Ghosh, N.N. Solar Light-Irradiated Photocatalytic Degradation of Model Dyes and Industrial Dyes by a Magnetic CoFe2O4-gC3N4 S-Scheme Heterojunction Photocatalyst. ACS Omega 2021, 6, 4831–4841. [Google Scholar] [CrossRef]
- Md Fauzi, M.A.F.; Razali, M.H.; Osman, M.U.; Mohd Azam, B. Synthesis and characterisation of TiO2/g-C3N4 as photocatalyst for photodegradation of dyes, phenol and caffeine. Adv. Mater. Process. Technol. 2022, 1–21. [Google Scholar] [CrossRef]
- Islam, M.R.; Chakraborty, A.K.; Gafur, M.A.; Rahman, M.A.; Rahman, M.H. Easy preparation of recyclable thermally stable visible-light-active graphitic-C3N4/TiO2 nanocomposite photocatalyst for efficient decomposition of hazardous organic industrial pollutants in aqueous medium. Res. Chem. Intermed. 2019, 45, 1753–1773. [Google Scholar] [CrossRef]
- Kocijan, M.; Ćurković, L.; Ljubas, D.; Mužina, K.; Bačić, I.; Radošević, T.; Podlogar, M.; Bdikin, I.; Otero-Irurueta, G.; Hortigüela, M.J.; et al. Graphene-Based TiO2 Nanocomposite for Photocatalytic Degradation of Dyes in Aqueous Solution under Solar-Like Radiation. Appl. Sci. 2021, 11, 3966. [Google Scholar] [CrossRef]








| Sample ID | Eg/eV |
|---|---|
| TiO2 | 3.14 |
| g-C3N4 | 2.85 |
| 4% g-C3N4@TiO2 | 3.05 |
| 8% g-C3N4@TiO2 | 2.99 |
| 16% g-C3N4@TiO2 | 2.95 |
| 32% g-C3N4@TiO2 | 2.94 |
| Sample Name | Degradation Efficiency after 90 min/% | k × 10−3, min−1 |
|---|---|---|
| TiO2 | 14 | 1.6 |
| g-C3N4 | 24 | 3.0 |
| 4% g-C3N4@TiO2 | 31 | 4.3 |
| 8% g-C3N4@TiO2 | 45 | 6.0 |
| 16% g-C3N4@TiO2 | 49 | 7.2 |
| 32% g-C3N4@TiO2 | 60 | 9.8 |
| Sample Name | Degradation Efficiency after 90 min/% | k × 10−3, min−1 |
|---|---|---|
| photolysis | 18 | 2.3 |
| TiO2 | 51 | 8.2 |
| g-C3N4 | 60 | 9.8 |
| 4% g-C3N4@TiO2 | 82 | 19.1 |
| 8% g-C3N4@TiO2 | 89 | 23.9 |
| 16% g-C3N4@TiO2 | 92 | 27.6 |
| 32% g-C3N4@TiO2 | 99 | 54.9 |
| Water Bodies | pH | Conductivity /µS·cm−1 | Degradation Efficiency after 90 min/% | k × 10−3 /min−1 |
|---|---|---|---|---|
| Ultrapure water | 6.20 | 0.378 | 99.4 | 54.9 |
| Sea water | 8.37 | 52,100 | 95.4 | 32.5 |
| Lake water | 7.95 | 351.2 | 98.1 | 42.7 |
| River water | 7.92 | 275.8 | 98.8 | 49.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kocijan, M.; Vukšić, M.; Kurtjak, M.; Ćurković, L.; Vengust, D.; Podlogar, M. TiO2-Based Heterostructure Containing g-C3N4 for an Effective Photocatalytic Treatment of a Textile Dye. Catalysts 2022, 12, 1554. https://doi.org/10.3390/catal12121554
Kocijan M, Vukšić M, Kurtjak M, Ćurković L, Vengust D, Podlogar M. TiO2-Based Heterostructure Containing g-C3N4 for an Effective Photocatalytic Treatment of a Textile Dye. Catalysts. 2022; 12(12):1554. https://doi.org/10.3390/catal12121554
Chicago/Turabian StyleKocijan, Martina, Milan Vukšić, Mario Kurtjak, Lidija Ćurković, Damjan Vengust, and Matejka Podlogar. 2022. "TiO2-Based Heterostructure Containing g-C3N4 for an Effective Photocatalytic Treatment of a Textile Dye" Catalysts 12, no. 12: 1554. https://doi.org/10.3390/catal12121554
APA StyleKocijan, M., Vukšić, M., Kurtjak, M., Ćurković, L., Vengust, D., & Podlogar, M. (2022). TiO2-Based Heterostructure Containing g-C3N4 for an Effective Photocatalytic Treatment of a Textile Dye. Catalysts, 12(12), 1554. https://doi.org/10.3390/catal12121554









