Biodesulfurization of Dibenzothiophene by Decorating Rhodococcus erythropolis IGTS8 Using Montmorillonite/Graphitic Carbon Nitride
Abstract
1. Introduction
2. Results and Discussion
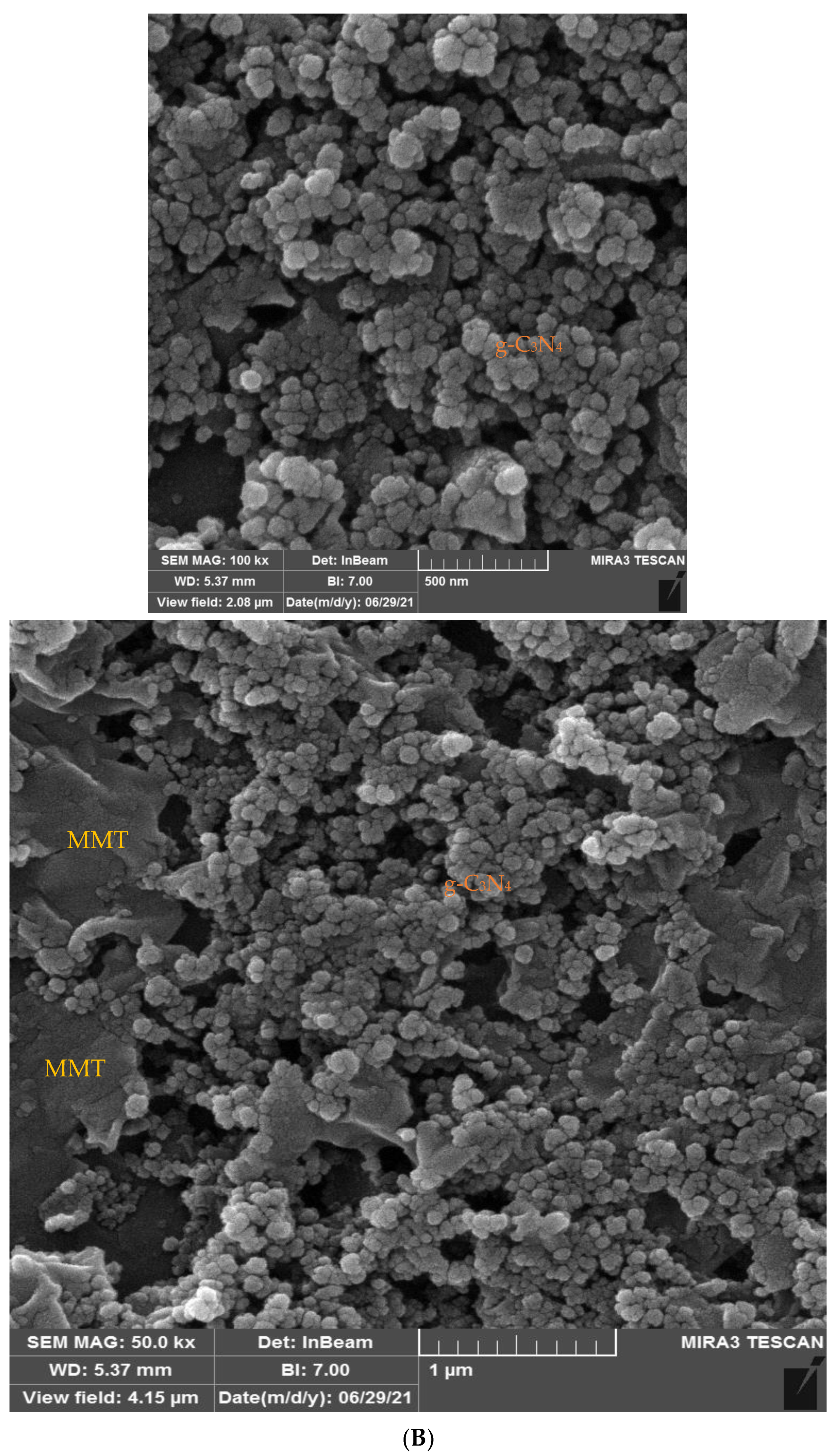
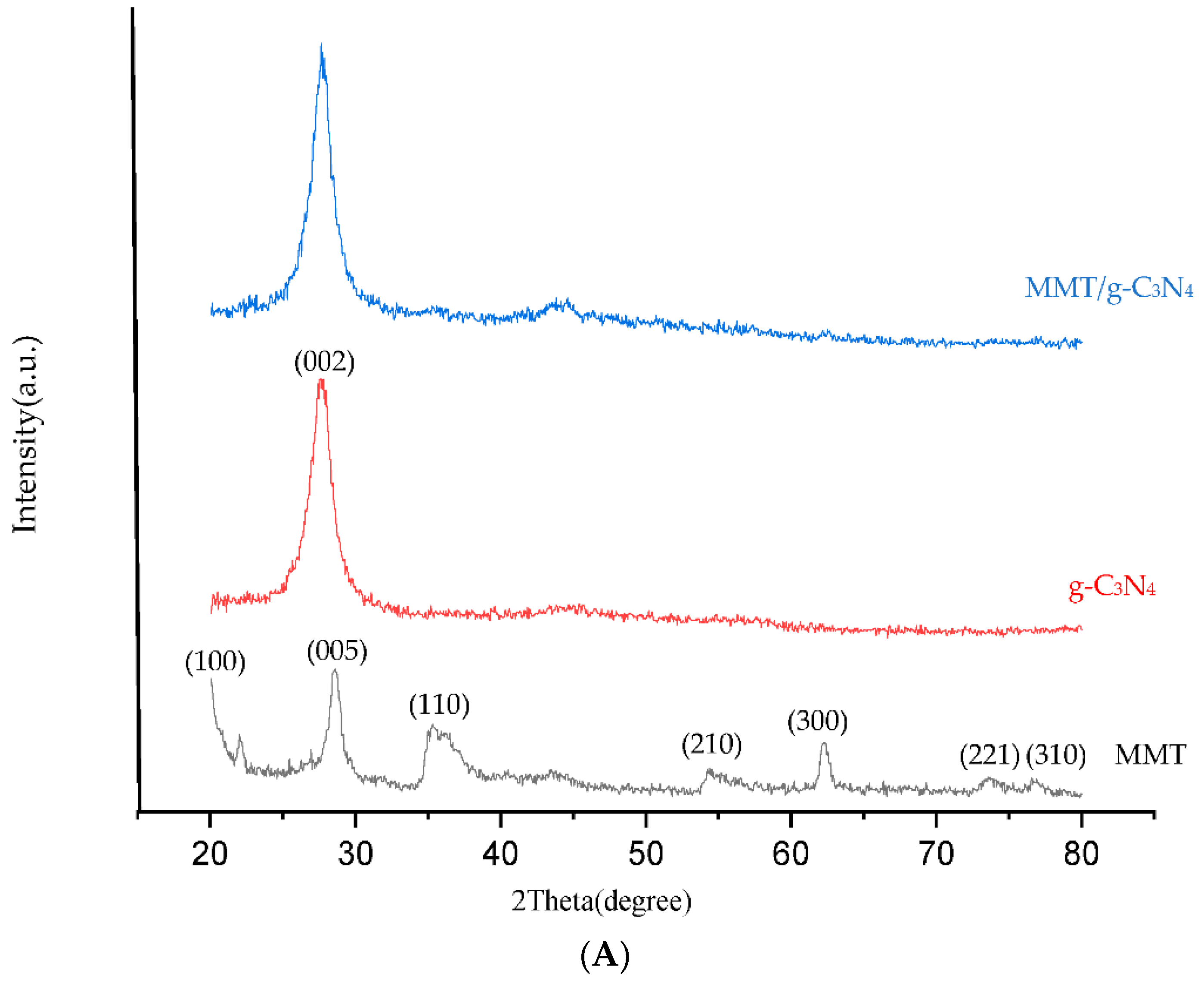
2.1. TEM and FESEM Analysis
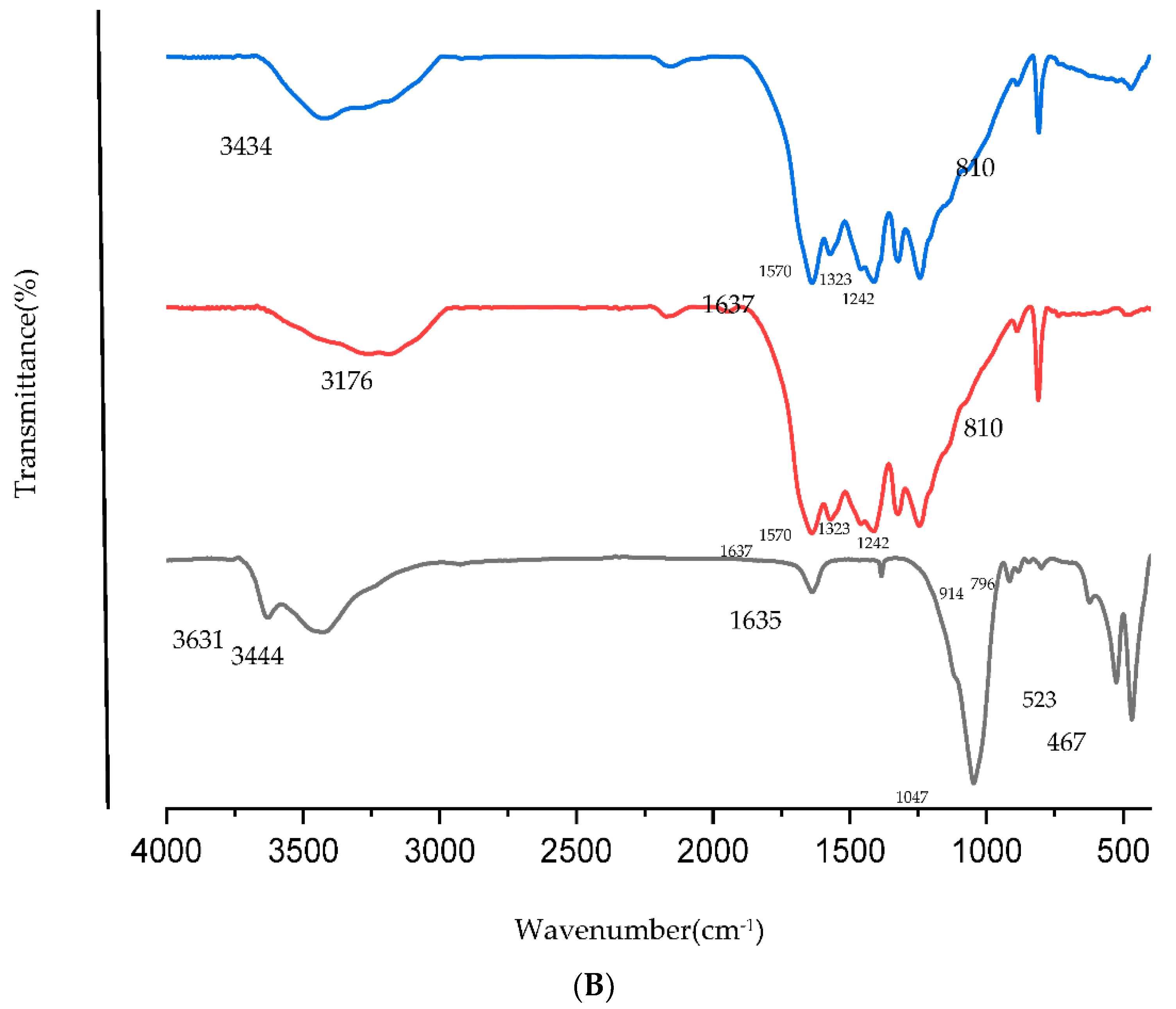
2.2. XRD and FTIR Analysis
2.3. Optimization of Effective Operating Factors
2.3.1. In the Absence of the Nanostructure
2.3.2. In the Presence of the Nanostructure
2.4. Statistical Methods
2.4.1. Statistical Methods in the Absence of the Nanostructure
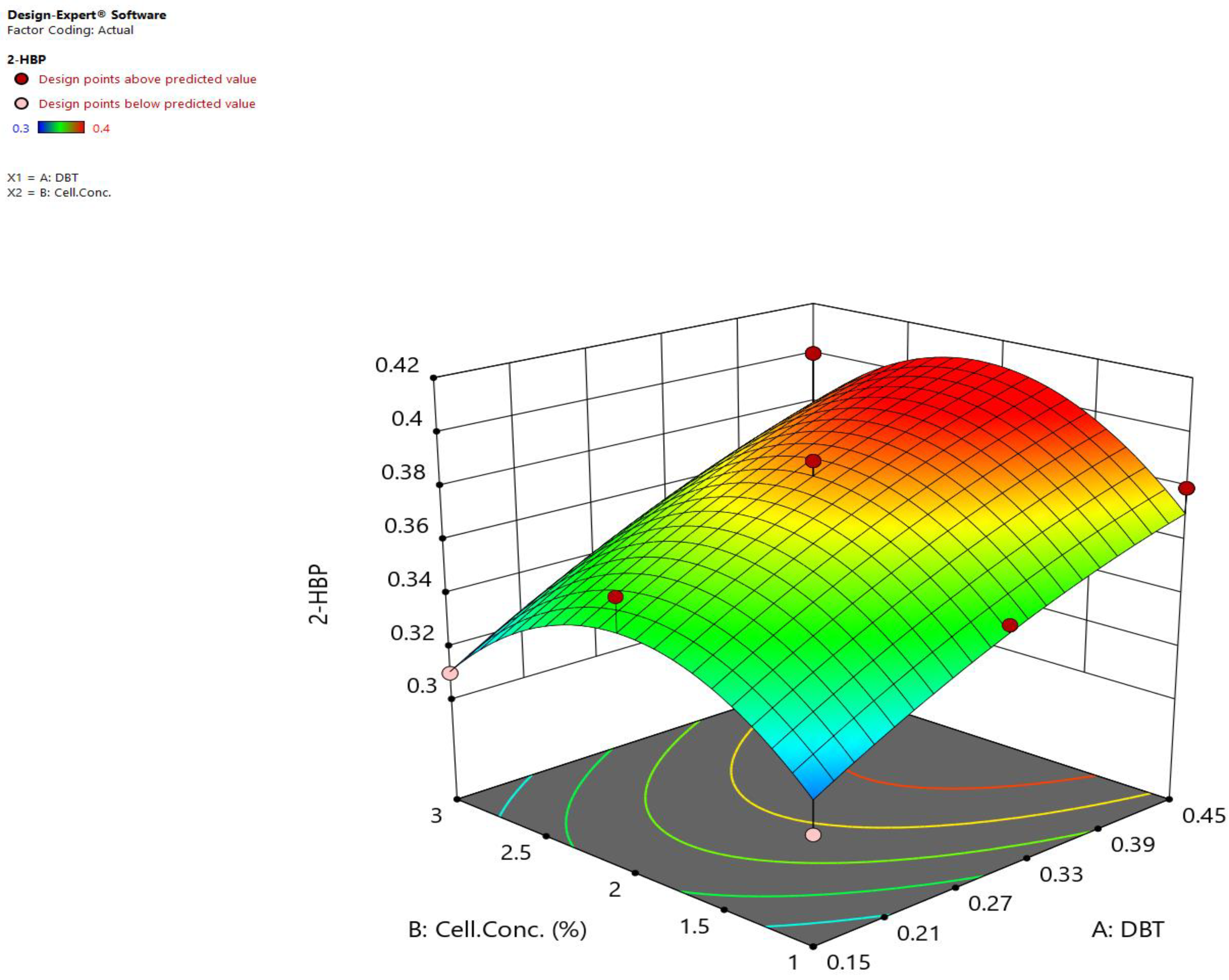
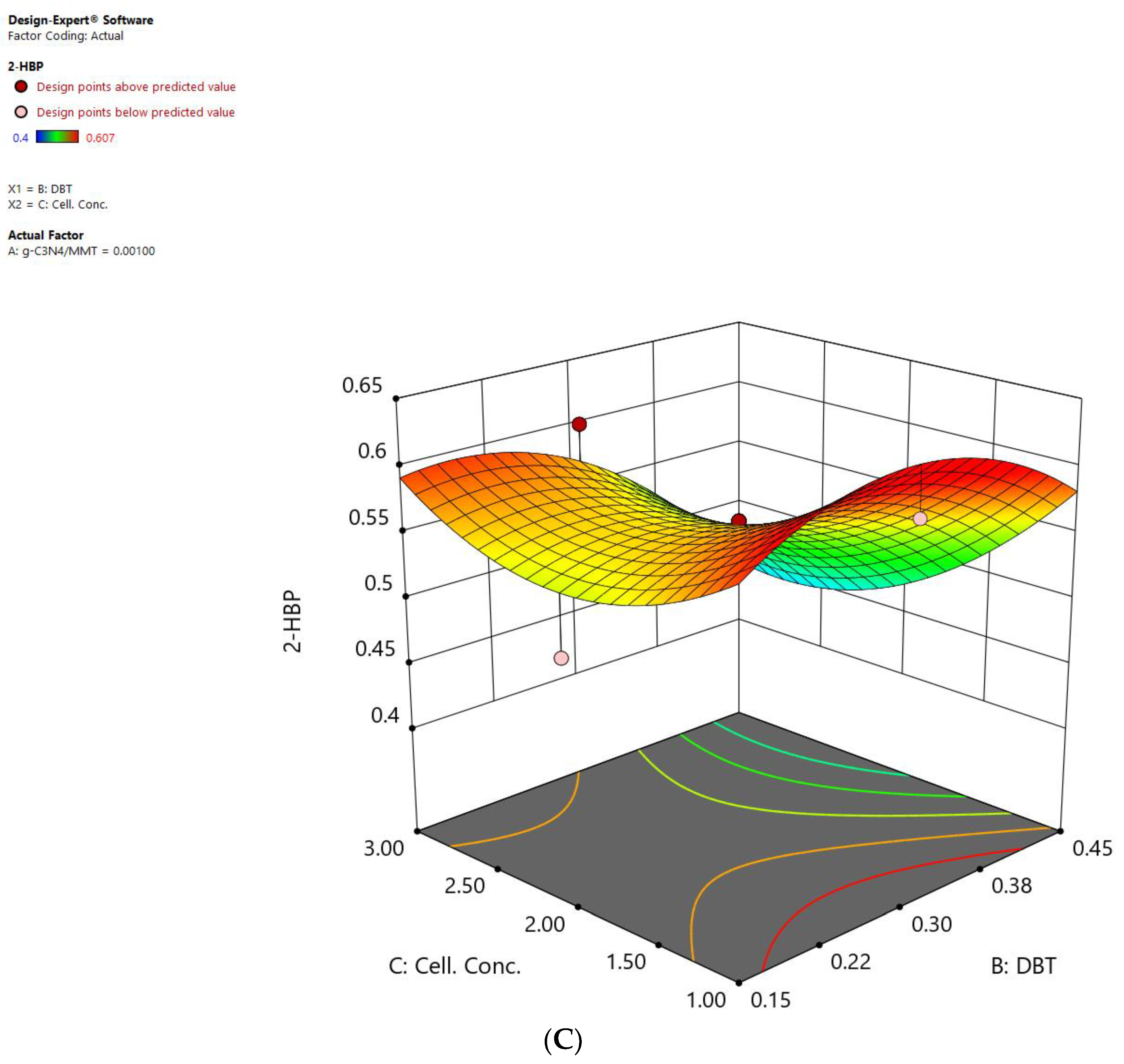
2.4.2. Statistical Methods in the Presence of the Nanostructure
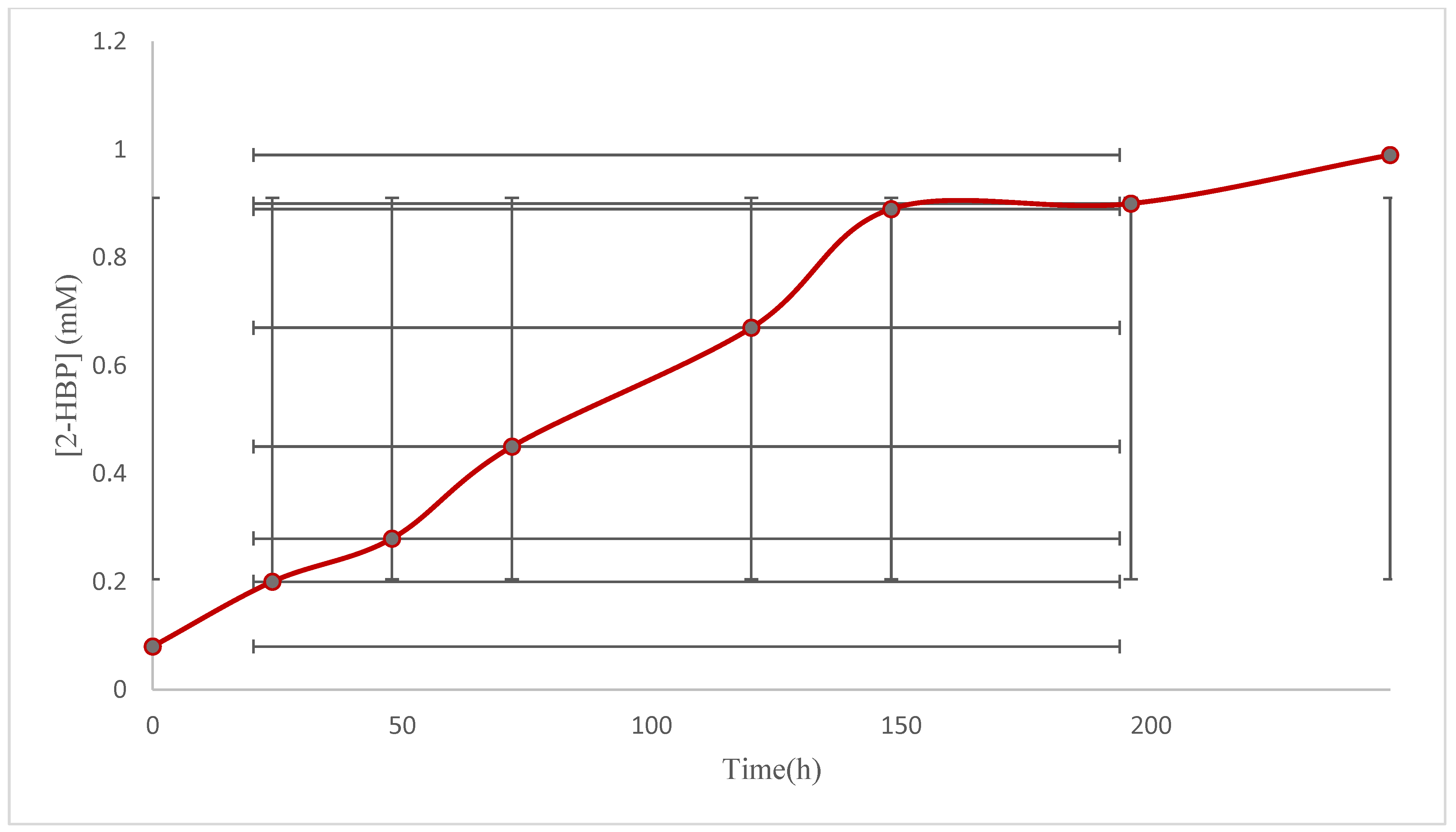
2.5. Biodesulfurization Activity
3. Materials and Methods
3.1. Materials
3.2. The Microorganism and Its Medium
3.3. Preparation of the Nanoparticles
3.3.1. Synthesis of Bulk Graphitic Carbon Nitride
3.3.2. Synthesis of Graphitic Carbon Nitride Nanosheets
3.3.3. Synthesis of Montmorillonite/Graphitic Carbon Nitride
3.4. Experimental Design
3.5. Analytical Methods
3.5.1. Determination of Biodesulfurization Activity of Microorganisms (Gibbs Assay)
3.5.2. Characterization of Nanostructures
3.6. Biodesulfurization Capacity of Decorated Microbial Cells in a Bioreactor
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gupta, N.; Roychoudhury, P.K.; Deb, J.K. Biotechnology of desulfurization of diesel: Prospects and challenges. Appl. Microbiol. Biotechnol. 2005, 66, 356–366. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, M.; Sadeghizadeh, M.; Khajeh, K.; Mohebali, G.; Zomorodipour, A. Genomic structure and promoter analysis of the dsz operon for dibenzothiophene biodesulfurization from Gordonia alkanivorans RIPI90A. Appl. Microbiol. Biotechnol. 2010, 87, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, M.; Kamyab, H.; Janalizadeh, N.; Huyop, F.Z. Bacterial Desulfurization of Organic Sulfur Compounds Exist in Fossil Fuels. J. Pure Appl. Microbiol. 2012, 6, 717–729. [Google Scholar]
- Soleimani, M.; Bassi, A.; Margaritis, A. Biodesulfurization of refractory organic sulfur compounds in fossil fuels. Biotechnol. Adv. 2007, 25, 570–596. [Google Scholar] [CrossRef]
- Sousa, J.P.M.; Neves, R.; Sousa, S.F.; Ramos, M.J.; Fernandes, P. Reaction Mechanism and Determinants for Efficient Catalysis by DszB, a Key Enzyme for Crude Oil Bio-desulfurization. ACS Catal. 2020, 10, 9545–9554. [Google Scholar] [CrossRef]
- Mohebali, G.; Ball, A.S. Biodesulfurization of diesel fuels—Past, present and future perspectives. Int. Biodeterior. Biodegrad. 2016, 110, 163–180. [Google Scholar] [CrossRef]
- Sadare, O.O.; Obazu, F.; Daramola, M.O. Biodesulfurization of Petroleum Distillates—Current Status, Opportunities and Future Challenges. Environments 2017, 4, 85. [Google Scholar] [CrossRef]
- Alves, L.; Marques, S.; Matos, J.; Tenreiro, R.; Gírio, F.M. Dibenzothiophene desulfurization by Gordonia alkanivorans strain 1B using recycled paper sludge hydrolyzate. Chemosphere 2008, 70, 967–973. [Google Scholar] [CrossRef]
- Mohebali, G.; Ball, A.S. Biocatalytic desulfurization (BDS) of petro diesel Fuels. Microbiology 2008, 154, 2169–2183. [Google Scholar] [CrossRef]
- Mohebali, G.; Ball, A.S.; Rasekh, B.; Kaytash, A. Biodesulfurization potential of a newly isolated bacterium Gordonia alkanivorans RIPI90A. Enzym. Microb. Technol. 2007, 40, 578–584. [Google Scholar] [CrossRef]
- Rahpeyma, S.S.; Dilmaghani, A.; Raheb, J. Evaluation of desulfurization activity of SPION nanoparticle-coated bacteria in the presence of magnetic field. Appl. Nanosci. 2018, 8, 1951–1972. [Google Scholar] [CrossRef]
- Karimi, E.; Jeffryes, C.; Yazdian, F.; Akhavan Sepahi, A. DBT desulfurization by decorating Rhodococcus erythropolis IGTS8 using magnetic Fe3O4 nanoparticles in a bioreactor. Eng. Life Sci. 2016, 17, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Bardania, H.; Raheb, J.; Mohammad-Beigi, H.; Rasekh, B.; Arpanaei, A. Desulfurization activity and reusability of magnetite nanoparticle–coated Rhodococcus erythropolis FMF and R. erythropolis IGTS8 bacterial cells. Biotechnol. Appl. Biochem. 2013, 60, 323–329. [Google Scholar] [CrossRef]
- Bell, K.S.; Philp, J.C.; Aw, D.W.J.; Christofi, N. The genus Rhodococcus. J. Appl. Microbiol. 1998, 85, 195–210. [Google Scholar] [CrossRef]
- Karimi, E.; Yazdian, F.; Rasekh, B.; Akhavan Sepahy, A. Biodesulfurization of Dibenzothiophene by Rhodococcus erythropolis IGTS8 in the Presence of Magnetic Nanoparticles and Carbon Nanotubes Surface modified Polyethylene Glycol. Modares J. Biotechnol. 2018, 9, 301–308. [Google Scholar]
- Prasoulas, G.; Dimos, K.; Glekas, P.; Kalantzi, S.; Sarris, S.; Templis, C.; Vavitsas, K.; Hatzinikolaou, D.G.; Papayannakos, N.; Kekos, D.; et al. Biodesulfurization of Dibenzothiophene and Its Alkylated Derivatives in a Two-Phase Bubble Column Bioreactor by Resting Cells of Rhodococcus erythropolis IGTS8. Processes 2021, 9, 2064. [Google Scholar] [CrossRef]
- Etemadi, N.; Akhavan Sepahi, A.; Mohebali, G.; Yazdian, F. The Isolation Desulfurizing Native Thermophilic Bacteria Bacillus thermoamylovorance and Optimization Culture Medium. J. Pet. Res. 2019, 29, 119–131. [Google Scholar]
- Monticello, D.; Finnerty, W. Microbial desulfurization of fossil fuels. Annu. Rev. Microbiol. 1985, 39, 371–389. [Google Scholar] [CrossRef]
- Wang, W.; Ma, T.; Lian, K.; Zhang, Y.; Tian, H.; Ji, K.; Li, G. Genetic Analysis of Benzothiophene Biodesulfurization Pathway of Gordonia terrae Strain C-6. PLoS ONE 2013, 8, e84386. [Google Scholar] [CrossRef]
- Shan, G.; Xing, J.; Zhang, H.; Liu, H. Biodesulfurization of dibenzothiophene by microbial cells coated with magnetite nanoparticles. Appl. Environ. Microbiol. 2005, 71, 4497–4502. [Google Scholar] [CrossRef]
- Ansari, F.; Grigoriev, P.; Libor, S.; Tothill, I.E.; Ramsden, J.J. DBT degradation enhancement by decorating Rhodococcus erythropolis IGST8 with magnetic Fe3O4 nanoparticles. Biotechnol. Bioeng. 2009, 102, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Li, W.-L.; Chen, X.-X.; Tang, H.; Li, Q.; Xing, J.-M.; Liu, H.-Z. Enhanced biodesulfurization by magnetic immobilized Rhodococcus erythropolis LSSE8-1-vgb assembled with nano-γ-Al2O3. World J. Microbiol. Biotechnol. 2011, 27, 299–305. [Google Scholar] [CrossRef]
- Etemadi, N.; Sepahy, A.A.; Mohebali, G.; Yazdian, F.; Omidi, M. Enhancement of bio-desulfurization capability of a newly isolated thermophilic bacterium using starch/iron nanoparticles in a controlled system. Int. J. Biol. Macromol. 2018, 120, 1801–1809. [Google Scholar] [CrossRef] [PubMed]
- Rahpeyma, S.S.; Mohammadi, M.; Raheb, J. Biodesulfurization of dibenzothiophene by two bacterial strains in cooperation with Fe3O4, ZnO and CuO nanoparticles. J. Microb. Biochem. Technol. 2017, 9, 587–591. [Google Scholar]
- Canales, C.; Eyzaguirre, J.; Baeza, P.; Aballay, P.; Ojeda, J. Kinetic Analysis for Biodesulfurization of Dibenzothiophene Using R. rhodochrous Adsorbed on Silica. Ecol. Chem. Eng. S 2018, 25, 549–556. [Google Scholar] [CrossRef]
- Nassar, H.N.; Abu Amr, S.S.; El-Gendy, N.S. Biodesulfurization of refractory sulfur compounds in petrodiesel by a novel hydrocarbon tolerable strain Paenibacillus glucanolyticus HN4. Environ. Sci. Pollut. Res. 2021, 28, 8102–8116. [Google Scholar] [CrossRef]
- Ahmad, W.; Ahmad, I.; Ishaq, M.; Ihsan, K. Adsorptive desulfurization of kerosene and diesel oil by Zn impregnated montmorollonite clay. Arab. J. Chem. 2017, 10, S3263–S3269. [Google Scholar] [CrossRef]
- Jia, C.; Zhang, M.; Lu, P. Preparation and characterization of polyurethane-/MMT nanocomposite-coated urea as controlled release fertilizers. Polym.-Plast. Technol. Mater. 2020, 59, 975–984. [Google Scholar] [CrossRef]
- Feng, R.; Zhang, Y.; Li, H.; Wu, D.; Xin, X.; Zhang, S.; Yu, H.; Wei, Q.; Du, B. Ultrasensitive electrochemical immunosensor for zeranol detection based on signal amplification strategy of nanoporous gold films and nano-montmorillonite as labels. Anal. Chim. Acta 2013, 758, 72–79. [Google Scholar] [CrossRef]
- Haseli, S.; Pourmadadi, M.; Samadi, A.; Yazdian, F.; Abdouss, M.; Rashedi, H.; Navaei-Nigjeh, M. A novel pH-responsive nanoniosomal emulsion for sustained release of curcumin from a chitosan-based nanocarrier: Emphasis on the concurrent improvement of loading, sustained release, and apoptosis induction. Biotechnol. Prog. 2022, 38, e3280. [Google Scholar] [CrossRef]
- Ahmadi, M.; Pourmadadi, M.; Ghorbanian, S.A.; Yazdian, F.; Rashedi, H. Ultra pH-sensitive nanocarrier based on Fe2O3/chitosan/montmorillonite for quercetin delivery. Int. J. Biol. Macromol. 2021, 191, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Nazir, M.S.; Kassim, M.H.M.; Mohapatra, L.; Gilani, M.A.; Raza, M.R.; Majeed, K. Characteristic Properties of Nanoclays and Characterization of Nanoparticulates and Nanocomposites. In Nanoclay Reinforced Polymer Composites; Springer: Singapore, 2016; pp. 35–55. [Google Scholar] [CrossRef]
- Takabatake, M.; Motokura, K. Montmorillonite-based heterogeneous catalysts for efficient organic reactions. Nano Express 2022, 3, 014004. [Google Scholar] [CrossRef]
- Pielichowski, K.; Majka, T.M. (Eds.) Polymer Composites with Functional Nanoparticles: Synthesis, Properties, and Applications; Woodhead Publishing: Thorston, UK, 2019. [Google Scholar]
- Zhang, Y.; Miao, B.; Chen, Q.; Bai, Z.; Cao, Y.; Davaa, B. Synthesis, Structure, and Photocatalytic Activity of TiO2-Montmorillonite Composites. Catalysts 2022, 12, 486. [Google Scholar] [CrossRef]
- Park, Y.; Ayoko, G.A.; Frost, R.L. Characterisation of organoclays and adsorption of p-nitrophenol: Environmental application. J. Colloid Interface Sci. 2011, 360, 440–456. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, X.; Yang, B.; Sun, R. Rapid modification of montmorillonite with novel cationic Gemini surfactants and its adsorption for methyl orange. Mater. Chem. Phys. 2011, 130, 1220–1226. [Google Scholar] [CrossRef]
- Zhou, Q.; Deng, S.; Yu, Q.; Zhang, Q.; Yu, G.; Huang, J.; He, H. Sorption of perfluorooctane sulfonate on organo-montmorillonites. Chemosphere 2010, 78, 688–694. [Google Scholar] [CrossRef]
- Tiwari, R.R.; Khilar, K.C.; Natarajan, U. Synthesis and characterization of novel organo-montmorillonites. Appl. Clay Sci. 2008, 38, 203–208. [Google Scholar] [CrossRef]
- Rezvani, M.A.; Khandan, S. Synthesis and characterization of a new nanocomposite (FeW11 V@CTAB-MMT) as an efficient heterogeneous catalyst for oxidative desulfurization of gasoline. Appl. Organomet. Chem. 2018, 32, e4524. [Google Scholar] [CrossRef]
- Wang, C.; Zhu, W.; Xu, Y.; Xu, H. Preparation of TiO2/g-C3N4 composites and their application in photocatalytic oxidative desulfurization. Ceram. Int. 2014, 40, 11627–11635. [Google Scholar] [CrossRef]
- Zhao, R.; Li, X.; Su, J.; Gao, X. Preparation of WO3/g-C3N4 composites and their application in oxidative desulfurization. Appl. Surf. Sci. 2017, 392, 810–816. [Google Scholar] [CrossRef]
- Alaghmandfard, A.; Ghandi, K. A Comprehensive Review of Graphitic Carbon Nitride (g-C3N4)–Metal Oxide-Based Nanocomposites: Potential for Photocatalysis and Sensing. Nanomaterials 2022, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Alnassar, M.A.; Alshehri, A.; Narasimharao, K. Visible Light Active Magnesium Silicate–Graphitic Carbon Nitride Nanocomposites for Methylene Blue Degradation and Pb2+ Adsorption. Catalysts 2022, 12, 1256. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Seza, A.; Soleimani, F.; Naseri, N.; Soltaninejad, M. Novel microwave-assisted synthesis of porous g-C3N4/SnO2 nanocomposite for solar water-splitting. Appl. Surf. Sci. 2018, 440, 153–161. [Google Scholar] [CrossRef]
- Lin, Q.; Li, H.; Wang, X.; Jian, L.; Ren, J.; Liu, C.; Sun, R. SO42−/Sn-MMT Solid Acid Catalyst for Xylose and Xylan Conversion into Furfural in the Biphasic System. Catalysts 2017, 7, 118. [Google Scholar] [CrossRef]
- Qamar, M.A.; Shahid, S.; Javed, M.; Iqbal, S. Highly efficient g-C3N4/Cr-ZnO nanocomposites with superior photo-catalytic and antibacterial activity. J. Photochem. Photobiol. A Chem. 2020, 401, 112776. [Google Scholar] [CrossRef]
- Shehap, A.; Nasr, R.; Mahfouz, M.A.; Ismail, A. Preparation and characterizations of high doping chitosan/MMT nanocomposites films for removing iron from ground water. J. Environ. Chem. Eng. 2021, 9, 104700. [Google Scholar] [CrossRef]
- Choudhary, N.; Yadav, V.K.; Yadav, K.K.; Almohana, A.I.; Almojil, S.F.; Gnanamoorthy, G.; Kim, D.-H.; Islam, S.; Kumar, P.; Jeon, B.-H. Application of Green Synthesized MMT/Ag Nanocomposite for Removal of Methylene Blue from Aqueous Solution. Water 2021, 13, 3206. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, Y.; Wang, M.; Shi, S. Biodesulfurization of dibenzothiophene and other organic sulfur compound by newly isolated Micobacterium strain ZD-M2. FEMS Microbiol. Lett. 2005, 247, 45–50. [Google Scholar]
- Karimi, E.; Yazdian, F.; Rasekh, B.; Jeffryes, C.; Rashedi, H.; Sepahi, A.A.; Shahmoradi, S.; Omidi, M.; Azizi, M.; Bidhendi, M.E.; et al. DBT desulfurization by decorating bacteria using modified carbon nanotube. Fuel 2018, 216, 787–795. [Google Scholar] [CrossRef]
- Hokmabadi, M.; Khosravinia, S.; Mahdavi, M.A.; Gheshlaghi, R. Enhancing the biodesulphurization capacity of Rhodococcus sp. FUM94 in a biphasic system through optimization of operational factors. J. Appl. Microbiol. 2022, 132, 3461–3475. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, N.N.; Hassani, K.H.; Kazemzad, M.; Ghavi, P.F.; Davoodi, D.F. Improvement of Desulfurization Performance of Rhodococcus erythropolis IGTS8 by Assembling Spherical Mesoporous Silica Nanosorbents on the Surface of the Bacterial Cells. J. Appl. Chem. Res. 2015, 9, 81–91. [Google Scholar]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A review of the current status of graphitic carbon nitride. Crit. Rev. Solid State Mater. Sci. 2021, 46, 189–217. [Google Scholar] [CrossRef]
- Zhang, M.; Lai, C.; Li, B.; Huang, D. Rational Design 2D/2D Biobr/Cds/g-C3N4 Z-Scheme Heterojunction Photocatalyst with Carbon Dots as Solid-State Electron Mediators for Enhanced Visible and Nir Photocatalytic Activity: Kinetics, Inter-mediates, and Mechanism Insight. J. Catal. 2019, 369, 469–481. [Google Scholar] [CrossRef]
- Talapaneni, S.N.; Lee, J.H.; Je, S.H.; Buyukcakir, O.; Kwon, T.-W.; Polychronopoulou, K.; Choi, J.W.; Coskun, A. Chemical Blowing Approach for Ultramicroporous Carbon Nitride Frameworks and Their Applications in Gas and Energy Storage. Adv. Funct. Mater. 2017, 27, 1604658. [Google Scholar] [CrossRef]
- Sun, S.; Li, J.; Cui, J.; Gou, X.; Yang, Q.; Jiang, Y.; Liang, S.; Yang, Z. Simultaneously engineering K-doping and exfoliation into graphitic carbon nitride (g-C3N4) for enhanced photocatalytic hydrogen production. Int. J. Hydrogen Energy 2019, 44, 778–787. [Google Scholar] [CrossRef]
- Mohamed, M.E.-S.; Al-Yacoub, Z.H.; Vedakumar, J.V. Biocatalytic desulfurization of thiophenic compounds and crude oil by newly isolated bacteria. Front. Microbiol. 2015, 6, 112. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.G.; Gao, H.S.; Li, W.L.; Xing, J.M.; Liu, H.Z. In situ magnetic separation and immobilization of dibenzothio-phene-desulfurizing bacteria. Bioresour. Technol. 2009, 100, 5092–5096. [Google Scholar] [CrossRef]
- Etemadi, N.; Akhavan Sepahi, A.; Yazdian, F.; Mohebali, G. The Optimization of Culture Medium Bacillus thermoamylo-vorance Strain EAMYO by RSM and Improvement Biodesulfurization Activity by Starch/Iron Nano Particles. J. Biotechnol. 2020, 11, 127–136. [Google Scholar]
- Sabaghian, S.; Yazdian, F.; Rasekh, B.; Shekarriz, M.; Mansouri, N. Investigating the effect of starch/Fe3O4 nanoparticles on biodesulfurization using molecular dynamic simulation. Environ. Sci. Pollut. Res. 2020, 27, 1667–1676. [Google Scholar] [CrossRef]
- Kalita, M.; Chutia, M.; Jha, D.K.; Subrahmanyam, G. Mechanistic Understanding of Gordonia sp. in Biodesulfurization of Organosulfur Compounds. Curr. Microbiol. 2022, 79, 82. [Google Scholar] [CrossRef] [PubMed]



| Experimental Run | Concentration of Sulfur (Dibenzothiophene) (mM) | Cell Concentration (v/v) | Concentration of 2-HBP (mM) |
|---|---|---|---|
| 1 | 0.15 | 1% | 0.3 |
| 2 | 0.45 | 1% | 0.38 |
| 3 | 0.15 | 3% | 0.31 |
| 4 | 0.45 | 3% | 0.4 |
| 5 | 0.15 | 2% | 0.36 |
| 6 | 0.45 | 2% | 0.38 |
| 7 | 0.3 | 1% | 0.35 |
| 8 | 0.3 | 3% | 0.33 |
| 9 | 0.3 | 2% | 0.39 |
| 10 | 0.3 | 2% | 0.38 |
| 11 | 0.3 | 2% | 0.39 |
| 12 | 0.3 | 2% | 0.39 |
| 13 | 0.3 | 2% | 0.39 |
| Experimental Run | Concentration of Nanostructure (MMT/g-C3N4) (mM) | Concentration of Sulfur (DBT) (mM) | Cell Concentration (v/v) | Concentration of 2-HBP (mM) |
|---|---|---|---|---|
| 1 | 0.0005 | 0.15 | 1% | 0.607 |
| 2 | 0.0015 | 0.15 | 1% | 0.556 |
| 3 | 0.0005 | 0.45 | 1% | 0.584 |
| 4 | 0.0015 | 0.45 | 1% | 0.512 |
| 5 | 0.0005 | 0.15 | 3% | 0.579 |
| 6 | 0.0015 | 0.15 | 3% | 0.546 |
| 7 | 0.0005 | 0.45 | 3% | 0.4 |
| 8 | 0.0015 | 0.45 | 3% | 0.4 |
| 9 | 0.0005 | 0.3 | 2% | 0.54 |
| 10 | 0.0015 | 0.3 | 2% | 0.5 |
| 11 | 0.001 | 0.15 | 2% | 0.5 |
| 12 | 0.001 | 0.45 | 2% | 0.508 |
| 13 | 0.001 | 0.3 | 1% | 0.599 |
| 14 | 0.001 | 0.3 | 3% | 0.599 |
| 15 | 0.001 | 0.3 | 2% | 0.56 |
| Source of Variance | The Sum of Squares Due to the Source (SS) | Degree of Freedom (df) | The Mean Sum of Squares Due to the Source (MS) | F Value | p-Value |
|---|---|---|---|---|---|
| Pattern | 0.011 | 5 | 2.156 × 10−3 | 6.43 | 0.0150 significant |
| A–A Concentration of sulfur (DBT) | 6.017 × 10−3 | 1 | 6.017 × 10−3 | 17.96 | 0.0039 |
| B–B Cell concentration | 1.677 × 10−5 | 1 | 1.677 × 10−5 | 0.050 | 0.8299 |
| AB | 2.500 × 10−5 | 1 | 2.500 × 10−5 | 0.075 | 0.7926 |
| A2 | 8.941 × 10−5 | 1 | 8.941 × 10−5 | 0.27 | 0.6213 |
| B2 | 3.518 × 10−3 | 1 | 3.518 × 10−3 | 10.50 | 0.0142 |
| A | B | R1 |
|---|---|---|
| Concentration of Sulfur (DBT) | Cell Concentration | Concentration of 2-HBP |
| 0.80 | −0.18 | 0.40437 |
| Sources of Variance | The Sum of Squares Due to the Source (SS) | Degree of Freedom (df) | The Mean Sum of Squares Due to the Source (MS) | F Value | p-Value |
|---|---|---|---|---|---|
| Pattern | 0.054 | 9 | 5.998 × 10−3 | 7.67 | 0.0019 significant |
| A–A Concentration of nanostructure (MMT/g-C3N4) | 3.842 × 10−3 | 1 | 3.842 × 10−3 | 4.91 | 0.0510 |
| B–B Concentration of sulfur (DBT) | 0.015 | 1 | 0.015 | 18.85 | 0.0015 |
| C–C Cell concentration | 0.011 | 1 | 0.011 | 14.26 | 0.0036 |
| AB | 1.800 × 10−5 | 1 | 1.800 × 10−5 | 0.023 | 0.8824 |
| AC | 1.013 × 10−3 | 1 | 1.013 × 10−3 | 1.29 | 0.2818 |
| BC | 8.320 × 10−3 | 1 | 8.320 × 10−3 | 10.64 | 0.0086 |
| A2 | 2.945 × 10−3 | 1 | 2.945 × 10−3 | 3.77 | 0.0810 |
| B2 | 6.529 × 10−3 | 1 | 6.529 × 10−3 | 8.35 | 0.0161 |
| C2 | 5.888 × 10−3 | 1 | 5.998 × 10−3 | 7.53 | 0.0207 |
| A | B | C | R1 |
|---|---|---|---|
| Concentration of Nanostructure (MMT/g-C3N4) | Concentration of Sulfur (DBT) | Cell Concentration | Concentration of Produced 2-HBP |
| −0.11 | −0.13 | −0.99 | 0.63808 |
| Strain | Nanoparticle | The Increase in Biodesulfurization Efficiency | Reference |
|---|---|---|---|
| Pseudomonas delafieldii | Magnetite nanoparticles (Fe3O4) | - | [20] |
| Rhodococcus erythropolis LSSE8-1 | Magnetite nanoparticles (Fe3O4) | - | [60] |
| Rhodococcus erythropolis LSSE8-1-vgb | Nano-γ-Al2O3 | 20% | [22] |
| Rhodococcus erythropolis FMF and R. erythropolis IGTS8 | Fe3O4 | - | [13] |
| Rhodococcus erythropolis IGTS8 | Magnetic Fe3O4 nanoparticles | 15.3% | [12] |
| Pseudomonas aeroginusa PTSOX4 | ZnO | 1.4-fold (40%) | [24] |
| Rhodococcus erythropolis IGTS8 | Modified carbon nanotube | 12% | [52] |
| Bacillus thermoamylovorance strain EAMYO | Starch/iron nanoparticles | For Fe0/starch it was increased by about 26.52% and for Fe3O4/starch it was increased by about 10.75% | [61] |
| Rhodococcus erythropolis IGTS8 | Starch/Fe3O4 | 50% | [62] |
| Rhodococcus sp. FUM94 | - | Not mentioned | [53] |
| Gordonia sp. | - | Not mentioned | [63] |
| Rhodococcus erythropolis IGTS8 | MMT/g-C3N4 | 52% | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasanbeik, N.Y.; Pourmadadi, M.; Ghadami, A.; Yazdian, F.; Rahdar, A.; Kyzas, G.Z. Biodesulfurization of Dibenzothiophene by Decorating Rhodococcus erythropolis IGTS8 Using Montmorillonite/Graphitic Carbon Nitride. Catalysts 2022, 12, 1450. https://doi.org/10.3390/catal12111450
Hasanbeik NY, Pourmadadi M, Ghadami A, Yazdian F, Rahdar A, Kyzas GZ. Biodesulfurization of Dibenzothiophene by Decorating Rhodococcus erythropolis IGTS8 Using Montmorillonite/Graphitic Carbon Nitride. Catalysts. 2022; 12(11):1450. https://doi.org/10.3390/catal12111450
Chicago/Turabian StyleHasanbeik, Nika Yavani, Mehrab Pourmadadi, Azam Ghadami, Fatemeh Yazdian, Abbas Rahdar, and George Z. Kyzas. 2022. "Biodesulfurization of Dibenzothiophene by Decorating Rhodococcus erythropolis IGTS8 Using Montmorillonite/Graphitic Carbon Nitride" Catalysts 12, no. 11: 1450. https://doi.org/10.3390/catal12111450
APA StyleHasanbeik, N. Y., Pourmadadi, M., Ghadami, A., Yazdian, F., Rahdar, A., & Kyzas, G. Z. (2022). Biodesulfurization of Dibenzothiophene by Decorating Rhodococcus erythropolis IGTS8 Using Montmorillonite/Graphitic Carbon Nitride. Catalysts, 12(11), 1450. https://doi.org/10.3390/catal12111450










