Critical Review of the Various Reaction Mechanisms for Glycerol Etherification
Abstract
1. Introduction
2. Glycerol Catalytic Conversion Pathways
3. Glycerol Etherification Experimental Setup
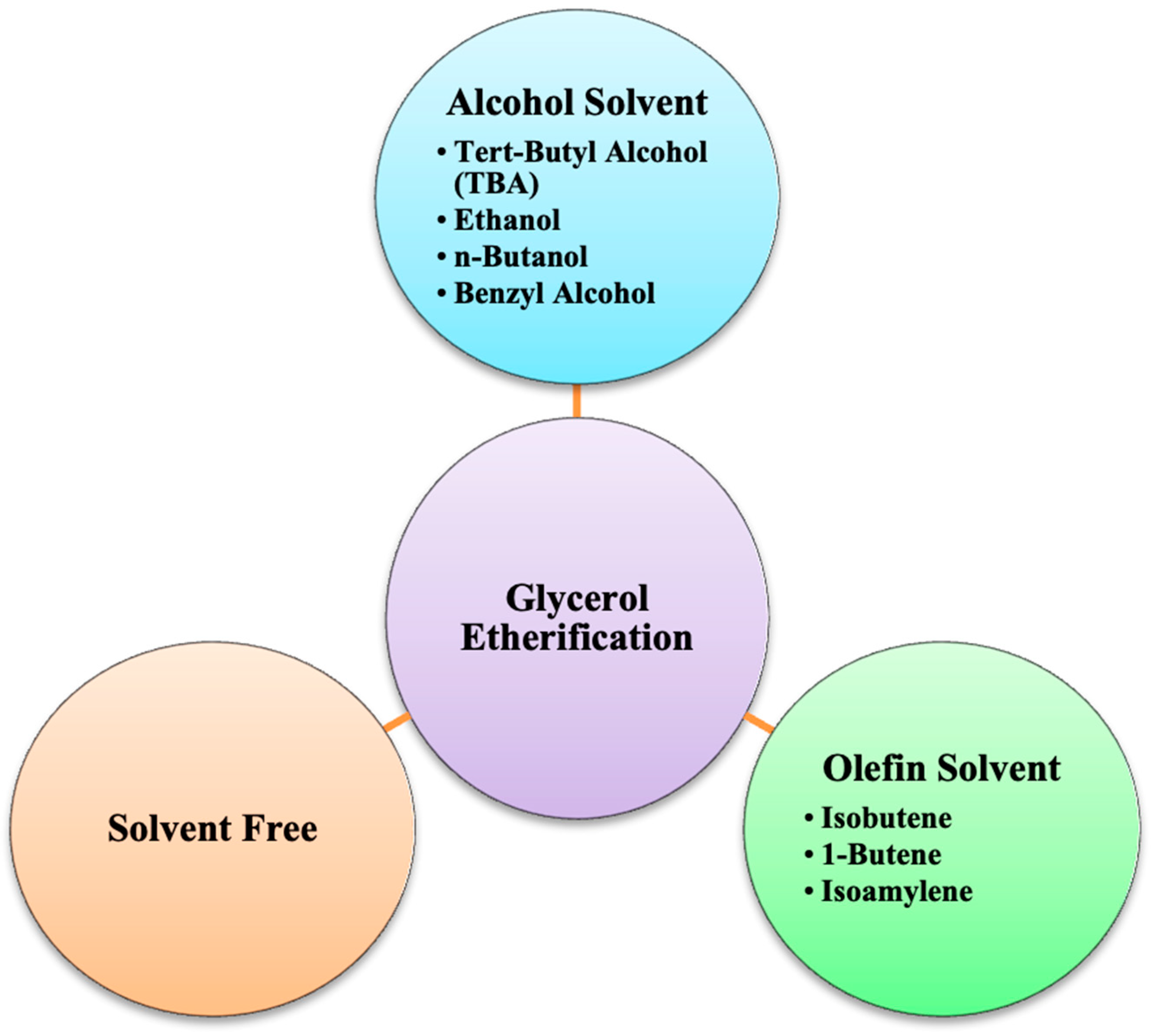
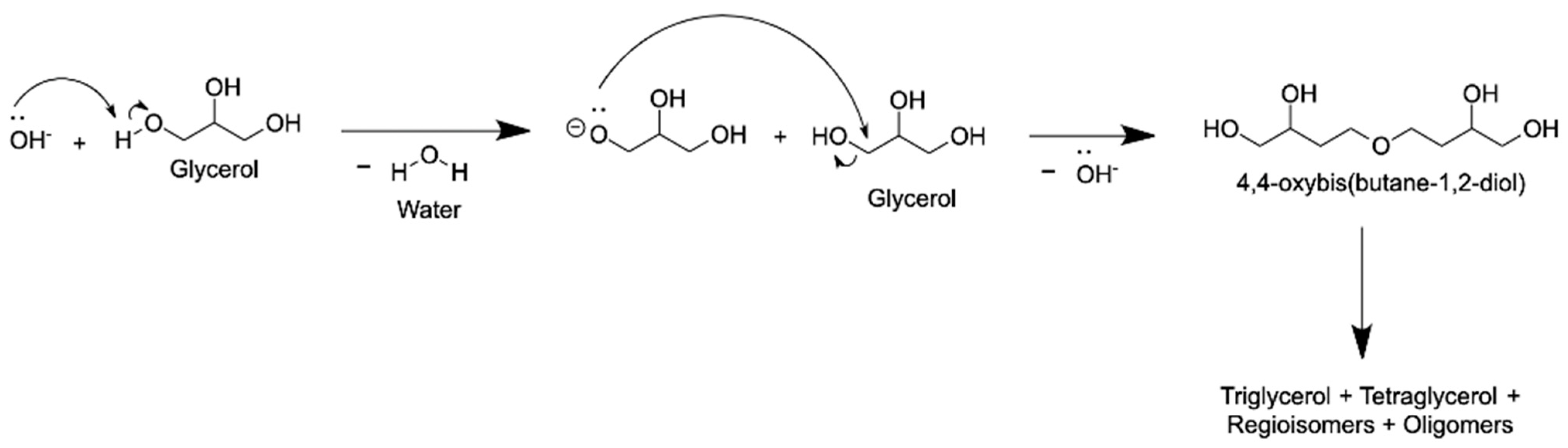
4. Reaction Mechanisms
4.1. Glycerol Etherification with Alcohol Solvent
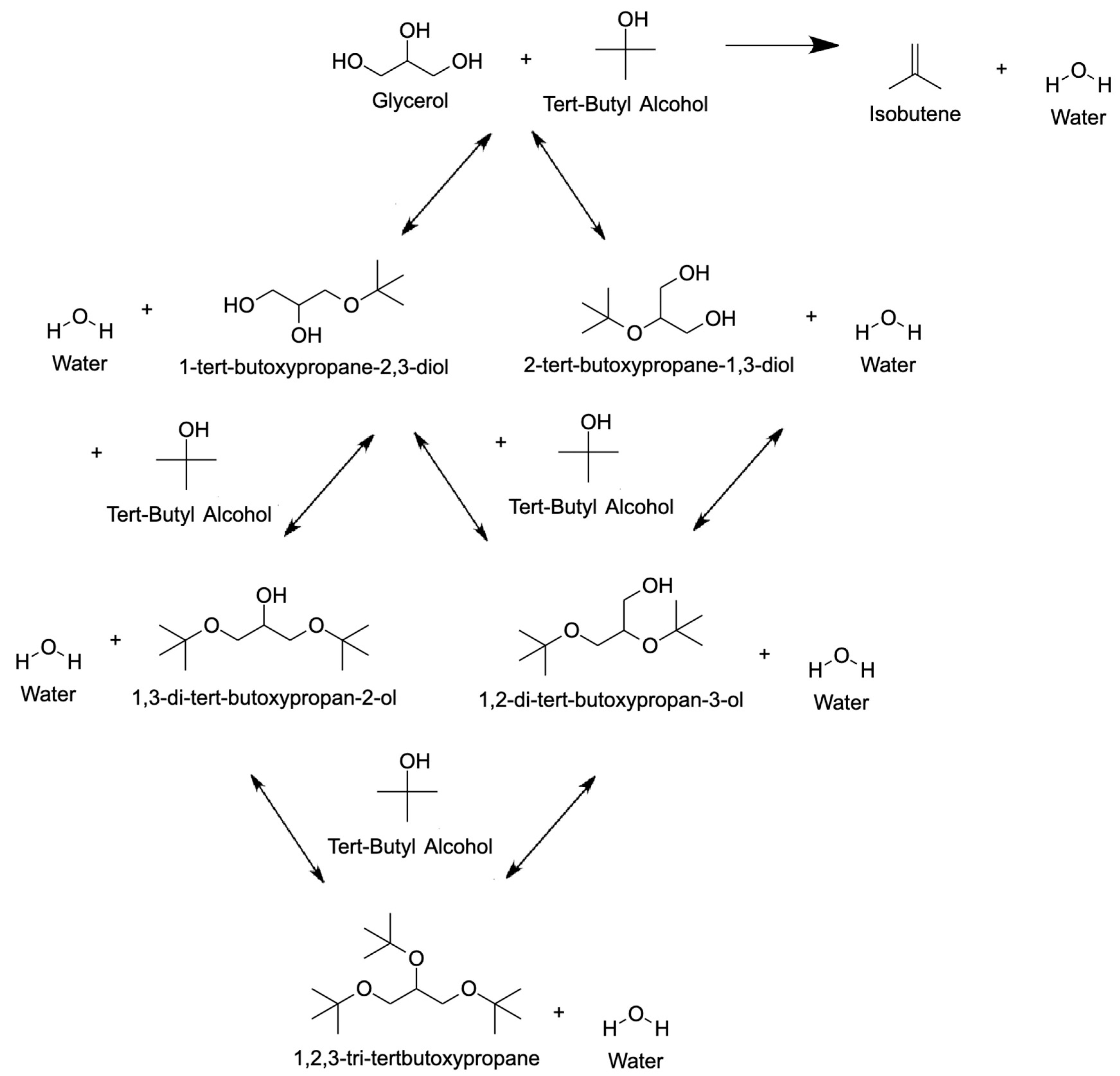
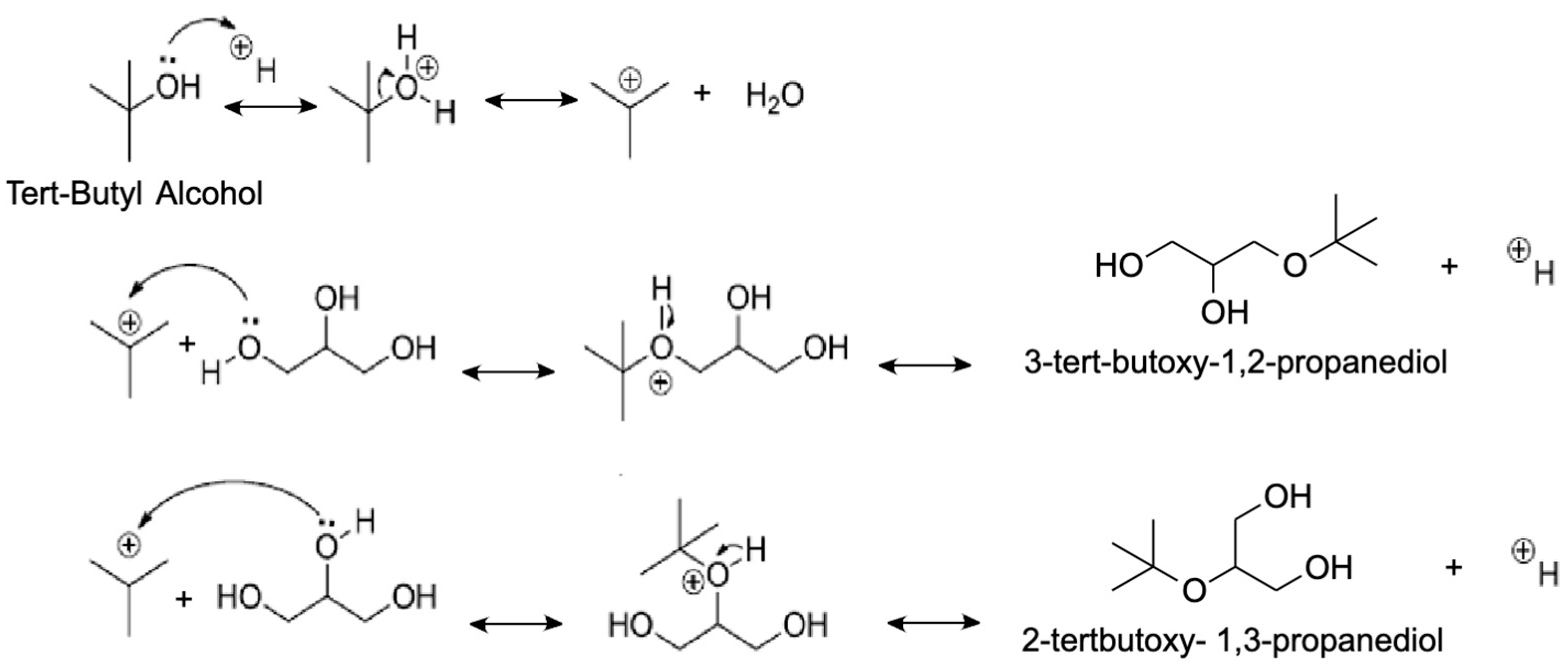
4.1.1. Tert-Butyl Alcohol (TBA)
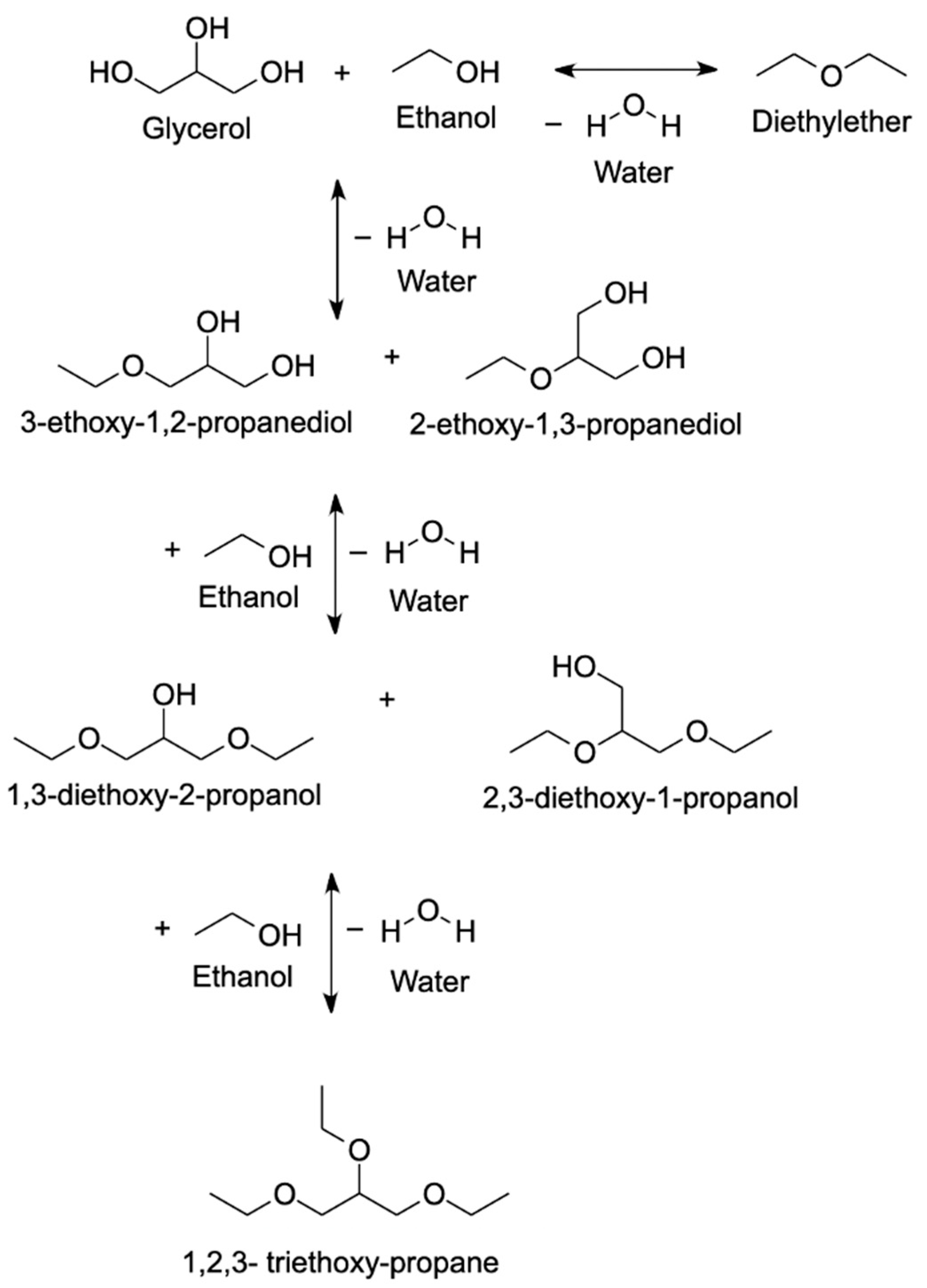
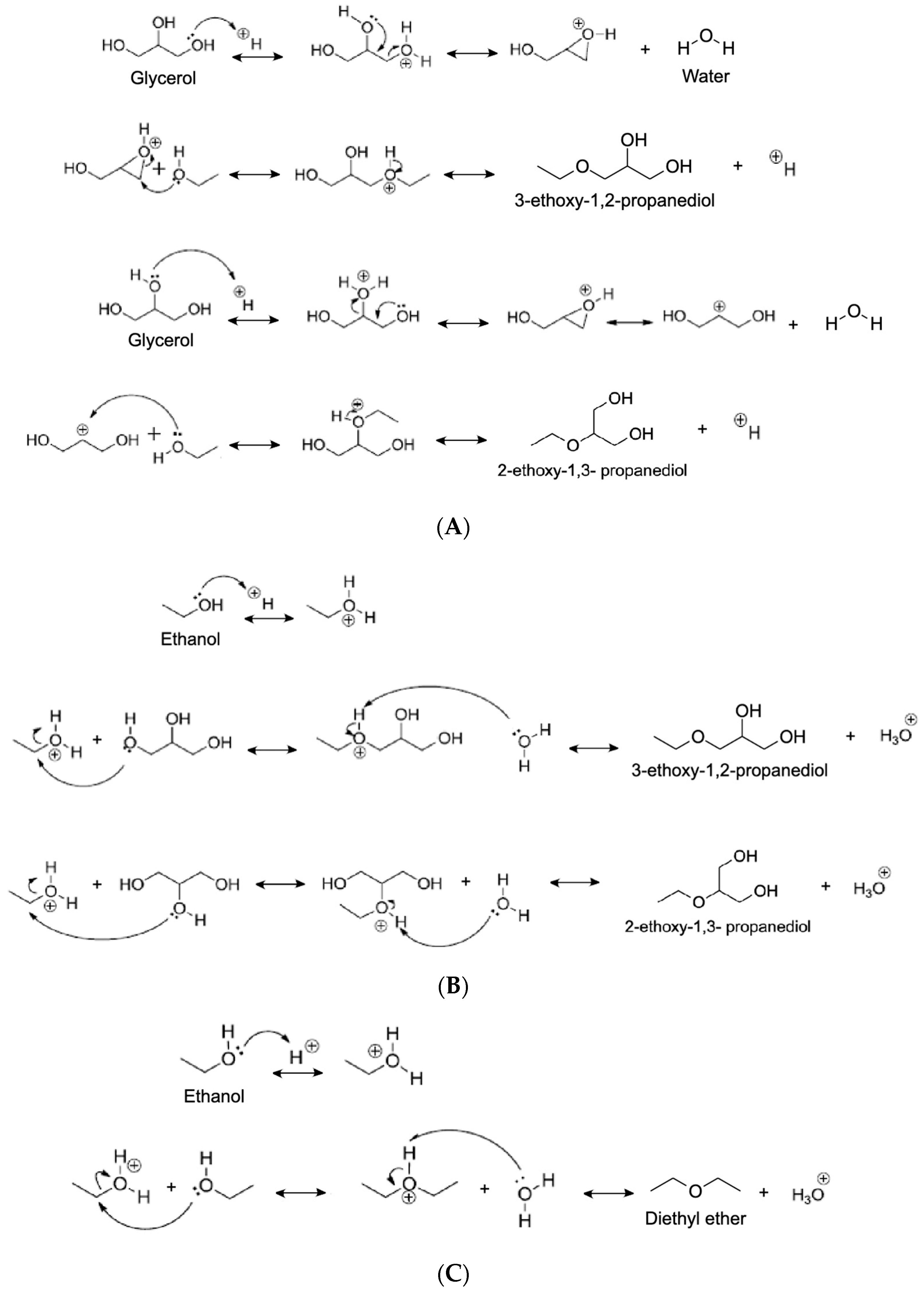
4.1.2. Ethanol
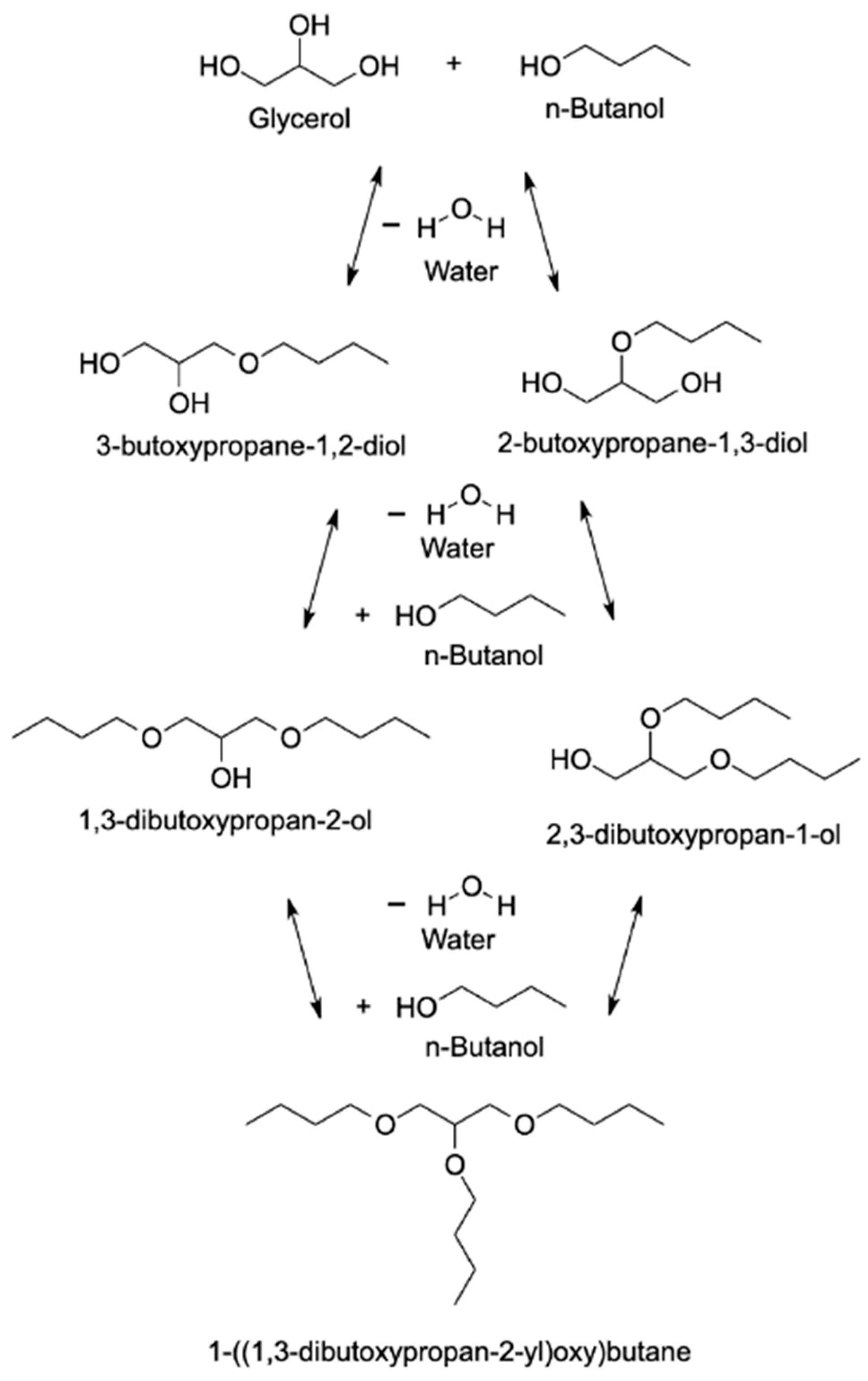
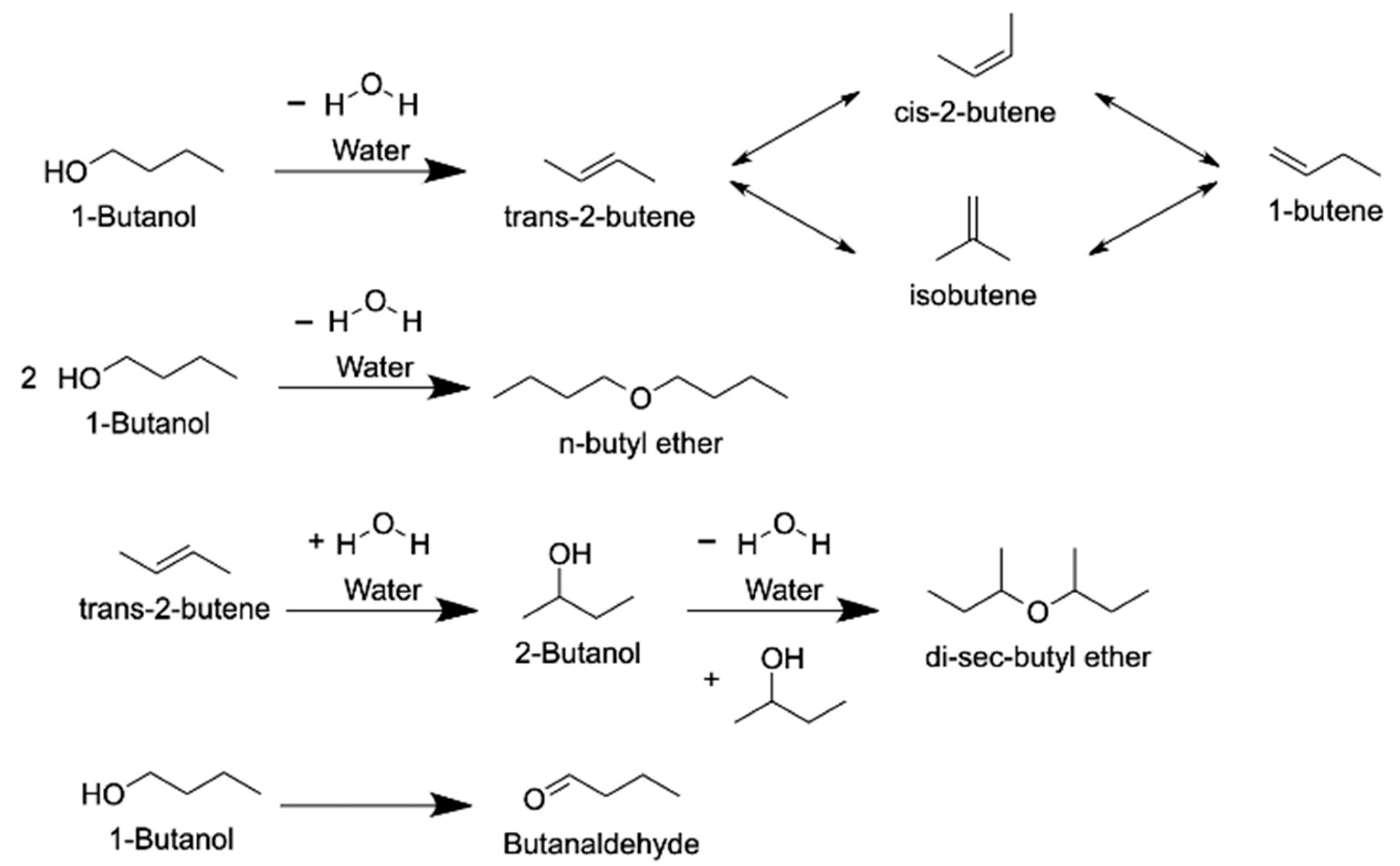
4.1.3. n-Butanol
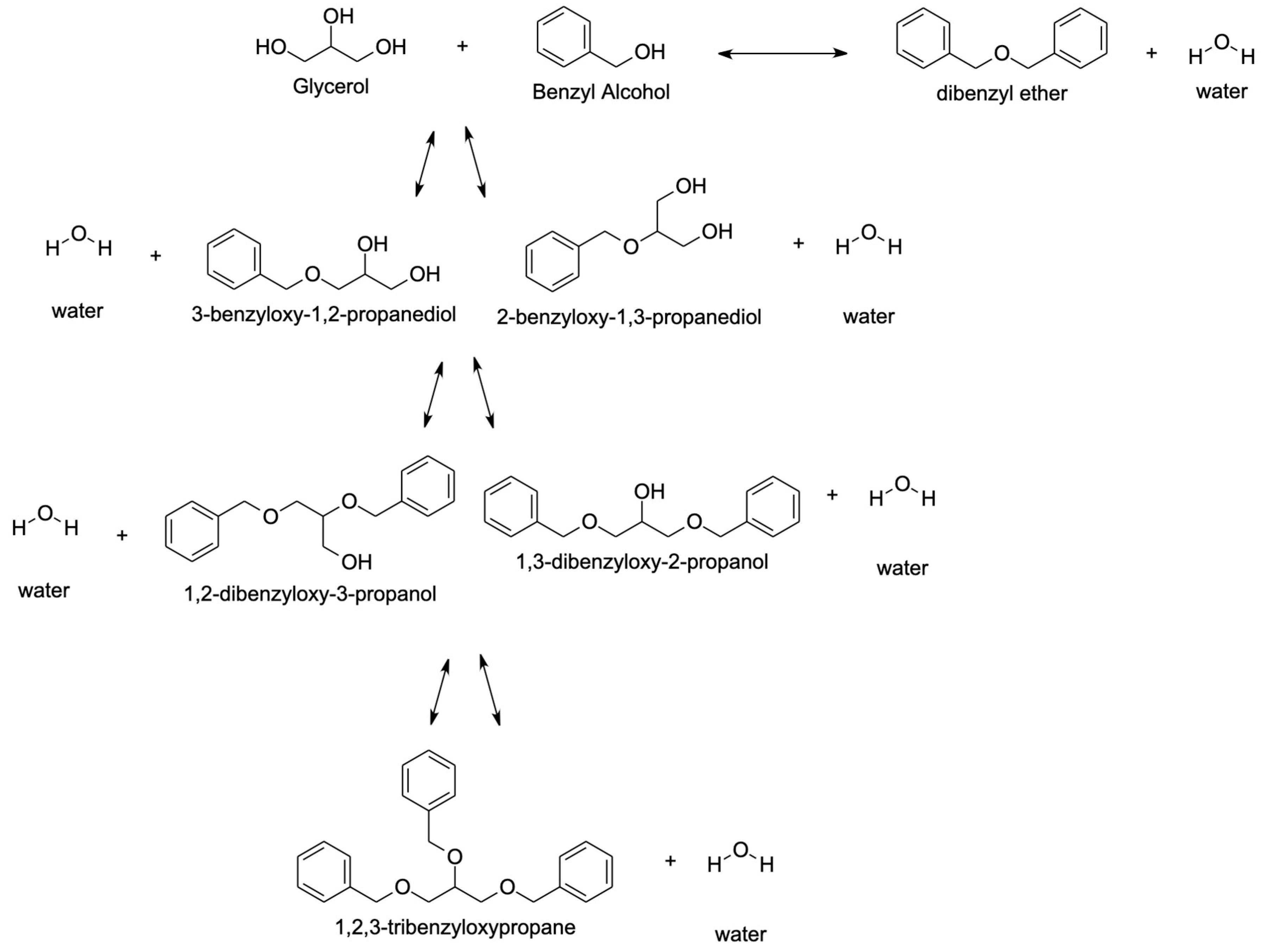
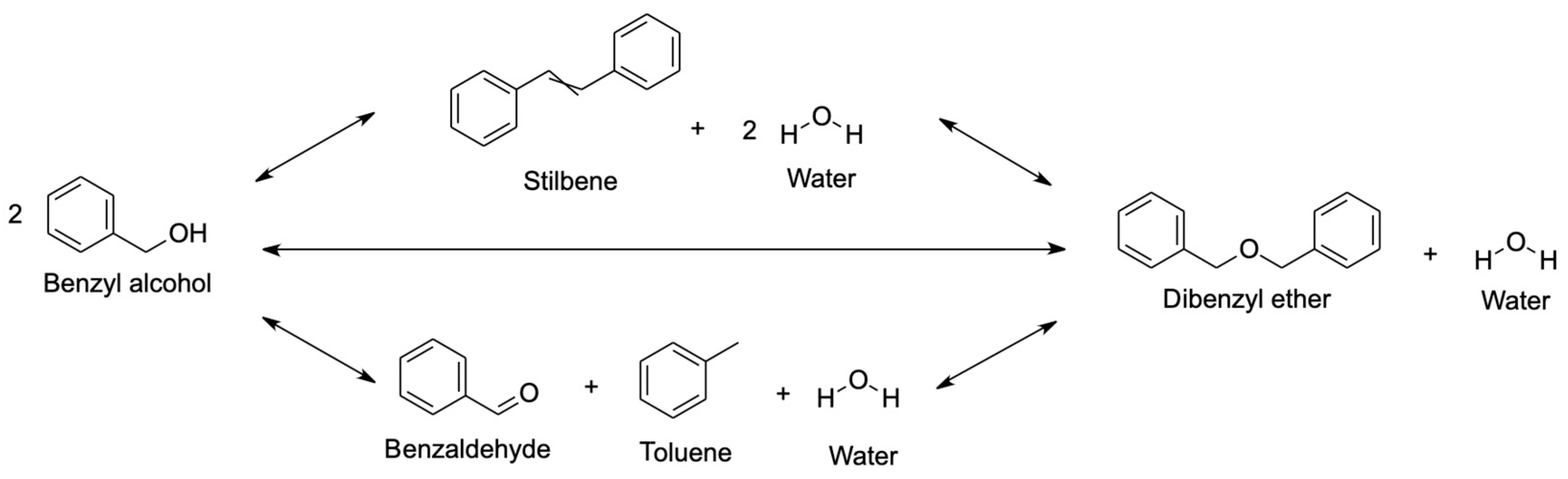
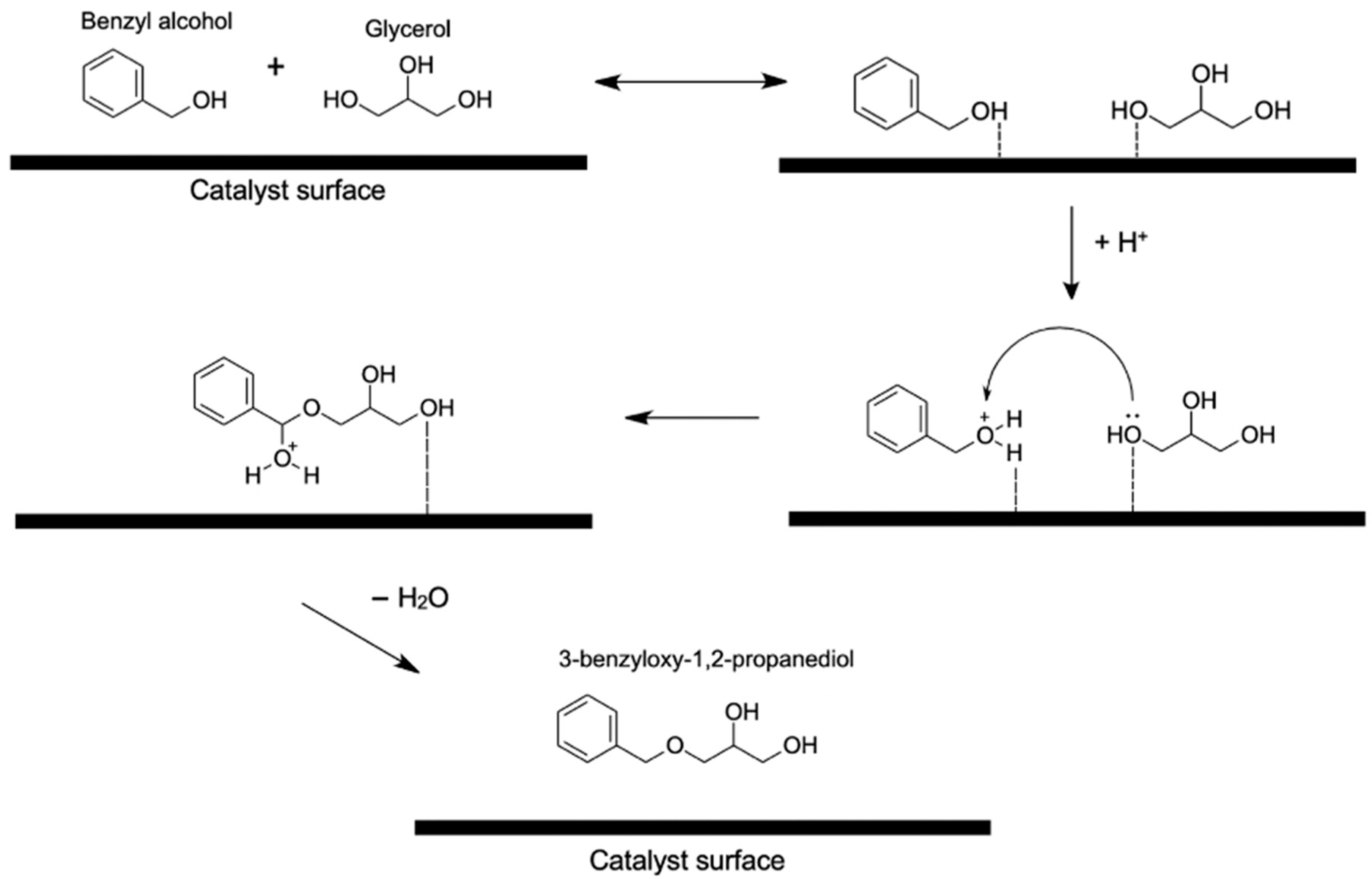
4.1.4. Benzyl Alcohol
4.2. Glycerol Etherification with Olefin Solvent
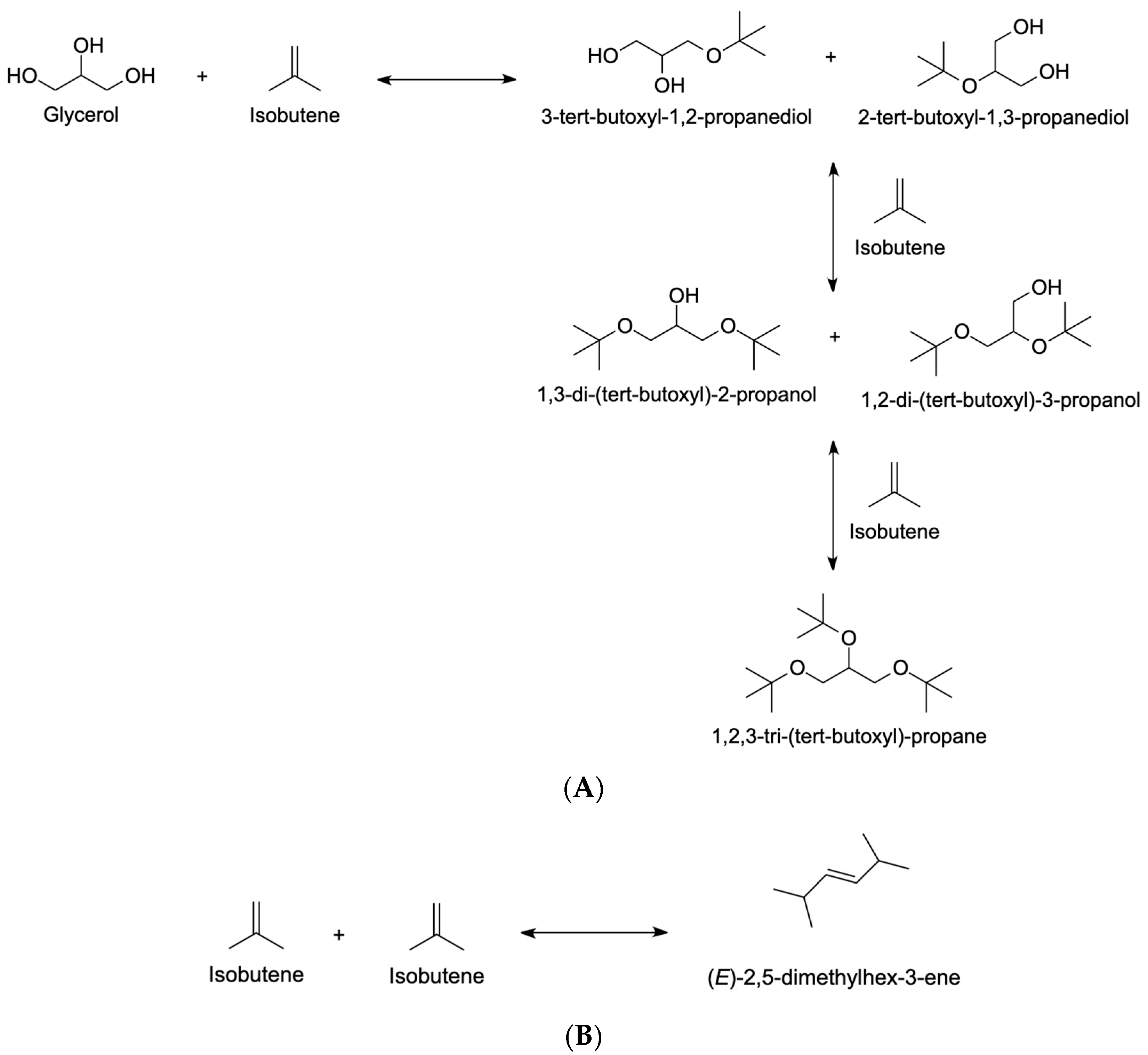
4.2.1. Isobutene
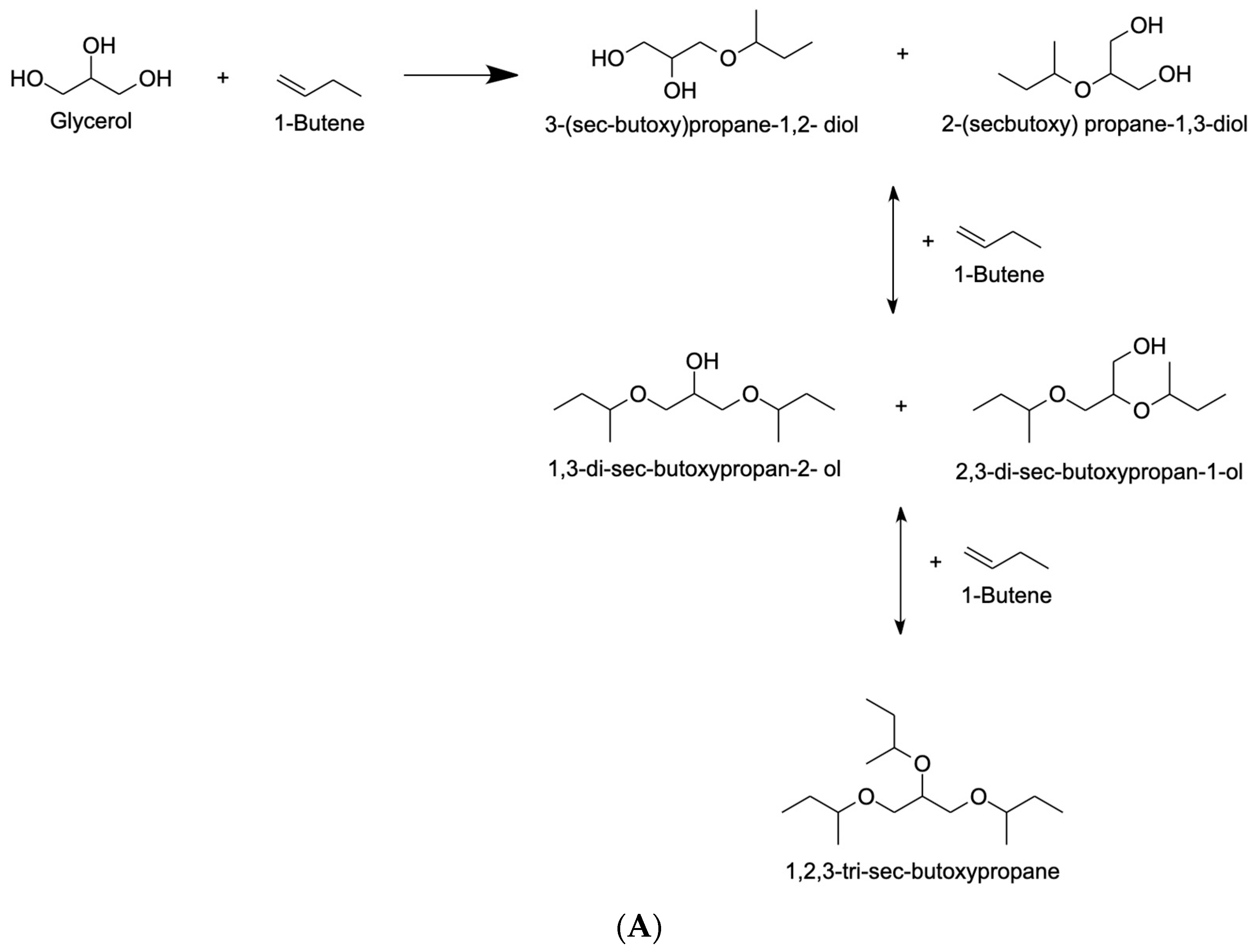
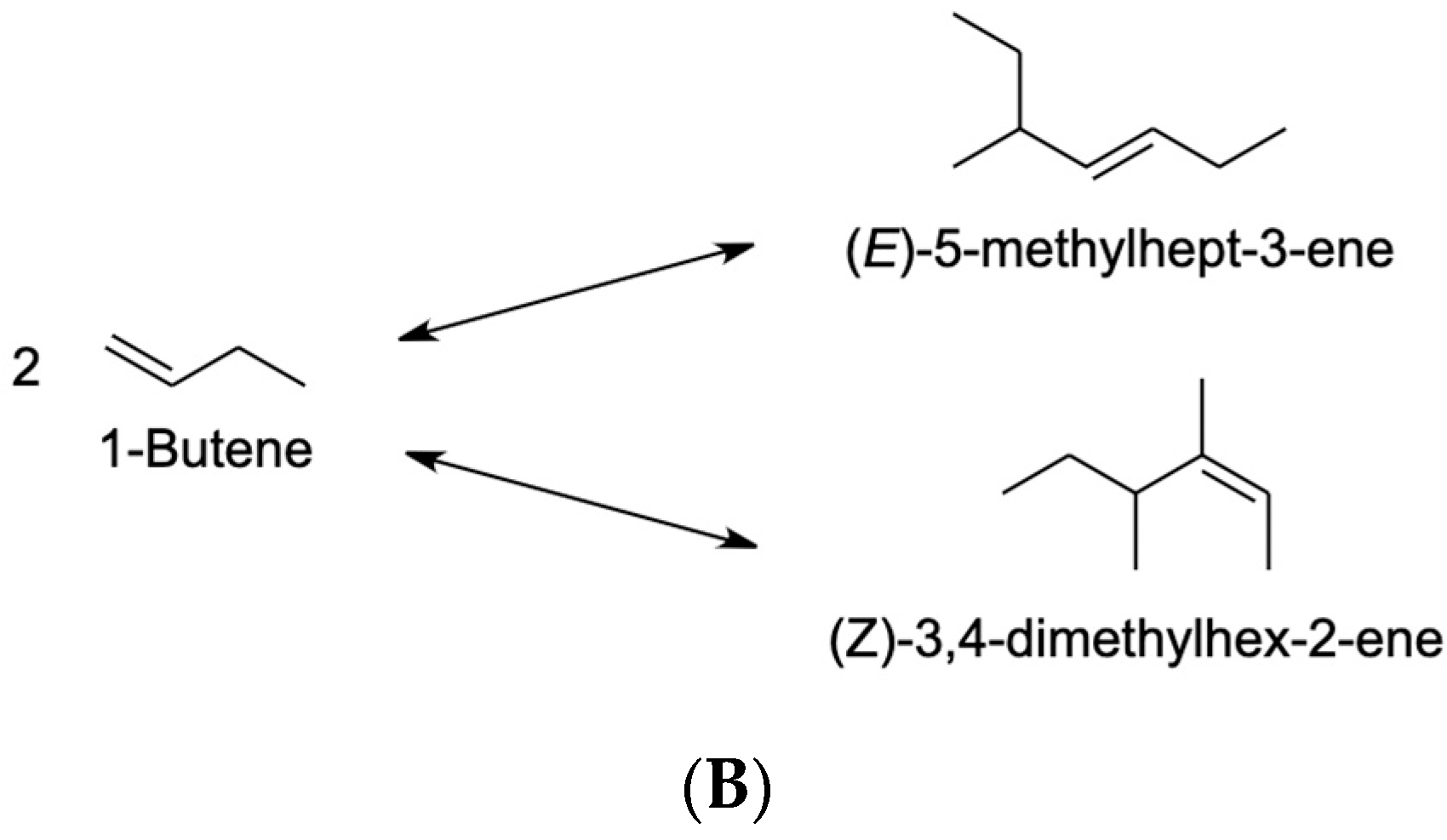
4.2.2. 1-Butene
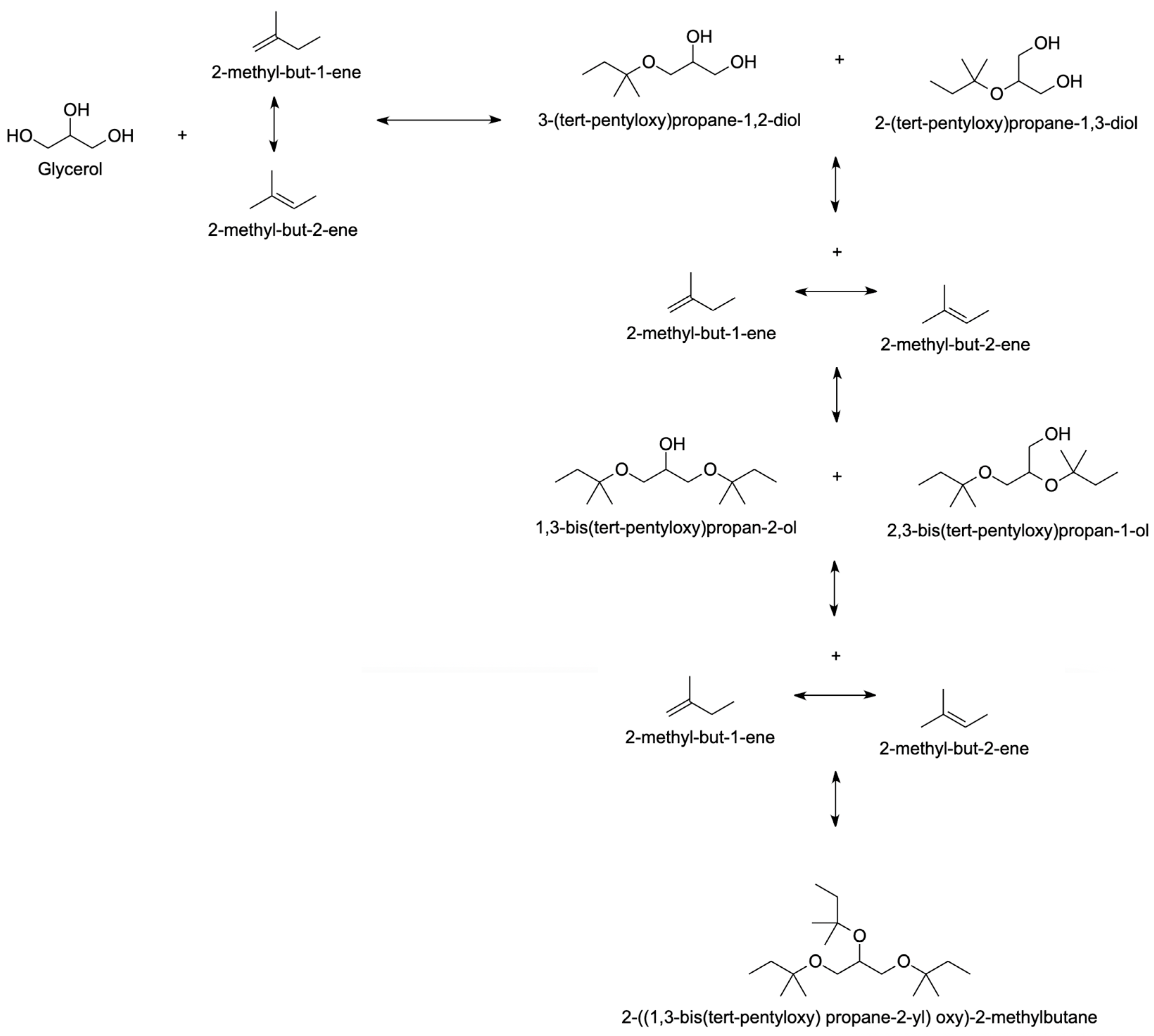
4.2.3. Isoamylene
4.3. Solvent-Free Glycerol Etherification
5. Future Recommendations
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Prati, L.; Hutchings, G.J. Glycerol Oxidation Using Gold-Containing Catalysts. Acc. Chem. Res. 2015, 48, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, S.; Li, Z.; Zhang, H.; Li, H.; Yang, S. Green Synthesis of Heterogeneous Polymeric Bio-Based Acid Decorated with Hydrophobic Regulator for Efficient Catalytic Production of Biodiesel at Low Temperatures. Fuel 2022, 329, 125467. [Google Scholar] [CrossRef]
- Saikia, K.; Saikia, K.; Rajkumari, K.; Moyon, N.S.; Basumatary, S.; Halder, G.; Rashid, U.; Rokhum, S.L. Sulphonated Biomass-Based Catalyst for Solketal Synthesis by Acetalization of Glycerol—A Byproduct of Biodiesel Production. Fuel Process. Technol. 2022, 238, 107482. [Google Scholar] [CrossRef]
- Foo, G.S.; Wei, D.; Sholl, D.S.; Sievers, C. Role of Lewis and Brønsted Acid Sites in the Dehydration of Glycerol over Niobia. ACS Catal. 2014, 4, 3180–3192. [Google Scholar] [CrossRef]
- Alashek, F.; Keshe, M.; Alhassan, G. Preparation of Glycerol Derivatives by Entered of Glycerol in Different Chemical Organic Reactions: A Review. Results Chem. 2022, 4, 100359. [Google Scholar] [CrossRef]
- Roy, D.; Subramaniam, B.; Chaudhari, R.v. Cu-Based Catalysts Show Low Temperature Activity for Glycerol Conversion to Lactic Acid. ACS Catal. 2011, 1, 548–551. [Google Scholar] [CrossRef]
- Chong, C.C.; Aqsha, A.; Ayoub, M.; Sajid, M.; Abdullah, A.Z.; Yusup, S.; Abdullah, B. A Review over the Role of Catalysts for Selective Short-Chain Polyglycerol Production from Biodiesel Derived Waste Glycerol. Environ. Technol. Innov. 2020, 19, 100859. [Google Scholar] [CrossRef]
- Fortune Business Insights Sample_Global Glycerine Market Analysis Insights and Forecast, 2021–2028. 2021. Available online: https://www.fortunebusinessinsights.com/glycerine-market-102168 (accessed on 5 October 2022).
- Yamamoto, K.; Kiyan, A.M.; Bagio, J.C.; Rossi, K.A.B.; Delabio Berezuk, F.; Berezuk, M.E. Green Cyclic Acetals Production by Glycerol Etherification Reaction with Benzaldehyde Using Cationic Acidic Resin. Green Process. Synth. 2019, 8, 183–190. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, L.; Jiang, Y.; Hunger, M.; Huang, J. Cooperativity of Brønsted and Lewis Acid Sites on Zeolite for Glycerol Dehydration. ACS Catal. 2014, 4, 1144–1147. [Google Scholar] [CrossRef]
- Nomanbhay, S.; Hussein, R.; Ong, M.Y. Sustainability of Biodiesel Production in Malaysia by Production of Bio-Oil from Crude Glycerol Using Microwave Pyrolysis: A Review. Green Chem. Lett. Rev. 2018, 11, 135–157. [Google Scholar] [CrossRef]
- Davies, T.E.; Kondrat, S.A.; Nowicka, E.; Graham, J.J.; Apperley, D.C.; Taylor, S.H.; Graham, A.E. Dehydrative Etherification Reactions of Glycerol with Alcohols Catalyzed by Recyclable Nanoporous Aluminosilicates: Telescoped Routes to Glyceryl Ethers. ACS Sustain. Chem. Eng. 2016, 4, 835–843. [Google Scholar] [CrossRef]
- Khumho, R.; Tocuweang, K.; Sangkhum, P.; Kuchonthara, P.; Ashokkumar, V.; Ngamcharussrivichai, C. Etherification of Glycerol into Short-Chain Polyglycerols over MgAl LDH/CaCO3 Nanocomposites as Heterogeneous Catalysts to Promote Circular Bioeconomy. Chemosphere 2022, 291, 133091. [Google Scholar] [CrossRef] [PubMed]
- Bhaduri, K.; Ghosh, A.; Auroux, A.; Chatterjee, S.; Bhaumik, A.; Chowdhury, B. Soft-Templating Routes for the Synthesis of Mesoporous Tantalum Phosphates and Their Catalytic Activity in Glycerol Dehydration and Carbonylation Reactions. Mol. Catal. 2022, 518, 112074. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Pasha, A.A.; Khan, H.W.; Imteyaz, B.; Irshad, K. Sustainable and Energy Efficient Hydrogen Production via Glycerol Reforming Techniques: A Review. Int. J. Hydrogen Energy, 2022; in press. [Google Scholar] [CrossRef]
- Pereira, V.S.; Nandenha, J.; Ramos, A.; Neto, A.O. Effects of TiO2 in Pd-TiO2/C for Glycerol Oxidation in a Direct Alkaline Fuel Cell. J. Fuel Chem. Technol. 2022, 50, 474–482. [Google Scholar] [CrossRef]
- Maquirriain, M.A.; Querini, C.A.; Pisarello, M.L. Glycerine Esterification with Free Fatty Acids: Homogeneous Catalysis. Chem. Eng. Res. Des. 2021, 171, 86–99. [Google Scholar] [CrossRef]
- Bagnato, G.; Iulianelli, A.; Sanna, A.; Basile, A. Glycerol Production and Transformation: A Critical Review with Particular Emphasis on Glycerol Reforming Reaction for Producing Hydrogen in Conventional and Membrane Reactors. Membranes 2017, 7, 17. [Google Scholar] [CrossRef]
- Chiosso, M.E.; Casella, M.L.; Merlo, A.B. Synthesis and Catalytic Evaluation of Acidic Carbons in the Etherification of Glycerol Obtained from Biodiesel Production. Catal. Today 2021, 372, 107–114. [Google Scholar] [CrossRef]
- da Silva, M.J.; Chaves, D.M.; Ferreira, S.O.; da Silva, R.C.; Gabriel Filho, J.B.; Bruziquesi, C.G.O.; Al-Rabiah, A.A. Impacts of Sn(II) Doping on the Keggin Heteropolyacid-Catalyzed Etherification of Glycerol with Tert-Butyl Alcohol. Chem. Eng. Sci. 2022, 247, 116913. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, Y.; Zhang, P.; Yang, B. Enhance Glycerol Conversion through Co-Etherification with Isobutene and Tert-Butanol. Fuel Process. Technol. 2021, 218, 106838. [Google Scholar] [CrossRef]
- Sakdasri, W.; Ngamprasertsith, S.; Saengsuk, P.; Sawangkeaw, R. Supercritical Reaction between Methanol and Glycerol: The Effects of Reaction Products on Biodiesel Properties. Energy Convers. Manag. X 2021, 12, 100145. [Google Scholar] [CrossRef]
- Cannilla, C.; Giacoppo, G.; Frusteri, L.; Todaro, S.; Bonura, G.; Frusteri, F. Techno-Economic Feasibility of Industrial Production of Biofuels by Glycerol Etherification Reaction with Isobutene or Tert-Butyl Alcohol Assisted by Vapor-Permeation Membrane. J. Ind. Eng. Chem. 2021, 98, 413–424. [Google Scholar] [CrossRef]
- Lopez-Suarez, F.E.; Riveros-Riveros, D.M.; Cesteros, Y.; Salagre, P. Raw Glycerol Re-Valuing through Etherification with Isobutylene: Process Design and Techno-Economical Assessment. J. Ind. Eng. Chem. 2021, 94, 159–165. [Google Scholar] [CrossRef]
- Andreeva, I.V.; Zaitsau, D.H.; Qian, S.; Turovtzev, V.V.; Pimerzin, A.A.; Bara, J.E.; Verevkin, S.P. Glycerol Valorisation towards Biofuel Additivities: Thermodynamic Studies of Glycerol Ethers. Chem. Eng. Sci. 2022, 247, 117032. [Google Scholar] [CrossRef]
- Wang, Z.-X.; Zhuge, J.; Fang, H.; Prior, B.A. Glycerol Production by Microbial Fermentation: A Review. Biotechnol. Adv. 2001, 19, 201–223. [Google Scholar] [CrossRef]
- Gupta, M.; Kumar, N. Scope and Opportunities of Using Glycerol as an Energy Source. Renew. Sustain. Energy Rev. 2012, 16, 4551–4556. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z. Critical Review on the Current Scenario and Significance of Crude Glycerol Resulting from Biodiesel Industry towards More Sustainable Renewable Energy Industry. Renew. Sustain. Energy Rev. 2012, 16, 2671–2686. [Google Scholar] [CrossRef]
- Yang, F.; Hanna, M.A.; Sun, R. Value-Added Uses for Crude Glycerol--a Byproduct of Biodiesel Production. Biotechnol. Biofuels 2012, 5, 13. [Google Scholar] [CrossRef]
- Tan, H.W.; Abdul Aziz, A.R.; Aroua, M.K. Glycerol Production and Its Applications as a Raw Material: A Review. Renew. Sustain. Energy Rev. 2013, 27, 118–127. [Google Scholar] [CrossRef]
- Veluturla, S.; Archna, N.; Subba Rao, D.; Hezil, N.; Indraja, I.S.; Spoorthi, S. Catalytic Valorization of Raw Glycerol Derived from Biodiesel: A Review. Biofuels 2018, 9, 305–314. [Google Scholar] [CrossRef]
- Cornejo, A.; Barrio, I.; Campoy, M.; Lázaro, J.; Navarrete, B. Oxygenated Fuel Additives from Glycerol Valorization. Main Production Pathways and Effects on Fuel Properties and Engine Performance: A Critical Review. Renew. Sustain. Energy Rev. 2017, 79, 1400–1413. [Google Scholar] [CrossRef]
- Dodekatos, G.; Schünemann, S.; Tüysüz, H. Recent Advances in Thermo-, Photo-, and Electrocatalytic Glycerol Oxidation. ACS Catal. 2018, 8, 6301–6333. [Google Scholar] [CrossRef]
- Adeniyi, A.G.; Ighalo, J.O. A Review of Steam Reforming of Glycerol. Chem. Pap. 2019, 73, 2619–2635. [Google Scholar] [CrossRef]
- Adnan, N.A.A.; Suhaimi, S.N.; Abd-Aziz, S.; Hassan, M.A.; Phang, L.Y. Optimization of Bioethanol Production from Glycerol by Escheric h Ia Coli SS1. Renew. Energy 2014, 66, 625–633. [Google Scholar] [CrossRef]
- Wan Isahak, W.N.R.; Che Ramli, Z.A.; Ismail, M.; Jahim, J.M.; Yarmo, M.A. Recovery and Purification of Crude Glycerol from Vegetable Oil Transesterification. Sep. Purif. Rev. 2015, 44, 250–267. [Google Scholar] [CrossRef]
- Bagheri, S.; Julkapli, N.M.; Yehye, W.A. Catalytic Conversion of Biodiesel Derived Raw Glycerol to Value Added Products. Renew. Sustain. Energy Rev. 2015, 41, 113–127. [Google Scholar] [CrossRef]
- Sudarsanam, P.; Mallesham, B.; Prasad, A.N.; Reddy, P.S.; Reddy, B.M. Synthesis of Bio-Additive Fuels from Acetalization of Glycerol with Benzaldehyde over Molybdenum Promoted Green Solid Acid Catalysts. Fuel Process. Technol. 2013, 106, 539–545. [Google Scholar] [CrossRef]
- Sadjadi, S.; Tarighi, S.; Moussavi, N.S.; Ahadi, N. Heteropolyacid Supported on the Composite of Bentonite and Ionic Liquid Containing Acidic Polymer: A Highly Selective Catalyst for Glycerol Acetalization to Solketal. J. Mol. Struct. 2022, 1256, 132556. [Google Scholar] [CrossRef]
- Hong, G.H.; Li, Z.; Park, J.S.; Li, Z.; Kim, K.Y.; Li, C.; Lee, J.; Jin, M.; Stucky, G.D.; Kim, J.M. Glycerol Acetalization over Highly Ordered Mesoporous Molybdenum Dioxide: Excellent Catalytic Performance, Recyclability and Water-Tolerance. J. Ind. Eng. Chem. 2022, 107, 354–364. [Google Scholar] [CrossRef]
- de Canck, E.; Dosuna-Rodríguez, I.; Gaigneaux, E.M.; van der Voort, P. Periodic Mesoporous Organosilica Functionalized with Sulfonic Acid Groups as Acid Catalyst for Glycerol Acetylation. Materials 2013, 6, 3556–3570. [Google Scholar] [CrossRef]
- Abida, K.; Ali, A. A Review on Catalytic Role of Heterogeneous Acidic Catalysts during Glycerol Acetylation to Yield Acetins. J. Indian Chem. Soc. 2022, 99, 100459. [Google Scholar] [CrossRef]
- Martin, A.; Kalevaru, V.N. Heterogeneously Catalyzed Ammoxidation: A Valuable Tool for One-Step Synthesis of Nitriles. ChemCatChem 2010, 2, 1504–1522. [Google Scholar] [CrossRef]
- da Silva, L.D.; Santos, R.C.; Silva, J.G.A.B.; de Paiva Alves, E.; Fréty, R.T.F.; Pontes, L.A.M. Direct Ammoxidation of Glycerol to Nitriles Using Mo/Alumina Catalysts. React. Kinet. Mech. Catal. 2022, 135, 271–285. [Google Scholar] [CrossRef]
- Ali, B.; Lan, X.; Arslan, M.T.; Gilani, S.Z.A.; Wang, H.; Wang, T. Controlling the Selectivity and Deactivation of H-ZSM-5 by Tuning b-Axis Channel Length for Glycerol Dehydration to Acrolein. J. Ind. Eng. Chem. 2020, 88, 127–136. [Google Scholar] [CrossRef]
- Reinoso, D.M.; Tonetto, G.M. Bioadditives Synthesis from Selective Glycerol Esterification over Acidic Ion Exchange Resin as Catalyst. J Environ. Chem Eng 2018, 6, 3399–3407. [Google Scholar] [CrossRef]
- Fukumura, T.; Toda, T.; Seki, Y.; Kubo, M.; Shibasaki-Kitakawa, N.; Yonemoto, T. Catalytic Synthesis of Glycerol Monoacetate Using a Continuous Expanded Bed Column Reactor Packed with Cation-Exchange Resin. Ind. Eng. Chem. Res. 2009, 48, 1816–1823. [Google Scholar] [CrossRef]
- Rodrigues, E.G.; Pereira, M.F.R.; Chen, X.; Delgado, J.J.; Órfão, J.J.M. Selective Oxidation of Glycerol over Platinum-Based Catalysts Supported on Carbon Nanotubes. Ind. Eng. Chem. Res. 2013, 52, 17390–17398. [Google Scholar] [CrossRef]
- Nakagawa, Y.; Tomishige, K. Heterogeneous Catalysis of the Glycerol Hydrogenolysis. Catal. Sci. Technol. 2011, 1, 179–190. [Google Scholar] [CrossRef]
- Cheng, S.; Fan, Y.; Zhang, X.; Zeng, Y.; Xie, S.; Pei, Y.; Zeng, G.; Qiao, M.; Zong, B. Tungsten-Doped Siliceous Mesocellular Foams-Supported Platinum Catalyst for Glycerol Hydrogenolysis to 1,3-Propanediol. Appl. Catal. B 2021, 297, 120428. [Google Scholar] [CrossRef]
- Qingli, X.; Zhengdong, Z.; Lifang, L.; Ping, L.; Rui, W.; Shoutao, C.; Pize, L.; Chenyang, Z. Hydrogen Production by Glycerol Reforming in a Two-Fixed-Bed Reactor. Int. J. Hydrogen Energy 2022, 47, 16805–16814. [Google Scholar] [CrossRef]
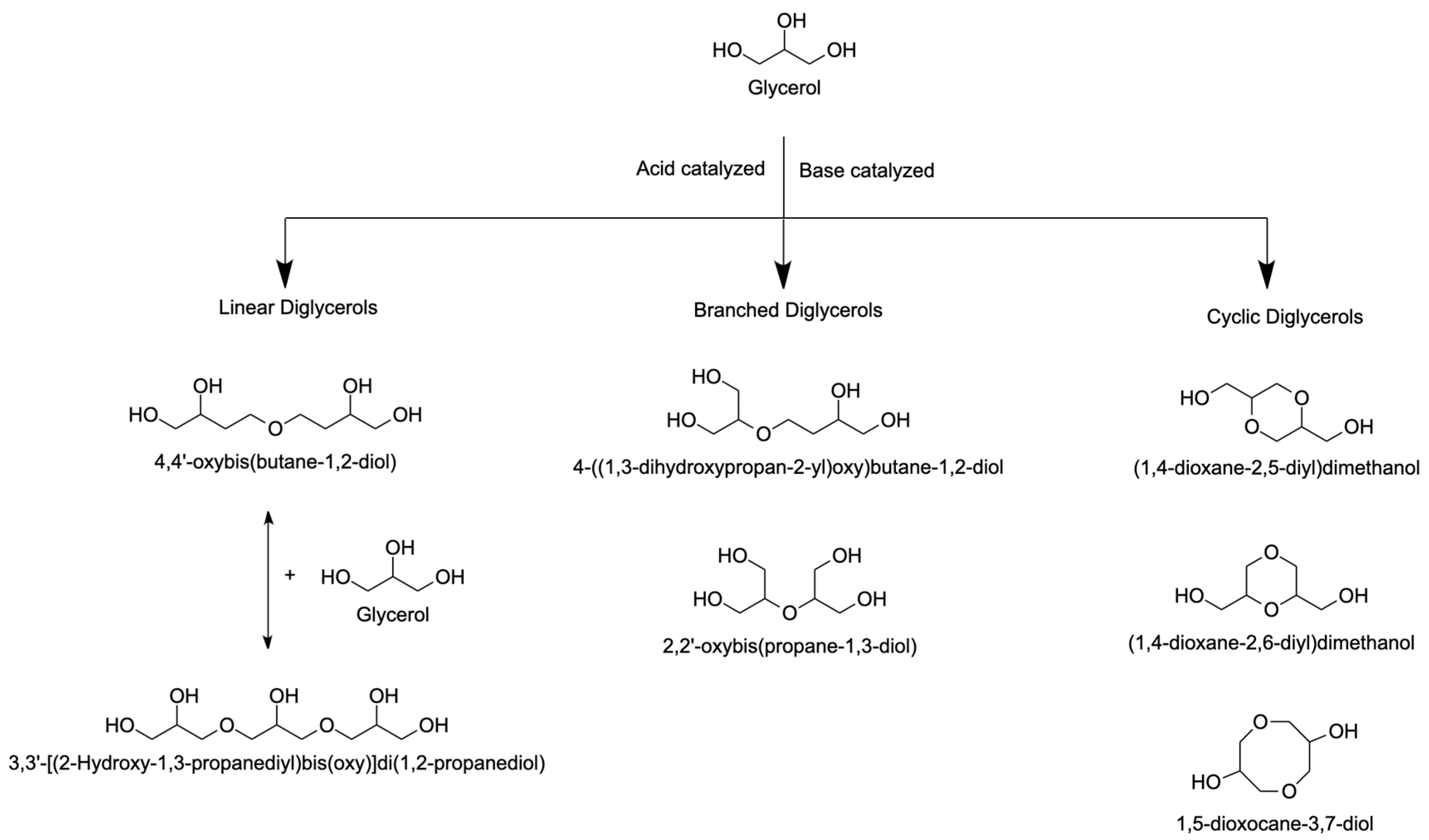
- Sivaiah, M.V.; Robles-Manuel, S.; Valange, S.; Barrault, J. Recent Developments in Acid and Base-Catalyzed Etherification of Glycerol to Polyglycerols. Catal. Today 2012, 198, 305–313. [Google Scholar] [CrossRef]
- Yuan, Z.; Xia, S.; Chen, P.; Hou, Z.; Zheng, X. Etherification of Biodiesel-Based Glycerol with Bioethanol over Tungstophosphoric Acid to Synthesize Glyceryl Ethers. Energy Fuels 2011, 25, 3186–3191. [Google Scholar] [CrossRef]
- Xiao, L.; Mao, J.; Zhou, J.; Guo, X.; Zhang, S. Enhanced Performance of HY Zeolites by Acid Wash for Glycerol Etherification with Isobutene. Appl. Catal. A Gen. 2011, 393, 88–95. [Google Scholar] [CrossRef]
- Frusteri, F.; Frusteri, L.; Cannilla, C.; Bonura, G. Catalytic Etherification of Glycerol to Produce Biofuels over Novel Spherical Silica Supported Hyflon® Catalysts. Bioresour. Technol. 2012, 118, 350–358. [Google Scholar] [CrossRef] [PubMed]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Catalytic Production of Oxygenated Additives by Glycerol Etherification. Open Chem. 2014, 12, 1248–1254. [Google Scholar] [CrossRef]
- Ozbay, N.; Oktar, N.; Dogu, G.; Dogu, T. Effects of Sorption Enhancement and Isobutene Formation on Etherification of Glycerol with Tert-Butyl Alcohol in a Flow Reactor. Ind. Eng. Chem. Res. 2012, 51, 8788–8795. [Google Scholar] [CrossRef]
- Pico, M.P.; Rodríguez, S.; Santos, A.; Romero, A. Etherification of Glycerol with Benzyl Alcohol. Ind. Eng. Chem. Res. 2013, 52, 14545–14555. [Google Scholar] [CrossRef]
- Izquierdo, J.F.; Iniesta, E.; Outón, P.R.; Izquierdo, M. Experimental Study of Glycerol Etherification with C5 Olefins to Produce Biodiesel Additives. Fuel Process. Technol. 2017, 160, 1–7. [Google Scholar] [CrossRef]
- Nandiwale, K.Y.; Patil, S.E.; Bokade, V.v. Glycerol Etherification Using N-Butanol to Produce Oxygenated Additives for Biodiesel Fuel over H-Beta Zeolite Catalysts. Energy Technol. 2014, 2, 446–452. [Google Scholar] [CrossRef]
- Mangourilos, V.; Filotti, L.; Ciuparu, D. Improving the Catalytic Performance of Ion Exchange Resins in the Etherification of Glycerol with Isobutene. Rev. Chim. 2016, 67, 930–934. [Google Scholar]
- Saengarun, C.; Petsom, A.; Tungasmita, D.N. Etherification of Glycerol with Propylene or 1-Butene for Fuel Additives. Sci. World J. 2017, 2017, 4089036. [Google Scholar] [CrossRef] [PubMed]
- Magar, S.; Kamble, S.; Mohanraj, G.T.; Jana, S.K.; Rode, C. Solid-Acid-Catalyzed Etherification of Glycerol to Potential Fuel Additives. Energy Fuels 2017, 31, 1227–1277. [Google Scholar] [CrossRef]
- de Souza, R.L.; Gonzales, W.A.; Essayem, N. Glycerol Etherification with Light Alcohols Promoted by Supported H3PW12O40. In Environmentally Benign Catalysts: For Clean Organic Reactions; Springer: Dordrecht, The Netherlands, 2013; pp. 141–152. ISBN 9789400767102. [Google Scholar]
- Ikizer, B.; Oktar, N.; Dogu, T. Etherification of Glycerol with C4 and C5 Reactive Olefins. Fuel Process. Technol. 2015, 138, 570–577. [Google Scholar] [CrossRef]
- Estevez, R.; Iglesias, I.; Luna, D.; Bautista, F.M. Sulfonic Acid Functionalization of Different Zeolites and Their Use as Catalysts in the Microwave-Assisted Etherification of Glycerol with Tert-Butyl Alcohol. Molecules 2017, 22, 2206. [Google Scholar] [CrossRef]
- Bookong, P.; Ruchirawat, S.; Boonyarattanakalin, S. Optimization of Microwave-Assisted Etherification of Glycerol to Polyglycerols by Sodium Carbonate as Catalyst. Chem. Eng. J. 2015, 275, 253–261. [Google Scholar] [CrossRef]
- Sajid, M.; Ayoub, M.; Uemura, Y.; Yusup, S.; Abdullah, B.B.; Ullah, S.; Aqsha, A. Catalytic Activity of Intercalated Montmorillonite Clay for Glycerol Conversion to Oligomers via Microwave Irradiation. J. Jpn. Inst. Energy 2020, 99, 16–19. [Google Scholar] [CrossRef]
- Ayoub, M.; Yusoff, M.H.M.; Yusup, S.B.; Danish, M.; Ullah, S.; Farrukh, S. Effect of Microwave Irradiation on the Etherification of Biodiesel-Derived Glycerol in a Solvent Free Process. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; Volume 460, p. 012043. [Google Scholar]
- Ayoub, M.; Jie Wei, W.; Ahmad, M.; Mathialagan, R.; Farrukh, S.; Danish, M.; Ullah, S.; Raza Naqvi, S. Glycerol Conversion to Diglycerol via Etherification under Microwave Irradiation. In Apolipoproteins, Triglycerides and Cholesterol; IntechOpen: London, UK, 2020. [Google Scholar]
- Aguado-Deblas, L.; Estevez, R.; Russo, M.; La Parola, V.; Bautista, F.M.; Testa, M.L. Microwave-Assisted Glycerol Etherification over Sulfonic Acid Catalysts. Materials 2020, 13, 1584. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Montes, V.; Caballero, A.; Bautista, F.M. Sulfonated Carbons from Olive Stones as Catalysts in the Microwave-Assisted Etherification of Glycerol with Tert-Butyl Alcohol. Molecular Catalysis 2020, 488, 110921. [Google Scholar] [CrossRef]
- Barros, F.J.S.; Moreno-Tost, R.; Cecilia, J.A.; Ledesma-Muñoz, A.L.; de Oliveira, L.C.C.; Luna, F.M.T.; Vieira, R.S. Glycerol Oligomers Production by Etherification Using Calcined Eggshell as Catalyst. Mol. Catal. 2017, 433, 282–290. [Google Scholar] [CrossRef]
- Ayoub, M.; Sufian, S.; Hailegiorgis, S.M.; Ullah, S.; Uemura, Y. Conversion of Glycerol to Polyglycerol over Waste Duck-Bones as a Catalyst in Solvent Free Etherification Process. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 226, p. 012073. [Google Scholar]
- Lee, J.H.; Park, S.K.; Ryu, J.B.; Lee, H.; Lee, J.S. Solventless Catalytic Etherification of Glycerol Using Acetate Salts as Efficient Catalysts. Bull. Korean Chem. Soc. 2018, 39, 722–725. [Google Scholar] [CrossRef]
- Kirby, F.; Nieuwelink, A.E.; Kuipers, B.W.M.; Kaiser, A.; Bruijnincx, P.C.A.; Weckhuysen, B.M. CaO as Drop-In Colloidal Catalysts for the Synthesis of Higher Polyglycerols. Chem. A Eur. J. 2015, 21, 5101–5109. [Google Scholar] [CrossRef] [PubMed]
- García-Sancho, C.; Moreno-Tost, R.; Mérida-Robles, J.M.; Santamaría-González, J.; Jiménez-López, A.; Torres, P.M. Etherification of Glycerol to Polyglycerols over MgAl Mixed Oxides. Catal. Today 2011, 167, 84–90. [Google Scholar] [CrossRef]
- Pérez-Barrado, E.; Pujol, M.C.; Aguiló, M.; Llorca, J.; Cesteros, Y.; Díaz, F.; Pallarès, J.; Marsal, L.F.; Salagre, P. Influence of Acid-Base Properties of Calcined MgAl and CaAl Layered Double Hydroxides on the Catalytic Glycerol Etherification to Short-Chain Polyglycerols. Chem. Eng. J. 2015, 264, 547–556. [Google Scholar] [CrossRef]
- Krisnandi, Y.K.; Eckelt, R.; Schneider, M.; Martin, A.; Richter, M. Glycerol Upgrading over Zeolites by Batch-Reactor Liquid-Phase Oligomerization: Heterogeneous versus Homogeneous Reaction. ChemSusChem 2008, 1, 835–844. [Google Scholar] [CrossRef]
- Gholami, Z.; Lee, K.T.; Abdullah, A.Z. Glycerol Etherification to Polyglycerols Using Ca1+xAlxLaxO3 Composite Catalysts in a Solventless Medium. J. Taiwan Inst. Chem. Eng. 2013, 44, 117–122. [Google Scholar] [CrossRef]
- Gonçalves, M.; Mantovani, M.; Carvalho, W.A.; Rodrigues, R.; Mandelli, D.; Silvestre Albero, J. Biodiesel Wastes: An Abundant and Promising Source for the Preparation of Acidic Catalysts for Utilization in Etherification Reaction. Chem. Eng. J. 2014, 256, 468–474. [Google Scholar] [CrossRef]
- Baroi, C.; Mahto, S.; Niu, C.; Dalai, A.K. Biofuel Production from Green Seed Canola Oil Using Zeolites. Appl. Catal. A Gen. 2014, 469, 18–32. [Google Scholar] [CrossRef]
- Manjunathan, P.; Kumar, M.; Churipard, S.R.; Sivasankaran, S.; Shanbhag, G.V.; Maradur, S.P. Catalytic Etherification of Glycerol to Tert-Butyl Glycerol Ethers Using Tert -Butanol over Sulfonic Acid Functionalized Mesoporous Polymer. RSC Adv. 2016, 6, 82654–82660. [Google Scholar] [CrossRef]
- Huang, R.; Kim, E.Y. Catalytic Synthesis of Glycerol Tert-Butyl Ethers as Fuel Additives from the Biodiesel by-Product Glycerol. J. Chem. 2015, 2015, 763854. [Google Scholar] [CrossRef]
- Srinivas, M.; Raveendra, G.; Parameswaram, G.; Prasad, P.S.S.; Lingaiah, N. Cesium Exchanged Tungstophosphoric Acid Supported on Tin Oxide: An Efficient Solid Acid Catalyst for Etherification of Glycerol with Tert-Butanol to Synthesize Biofuel Additives. J. Mol. Catal. A Chem. 2016, 413, 7–14. [Google Scholar] [CrossRef]
- Estevez, R.; López, M.I.; Jiménez-Sanchidrián, C.; Luna, D.; Romero-Salguero, F.J.; Bautista, F.M. Etherification of Glycerol with Tert-Butyl Alcohol over Sulfonated Hybrid Silicas. Appl. Catal. A Gen. 2016, 526, 155–163. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Glycerol Etherification with TBA: High Yield to Poly-Ethers Using a Membrane Assisted Batch Reactor. Environ. Sci. Technol. 2014, 48, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, M.; Castro, C.S.; Oliveira, L.C.A.; Carvalho, W.A. Green Acid Catalyst Obtained from Industrial Wastes for Glycerol Etherification. Fuel Process. Technol. 2015, 138, 695–703. [Google Scholar] [CrossRef]
- Estevez, R.; Lopez-Pedrajas, S.; Luna, D.; Bautista, F.M. Microwave-Assisted Etherification of Glycerol with Tert-Butyl Alcohol over Amorphous Organosilica-Aluminum Phosphates. Appl. Catal. B 2017, 213, 42–52. [Google Scholar] [CrossRef]
- Galhardo, T.S.; Simone, N.; Gonçalves, M.; Figueiredo, F.C.A.; Mandelli, D.; Carvalho, W.A. Preparation of Sulfonated Carbons from Rice Husk and Their Application in Catalytic Conversion of Glycerol. ACS Sustain. Chem. Eng. 2013, 1, 1381–1389. [Google Scholar] [CrossRef]
- Celdeira, P.A.; Gonçalves, M.; Figueiredo, F.C.A.; Bosco, S.M.D.; Mandelli, D.; Carvalho, W.A. Sulfonated Niobia and Pillared Clay as Catalysts in Etherification Reaction of Glycerol. Appl. Catal. A Gen. 2014, 478, 98–106. [Google Scholar] [CrossRef]
- Mravec, D.; Turan, A.; Filková, A.; Mikesková, N.; Volkovicsová, E.; Onyestyák, G.; Harnos, S.; Lónyi, F.; Valyon, J.; Kaszonyi, A. Catalytic Etherification of Bioglycerol with Bioethanol over H-Beta, H-Y and H-MOR Zeolites. Fuel Process. Technol. 2017, 159, 111–117. [Google Scholar] [CrossRef]
- Yadav, V.P.; Maity, S.K.; Shee, D. Etherification of Glycerol with Ethanol over Solid Acid Catalysts: Kinetic Study Using Cation Exchange Resin. Indian Chem. Eng. 2017, 59, 117–135. [Google Scholar] [CrossRef]
- Pinto, B.P.; de Lyra, J.T.; Nascimento, J.A.C.; Mota, C.J.A. Ethers of Glycerol and Ethanol as Bioadditives for Biodiesel. Fuel 2016, 168, 76–80. [Google Scholar] [CrossRef]
- Lemos, C.O.T.; Rade, L.L.; Barrozo, M.A.D.S.; Cardozo-Filho, L.; Hori, C.E. Study of Glycerol Etherification with Ethanol in Fixed Bed Reactor under High Pressure. Fuel Process. Technol. 2018, 178, 1–6. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Maisano, S.; Frusteri, L.; Migliori, M.; Giordano, G.; Todaro, S.; Frusteri, F. Zeolite-Assisted Etherification of Glycerol with Butanol for Biodiesel Oxygenated Additives Production. J. Energy Chem. 2020, 48, 136–144. [Google Scholar] [CrossRef]
- Fang, W.; Wang, S.; Liebens, A.; de Campo, F.; Xu, H.; Shen, W.; Pera-Titus, M.; Clacens, J.M. Silica-Immobilized Aquivion PFSA Superacid: Application to Heterogeneous Direct Etherification of Glycerol with n-Butanol. Catal. Sci. Technol. 2015, 5, 3980–3990. [Google Scholar] [CrossRef]
- Samoilov, V.O.; Ramazanov, D.N.; Nekhaev, A.I.; Maksimov, A.L. Heterogeneous Catalytic Conversion of Glycerol with N-Butyl Alcohol. Pet. Chem. 2016, 56, 125–130. [Google Scholar] [CrossRef]
- Gonzalez-Arellano, C.; Grau-Atienza, A.; Serrano, E.; Romero, A.A.; Garcia-Martinez, J.; Luque, R. The Role of Mesoporosity and Si/Al Ratio in the Catalytic Etherification of Glycerol with Benzyl Alcohol Using ZSM-5 Zeolites. J. Mol. Catal. A Chem. 2015, 406, 40–45. [Google Scholar] [CrossRef]
- Tekale, D.P.; Yadav, G.D.; Dalai, A.K. Solvent-Free Benzylation of Glycerol by Benzyl Alcohol Using Heteropoly Acid Impregnated on k-10 Clay as Catalyst. Catalysts 2021, 11, 34. [Google Scholar] [CrossRef]
- Frusteri, L.; Cannilla, C.; Bonura, G.; Chuvilin, A.L.; Perathoner, S.; Centi, G.; Frusteri, F. Carbon Microspheres Preparation, Graphitization and Surface Functionalization for Glycerol Etherification. Catal. Today 2016, 277, 68–77. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Linares, M.; García, R.; Serrano, D.; Cesteros, Y. Effect of Hierarchical Porosity and Fluorination on the Catalytic Properties of Zeolite Beta for Glycerol Etherification. Appl. Catal. A Gen. 2014, 473, 75–82. [Google Scholar] [CrossRef]
- Zhao, W.; Yi, C.; Yang, B.; Hu, J.; Huang, X. Etherification of Glycerol and Isobutylene Catalyzed over Rare Earth Modified Hβ-Zeolite. Fuel Process. Technol. 2013, 112, 70–75. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Guo, X.; Mao, J.; Zhang, S. Etherification of Glycerol with Isobutene on Sulfonated Graphene: Reaction and Separation. Green Chem. 2014, 16, 4669–4679. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B.; Yi, C. Kinetic Study of Glycerol Etherification with Isobutene. Ind. Eng. Chem. Res. 2013, 52, 3742–3751. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Taboada, E.; Llorca, J.; Cesteros, Y. Microwave-Assisted Synthesis of Sulfonic Acid-Functionalized Microporous Materials for the Catalytic Etherification of Glycerol with Isobutene. Green Chem. 2013, 15, 2230–2239. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Taboada, E.; Llorca, J.; Molins, E.; Cesteros, Y. Sulfonic Acid-Functionalized Aerogels as High Resistant to Deactivation Catalysts for the Etherification of Glycerol with Isobutene. Appl. Catal. B 2013, 136–137, 287–293. [Google Scholar] [CrossRef]
- González, M.D.; Salagre, P.; Mokaya, R.; Cesteros, Y. Tuning the Acidic and Textural Properties of Ordered Mesoporous Silicas for Their Application as Catalysts in the Etherification of Glycerol with Isobutene. Catal. Today 2014, 227, 171–178. [Google Scholar] [CrossRef]
- Bozkurt, Ö.D.; Bağlar, N.; Çelebi, S.; Uzun, A. Screening of Solid Acid Catalysts for Etherification of Glycerol with Isobutene under Identical Conditions. Catal. Today 2020, 357, 483–494. [Google Scholar] [CrossRef]
- Martín, M.; Grossmann, I.E. Simultaneous Optimization and Heat Integration for the Coproduction of Diesel Substitutes: Biodiesel (FAME and FAEE) and Glycerol Ethers from Algae Oil. Ind. Eng. Chem. Res. 2014, 53, 11371–11383. [Google Scholar] [CrossRef]
- Izquierdo, J.F.; Outón, P.R.; Galán, M.; Jutglar, L.; Villarrubia, M.; Ariza, X. New Biodiesel Additives from Glycerol and Isoamylenes. Biofuels Bioprod. Biorefining 2014, 8, 658–669. [Google Scholar] [CrossRef]
- Sutter, M.; da Silva, E.; Duguet, N.; Raoul, Y.; Métay, E.; Lemaire, M. Glycerol Ether Synthesis: A Bench Test for Green Chemistry Concepts and Technologies. Chem. Rev. 2015, 115, 8609–8651. [Google Scholar] [CrossRef]
- Pico, M.P.; Romero, A.; Rodríguez, S.; Santos, A. Etherification of Glycerol by Tert-Butyl Alcohol: Kinetic Model. Ind. Eng. Chem. Res. 2012, 51, 9500–9509. [Google Scholar] [CrossRef]
- da Silva, M.J.; Chaves, D.M.; Júlio, A.A.; Rodrigues, F.A.; Bruziquesi, C.G.O. Sn(II)-Exchanged Keggin Silicotungstic Acid-Catalyzed Etherification of Glycerol and Ethylene Glycol with Alkyl Alcohols. Ind. Eng. Chem. Res. 2020, 59, 9858–9868. [Google Scholar] [CrossRef]
- Miranda, C.; Urresta, J.; Cruchade, H.; Tran, A.; Benghalem, M.; Astafan, A.; Gaudin, P.; Daou, T.J.; Ramírez, A.; Pouilloux, Y.; et al. Exploring the Impact of Zeolite Porous Voids in Liquid Phase Reactions: The Case of Glycerol Etherification by Tert-Butyl Alcohol. J. Catal. 2018, 365, 249–260. [Google Scholar] [CrossRef]
- Hieu, D.T.; Kosslick, H.; Riaz, M.; Schulz, A.; Springer, A.; Frank, M.; Jaeger, C.; Thu, N.T.M.; Son, L.T. Acidity and Stability of Brønsted Acid Sites in Green Clinoptilolite Catalysts and Catalytic Performance in the Etherification of Glycerol. Catalysts 2022, 12, 253. [Google Scholar] [CrossRef]
- Dominguez, C.M.; Romero, A.; Santos, A. Improved Etherification of Glycerol with Tert-Butyl Alcohol by the Addition of Dibutyl Ether as Solvent. Catalysts 2019, 9, 378. [Google Scholar] [CrossRef]
- Veiga, P.M.; Gomes, A.C.L.; Veloso, C.O.; Henriques, C.A. Acid Zeolites for Glycerol Etherification with Ethyl Alcohol: Catalytic Activity and Catalyst Properties. Appl Catal A Gen 2017, 548, 2–15. [Google Scholar] [CrossRef]
- Marinho, C.M.; Marcos, M.A.; Hori, C.E. Optimization of Glycerol Etherification with Ethanol in Fixed Bed Reactor under Various Pressures. Energy 2020, 207, 118301. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, L.; Frusteri, F. Batch Reactor Coupled with Water Permselective Membrane: Study of Glycerol Etherification Reaction with Butanol. Chem. Eng. J. 2015, 282, 187–193. [Google Scholar] [CrossRef]
- Jaworski, M.A.; Rodríguez Vega, S.; Siri, G.J.; Casella, M.L.; Romero Salvador, A.; Santos López, A. Glycerol Etherification with Benzyl Alcohol over Sulfated Zirconia Catalysts. Appl. Catal. A Gen. 2015, 505, 36–43. [Google Scholar] [CrossRef]
- Chiosso, M.E.; Lick, I.D.; Casella, M.L.; Merlo, A.B. Acid Functionalized Carbons as Catalyst for Glycerol Etherification with Benzyl Alcohol. Braz. J. Chem. Eng. 2020, 37, 129–137. [Google Scholar] [CrossRef]
- Liu, J.; Yang, B. Liquid–Liquid–Solid Mass Transfer and Phase Behavior of Heterogeneous Etherification of Glycerol with Isobutene. AIChE J. 2018, 64, 2526–2535. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Z.; Zhang, P.; Yang, B. On the Kinetics of Multiphase Etherification of Glycerol with Isobutene. Chem. Eng. J. 2019, 375, 122039. [Google Scholar] [CrossRef]
- Estevez, R.; Aguado-Deblas, L.; Luna, D.; Bautista, F.M. An Overview of the Production of Oxygenated Fuel Additives by Glycerol Etherification, Either with Isobutene or Tert-Butyl Alcohol, over Heterogeneous Catalysts. Energies 2019, 12, 2364. [Google Scholar] [CrossRef]
- Vlad, E.; Bildea, C.S.; Zaharia, E.; Bozga, G. Conceptual Design of Glycerol Etherification Processes. In Computer Aided Chemical Engineering; Elsevier: Amsterdam, The Netherlands, 2011; Volume 29, pp. 331–335. [Google Scholar]
- Soto, R.; Fité, C.; Ramírez, E.; Bringué, R.; Cunill, F. Equilibrium of the Simultaneous Etherification of Isobutene and Isoamylenes with Ethanol in Liquid-Phase. Chem. Eng. Res. Des. 2014, 92, 644–656. [Google Scholar] [CrossRef]
- Felipe Izquierdo, J.; Outón, P.R.; Galán, M.; Jutglar, L.; Villarrubia, M.; Hermo, M.P.; Ariza, X.; Fernández, I. Ethers of Glycerol and Isoamylenes as Biodiesel Additives: Synthesis and Characterization. Chem. Eng. Trans. 2013, 32, 877–882. [Google Scholar]
- Medeiros, M.D.A.; Rezende, J.D.C.; Araújo, M.H.; Lago, R.M. Influência Da Temperatura e Da Natureza Do Catalisador Na Polimerização Do Glicerol Influence of Temperature and Nature of the Catalyst on Glycerol Polymerization. Polímeros 2010, 20, 188–193. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; della Pina, C. From Glycerol to Value-Added Products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, M.; Khayoon, M.S.; Abdullah, A.Z. Synthesis of Oxygenated Fuel Additives via the Solventless Etherification of Glycerol. Bioresour. Technol. 2012, 112, 308–312. [Google Scholar] [CrossRef] [PubMed]
- Ruppert, A.M.; Meeldijk, J.D.; Kuipers, B.W.M.; Erné, B.H.; Weckhuysen, B.M. Glycerol Etherification over Highly Active CaO-Based Materials: New Mechanistic Aspects and Related Colloidal Particle Formation. Chem. A Eur. J. 2008, 14, 2016–2024. [Google Scholar] [CrossRef]
- Gholami, Z.; Abdullah, A.Z.; Lee, K.T. Dealing with the Surplus of Glycerol Production from Biodiesel Industry through Catalytic Upgrading to Polyglycerols and Other Value-Added Products. Renew. Sustain. Energy Rev. 2014, 39, 327–341. [Google Scholar] [CrossRef]
- Calatayud, M.; Ruppert, A.M.; Weckhuysen, B.M. Theoretical Study on the Role of Surface Basicity and Lewis Acidity on the Etherification of Glycerol over Alkaline Earth Metal Oxides. Chem. A Eur. J. 2009, 15, 10864–10870. [Google Scholar] [CrossRef]
- French, J.-M.C.; Centre, N.; Universit, Y.P.; Barrault, J.; Carbone, V. Préparation de Diglydérol et Triglycérol Par Polymérisation Directe Du Glycérol En Présence de Catalyseurs Solides. Oilseeds Fats Crop. Lipids 2003, 10, 74–82. [Google Scholar]
- Sing, K.S.W. Reporting Physisorption Data For Gas/Solid Systems with Special Reference to the Determination of Surface Area and Porosity. International Union of Pure and Applied Chemistry Physical Chemistry Division Commission on Colloid and Surface Chemistry Including Catalysis. Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Ayoub, M.; Abdullah, A.Z.; Ahmad, M.; Sultana, S. Performance of Lithium Modified Zeolite Y Catalyst in Solvent-Free Conversion of Glycerol to Polyglycerols. J. Taibah Univ. Sci. 2014, 8, 231–235. [Google Scholar] [CrossRef]
- Park, S.K.; Kim, D.W.; Lee, S.Y.; Lee, J.S. Direct Etherification Reaction of Glycerol Using Alkali Metal Cation (Li+, Na+ and K+) Containing X-Type Zeolites as Heterogeneous Catalysts: Optimization of the Reaction Conditions. Catalysts 2021, 11, 1323. [Google Scholar] [CrossRef]
- Gholami, Z.; Abdullah, A.Z.; Lee, K.T. Catalytic Etherification of Glycerol to Diglycerol Over Heterogeneous Calcium-Based Mixed-Oxide Catalyst: Reusability and Stability. Chem Eng Commun 2015, 202, 1397–1405. [Google Scholar] [CrossRef]
- Han, T.; Lee, J.S. Positive Effect of Antagonistic Additives on the Homogeneous Catalytic Etherification Reaction of Glycerol. Catalysts 2021, 11, 1000. [Google Scholar] [CrossRef]
- Lee, J.S.; Jang, E.; Kim, D.W.; Park, S.K. Tuning the Catalytic Activity of Recyclable Heterogeneous Catalysts for the Direct Etherification Reaction of Glycerol Using Antagonistic Additives. Catalysts 2022, 12, 220. [Google Scholar] [CrossRef]
- Aloui, M.; Cecilia, J.A.; Moreno-Tost, R.; Ghorbel, S.B.; Saïd Zina, M.; Rodríguez-Castellón, E. Glycerol Etherification towards Selective Diglycerol over Mixed Oxides Derived from Hydrotalcites: Effect of Ni Loading. J. Solgel. Sci. Technol. 2021, 97, 351–364. [Google Scholar] [CrossRef]

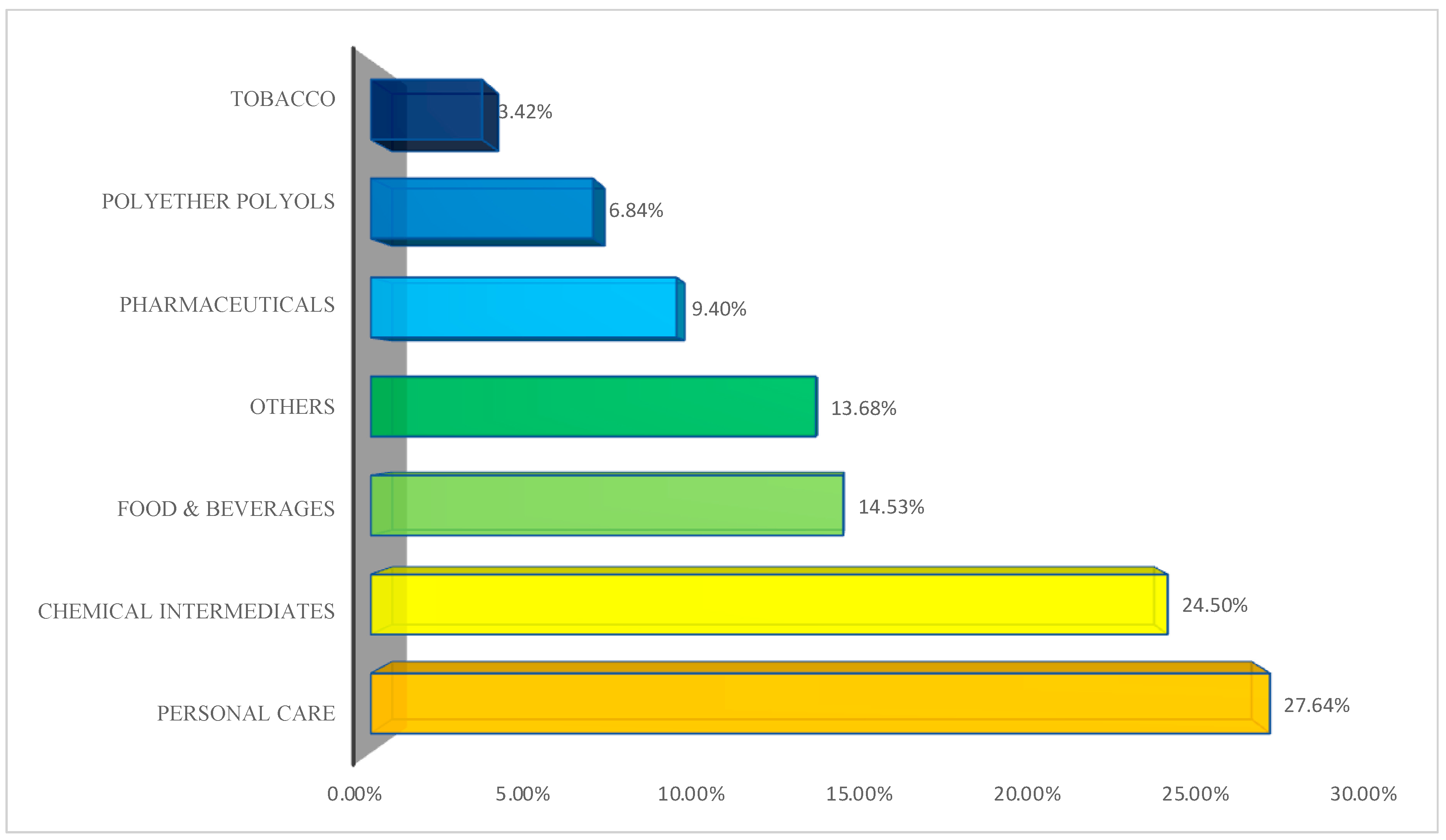


| Catalyzed Glycerol Conversion Pathways | Advantages | Disadvantages | References |
|---|---|---|---|
| Acetalization |
|
| [38,39] |
| Acetylation |
|
| [42] |
| Ammoxidation |
|
| [43] |
| Dehydration |
|
| [14,45] |
| Esterification |
|
| [46,47] |
| Etherification |
|
| [7,13,53,54] |
| Oxidation |
|
| [16] |
| Reduction |
|
| [49] |
| Reforming |
|
| [51] |
| Types of Glycerol Etherification Mechanisms | Types of Solvent Used | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Alcohol Solvent |
|
|
| [56,66,81,82,83,84,85,86,87,88,89,90,91] |
|
|
| [92,93,94,95] | |
|
|
| [96,97,98] | |
|
|
| [99,100] | |
| Olefin Solvent |
|
|
| [56,101,102,103,104,105,106,107,108,109] |
| Dimerization reactions are not facilitated by shorter reaction times. | The side reaction that results in the dimerization of 1-butene consumes a significant percentage of the solvent. | [110] | |
|
|
| [111] | |
| Solvent Free | - |
|
| [7,28,112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palanychamy, P.; Lim, S.; Yap, Y.H.; Leong, L.K. Critical Review of the Various Reaction Mechanisms for Glycerol Etherification. Catalysts 2022, 12, 1487. https://doi.org/10.3390/catal12111487
Palanychamy P, Lim S, Yap YH, Leong LK. Critical Review of the Various Reaction Mechanisms for Glycerol Etherification. Catalysts. 2022; 12(11):1487. https://doi.org/10.3390/catal12111487
Chicago/Turabian StylePalanychamy, Prakas, Steven Lim, Yeow Hong Yap, and Loong Kong Leong. 2022. "Critical Review of the Various Reaction Mechanisms for Glycerol Etherification" Catalysts 12, no. 11: 1487. https://doi.org/10.3390/catal12111487
APA StylePalanychamy, P., Lim, S., Yap, Y. H., & Leong, L. K. (2022). Critical Review of the Various Reaction Mechanisms for Glycerol Etherification. Catalysts, 12(11), 1487. https://doi.org/10.3390/catal12111487


_Xu.png)





