Enhancement and Mechanism of Rhodamine B Decomposition in Cavitation-Assisted Plasma Treatment Combined with Fenton Reactions
Abstract
:1. Introduction
2. Experimental
2.1. Experimental Setup
2.2. Experimental Conditions
2.3. Methods for Measuring H2O2 Concentration
3. Results
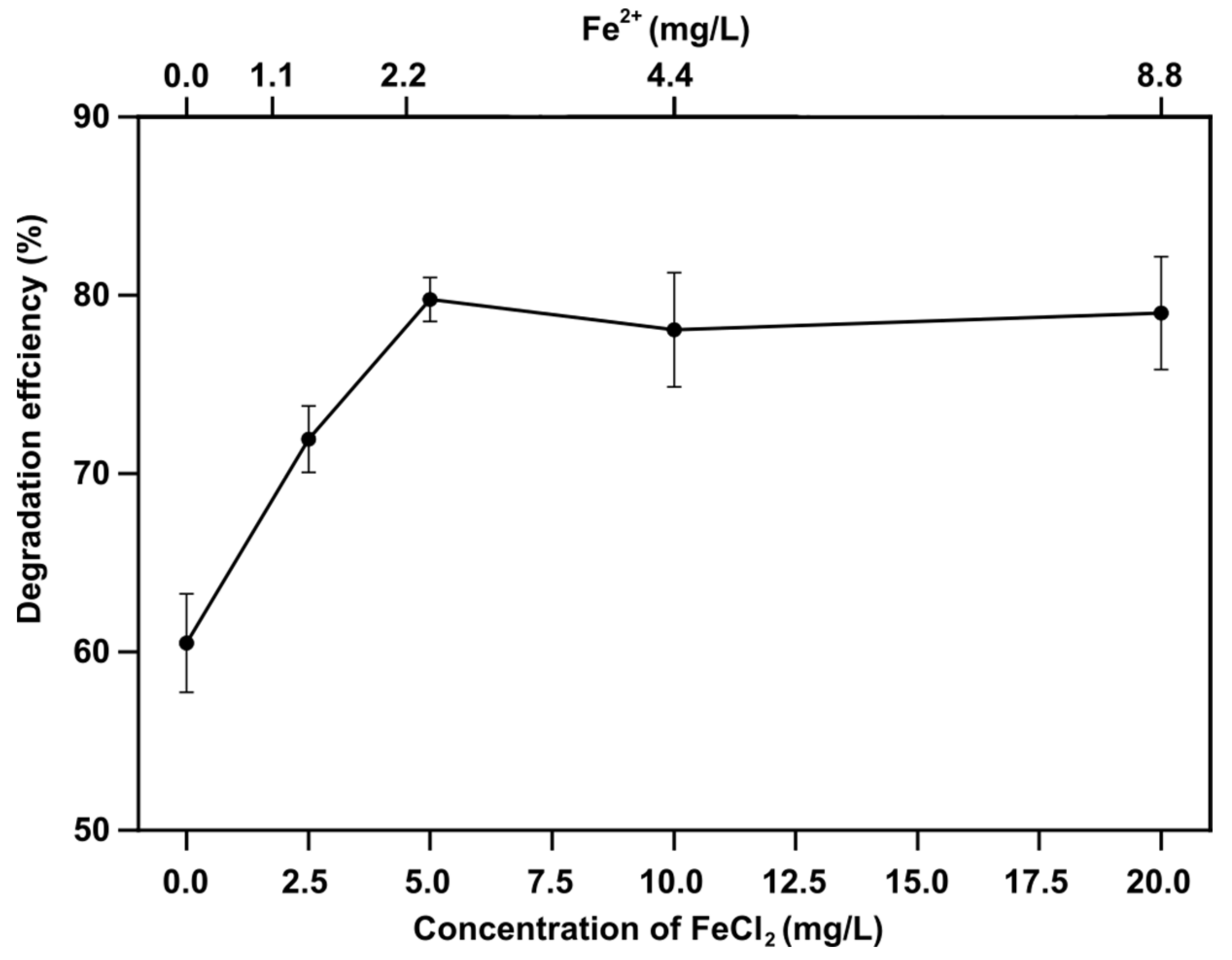
3.1. Effect of Temperature and FeCl2 Addition on Decomposition Efficiency
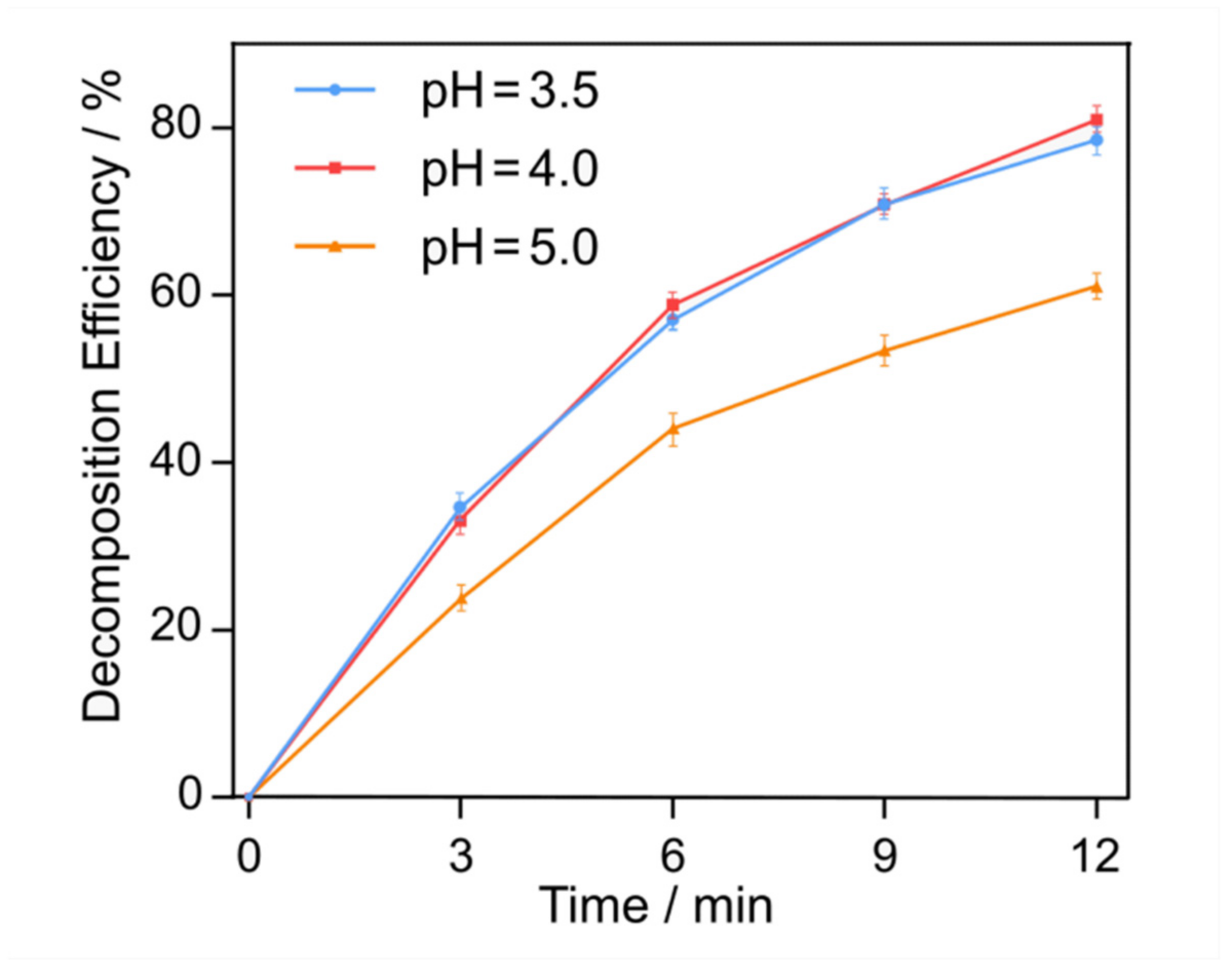
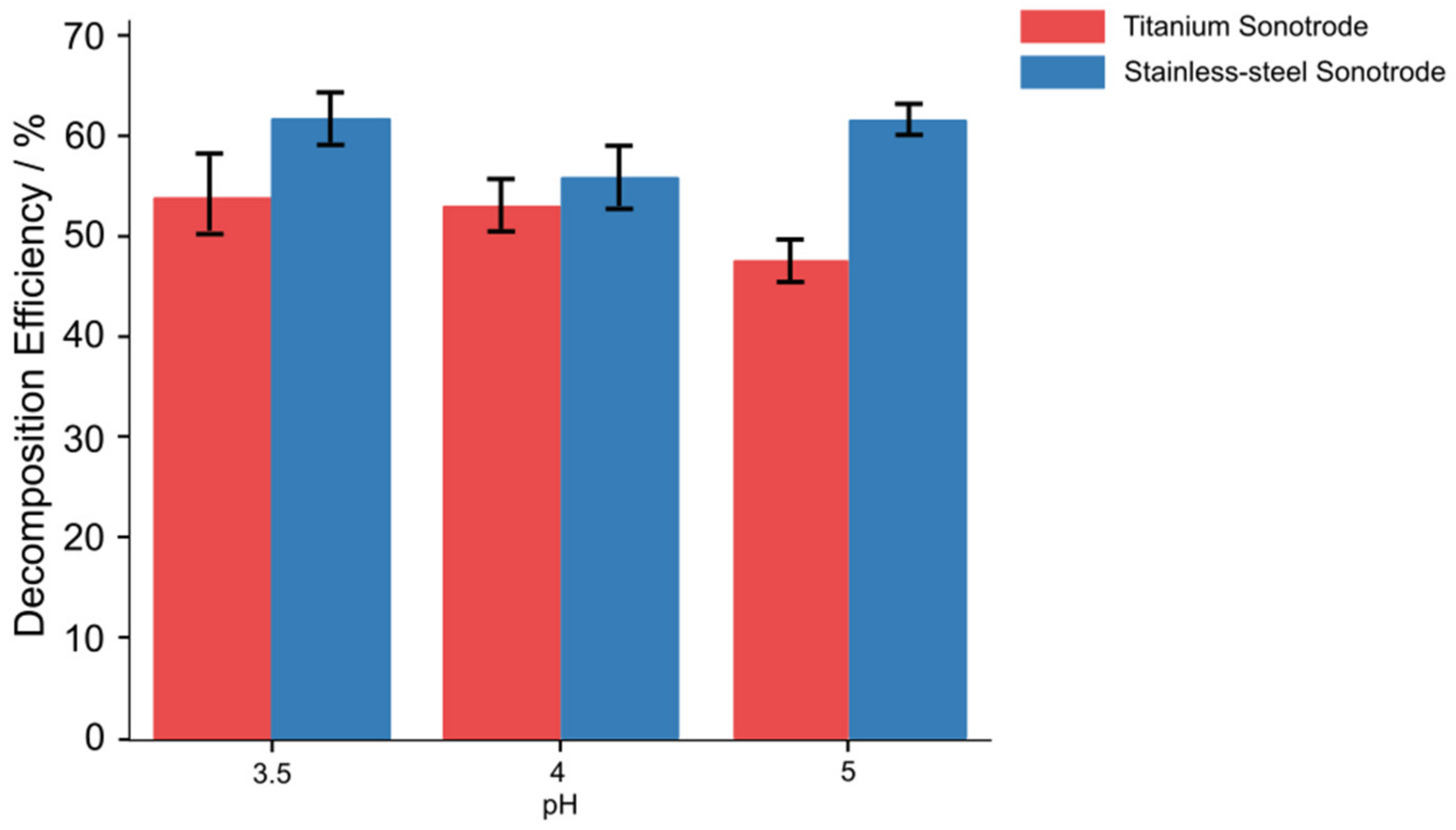
3.2. Effect of Initial pH Level on Decomposition Efficiency
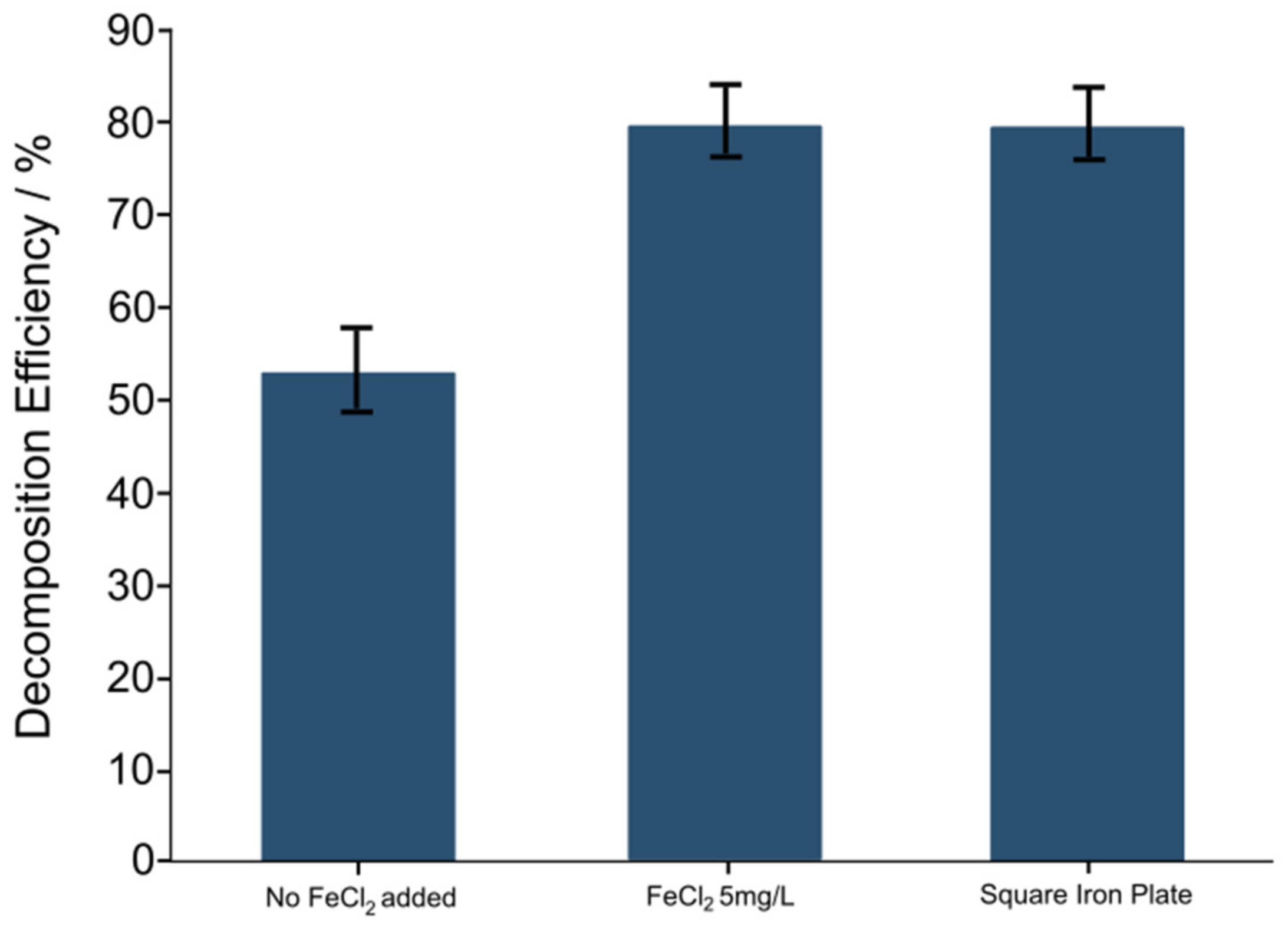
3.3. Decomposition Efficiency in the Presence of Steel Parts
4. Discussion
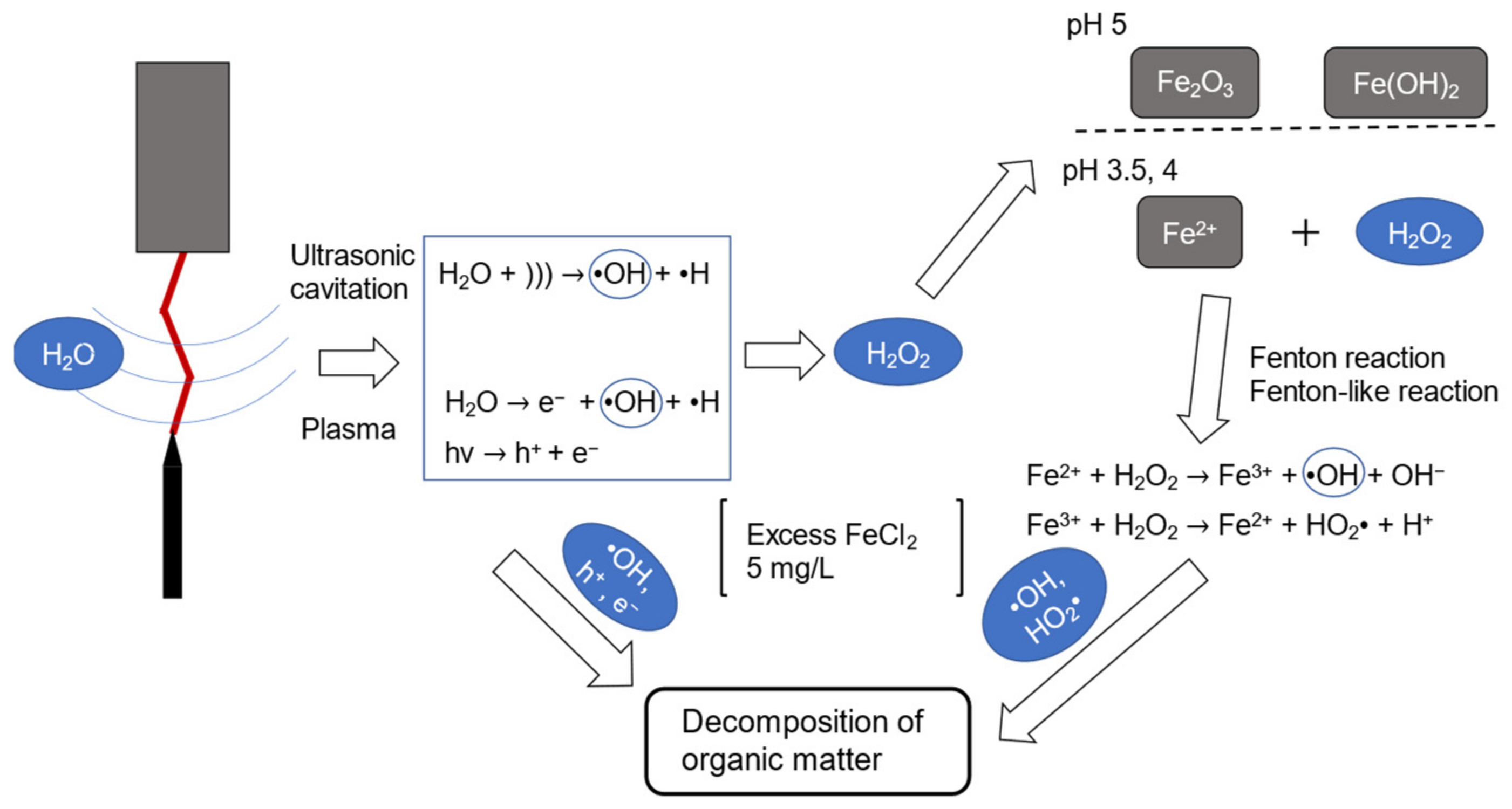
4.1. Possible Chemical Reactions with FeCl2 Addition
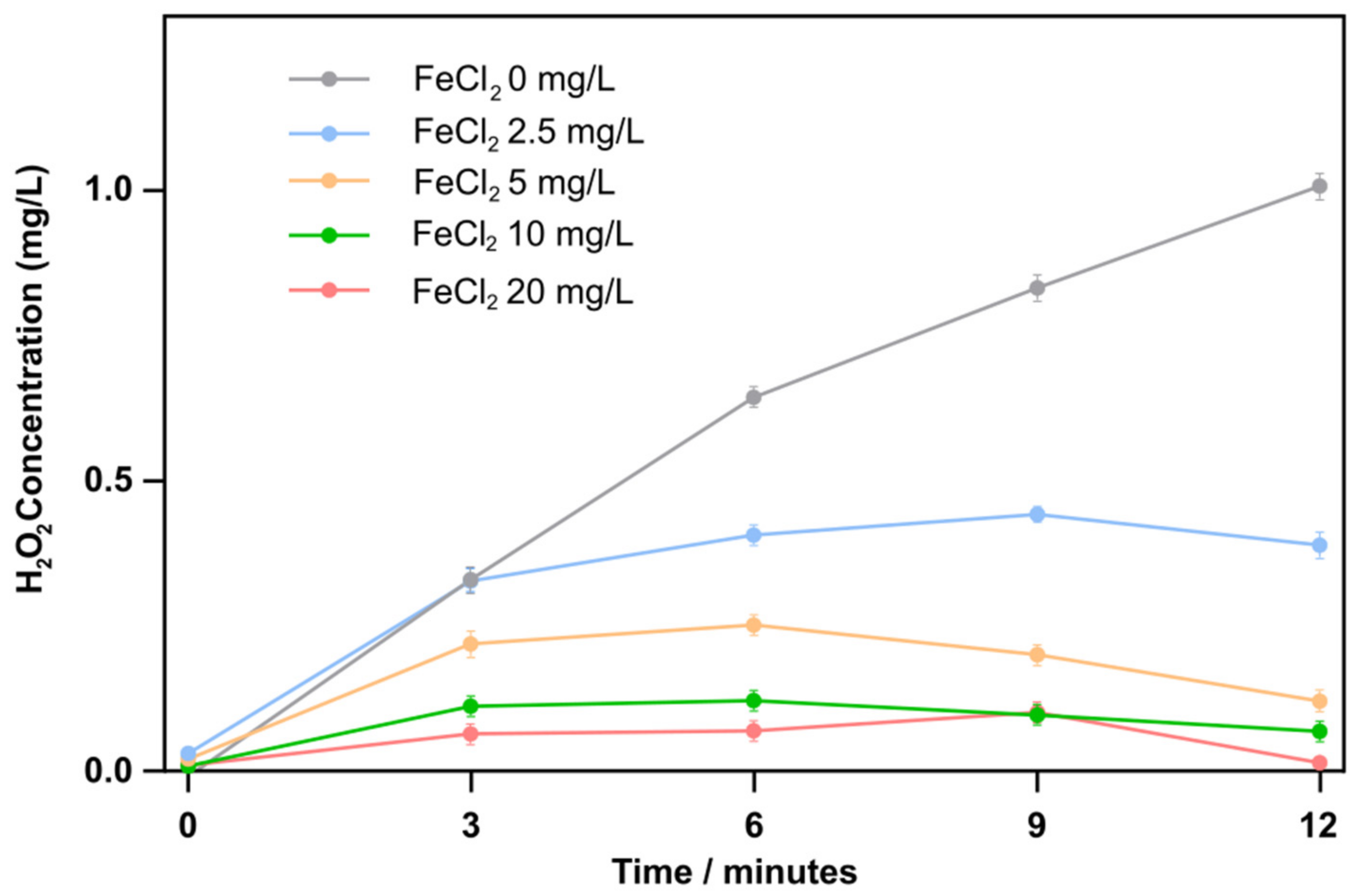
4.2. Production of Hydrogen Peroxide in the ACAP Reactor
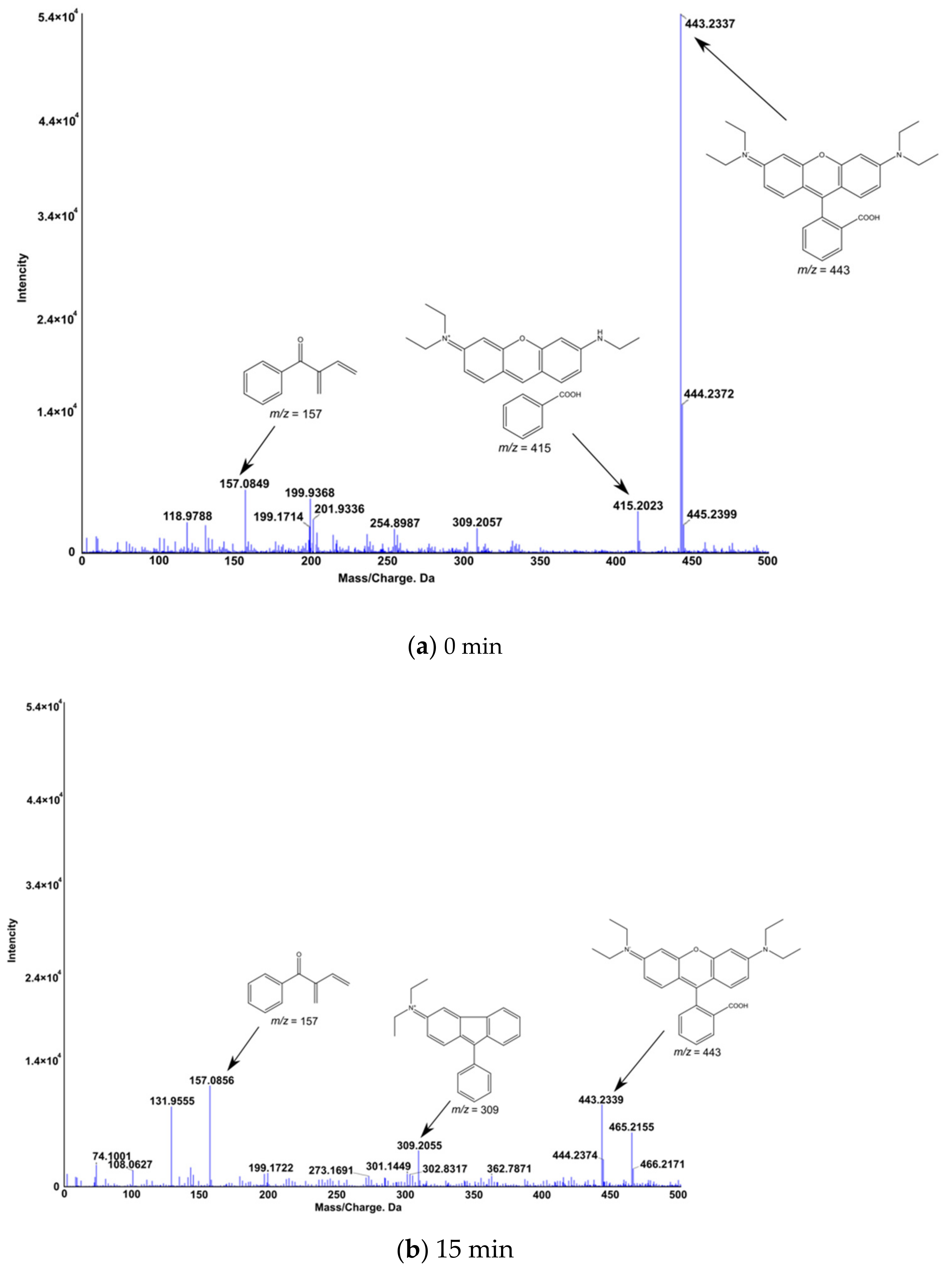
4.3. Mechanisms of RhB Decomposition
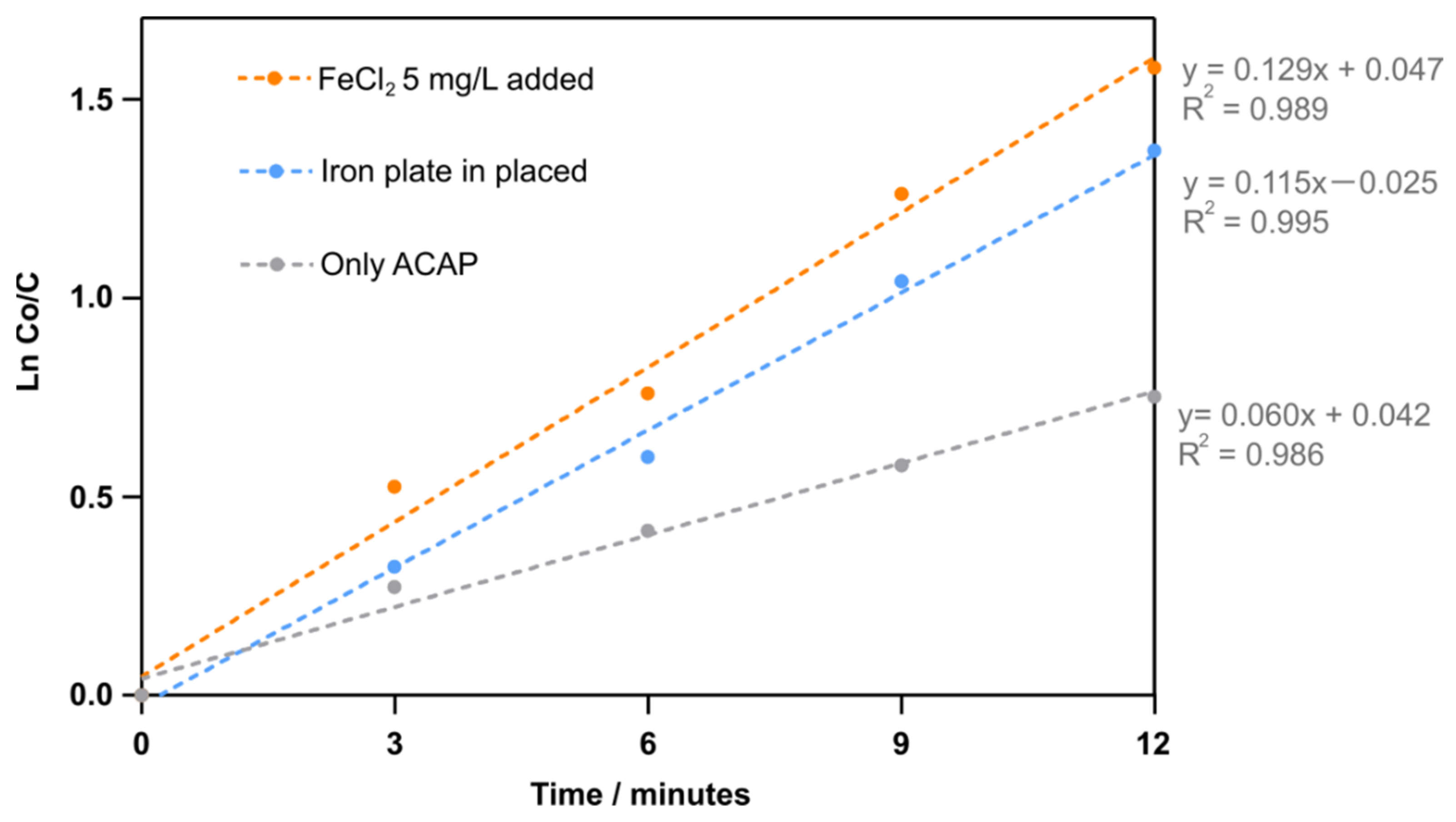
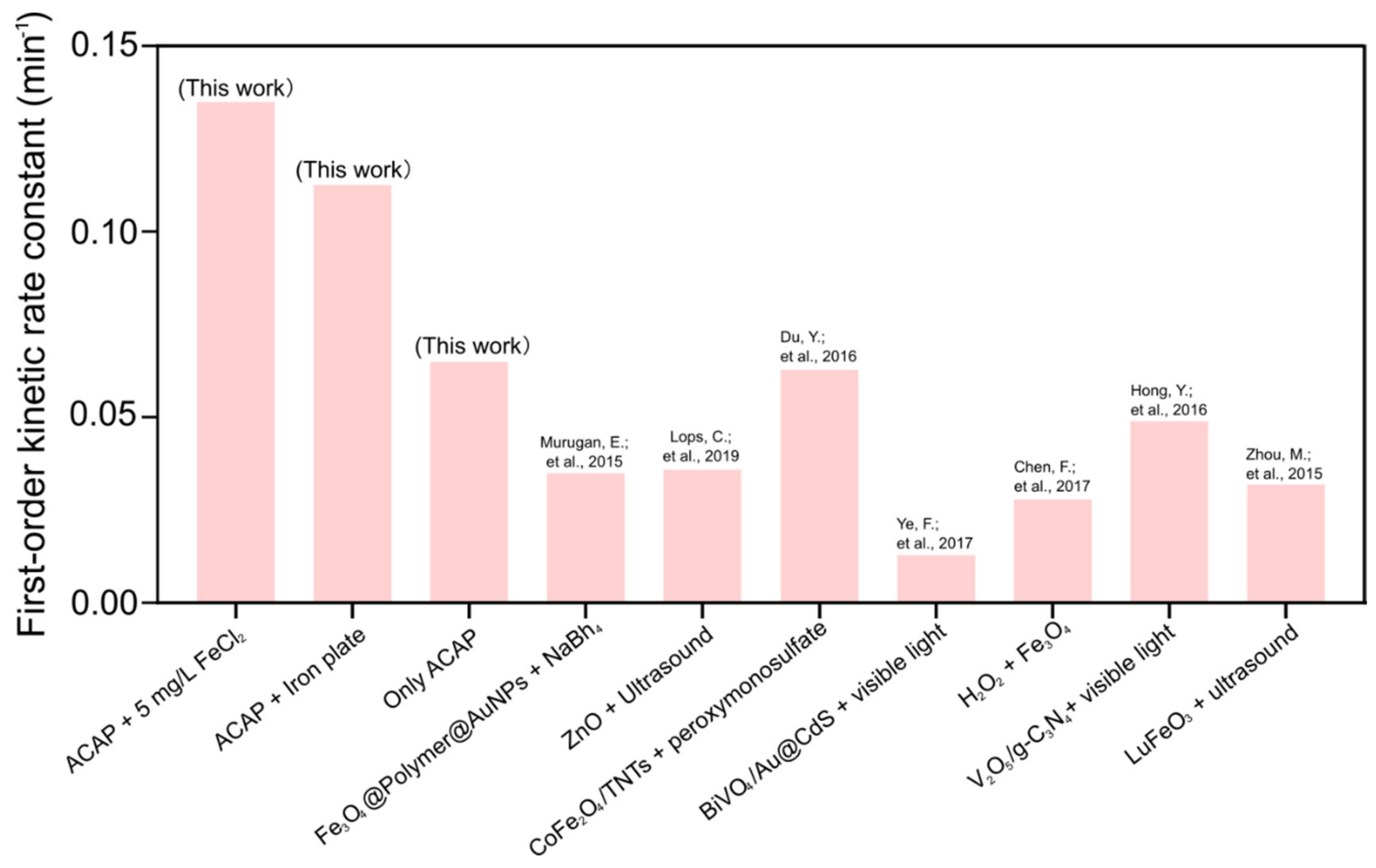
4.4. Reaction Kinetics and Comparison of Various Treatment Methods
5. Conclusions
- (1)
- The Fenton reactions greatly enhanced the efficiency of the acoustic cavitation-assisted plasma decomposition of rhodamine B. Under the present experimental conditions, the decomposition efficiency reached almost 80%, which is 20% greater compared to the case without Fenton reactions.
- (2)
- When FeCl2 was added to the solution, the RhB decomposition efficiency was affected by the pH and the concentration of Fe2+ ions. At pH = 4, the degradation efficiency increased up to 80% as the amount of added FeCl2 increased from 0 to 5 mg/L, and then it remained approximately at the same level with further addition of FeCl2.
- (3)
- Placing iron or steel components inside the ACAP reactor could also improve the efficiency of RhB decomposition, although the effect was slightly less than in the case of ferrous ion addition, due to the slow dissolution rate of steel parts in the aqueous solution.
- (4)
- The mechanism of enhanced Fenton-assisted degradation of RhB is suggested to be as follows: ultrasonic cavitation and plasma discharge generate HO• radicals but a portion recombines to produce H2O2, which reacts with Fe2+ ions to produce HO• radicals again. Under the present experimental conditions, a 5 mg/L addition of FeCl2 was sufficient to convert all H2O2 to the hydroxyl radicals.
- (5)
- The first order kinetics rate constants of RhB decomposition obtained in our experiments were compared with other studies. The results indicated that the ACAP with FeCl2 5 mg/L added to solution directly or with an iron plate installed under the sonotrode tip both had an enhanced promoting effect on RhB decomposition.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A

References
- Vanraes, P.; Nikiforov, A.Y.; Leys, C. Electrical discharge in water treatment technology for micropollutant decomposition. In Plasma Science and Technology–Progress in Physical States and Chemical Reactions; IntechOpen: London, UK, 2016; pp. 428–478. [Google Scholar]
- Stratton, G.R.; Dai, F.; Bellona, C.L.; Holsen, T.M.; Dickenson, E.R.; Mededovic Thagard, S. Plasma-based water treatment: Efficient transformation of perfluoroalkyl substances in prepared solutions and contaminated groundwater. Environ. Sci. Technol. 2017, 51, 1643–1648. [Google Scholar] [CrossRef] [PubMed]
- Stratton, G.R.; Bellona, C.L.; Dai, F.; Holsen, T.M.; Thagard, S.M. Plasma-based water treatment: Conception and application of a new general principle for reactor design. Chem. Eng. J. 2015, 273, 543–550. [Google Scholar] [CrossRef]
- Shen, Y.; Lei, L.; Zhang, X.; Zhou, M.; Zhang, Y. Improvement of diagnostic techniques and electrical circuit in azo dye degradation by high voltage electrical discharge. Energy Convers. Manag. 2008, 49, 2254–2263. [Google Scholar] [CrossRef]
- Sato, M. Degradation of organic contaminants in water by plasma. Int. J. Plasma Environ. Sci. Technol. 2009, 3, 8–14. [Google Scholar]
- Peng, J.; Wang, Z.; Wang, S.; Liu, J.; Zhang, Y.; Wang, B.; Gong, Z.; Wang, M.; Dong, H.; Cao, Z.; et al. Enhanced removal of methylparaben mediated by cobalt/carbon nanotubes (Co/CNTs) activated peroxymonosulfate in chloride-containing water: Reaction kinetics, mechanisms and pathways. Chem. Eng. J. 2021, 409, 128176. [Google Scholar] [CrossRef]
- Takahashi, T.; Takada, N.; Toyoda, H. 3p4–6 effect of superposing ultrasonic wave on microwave plasma under water. Proc. Symp. Ultrason. Electron. 2013, 34, 441–442. [Google Scholar]
- Komarov, S.; Yamamoto, T.; Fang, Y.; Hariu, D. Combined effect of acoustic cavitation and pulsed discharge plasma on wastewater treatment efficiency in a circulating reactor: A case study of rhodamine b. Ultrason. Sonochem. 2020, 68, 105236. [Google Scholar] [CrossRef]
- Fang, Y.; Hariu, D.; Yamamoto, T.; Komarov, S. Acoustic cavitation assisted plasma for wastewater treatment: Degradation of rhodamine b in aqueous solution. Ultrason. Sonochem. 2019, 52, 318–325. [Google Scholar] [CrossRef]
- Xu, Y.; Yamamoto, T.; Hariu, D.; Komarov, S. Effect of gas injection on cavitation-assisted plasma treatment efficiency of wastewater. Ultrason. Sonochem. 2022, 83, 105941. [Google Scholar] [CrossRef] [PubMed]
- Qi, C.; Wen, Y.; Zhao, Y.; Dai, Y.; Li, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of organic contaminants by Fe (III)/peroxymonosulfate process with L-cysteine. Chin. Chem. Lett. 2022, 33, 2125–2128. [Google Scholar] [CrossRef]
- Dai, Y.; Qi, C.; Cao, H.; Wen, Y.; Zhao, Y.; Xu, C.; Yang, S.; He, H. Enhanced degradation of sulfamethoxazole by microwave-activated peracetic acid under alkaline condition: Influencing factors and mechanism. Sep. Purif. Technol. 2022, 288, 120716. [Google Scholar] [CrossRef]
- Pirsaheb, M.; Hossaini, H.; Raad, N.K.; Kianpour, S.; Hossini, H. A systematic review on photo-fenton process as an efficient advanced oxidation for degradation of amoxicillin in aqueous environments. Rev. Environ. Health 2022. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, S.M. High-performance liquid chromatography method for determination of hydrogen peroxide in aqueous solution and application to simulated martian soil and related materials. Environ. Monit. Assess. 2013, 185, 3749–3757. [Google Scholar] [CrossRef] [PubMed]
- Kormann, C.; Bahnemann, D.W.; Hoffmann, M.R. Photocatalytic production of hydrogen peroxides and organic peroxides in aqueous suspensions of titanium dioxide, zinc oxide, and desert sand. Environ. Sci. Technol. 1988, 22, 798–806. [Google Scholar] [CrossRef]
- Ustün, G.E.; Solmaz, S.K.; Morsünbül, T.; Azak, H.S. Advanced oxidation and mineralization of 3-indole butyric acid (iba) by fenton and fenton-like processes. J. Hazard. Mater. 2010, 180, 508–513. [Google Scholar] [CrossRef]
- Eary, L.E.; Rai, D. Chromate removal from aqueous wastes by reduction with ferrous ion. Environ. Sci. Technol. 1988, 22, 972–977. [Google Scholar] [CrossRef]
- Chang, S.H.; Wang, K.S.; Li, H.C.; Wey, M.Y.; Chou, J.D. Enhancement of rhodamine b removal by low-cost fly ash sorption with fenton pre-oxidation. J. Hazard. Mater. 2009, 172, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Viraraghavan, T. Sonochemical degradation of chlorinated organic compounds, phenolic compounds and organic dyes—A review. Sci. Total. Environ. 2009, 407, 2474–2492. [Google Scholar] [CrossRef] [PubMed]
- Babuponnusami, A.; Muthukumar, K. A review on fenton and improvements to the fenton process for wastewater treatment. J. Environ. Chem. Eng. 2014, 2, 557–572. [Google Scholar] [CrossRef]
- Nikitenko, S.I.; Pflieger, R. Toward a new paradigm for sonochemistry: Short review on nonequilibrium plasma observations by means of MBSL spectroscopy in aqueous solutions. Ultrason. Sonochemistry 2017, 35, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Yuan, X.; Huang, L.; Shang, S.; Xu, D. Fe-based metal-organic frameworks as heterogeneous catalysts for highly efficient degradation of wastewater in plasma/fenton-like systems. RSC Adv. 2020, 10, 36363–36370. [Google Scholar] [CrossRef] [PubMed]
- Wahono, J.Z.; Yusharyahya, R.D.; Harianingsih; Saksono, N. Phenol degradation by fenton reaction in air injection using plasma electrolysis method. IOP Conf. Ser. Mater. Sci. Eng. 2020, 980, 012051. [Google Scholar] [CrossRef]
- Ma, Y.; Chang, C.; Chao, C. Decolorization of rhodamine b by a photo-fenton process: Effect of system parameters and kinetic study. Int. J. Environ. Resour. 2012, 1, 73–80. [Google Scholar]
- Natarajan, T.S.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Enhanced photocatalytic activity of bismuth-doped tio2 nanotubes under direct sunlight irradiation for degradation of rhodamine b dye. J. Nanopart. Res. 2013, 15, 1669. [Google Scholar] [CrossRef]
- Natarajan, T.S.; Thomas, M.; Natarajan, K.; Bajaj, H.C.; Tayade, R.J. Study on uv-led/tio2 process for degradation of rhodamine b dye. Chem. Eng. J. 2011, 169, 126–134. [Google Scholar] [CrossRef]
- Ferreira, B.R.; Correa, D.N.; Eberlin, M.N.; Vendramini, P.H. Fragmentation reactions of rhodamine b and 6g as revealed by high accuracy orbitrap tandem mass spectrometry. J. Braz. Chem. Soc. 2017, 28, 136–142. [Google Scholar] [CrossRef]
- Bai, Y.; Wu, D.; Wang, W.; Chen, P.; Tan, F.; Wang, X.; Qiao, X.; Wong, P.K. Dramatically enhanced degradation of recalcitrant organic contaminants in mgo(2)/fe(iii) fenton-like system by organic chelating agents. Environ. Res. 2021, 192, 110242. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yang, H.; Xian, T.; Li, R.S.; Zhang, H.M.; Wang, X.X. Sonocatalytic degradation of rhb over lufeo3 particles under ultrasonic irradiation. J. Hazard. Mater. 2015, 289, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Murugan, E.; Jebaranjitham, J.N. Dendrimer grafted core–shell fe3o4–polymer magnetic nanocomposites stabilized with aunps for enhanced catalytic degradation of rhodamine b—A kinetic study. Chem. Eng. J. 2015, 259, 266–276. [Google Scholar] [CrossRef]
- Lops, C.; Ancona, A.; Di Cesare, K.; Dumontel, B.; Garino, N.; Canavese, G.; Hérnandez, S.; Cauda, V. Sonophotocatalytic degradation mechanisms of rhodamine b dye via radicals generation by micro- and nano-particles of zno. Appl. Catal. B Environ. 2019, 243, 629–640. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ma, W.; Liu, P.; Zou, B.; Ma, J. Magnetic cofe2o4 nanoparticles supported on titanate nanotubes (cofe2o4/tnts) as a novel heterogeneous catalyst for peroxymonosulfate activation and degradation of organic pollutants. J. Hazard. Mater. 2016, 308, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Li, H.; Yu, H.; Chen, S.; Quan, X. Constructing bivo4-au@cds photocatalyst with energic charge-carrier-separation capacity derived from facet induction and z-scheme bridge for degradation of organic pollutants. Appl. Catal. B Environ. 2018, 227, 258–265. [Google Scholar] [CrossRef]
- Chen, F.; Xie, S.; Huang, X.; Qiu, X. Ionothermal synthesis of fe(3)o(4) magnetic nanoparticles as efficient heterogeneous fenton-like catalysts for degradation of organic pollutants with h(2)o(2). J. Hazard. Mater. 2017, 322, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Jiang, Y.; Li, C.; Fan, W.; Yan, X.; Yan, M.; Shi, W. In-situ synthesis of direct solid-state z-scheme v2o5/g-c3n4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B Environ. 2016, 180, 663–673. [Google Scholar] [CrossRef]


| Temperature (°C) | RhB Decomposition Efficiency (%) | |
|---|---|---|
| With temperature control | 20 | 77.7 |
| Without temperature control | 20–40 | 77.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Y.; Komarov, S.; Yamamoto, T.; Kutsuzawa, T. Enhancement and Mechanism of Rhodamine B Decomposition in Cavitation-Assisted Plasma Treatment Combined with Fenton Reactions. Catalysts 2022, 12, 1491. https://doi.org/10.3390/catal12121491
Xu Y, Komarov S, Yamamoto T, Kutsuzawa T. Enhancement and Mechanism of Rhodamine B Decomposition in Cavitation-Assisted Plasma Treatment Combined with Fenton Reactions. Catalysts. 2022; 12(12):1491. https://doi.org/10.3390/catal12121491
Chicago/Turabian StyleXu, Yifan, Sergey Komarov, Takuya Yamamoto, and Takaaki Kutsuzawa. 2022. "Enhancement and Mechanism of Rhodamine B Decomposition in Cavitation-Assisted Plasma Treatment Combined with Fenton Reactions" Catalysts 12, no. 12: 1491. https://doi.org/10.3390/catal12121491
APA StyleXu, Y., Komarov, S., Yamamoto, T., & Kutsuzawa, T. (2022). Enhancement and Mechanism of Rhodamine B Decomposition in Cavitation-Assisted Plasma Treatment Combined with Fenton Reactions. Catalysts, 12(12), 1491. https://doi.org/10.3390/catal12121491





