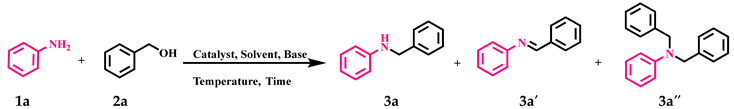
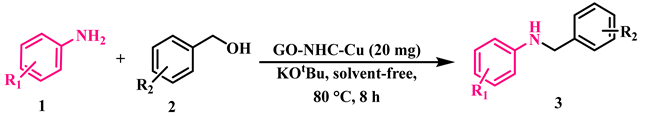
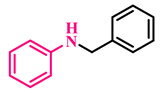
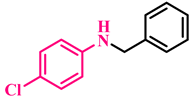
Abstract
A new N-heterocyclic carbene (NHC) copper(I) complex supported on graphene oxide (GO-NHC-Cu) was synthesised and thoroughly characterised by various instrumental techniques such as FT-IR, FT-Raman, PXRD, XPS, FESEM, EDX, HRTEM, TGA and ICP-OES. The catalytic activity of the supported complex was explored in the N-alkylation of anilines with alcohols under solvent-free and aerobic conditions to afford monoalkylated products in good to excellent yields (20 products, 83–96%). All products were isolated and characterised by 1H and 13C{1H} NMR spectroscopy. The catalyst was recuperated from the reaction mixture by simple filtration and reused for up to five successive cycles with insignificant loss in the catalytic activity. The control experiments showed that the reaction proceeded in aerobic conditions. The green chemistry metrics for the reaction were found to be fairly close to the ideal values: carbon efficiency (95.9%), E-factor (0.15), atom economy (92.14%), process mass intensity (1.15) and reaction mass efficiency (86.80%). The air stability, selectivity, recyclability of the catalyst, and the high yields of the products under solvent-free conditions are some of the salient features of the reported methodology.
1. Introduction
Amines are an important class of organic compounds and find miscellaneous applications in biological and synthetic chemistry [1,2]. N-alkylamines have been used as synthetic intermediates for pharmaceuticals [3], fungicides [4], dyes [5] and agrochemicals [5]. Conventionally, N-alkylated amines have been synthesised by the reaction of amines with alkyl halides in the presence of a base [6,7] or by reductive amination [8]. However, the reported protocols require a stoichiometric amount of the alkylating reagents and often lead to undesired side products. An alternative approach for the synthesis of alkylated amines entails the application of primary alcohols instead of alky halides as the alkylating agents via hydrogen borrowing or the hydrogen auto-transfer (HB/HAT) strategy, as evidenced by a plethora of work on these reactions [9].
A scrutiny of the literature shows that N-alkylation reactions are carried out by various methods such as non-metal acid–base catalysed alkylation of amines by substitution reaction (SN) and homo- and heterogeneous catalytic systems based on Rh, Pt, Pd, Ru, Mn, Fe etc. via BH/HAT [10,11,12,13,14,15,16,17,18]. Although these methods were found to be efficient, they suffer from several limitations, such as the use of expensive ligands, poor thermal steadiness, high catalyst loading, limited recyclability of the catalyst, unselective alkylation, imine formation and so forth. A base-promoted alkylation of amines under metal-free conditions was also reported [19]. However, the substrate scope was limited. It required an inert atmosphere, a longer reaction time and a surplus amount of base. To avoid these shortcomings, there is a need to develop economic, green and sustainable catalysts.
Copper is the 25th most abundant metal that is commercially available; it is cheap and can undergo a variety of reactions due to variable oxidation states (0 to +3) [20]. Copper-based complexes have been used as catalysts for several organic transformations, particularly in the area of electrocatalysis and photocatalysis. [21,22,23,24].
In recent years, N-heterocyclic carbenes (NHCs) transition metal complexes have been widely used as both homo- and heterogeneous catalysts with spectacular success [25,26,27,28]. The superiority of NHCs ligands over other ligands is ascribed to the better σ-donating properties and steric hindrance of NHC ligands [29,30]. The catalytic potential of Cu-NHC complexes has been explored in hydroboration [31], oxidative coupling [32], hydrosilylation [33] and carboxylation reactions [34]. However, N-alkylation reactions by Cu-based NHC complexes has remained a relatively unexplored area in synthetic chemistry [35]. Therefore, we envisioned to explore the catalytic potential of the synthesized Cu-NHC complex in selective N-alkylation reactions. The covalent attachment of metal complexes to a support not only minimizes metal leaching but also provides avenues to harvest the benefits of both homo- and heterogeneous catalysis [36].
Amongst a multitude of solid supports listed in the literature, graphene oxide (GO) has been considered to be an excellent support due to its high thermal stability, good accessibility and large surface area [37,38,39,40,41]. GO has also been christened as a starburst 2D material or a nanoreactor [42]. In addition, GO sheets are amenable to facile surface engineering due to the presence of various functionalities such as carboxyl groups on the edges and epoxy and hydroxy groups on the basal plane of GO. It appears that only four examples of GO-based covalently linked NHC complexes of transition metals have been reported [43,44,45,46]. Prompted by these reports, we herein report the synthesis and characterisation of a new GO-NHC-Cu(I) complex, in which a benzimidazole-derived-NHC ligand is coordinated to Cu(I) and covalently attached to the surface of graphene oxide (Scheme 1). The catalytic potential of the synthesized complex was explored in the selective N-monoalkylation of a series of amines with alcohols via a hydrogen-borrowing strategy under solvent-free conditions. To the best of our knowledge, this is the first report on the selective N-monoalkylation of amines using a GO-supported Cu(I)-NHC complex as a heterogeneous catalyst.
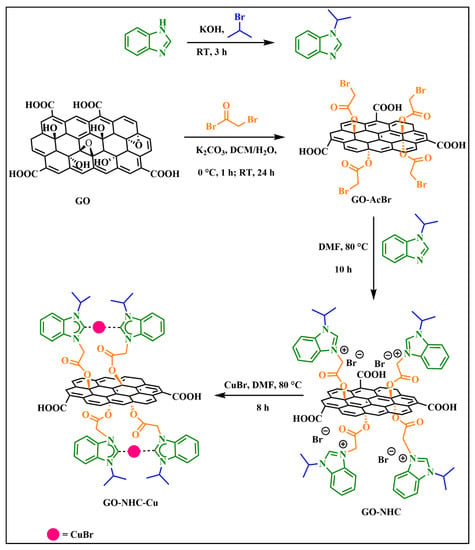
Scheme 1.
Schematic illustration of the synthesis of GO-NHC-Cu.
2. Results and Discussion
The presence of oxygen functionalities on the surface of GO makes it a suitable support for catalysis. Considering the hydrophilic oxygen functionalities on the surface of GO, we synthesised an N-heterocyclic carbene copper(I) complex supported on GO, GO-NHC-Cu (Scheme 1). The supported complex GO-NHC-Cu was thoroughly characterised by various sophisticated techniques such as FT-IR, FT-Raman, PXRD, XPS, FESEM, EDX, HRTEM, TGA and ICP-OES. All the synthesised N-alkylated amines were isolated as colourless to pale yellow liquids and characterised by 1H and 13C{1H} NMR spectroscopy.
2.1. Characterisation of GO-NH--Cu
The FT-IR spectra of GO, GO-AcBr, GO-NHC and GO-NHC-Cu are shown in Figure 1. The GO spectrum exhibited bands at 3420, 1735, 1620, 1400, 1225 and 1048 cm−1 associated with ν(OH), ν(C=O), ν(C=C), ν(C-OH), δ(O-H) and ν(C-O), respectively [47]. The FT-IR of GO-AcBr displayed the characteristic peaks of GO with minimal changes in the intensities. However, a decrease in the intensities of ν-OH and ν-CO as compared to GO, indicated the successful attachment of bromoacetyl bromide by reacting with -OH and C-O-C functionalities [48]. The spectrum of GO-NHC displayed two new bands at 1578 and 1377 cm−1, which can be assigned to ν(C=N) and ν(C-N), indicating the attachment of 1-isopropyl-1H-benzimidazole to GO-AcBr [49]. The spectrum of GO-NHC-Cu showed peaks similar to those of GO-NHC, with a slight alteration in the frequency of ν(C=N) and ν(C-N), from 1578 cm−1 to 1582 and from 1377 cm−1 to 1389 cm−1. This may be taken as indirect evidence of the coordination of NHC to copper [49].
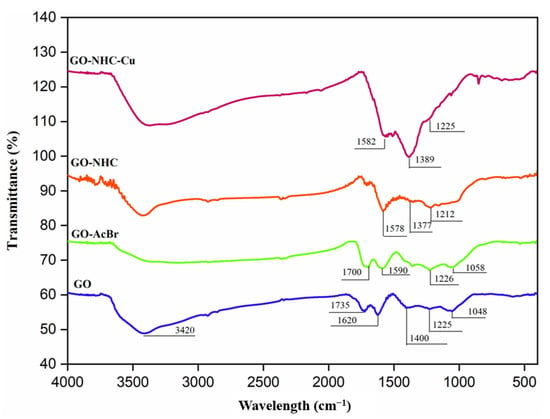
Figure 1.
FT-IR spectra of GO, GO-AcBr, GO-NHC and GO-NHC-Cu.
The FT-Raman spectrum of GO, GO-AcBr, GO-NHC and GO-NHC-Cu were recorded to study the disorderedness in the complex (Figure S1). The FT-Raman spectra data of GO, GO-AcBr, GO-NHC and GO-NHC-Cu displayed two distinct peaks consistent with a defective (D band) and a graphitic band (G band), and the corresponding data are tabulated in Table 1. The G band is associated with the E2g vibration mode of sp2 carbon domains, the D band is associated with defects in the carbon framework, and the ID/IG ratio indicates the disorder due to structural imperfections in the graphene lattice [50]. It can be inferred from Table 1 that the ID/IG ratio increased with each functionalization step (GO to GO-NHC-Cu), indicating the increment in disorderedness in the graphene lattice.

Table 1.
FT-Raman spectral data of GO, GO-AcBr, GO-NHC and GO-NHC-Cu.
The PXRD analysis of GO, GO-AcBr and GO-NHC-Cu was recorded to examine the changes that took place upon the surface functionalization of GO; the spectra are summarised in Figure 2. The PXRD spectrum of GO showed a sharp peak at 2θ = 11.86° corresponding to the 001 plane, with an interlayer spacing of 0.77 nm and a small peak at 42° due to residual MnO2 formed by the reaction of KMnO4 during the Hummers process [51]. However, in GO-AcBr a new peak at 2θ = 13.84° was observed corresponding to an interplanar distance of 0.64 nm, suggesting the grafting of bromoacetyl bromide on the surface of GO (Figure 2b) [48]. The PXRD spectrum of the complex GO-NHC-Cu did not exhibit any peak, as almost a flat baseline spectrum was obtained (Figure 2c). It can be inferred from the spectrum that almost all functionalities on the surface of GO were consumed in the functionalization of the immobilized complex, and the nature of the complex was amorphous.
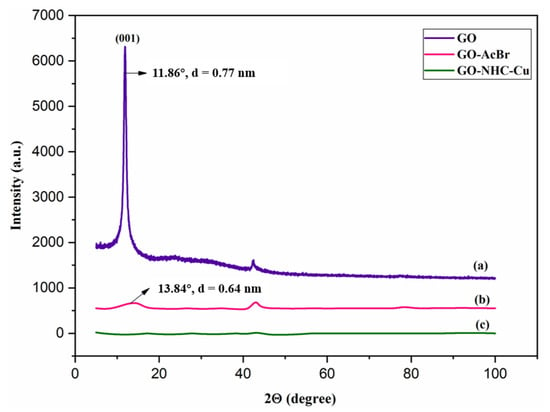
Figure 2.
Powder XRD spectrum of (a) GO, (b) GO-AcBr and (c) GO-NHC-Cu.
The XPS spectra of GO-NHC-Cu are depicted in Figure 3. The XPS analysis was carried out to analyse the components on the surface of GO-NHC-Cu and the oxidation state of the metal ion. The survey spectrum of GO-NHC-Cu (Figure 3a) showed the presence of carbon, oxygen, nitrogen and copper. The deconvoluted Cu 2p spectrum showed two peaks at 933.28 eV and 953.18 eV, corresponding to Cu 2p3/2 and Cu 2p1/2 of Cu(I) (Figure 3b) [52,53]. The C 1s deconvoluted spectrum showed four peaks centred at 283.52, 284.59, 286.06 and 289.15 eV due to C=C, C-O/C-N, C=O/C=N and O-C=O, respectively (Figure 3c). The N 1s deconvoluted spectrum showed two peaks due to C-N and C=N at 401.21 and 398.94 eV, respectively (Figure 3d). The deconvoluted spectrum of O 1s showed five peaks at 529.20, 530.17, 531.08, 531.92 and 533.06 eV due to C(O)O, C=O, COOH, C-OH and C-O, respectively (Figure 3e) [48].
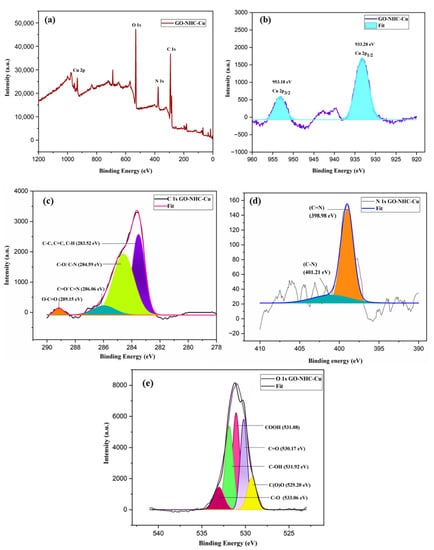
Figure 3.
XPS survey spectrum of (a) GO-NHC-Cu and deconvoluted spectra of (b) Cu 2p for GO-NHC-Cu, (c) C 1s for GO-NHC-Cu and (d) N 1s for GO-NHC-Cu, (e) O 1s for GO-NHC-Cu.
The FESEM images of GO, GO-AcBr, GO-NHC and GO-NHC-Cu are shown in Figure 4. The FESEM image of GO showed a wrinkled sheet-like morphology (Figure 4a). After the functionalization of GO with bromoacetyl bromide, the crumpling in the sheets further increased (Figure 4b). Further, the FESEM of GO-NHC and GO-NHC-Cu also showed crumpling, with an increase in protrusion and surface roughness (Figure 4c,d).

Figure 4.
FESEM images of (a) GO, (b) GO–Ac-Br, (c) GO-NHC and (d) GO-NHC-Cu.
The EDX data of GO-NHC-Cu are shown in Figure S2. The EDX data confirmed the presence of all expected elements, C, O, N, Cu and Br, in the composite in 82.67%, 4.76%, 1.42%, 11.09% and 0.03% (atomic%), respectively.
The HRTEM micrograph of GO-NHC-Cu is depicted in Figure S3. The HRTEM micrograph shows light and dark patches which may be attributed to the GO sheet and the attachment of Cu-NHC.
The Cu content in GO-NHC-Cu was found to be 0.17 mmol/g or 0.16 mol%, based on ICP analysis.
The thermal stability of the composite was determined by TGA. The TGA thermogram of GO-NHC-Cu is shown in Figure S4. GO-NHC-Cu exhibited a weight loss of ~4% at 150 °C, which can be attributed to the loss of various oxygen-containing groups. It can be observed that above 200 °C, the complex started to decompose rapidly, up to 500 °C. There was no discernible weight loss above this temperature.
2.2. Catalytic Application of GO-NHC-Cu in the N-Alkylation of Amines with Alcohols
The catalytic activity of GO-NHC-Cu was explored in the N-alkylation of anilines with alcohols. Initially, aniline (1a) and benzyl alcohol (2a) were used as model substrates for optimization studies. To determine the best reaction conditions, different parameters such as solvent, catalyst amount, base, time, and temperature were optimized, and the results are summarised in Table 2. At first, a reaction in the absence of a catalyst was carried out in toluene, using KOtBu as a base, at 110 °C for 24 h, but no desired product (3a) was obtained (Table 2, entry 1). To determine the role played by the GO support, the reaction was repeated under similar conditions using 30 mg of GO as a catalyst. Again, no product was obtained (Table 2, entry 2). This ruled out the possibility of the support participating in the catalysis. Next, the reaction was carried out in toluene using 30 mg of GO-NHC-Cu as the catalyst and KOtBu as the base, at 110 °C for 24 h; it afforded 3a with 88% yield (Table 2, entry 3). The reaction was further performed using various solvents such as toluene, 1,4-dioxane, THF and DMSO, for 24 h in the presence of 30 mg of the catalyst and afforded 3a in moderate to good yield (Table 2, entries 3–6). Since the solvent screening results were discouraging, the reaction was performed under solvent-free conditions at 110 °C for 24 h. Surprisingly, the solvent-free conditions afforded the best yield of 3a (93%) (Table 2, entry 7). Next, optimization of the catalyst loading was carried out using 30, 25, 20 and 15 mg of the catalyst to afford 3a in 93%, 93%, 93% and 85% yields (Table 2, entries 7–10). It is apparent from Table 2 that the yield of 3a was affected when the catalyst loading was reduced to 15 mg; however, the product yield remained unaffected at 93% with 30, 25 and 20 mg of catalyst. Therefore, 20 mg of catalyst was found to be the optimum amount for this reaction (Table 2, entry 9). Further, the reaction was carried out using four bases, i.e., KOtBu, KOH, Cs2CO3 and K2CO3, at 110 °C to give 3a in 93%, 82%, 66% and 48% yield, respectively (Table 2, entries 7,11–13). It is apparent from Table 2 that the best result was obtained with KOtBu (93%). A reaction in the absence of a base gave a product yield of only 41% (Table 2, entry 14). This indicated that the base also played a vital role in product formation. At last, both temperature and time were optimized for the model reaction (Table 2, entries 15–22). As evident in Table 2, the best yield of the product was obtained at 80 °C in 8 h (Table 2, entry 21). Additionally, the model reaction was carried out in an inert atmosphere. To our surprise, the yield of 3a dropped drastically, as only a trace amount of product formation could be observed (Table 2, entry 23). This showed that air played an essential role in this transformation. At last, to analyse the selectivity of GO-NHC-Cu, the model reaction was carried out with CuBr (5 mg, 0.034 mmol) under identical conditions, and surprisingly even after 24 h, only 10% of 3a and 30% of 3a′ were obtained (Table 2, entry 24). This showed the selectivity of GO-NHC-Cu for N-monoalkylated amines.

Table 2.
Optimization of the reaction parameters for the synthesis of N-benzylaniline a.
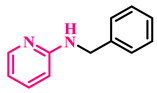
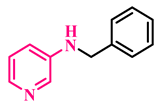
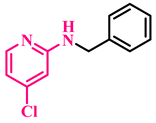
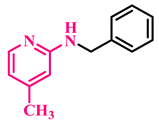
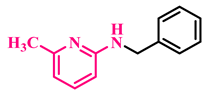
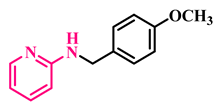
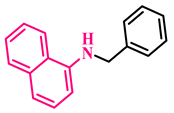
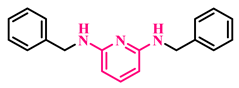
Under the optimized conditions, various substituted anilines and alcohols were examined (Table 3). The reactions were executed with anilines bearing either electron-donating (ED) or electron-withdrawing (EW) groups. As shown in Table 3, the reaction of ED and EW anilines proceeded smoothly to give the desired products (3a–3t) in good yields. However, anilines with the ED group afforded a slightly higher yield in comparison to those with the EW group (Table 3, entry 3e). Para-substituted EW groups on anilines (-Cl, -Br, -F) afforded the desired product in 91%, 92% and 88% yield, respectively (Table 3, entries 3b–3d). The reaction with para-substituted ED aniline (-OCH3) gave the corresponding product in 96% yield (Table 3, entry 3e). This may be ascribed to the facile formation of the imine intermediate by ED anilines in comparison to EW anilines. The reactions with meta-substituted anilines (-CF3, -Cl, -OCH3) also afforded the desired products in 91%, 92% and 94% yields (Table 3, entries 3f-3h). Delightedly, the reactions with ortho-substituted anilines (-Cl, -Br) also proceeded well to afford the desired products in 90% and 87% yields (Table 3, entries 3i–3j). Notably, heteroaromatic amines gave the corresponding N-alkylated products in good yields (89–96%) (Table 3, entries 3k–3p). Even challenging substrates such as 1-napthylamine and 2,6-diaminopyridine gave the corresponding product in 86% and 83% yield, respectively (Table 3, entries 3q–3r). However, a reaction of aliphatic amines with benzyl alcohol was found to be sluggish for our reaction conditions, and no product could be isolated. This may be ascribed to the low nucleophilicity of aliphatic amines with respect to aromatic amines. Furthermore, the present catalytic system was successfully explored using substituted alcohols. Gratifyingly, both ED- and EW-bearing benzylic alcohols gave the corresponding product in good yields (Table 3, entries 3p, 3s and 3t). However, alcohols containing the ED group (-CH3, or -OCH3) performed comparatively better, giving 95% and 96% yields, respectively (Table 3, entries 3s and 3p). On the other hand, 4-chlorobenzyl alcohol gave a slightly lower yield (92%, 3t).

Table 3.
Alcohols and amines used for N-alkylation a,b.
2.3. Discussion on the Reaction Mechanism
In order to gain insight into the reaction mechanism, control experiments were carried out, as depicted in Scheme 2. At first, a trial reaction was carried out under different conditions, with benzyl alcohol (1 mmol) as the model substrate. When the reaction was carried out in the presence of the synthesized catalyst (GO-NHC-Cu) under an N2 atmosphere at 80 °C for 8 h, no product (4a) could be isolated (Scheme 2, equation (i)), but traces of the product could be visualized on a TLC plate. However, the same reaction in air under identical conditions afforded 4a in 52% yield (Scheme 2, equation (ii)). These results support our results reported in Table 2, entry 23. Further, a reaction with only KOtBu (1 mmol) was carried out under an N2 atmosphere and afforded 4a in trace yields (Scheme 2, equation (iii)). A similar reaction was repeated in air, but again, only trace amounts of 4a were observed (Scheme 2, equation (iv)). However, when the reaction was performed in the presence of the catalyst and KOtBu (1 mmol) in air, the yield of 4a was improved to 75% (Scheme 2, equation (vi)), whereas a similar reaction under N2 gave a trace amount of 4a (Scheme 2, equation (v)). Therefore, it can be concluded from the control experiments that the reaction was likely to proceed via the dehydrogenation of the alcohol in the presence of GO-NHC-Cu and a base under aerobic conditions.
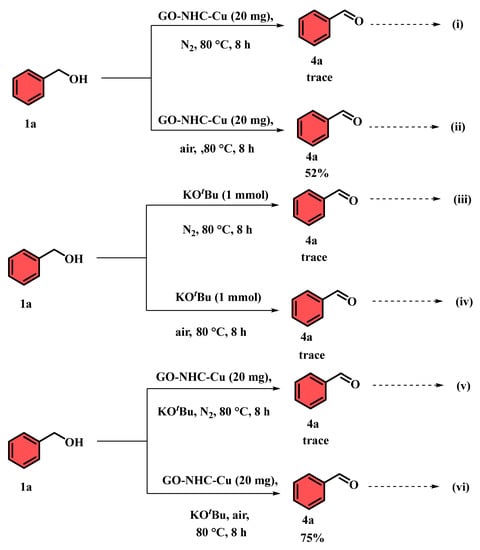
Scheme 2.
Control experiment for GO-NHC-Cu-mediated oxidation.
2.4. Plausible Mechanism
Based on the above mechanistic studies and literature reports, a plausible mechanism for N-alkylation catalysed by GO-NHC-Cu is depicted in Scheme 3 [17,53,54,55]. Initially, Cu-NHC-GO reacts with the alcohol in the presence of a base to give a Cu-coordinated alkoxide intermediate (I). The intermediate (I) generates a Cu-hydride intermediate (II), with the concomitant elimination of the corresponding aldehyde in the presence of air. The in situ formed aldehyde then undergoes condensation with an amine to form the imine intermediate (III) and water. The imine intermediate (III) and the Cu-hydride intermediate (II) react together, by insertion of the imine into the Cu-hydride, to generate the Cu-bound alkylated amine intermediate (IV). The intermediate (IV) undergoes a reaction with another molecule of alcohol to give the final product, followed by the regeneration of Cu-coordinated alkoxide species (I) to sustain the catalytic cycle.
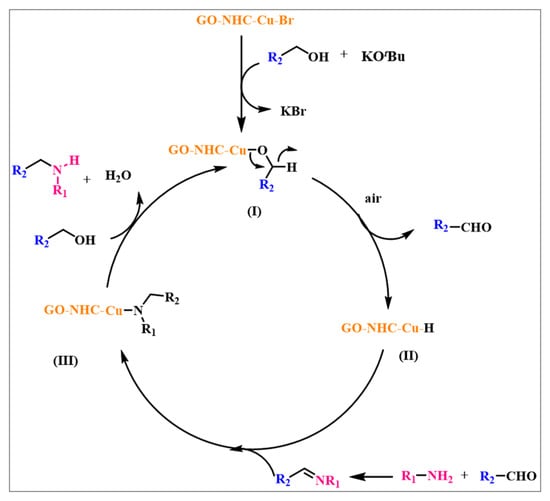
Scheme 3.
A plausible mechanism for the N-alkylation of aniline and alcohol.
2.5. Recyclability and Hot Filtration Test
Robustness and recyclability are key factors for a heterogeneous catalyst. To study the recyclability of GO-NHC-Cu, the catalyst recovered from the reaction mixture was thoroughly washed with distilled water and ethanol and then vacuum-dried at 80 °C. The dried catalyst was then used for the next cycle of N-alkylation. The recyclability plot showed that the catalytic system was recyclable under the desired conditions and could be reused for up to five successive cycles (Figure 5). The identity of the retrieved catalyst was examined by FT-IR, FESEM and ICP-OES. The FT-IR spectra showed all characteristic peaks after the fifth cycle (Figure S5). The FESEM image showed no discernible change in the surface morphology after the fifth cycle (Figure S6). The content of Cu in the fresh catalyst was found to be 0.17 g/mol, whereas the catalyst retrieved after the fifth cycle was found to be 0.14 g/mol. It is clear from ICP-OES that negligible leaching of Cu took place.
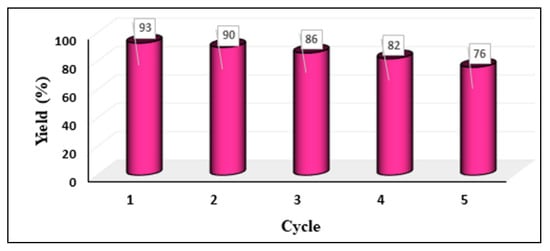
Figure 5.
Recyclability of GO-NHC-Cu.
A hot filtration test was carried out to ascertain the heterogeneous nature of the catalyst [56]. The reaction of aniline with benzyl alcohol was carried out under optimized conditions. The reaction was stopped mid-way (4 h), and the catalyst was separated from the reaction mixture. Only a 42% product yield was achieved up until this point. Afterwards, a new reaction was carried out under the optimal conditions for 4 h, after which the catalyst was filtered, and the reaction was continued for another 4 h. It was found that there was no further increase in product yield even after prolonged heating. This confirmed the heterogeneous nature of the catalyst.
2.6. Comparison of GO-NHC-Cu with Reported Heterogeneous Catalysts
The catalytic activity of the synthesized catalyst GO-NHC-Cu was compared with that of previously reported catalysts for the reaction, and the results are summarised in Table 4. It can be seen in the table that our catalyst afforded comparable or even better results at low temperatures.

Table 4.
Comparison of GO-NHC-Cu with formerly reported catalysts for the N-alkylation of anilines with benzyl alcohol.
2.7. Calculation of Green Chemistry Metrics
An industrially viable reaction must meet the green chemistry metrics. The green chemistry (GC) metrics estimate the efficacy of the environmental performance of a chemical process. The GC metrics were calculated (Supplementary Materials, Figure S49) for product 3e (its product yield was the highest), and the results are presenetd in Table 5. The GC metrics were found close to the ideal values [61].

Table 5.
Green chemistry metrics.
3. Experimental Section
As illustrated in Scheme 1, the GO-supported NHC-Cu complex was prepared in four steps.
3.1. Synthesis of GO and GO-AcBr
GO was synthesised by the modified hummers’ method [62]. GO (200 mg) was dispersed in distilled water (80 mL) and sonicated for 45 min at room temperature (RT). To it, K2CO3 (1.36 g) and DCM (20 mL) were added, and the mixture was stirred for 15 min. This mixture was transferred to an ice bath and was kept under constant stirring. To it 2 g of bromoacetyl bromide dissolved in DCM (40 mL) was dropwise added within 1 h under continuous stirring. Further, this mixture was stirred at RT for 24 h. Next, DCM was removed from the mixture under reduced pressure, and the product (GO-AcBr) was collected by centrifugation and washed with a copious amount of distilled water followed by ethanol and then was vacuum-dried at 80 °C [48].
3.2. Synthesis of GO-NHC and GO-NHC-Cu
The compound 1-isopropyl-1H-benzimidazole was synthesised by a previously reported method [63]. GO-AcBr (200 mg) was dispersed in 50 mL of DMF. To the dispersed solution of GO-AcBr, 1-isopropyl-1H-benzimidazole (3 mL) was added, and the mixture was heated at 80 °C under constant stirring for 10 h. The prepared GO-NHC was filtered and washed with ethanol (2 × 10 mL), followed by vacuum heating at 80 °C. Next, a mixture of GO-AcBr (200 mg) and CuBr (2 mmol) was poured into a 100 mL round bottom flask containing 50 mL of DMF. The mixture was heated at 80 °C for 8 h. The product (GO-NHC-Cu) was then filtered and washed with a profuse amount of ethanol followed by vacuum drying at 80 °C.
3.3. N-Monoalkylation of Amines with Alcohol Using GO-NHC-Cu
In a 20 mL sealed tube, we placed the amine (1 mmol), the alcohol (1 mmol), 20 mg of GO-NHC-Cu as the catalyst and KOtBu (1 mmol) as the base. This mixture was stirred in air under solvent-free conditions at 80 °C for 8 h. The reaction progression was monitored by TLC. Upon completion, the reaction mixture was cooled, ethyl acetate was added as a solvent, and the catalyst and product were filtered. The product was diluted with distilled water and extracted with ethyl acetate (3 × 15 mL). The ethyl acetate layer was then separated and dried over anhydrous NaSO4. The solvent was removed under reduced pressure, and the crude product obtained was purified by column chromatography.
4. Conclusions
In conclusion, a new benzimidazole-derived NHC-Cu complex supported on GO (GO-NHC-Cu) was synthesised and thoroughly characterized by various physicochemical techniques such as FT-IR, FT-Raman, PXRD, XPS, FESEM, EDX, HRTEM, TGA and ICP-OES. The synthesised complex was found to exhibit high catalytic activity in the selective N-monoalkylation of amines with alcohols in air under solvent-free conditions, with good to excellent yield (83–96%) and ideal green chemistry metrics, as shown in Table 4. It is evident from this work, that the reaction afforded the best yield of products in the presence of atmospheric oxygen. Furthermore, the catalyst could be readily separated and recycled up to five times, with insignificant loss in its catalytic activity. To the best of our knowledge, this is the first heterogeneous approach for the N-alkylation of amines with a heterogeneous catalyst containing the NHC ligand, according to a sustainable and green protocol.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal12111458/s1, Figure S1: FT-Raman spectra of GO, GO-AcBr, GO-NHC and GO-NHC-Cu; Figure S2: EDX spectra of GO-NHC-Cu; Figure S3: HRTEM micrograph of G-NHC-Cu(I); Figure S4: TGA thermogram of GO-NHC-Cu; Figure S5: FTIR plot of (a) GO-NHC-Cu (I) fresh and (b) GO-NHC-Cu (I) after the fifth cycle.; Figure S6: FESEM image of GO-NHC-Cu after the fifth cycle; Figure S7: 1H NMR of 1-isopropyl-1H-benzimidazole (L1); Figure S8: 13C NMR of 1-isopropyl-1H-benzimidazole (L1); Figure S9: 1H NMR of N-benzylaniline; Figure S10: 13C NMR of N-benzylaniline; Figure S11: 1H NMR of N-benzyl-4-chloroaniline; Figure S12: 13C NMR of N-benzyl-4-chloroaniline; Figure S13: 1H NMR of N-benzyl-4-bromoaniline; Figure S14: 13C NMR of N-benzyl-4-bromoaniline; Figure S15: 1H NMR of N-benzyl-4-fluoroaniline; Figure S16: 13C NMR of N-benzyl-4-fluoroaniline; Figure S17: 1H NMR of N-benzyl-4-methoxyaniline; Figure S18: 13C NMR of N-benzyl-4-methoxyaniline; Figure S19: 1H NMR of N-benzyl-3-(trifluroylmethyl)aniline; Figure S20: 13C NMR of N-benzyl-3-(trifluroylmethyl)aniline; Figure S21: 1H NMR of N-benzyl-3-chloroaniline; Figure S22: 13C NMR of N-benzyl-3-chloroaniline; Figure S23: 1H NMR of N-benzyl-3-methoxyaniline; Figure S24: 13C NMR of N-benzyl-3-methoxyaniline; Figure S25: 1H NMR of N-benzyl-2-bromoaniline; Figure S26: 13C NMR of N-benzyl-2-bromoaniline; Figure S27: 1H NMR of N-benzyl-2-chloroaniline; Figure S28: 13C NMR of N-benzyl-2-chloroaniline; Figure S29: 1H NMR of N-benzyl-(2-pyridyl)amine; Figure S30: 13C NMR of N-benzyl-(2-pyridyl)amine; Figure S31: 1H NMR of N-benzyl-(3-pyridyl)amine; Figure S32: 13C NMR of N-benzyl-(3-pyridyl)amine; Figure S33: 1H NMR of N-benzyl-4-chloropyridin-2-amine; Figure S34: 13C NMR of N-benzyl-4-chloropyridin-2-amine; Figure S35: 1H NMR of N-benzyl-4-methylpyridin-2-amine; Figure S36: 13C NMR of N-benzyl-4-methylpyridin-2-amine; Figure S37: 1H NMR of N-benzyl-6-methylpyridin-2-amine; Figure S38: 13C NMR of N-benzyl-6-methylpyridin-2-amine; Figure S39: 1H NMR of N-(4-methoxybenzyl)pyridine-3-amine; Figure S40: 13C NMR of N-(4-methoxybenzyl)pyridine-3-amine; Figure S41: 1H NMR of N-Benzylnaphthalen-1-amine; Figure S42: 13C NMR of N-Benzylnaphthalen-1-amine; Figure S43: 1H NMR of N,N′-Dibenzylpyridine-2,6-diamine; Figure S44: 13C NMR of N,N′-Dibenzylpyridine-2,6-diamine; Figure S45: 1H NMR of N-(4-methyl)benzylaniline; Figure S46: 13C NMR of N-(4-methyl)benzylaniline; Figure S47: 1H NMR of N-(4-chloro)benzylaniline; Figure S48: 13C NMR of N-(4-chloro)benzylaniline; Figrue S49: Green Chemistry Metric calculation; Physical and spectral data of N-alkylation. References [64,65,66,67,68,69] are cited in the Supplementary Materials.
Author Contributions
S.K.: Conceptualization, writing-original draft preparation, data curation, methodology. S.V.: Writing—Review and Editing, data curation. D.D.P.: Supervision, Conceptualization. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to CRF IIT(ISM) Dhanbad, STIC Cochin, for providing help in the analysis of the samples S. Kujur and S.Verma acknowledge the receipt of an IIT(ISM) fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vitaku, E.; Smith, D.T.; Njardarson, J.T. Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257–10274. [Google Scholar] [CrossRef] [PubMed]
- Kaloğlu, M.; Gürbüz, N.; Sémeril, D.; Özdemir, İ. Ruthenium(II)-(p-Cymene)-N-Heterocyclic Carbene Complexes for the N-Alkylation of Amine Using the Green Hydrogen Borrowing Methodology. Eur. J. Inorg. Chem. 2018, 2018, 1236–1243. [Google Scholar] [CrossRef]
- Guillena, G.; Ramón, D.J.; Yus, M. Hydrogen Autotransfer in the N-Alkylation of Amines and Related Compounds Using Alcohols and Amines as Electrophiles. Chem. Rev. 2010, 110, 1611–1641. [Google Scholar] [CrossRef]
- Salvatore, R.N.; Yoon, C.H.; Jung, K.W. Synthesis of Secondary Amines. Tetrahedron 2001, 57, 7785–7811. [Google Scholar] [CrossRef]
- Yan, L.; Liu, X.X.; Fu, Y. N-Alkylation of Amines with Phenols over Highly Active Heterogeneous Palladium Hydride Catalysts. RSC Adv. 2016, 6, 109702–109705. [Google Scholar] [CrossRef]
- Ju, Y.; Varma, R.S. Aqueous N-Alkylation of Amines Using Alkyl Halides: Direct Generation of Tertiary Amines under Microwave Irradiation. Green Chem. 2004, 6, 219–221. [Google Scholar] [CrossRef]
- Bissember, A.C.; Lundgren, R.J.; Creutz, S.E.; Peters, J.C.; Fu, G.C. Transition-Metal-Catalyzed Alkylations of Amines with Alkyl Halides: Photoinduced, Copper-Catalyzed Couplings of Carbazoles. Angew. Chem. Int. Ed. 2013, 52, 5129–5133. [Google Scholar] [CrossRef]
- Tararov, V.I.; Börner, A. Approaching Highly Enantioselective Reductive Animation. Synlett 2005, 2005, 203–211. [Google Scholar] [CrossRef]
- Reed-Berendt, B.G.; Latham, D.E.; Dambatta, M.B.; Morrill, L.C. Borrowing Hydrogen for Organic Synthesis. ACS Cent. Sci. 2021, 7, 570–585. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. Transition-Metal-Catalyzed Reductive Amination Employing Hydrogen. Chem. Rev. 2020, 120, 9583–9674. [Google Scholar] [CrossRef]
- Sankar, V.; Kathiresan, M.; Sivakumar, B.; Mannathan, S. Zinc-Catalyzed N-Alkylation of Aromatic Amines with Alcohols: A Ligand-Free Approach. Adv. Synth. Catal. 2020, 362, 4409–4414. [Google Scholar] [CrossRef]
- Bains, A.K.; Kundu, A.; Yadav, S.; Adhikari, D. Borrowing Hydrogen-Mediated N-Alkylation Reactions by a Well-Defined Homogeneous Nickel Catalyst. ACS Catal. 2019, 9, 9051–9059. [Google Scholar] [CrossRef]
- Irrgang, T.; Kempe, R. 3d-Metal Catalyzed N- and C-Alkylation Reactions via Borrowing Hydrogen or Hydrogen Autotransfer. Chem. Rev. 2019, 119, 2524–2549. [Google Scholar] [CrossRef] [PubMed]
- Dang, T.T.; Shan, S.P.; Ramalingam, B.; Seayad, A.M. An Efficient Heterogenized Palladium Catalyst for N-Alkylation of Amines and α-Alkylation of Ketones Using Alcohols. RSC Adv. 2015, 5, 42399–42406. [Google Scholar] [CrossRef]
- Prades, A.; Corberán, R.; Poyatos, M.; Peris, E. [IrCl2Cp*(NHC)] Complexes as Highly Versatile Efficient Catalysts for the Cross-Coupling of Alcohols and Amines. Chem. A Eur. J. 2008, 14, 11474–11479. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Lenormand, M.; Martín-Matute, B. Selective Alkylation of (Hetero)Aromatic Amines with Alcohols Catalyzed by a Ruthenium Pincer Complex. Org. Lett. 2012, 14, 1456–1459. [Google Scholar] [CrossRef] [PubMed]
- Putro, W.S.; Hara, T.; Ichikuni, N.; Shimazu, S. One-Pot Synthesis of Aniline N-Alkylation from Benzyl Alcohol over Cu-Fe Catalyst. Appl. Catal. A Gen. 2020, 602, 117519. [Google Scholar] [CrossRef]
- Lu, G.P.; Shan, H.; Lin, Y.; Zhang, K.; Zhou, B.; Zhong, Q.; Wang, P. A Fe Single Atom on N,S-Doped Carbon Catalyst for Performing N-Alkylation of Aromatic Amines under Solvent-Free Conditions. J. Mater. Chem. A Mater. 2021, 9, 25128–25135. [Google Scholar] [CrossRef]
- Li, Q.Q.; Xiao, Z.F.; Yao, C.Z.; Zheng, H.X.; Kang, Y.B. Direct Alkylation of Amines with Alcohols Catalyzed by Base. Org. Lett. 2015, 17, 5328–5331. [Google Scholar] [CrossRef]
- Allen, S.E.; Walvoord, R.R.; Padilla-Salinas, R.; Kozlowski, M.C. Aerobic Copper-Catalyzed Organic Reactions. Chem. Rev. 2013, 113, 6234–6458. [Google Scholar] [CrossRef]
- Pawar, R.C.; Choi, D.H.; Lee, J.S.; Lee, C.S. Formation of Polar Surfaces in Microstructured ZnO by Doping with Cu and Applications in Photocatalysis Using Visible Light. Mater. Chem. Phys. 2015, 151, 167–180. [Google Scholar] [CrossRef]
- Shih, Z.Y.; Periasamy, A.P.; Hsu, P.C.; Chang, H.T. Synthesis and Catalysis of Copper Sulfide/Carbon Nanodots for Oxygen Reduction in Direct Methanol Fuel Cells. Appl. Catal. B 2013, 132, 363–369. [Google Scholar] [CrossRef]
- Aneeja, T.; Neetha, M.; Afsina, C.M.A.; Anilkumar, G. Progress and Prospects in Copper-Catalyzed C–H Functionalization. RSC Adv. 2020, 10, 34429–34458. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Wu, X.; Sun, L. Copper-Based Homogeneous and Heterogeneous Catalysts for Electrochemical Water Oxidation. Nanoscale 2020, 12, 4187–4218. [Google Scholar] [CrossRef]
- Koy, M.; Bellotti, P.; Das, M.; Glorius, F. N-Heterocyclic Carbenes as Tunable Ligands for Catalytic Metal Surfaces. Nat. Catal. 2021, 4, 352–363. [Google Scholar] [CrossRef]
- Ranganath, K.V.S.; Onitsuka, S.; Kumar, A.K.; Inanaga, J. Recent Progress of N-Heterocyclic Carbenes in Heterogeneous Catalysis. Catal. Sci. Technol. 2013, 3, 2161–2181. [Google Scholar] [CrossRef]
- Jalal, M.; Hammouti, B.; Touzani, R.; Aouniti, A.; Ozdemir, I. Metal-NHC Heterocycle Complexes in Catalysis and Biological Applications: Systematic Review. Mater. Today Proc. 2020, 31, S122–S129. [Google Scholar] [CrossRef]
- Lazreg, F.; Nahra, F.; Cazin, C.S.J. Copper–NHC Complexes in Catalysis. Coord. Chem. Rev. 2015, 293–294, 48–79. [Google Scholar] [CrossRef]
- Herrmann, W.A.; Köcher, C. N-Heterocyclic Carbenes. Angew. Chem. Int. Ed. Engl. 1997, 36, 2162–2187. [Google Scholar] [CrossRef]
- Peris, E.; Crabtree, R.H. Recent Homogeneous Catalytic Applications of Chelate and Pincer N-Heterocyclic Carbenes. Coord. Chem. Rev. 2004, 248, 2239–2246. [Google Scholar] [CrossRef]
- Lee, Y.; Hoveyda, A.H. Efficient Boron-Copper Additions to Aryl-Substituted Alkenes Promoted by NHC-Based Catalysts. Enantioselective Cu-Catalyzed Hydroboration Reactions. J. Am. Chem. Soc. 2009, 131, 3160–3161. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Yang, J.; Li, P.; Wang, L. An Efficient and Recyclable Silica-Supported Carbene–Cu(II) Catalyst for the Oxidative Coupling Reaction of Terminal Alkynes with H-Phosphonates under Base-Free Reaction Conditions. Appl. Organomet. Chem. 2011, 25, 830–835. [Google Scholar] [CrossRef]
- Kaur, H.; Zinn, F.K.; Stevens, E.D.; Nolan, S.P. (NHC) CuI (NHC = N-Heterocyclic Carbene) Complexes as Efficient Catalysts for the Reduction of Carbonyl Compounds. Organometallics 2004, 23, 1157–1160. [Google Scholar] [CrossRef]
- Ohishi, T.; Zhang, L.; Nishiura, M.; Hou, Z. Carboxylation of Alkylboranes by N-Heterocyclic Carbene Copper Catalysts: Synthesis of Carboxylic Acids from Terminal Alkenes and Carbon Dioxide. Angew. Chem. Int. Ed. 2011, 50, 8114–8117. [Google Scholar] [CrossRef] [PubMed]
- Neshat, A.; Mastrorilli, P.; Mobarakeh, A.M. Recent Advances in Catalysis Involving Bidentate N-Heterocyclic Carbene Ligands. Molecules 2021, 27, 95. [Google Scholar] [CrossRef]
- Fadhel, A.Z.; Pollet, P.; Liotta, C.L.; Eckert, C.A. Combining the Benefits of Homogeneous and Heterogeneous Catalysis with Tunable Solvents and Nearcritical Water. Molecules 2010, 15, 8400–8424. [Google Scholar] [CrossRef]
- Brisebois, P.P.; Siaj, M. Harvesting Graphene Oxide—Years 1859 to 2019: A Review of Its Structure, Synthesis, Properties and Exfoliation. J. Mater. Chem. C 2020, 8, 1517–1547. [Google Scholar] [CrossRef]
- Cheng, Z.; Liao, J.; He, B.; Zhang, F.; Zhang, F.; Huang, X.; Zhou, L. One-Step Fabrication of Graphene Oxide Enhanced Magnetic Composite Gel for Highly Efficient Dye Adsorption and Catalysis. ACS Sustain. Chem. Eng. 2015, 3, 1677–1685. [Google Scholar] [CrossRef]
- Shangguan, Q.; Chen, Z.; Yang, H.; Cheng, S.; Yang, W.; Yi, Z.; Wu, X.; Wang, S.; Yi, Y.; Wu, P. Design of Ultra-Narrow Band Graphene Refractive Index Sensor. Sensors 2022, 22, 6483. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Z.; Yang, H.; Wen, L.; Yi, Z.; Zhou, Z.; Dai, B.; Zhang, J.; Wu, X.; Wu, P. Multi-Mode Surface Plasmon Resonance Absorber Based on Dart-Type Single-Layer Graphene. RSC Adv. 2022, 12, 7821–7829. [Google Scholar] [CrossRef]
- Zhang, Z.; Cai, R.; Long, F.; Wang, J. Development and Application of Tetrabromobisphenol A Imprinted Electrochemical Sensor Based on Graphene/Carbon Nanotubes Three-Dimensional Nanocomposites Modified Carbon Electrode. Talanta 2015, 134, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Banerjee, A. Tailored Synthesis of Various Nanomaterials by Using a Graphene-Oxide-Based Gel as a Nanoreactor and Nanohybrid-Catalyzed C-C Bond Formation. Chem. Asian J. 2014, 9, 3451–3456. [Google Scholar] [CrossRef] [PubMed]
- Navalón, S.; Herance, J.R.; Álvaro, M.; García, H. Covalently Modified Graphenes in Catalysis, Electrocatalysis and Photoresponsive Materials. Chem. A Eur. J. 2017, 23, 15244–15275. [Google Scholar] [CrossRef] [PubMed]
- Kandathil, V.; Siddiqa, A.; Patra, A.; Kulkarni, B.; Kempasiddaiah, M.; Sasidhar, B.S.; Patil, S.A.; Rout, C.S.; Patil, S.A. NHC-Pd Complex Heterogenized on Graphene Oxide for Cross-Coupling Reactions and Supercapacitor Applications. Appl. Organomet. Chem. 2020, 34, e5924. [Google Scholar] [CrossRef]
- Park, J.H.; Raza, F.; Jeon, S.J.; Kim, H.I.; Kang, T.W.; Yim, D.; Kim, J.H. Recyclable N-Heterocyclic Carbene/Palladium Catalyst on Graphene Oxide for the Aqueous-Phase Suzuki Reaction. Tetrahedron Lett. 2014, 55, 3426–3430. [Google Scholar] [CrossRef]
- Qian, Y.; So, J.; Jung, S.Y.; Hwang, S.; Jin, M.J.; Shim, S.E. A Graphene Oxide Nanosheet Supported NHC–Palladium Complex as a Highly Efficient and Recyclable Suzuki Coupling Catalyst. Synthesis 2019, 51, 2287–2292. [Google Scholar] [CrossRef]
- Coman, S.M.; Podolean, I.; Tudorache, M.; Cojocaru, B.; Parvulescu, V.I.; Puche, M.; Garcia, H. Graphene Oxide as a Catalyst for the Diastereoselective Transfer Hydrogenation in the Synthesis of Prostaglandin Derivatives. Chem. Commun. 2017, 53, 10271–10274. [Google Scholar] [CrossRef]
- Ding, J.; Tang, S.; Chen, X.; Ding, M.; Kang, J.; Wu, R.; Fu, Z.; Jin, Y.; Li, L.; Feng, X.; et al. Introduction of Benzotriazole into Graphene Oxide for Highly Selective Coadsorption of An and Ln: Facile Synthesis and Theoretical Study. Chem. Eng. J. 2018, 344, 594–603. [Google Scholar] [CrossRef]
- Pawar, A.; Gajare, S.; Jagdale, A.; Patil, S.; Chandane, W.; Rashinkar, G.; Patil, S. Supported NHC-Benzimi@Cu Complex as a Magnetically Separable and Reusable Catalyst for the Multicomponent and Click Synthesis of 1,4-Disubstituted 1,2,3-Triazoles via Huisgen 1,3-Dipolar Cycloaddition. Catal. Lett. 2022, 152, 1854–1868. [Google Scholar] [CrossRef]
- Vinoth, R.; Babu, S.G.; Bharti, V.; Gupta, V.; Navaneethan, M.; Bhat, S.V.; Muthamizhchelvan, C.; Ramamurthy, P.C.; Sharma, C.; Aswal, D.K.; et al. Ruthenium Based Metallopolymer Grafted Reduced Graphene Oxide as a New Hybrid Solar Light Harvester in Polymer Solar Cells. Sci. Rep. 2017, 7, 43133. [Google Scholar] [CrossRef]
- Gou, F.; Liu, J.; Ye, N.; Jiang, X.; Qi, C. Cobalt-Porphyrin Modified Graphene Oxide as a Heterogeneous Catalyst for Solvent-Free CO2 Fixation to Cyclic Carbonates. J. CO2 Util. 2021, 48, 101534. [Google Scholar] [CrossRef]
- Zhao, Q.; Bai, C.; Zhang, W.; Li, Y.; Zhang, G.; Zhang, F.; Fan, X. Catalytic Epoxidation of Olefins with Graphene Oxide Supported Copper (Salen) Complex. Ind. Eng. Chem. Res. 2014, 53, 4232–4238. [Google Scholar] [CrossRef]
- Sharma, R.K.; Sharma, A.; Sharma, S.; Dutta, S.; Yadav, S.; Arora, B. Design and Exploration of Catalytic Activity of Two-Dimensional Surface-Engineered Graphene Oxide Nanosheets in the Transannulation of N-Heterocyclic Aldehydes or Ketones with Alkylamines. ACS Omega 2019, 4, 3146–3158. [Google Scholar] [CrossRef]
- Prabha, D.; Pachisia, S.; Gupta, R. Cobalt Mediated N-Alkylation of Amines by Alcohols: Role of Hydrogen Bonding Pocket. Inorg. Chem. Front. 2021, 8, 1599–1609. [Google Scholar] [CrossRef]
- Wang, L.; Jv, X.; Wang, R.; Ma, L.; Liu, J.; Sun, J.; Shi, T.; Zhao, L.; Zhang, X.; Wang, B. Highly Selective Synergistic N-Alkylation of Amines with ROH Catalyzed by Nickel–Ruthenium. ACS Sustain. Chem. Eng. 2022, 10, 8342–8349. [Google Scholar] [CrossRef]
- Verma, S.; Kujur, S.; Sharma, R.; Pathak, D.D. Cucurbit[6]Uril-Supported Fe3O4 Magnetic Nanoparticles Catalyzed Green and Sustainable Synthesis of 2-Substituted Benzimidazoles via Acceptorless Dehydrogenative Coupling. ACS Omega 2022, 7, 9754–9764. [Google Scholar] [CrossRef]
- Nallagangula, M.; Sujatha, C.; Bhat, V.T.; Namitharan, K. A Nanoscale Iron Catalyst for Heterogeneous Direct N- and C-Alkylations of Anilines and Ketones Using Alcohols under Hydrogen Autotransfer Conditions. Chem. Commun. 2019, 55, 8490–8493. [Google Scholar] [CrossRef]
- Goyal, V.; Sarki, N.; Poddar, M.K.; Narani, A.; Tripathi, D.; Ray, A.; Natte, K. Biorenewable Carbon-Supported Ru Catalyst for N -Alkylation of Amines with Alcohols and Selective Hydrogenation of Nitroarenes. New J. Chem. 2021, 45, 14687–14694. [Google Scholar] [CrossRef]
- Takahashi, Y.; Kondo, R.; Utsunomiya, M.; Suzuki, T.; Takeshita, H.T.; Obora, Y. Ti−Pd Alloys as Heterogeneous Catalysts for the Hydrogen Autotransfer Reaction and Catalytic Improvement by Hydrogenation Effects. ChemCatChem 2019, 11, 2432–2437. [Google Scholar] [CrossRef]
- Rasero-Almansa, A.M.; Corma, A.; Iglesias, M.; Sánchez, F. Design of a Bifunctional Ir–Zr Based Metal–Organic Framework Heterogeneous Catalyst for the N-Alkylation of Amines with Alcohols. ChemCatChem 2014, 6, 1794–1800. [Google Scholar] [CrossRef]
- Kumar, G.; Mogha, N.K.; Kumar, M.; Masram, D.T. NiO Nanocomposites/RGO as a Heterogeneous Catalyst for Imidazole Scaffolds with Applications in Inhibiting the DNA Binding Activity. Dalton Trans. 2020, 49, 1963–1974. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.-J. The Chemical and Structural Analysis of Graphene Oxide with Different Degrees of Oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Lin, Y.R.; Chiu, C.C.; Chiu, H.T.; Lee, D.S.; Lu, T.J. Bis-Benzimidazolium-Palladium System Catalyzed Suzuki-Miyaura Coupling Reaction of Aryl Bromides under Mild Conditions. Appl. Organomet. Chem. 2018, 32, e3896. [Google Scholar] [CrossRef]
- Li, Q.; Fan, S.J.; Sun, Q.; Tian, H.W.; Yu, X.C.; Xu, Q. Copper-Catalyzed N-alkylation of Amides and Amines with Alcohols Employing the Aerobic Relay Race Methodology. Org. Biomol. Chem. 2012, 10, 2966–2972. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Jiang, L.; Li, Q.; Xie, Y.; Xu, Q. Palladium-Catalyzed N-Alkylation of Amides and Amines with Alcohols Employing the Aerobic Relay Race Methodology. Chin. J. Chem. 2012, 30, 2322–2332. [Google Scholar] [CrossRef]
- Liu, C.; Liao, S.; Li, Q.; Feng, S.; Sun, Q.; Yu, X.; Xu, Q. Discovery and Mechanistic Studies of a General Air-Promoted Metal-Catalyzed Aerobic N-Alkylation Reaction of Amides and Amines with Alcohols. J. Org. Chem 2011, 76, 5759–5773. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.B.; Ye, Z.; Yang, C.; Li, W.; Liu, J.; Huang, M.; Liu, Y.; Ke, Z. Tungsten-Catalyzed Direct N-Alkylation of Anilines with Alcohols. ChemSusChem 2021, 14, 860–865. [Google Scholar] [CrossRef]
- Langde, V.G.; Mondal, A.; Kumar, V.; Nandakumar, A.; Balaram, E. Manganese Catalyzed N-Alkylation of Anilines with Alcohols: Ligand Enabled Selectivity. Org. Biomol. Chem. 2018, 16, 8175–8180. [Google Scholar] [CrossRef]
- Ramachandran, R.; Prakash, G.; Selvamurugan, S.; Viswanathamurthi, P.; Malecki, J.G.; Ramkumar, V. Efficient and Versatile Catalysis of N-Alkylation of Heterocyclic Amines with Alcohols and One-Pot Synthesis of 2-Aryl Substituted Benzazoles with Newly Designed Ruthenium (II) Complexes of PNS Thiosemicarbazones. Dalton Trans. 2014, 43, 7889–7902. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).