Ab Initio Investigation of the Adsorption and Dissociation of O2 on Cu-Skin Cu3Au(111) Surface
Abstract
1. Introduction
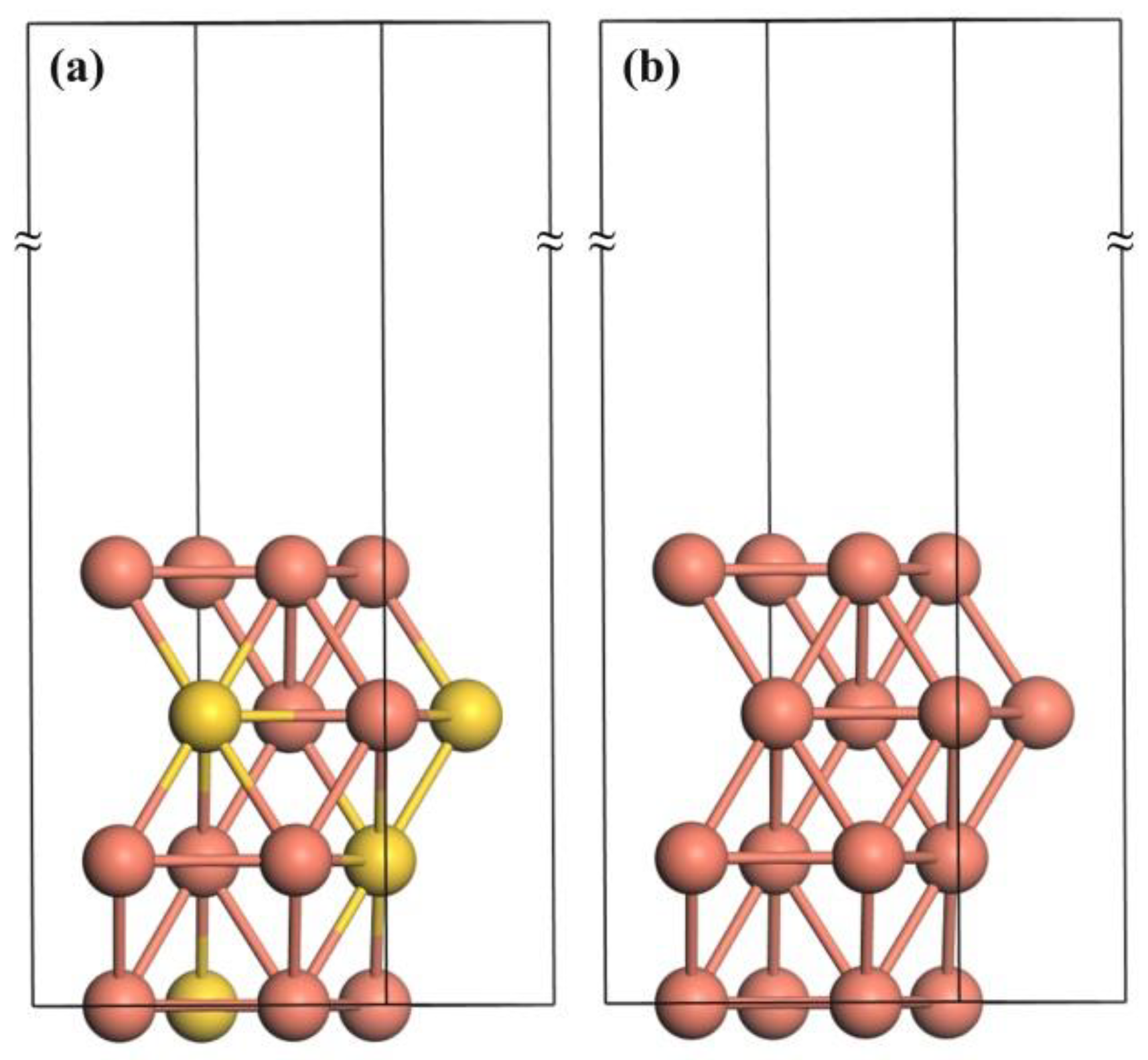
2. Computational Methods
3. Theoretical Results
3.1. The Adsorption of O2
3.2. The Electronic Structure of Cu-Skin Cu3Au Surface
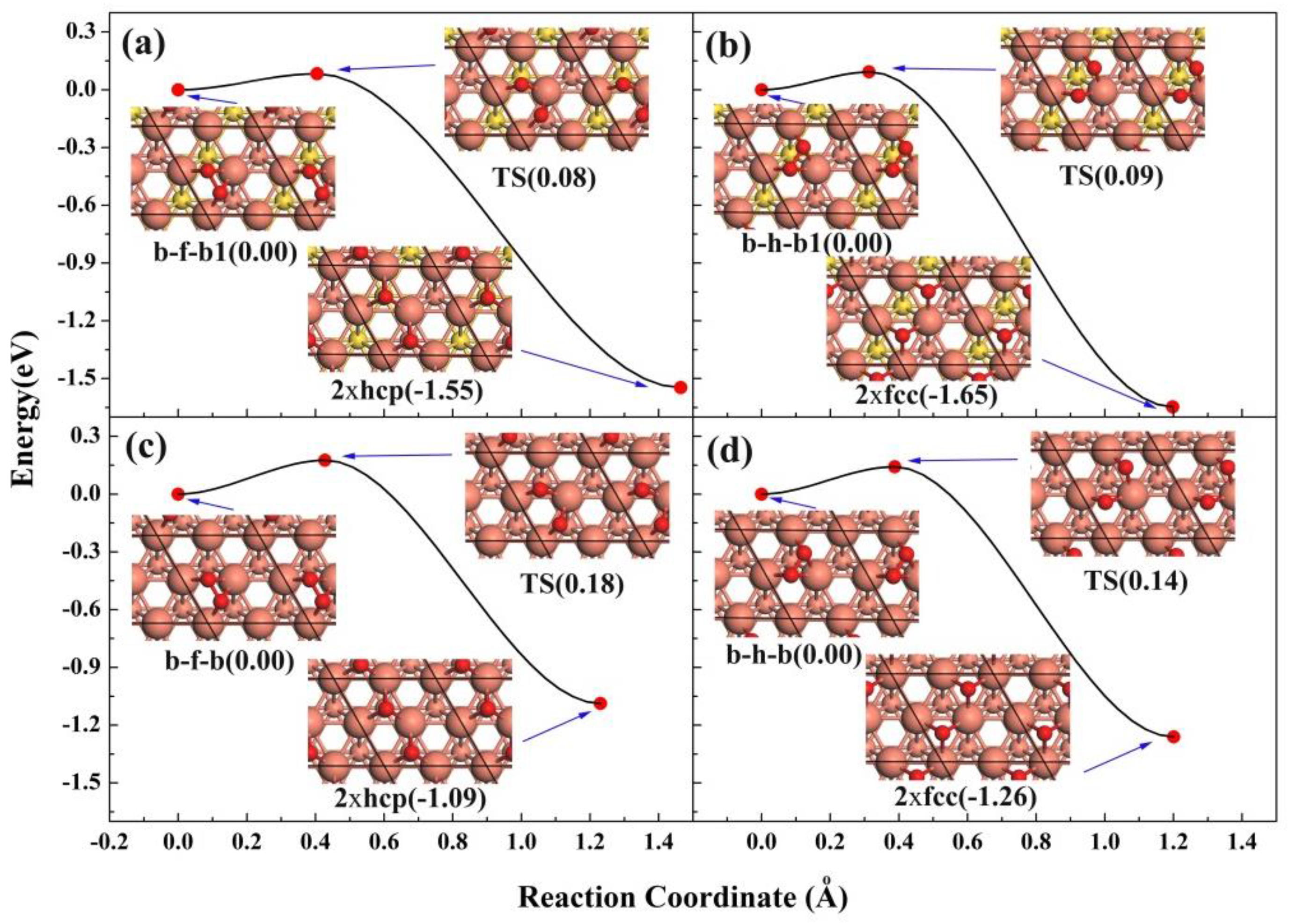
3.3. The Dissociation of O2
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stamenkovic, V.R.; Mun, B.S.; Arenz, M.; Mayrhofer, K.J.; Lucas, C.A.; Wang, G.; Ross, P.N.; Markovic, N.M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces. Nat. Mater. 2007, 6, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Zhao, J.; Tian, Y.; Zhang, B.; Wu, Y. Preparation and performances of ZIF-67-derived FeCo bimetallic catalysts for CO2 hydrogenation to light olefins. Catalysts 2020, 10, 455. [Google Scholar] [CrossRef]
- Montemore, M.M.; van Spronsen, M.A.; Madix, R.J.; Friend, C.M. O2 activation by metal surfaces: Implications for bonding and reactivity on heterogeneous catalysts. Chem. Rev. 2017, 118, 2816–2862. [Google Scholar] [CrossRef] [PubMed]
- Murillo, L.E.; Goda, A.M.; Chen, J.G. Selective hydrogenation of the CO bond in acrolein through the architecture of bimetallic surface structures. J. Am. Chem. Soc. 2007, 129, 7101–7105. [Google Scholar] [CrossRef] [PubMed]
- Tripkovic, V.; Hansen, H.A.; Rossmeisl, J.; Vegge, T. First principles investigation of the activity of thin film Pt, Pd and Au surface alloys for oxygen reduction. Phys. Chem. Chem. Phys. 2015, 17, 11647–11657. [Google Scholar] [CrossRef]
- Pompeo, F.; Nichio, N.N.; Ferretti, O.A.; Resasco, D. Study of Ni catalysts on different supports to obtain synthesis gas. Int. J. Hydrog. Energy 2005, 30, 1399–1405. [Google Scholar] [CrossRef]
- Chen, M.; Kumar, D.; Yi, C.-W.; Goodman, D.W. The promotional effect of gold in catalysis by palladium-gold. Science 2005, 310, 291–293. [Google Scholar] [CrossRef]
- Feng, M.; Huang, J.; Peng, Y.; Huang, C.; Yue, X.; Huang, S. Tuning the electronic structures of cobalt-molybdenum bimetallic carbides to boost the hydrogen oxidation reaction in alkaline medium. Chem. Eng. J. 2022, 428, 131206. [Google Scholar] [CrossRef]
- Shin, K.; Kim, D.H.; Lee, H.M. Catalytic characteristics of AgCu bimetallic nanoparticles in the oxygen reduction reaction. ChemSusChem 2013, 6, 1044–1049. [Google Scholar] [CrossRef]
- Liu, X.; Wang, A.; Wang, X.; Mou, C.-Y.; Zhang, T. Au–Cu alloy nanoparticles confined in SBA-15 as a highly efficient catalyst for CO oxidation. Chem. Commun. 2008, 27, 3187–3189. [Google Scholar] [CrossRef]
- Yong, S.T.; Ooi, C.W.; Chai, S.-P.; Wu, X. Review of methanol reforming-Cu-based catalysts, surface reaction mechanisms, and reaction schemes. Int. J. Hydrog. Energy 2013, 38, 9541–9552. [Google Scholar] [CrossRef]
- Graciani, J.; Mudiyanselage, K.; Xu, F.; Baber, A.E.; Evans, J.; Senanayake, S.D.; Stacchiola, D.J.; Liu, P.; Hrbek, J.; Sanz, J.F. Highly active copper-ceria and copper-ceria-titania catalysts for methanol synthesis from CO2. Science 2014, 345, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhu, P.; Ma, H.; Jia, X.; Wakatsuki, M.; Zou, G. Effects of copper-based alloy on the synthesis of single-crystal diamond. J. Phys. Condens. Matter 2002, 141, 10957–10961. [Google Scholar] [CrossRef]
- Menumerov, E.; Gilroy, K.D.; Hajfathalian, M.; Murphy, C.J.; Neretina, S. Plastically deformed Cu-based alloys as high-performance catalysts for the reduction of 4-nitrophenol. Catal. Sci. Technol. 2016, 6, 5737–5745. [Google Scholar] [CrossRef]
- Bauer, J.C.; Mullins, D.; Li, M.; Wu, Z.; Payzant, E.A.; Overbury, S.H.; Dai, S. Synthesis of silica supported AuCu nanoparticle catalysts and the effects of pretreatment conditions for the CO oxidation reaction. Phys. Chem. Chem. Phys. 2011, 13, 2571–2581. [Google Scholar] [CrossRef]
- Hashmi, A.S.K.; Hutchings, G.J. Gold catalysis. Angew. Chem. Int. Ed. 2006, 45, 7896–7936. [Google Scholar] [CrossRef]
- Bond, G.C.; Thompson, D.T. Catalysis by gold. Catal. Rev.-Sci. Eng. 1999, 41, 319–388. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B Condens. Matter 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1993, 49, 14251–14269. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter 1994, 50, 2665–2668. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Monkhorst, H.J.; Hendrik, J.; James, D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Dhifallah, M.; Dhouib, A.; Aldulaijan, S.; Di Renzo, F.; Guesmi, H. First-principles study of Au–Cu alloy surface changes induced by gas adsorption of CO, NO, or O2. J. Chem. Phys. 2016, 145, 024701. [Google Scholar] [CrossRef]
- Hirunsit, P.; Soodsawang, W.; Limtrakul, J. CO2 Electrochemical Reduction to Methane and Methanol on Copper-Based Alloys: Theoretical Insight. J. Phys. Chem. C 2015, 119, 8238–8249. [Google Scholar] [CrossRef]
- Hirunsit, P. Electroreduction of Carbon Dioxide to Methane on Copper, Copper–Silver, and Copper–Gold Catalysts: A DFT Study. J. Phys. Chem. C 2013, 117, 8262–8268. [Google Scholar] [CrossRef]
- Oka, K.; Tsuda, Y.; Makino, T.; Okada, M.; Hashinokuchi, M.; Yoshigoe, A.; Teraoka, Y.; Kasai, H. The effects of alloying and segregation for the reactivity and diffusion of oxygen on Cu3Au (111). Phys. Chem. Chem. Phys. 2014, 16, 19702–19711. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, W.; Liu, Z.; Hu, Z.; Wang, L. First-principles study of surface segregation in bimetallic Cu3M (1 1 1)(M = Au, Ag, and Zn) alloys in presence of adsorbed CO. Comput. Mater. Sci. 2022, 212, 111550. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Hirschl, R.; Delbecq, F.; Sautet, P.; Hafner, J. Pt80Fe20 surface from first principles: Electronic structure and adsorption of CO and atomic H. Phys. Rev. B 2002, 66, 155438. [Google Scholar] [CrossRef]
- Anderson, A.B. Theory for the Potential Shift for OH ads Formation on the Pt Skin on Pt3Cr (111) in Acid. J. Electrochem. Soc. 2004, 151, E85. [Google Scholar]
- Xu, Y.; Mavrikakis, M. Adsorption and dissociation of O2 on Cu (111): Thermochemistry, reaction barrier and the effect of strain. Surf. Sci. 2001, 494, 131–144. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Yu, X. The adsorption, diffusion and dissociation of O2 on Pt-skin Pt3Ni (111): A density functional theory study. Chem. Phys. Lett. 2010, 499, 83–88. [Google Scholar] [CrossRef]
- Hammer, B.; Norskov, J.K. Why gold is the noblest of all the metals. Nature 1995, 376, 238–240. [Google Scholar] [CrossRef]
- Hammer, B.; Nørskov, J.K. Electronic factors determining the reactivity of metal surfaces. Surf. Sci. 1995, 343, 211–220. [Google Scholar] [CrossRef]
- Kitchin, J.; Nørskov, J.K.; Barteau, M.; Chen, J. Modification of the surface electronic and chemical properties of Pt (111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246. [Google Scholar] [CrossRef]
- Hensley, A.J.R.; Zhang, R.; Wang, Y.; Mcewen, J.S. Tailoring the Adsorption of Benzene on PdFe Surfaces: A Density Functional Theory Study. J. Phys. Chem. C 2013, 117, 24317–24328. [Google Scholar] [CrossRef]
- Schweitzer, N.; Xin, H.; Nikolla, E.; Miller, J.T.; Linic, S. Establishing relationships between the geometric structure and chemical reactivity of alloy catalysts based on their measured electronic structure. Top. Catal. 2010, 53, 348–356. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Yu, X. Density functional theory studies on the adsorption, diffusion and dissociation of O2 on Pt(111). Phys. Lett. A 2010, 374, 4713–4717. [Google Scholar] [CrossRef]
- Xu, Y.; Ruban, A.V.; Mavrikakis, M. Adsorption and dissociation of O2 on Pt−Co and Pt−Fe alloys. J. Am. Chem. Soc. 2004, 126, 4717–4725. [Google Scholar] [CrossRef] [PubMed]
- Diao, Z.Y.; Han, L.L.; Wang, Z.X.; Dong, C.C. The adsorption and dissociation of O2 on Cu low-index surfaces. J. Phys. Chem. B 2005, 109, 5739–5745. [Google Scholar] [CrossRef] [PubMed]
- Okada, M.; Hashinokuchi, M.; Fukuoka, M.; Kasai, T.; Moritani, K.; Teraoka, Y. Protective layer formation during oxidation of Cu3Au(100) using hyperthermal O2 molecular beam. Appl. Phys. Lett. 2006, 89, 201912. [Google Scholar] [CrossRef]
- Habraken, F.; Kieffer, E.P.; Bootsma, G. A study of the kinetics of the interactions of O2 and N2O with a Cu(111) surface and of the reaction of CO with adsorbed oxygen using AES, LEED and ellipsometry. Surf. Sci. 1979, 83, 45–59. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, C.; Cen, W.; Li, H. Design strategy of bifunctional catalysts for CO oxidation. Fuel 2022, 320, 123909. [Google Scholar] [CrossRef]
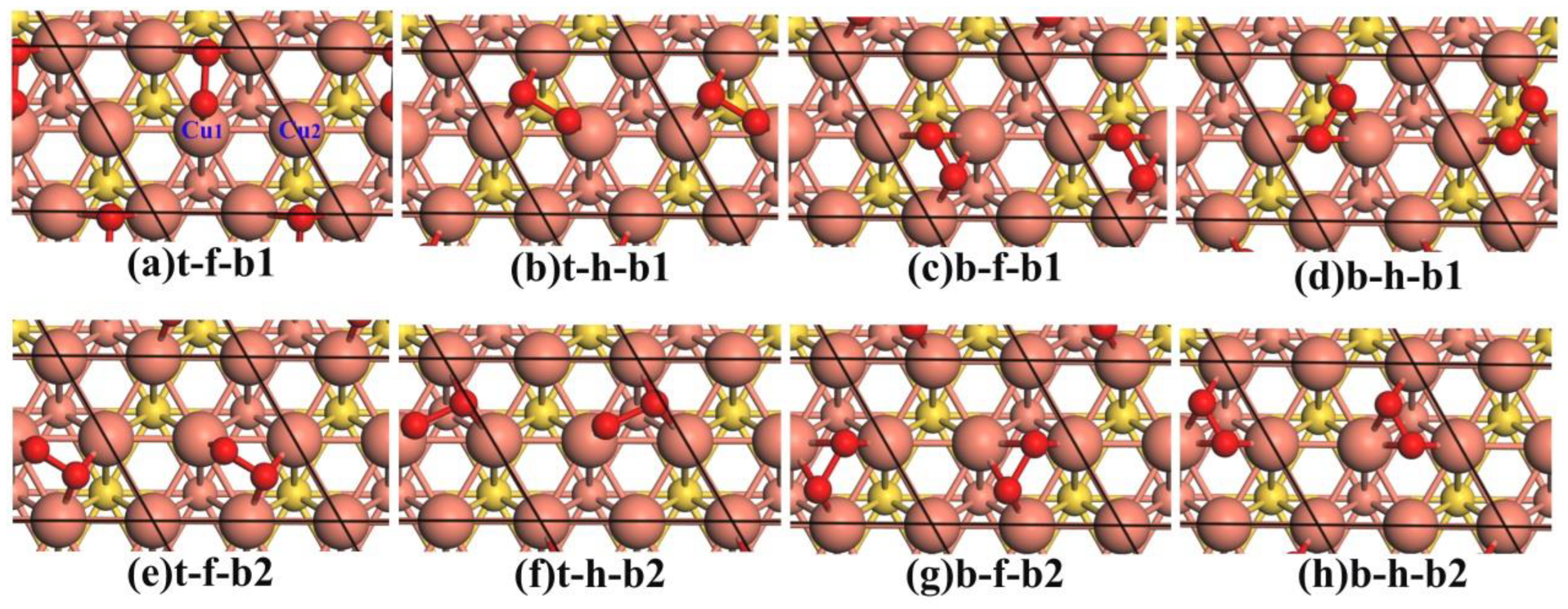

| Cu3Au(111) | Cu(111) | ||||
|---|---|---|---|---|---|
| Site | Eads | Nchg | Site | Eads | Nchg |
| t-f-b1 | −1.08 | 0.92 | t-f-b | −0.71 | 0.87 |
| t-f-b2 | −1.08 | 0.93 | t-h-b | −0.71 | 0.87 |
| t-h-b1 | −1.09 | 0.91 | t-b-t | −0.50 | 0.70 |
| t-h-b2 | −1.07 | 0.92 | b-f-b | −0.76 | 0.94 |
| b-f-b1 | −1.14 | 1.01 | b-h-b | −0.76 | 0.94 |
| b-f-b2 | −1.12 | 1.00 | |||
| b-h-b1 | −1.15 | 1.01 | |||
| b-h-b2 | −1.11 | 1.00 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Liu, Z.; Huang, W.; Zhou, S.; Hu, Z.; Wang, L. Ab Initio Investigation of the Adsorption and Dissociation of O2 on Cu-Skin Cu3Au(111) Surface. Catalysts 2022, 12, 1407. https://doi.org/10.3390/catal12111407
Yu Y, Liu Z, Huang W, Zhou S, Hu Z, Wang L. Ab Initio Investigation of the Adsorption and Dissociation of O2 on Cu-Skin Cu3Au(111) Surface. Catalysts. 2022; 12(11):1407. https://doi.org/10.3390/catal12111407
Chicago/Turabian StyleYu, Yanlin, Zhiming Liu, Wenxian Huang, Shan Zhou, Zuofu Hu, and Ligen Wang. 2022. "Ab Initio Investigation of the Adsorption and Dissociation of O2 on Cu-Skin Cu3Au(111) Surface" Catalysts 12, no. 11: 1407. https://doi.org/10.3390/catal12111407
APA StyleYu, Y., Liu, Z., Huang, W., Zhou, S., Hu, Z., & Wang, L. (2022). Ab Initio Investigation of the Adsorption and Dissociation of O2 on Cu-Skin Cu3Au(111) Surface. Catalysts, 12(11), 1407. https://doi.org/10.3390/catal12111407





