Abstract
Chalcogenides are essential in the conversion of solar energy into hydrogen fuel due to their narrow band gap energy. Hydrogen fuel could resolve future energy crises by substituting carbon fuels owing to zero-emission carbon-free gas and its eco-friendliness. The fabrication of different metal chalcogenide-based photocatalysts with enhanced photocatalytic water splitting have been summarized in this review. Different modifications of these chalcogenides, including coupling with another semiconductor, metal loading, and doping, are fabricated with different synthetic routes that can remarkably improve the photo-exciton separation and have been extensively investigated for photocatalytic hydrogen generation. In this direction, this review is undertaken to provide an overview of the enhanced photocatalytic performance of the binary and ternary chalcogenide heterostructures and their mechanisms for hydrogen production under irradiation of light.
1. Introduction
Metal oxides and chalcogenides are semiconductors that possess conductivities between those of conductors and insulators and these materials are widely researched semiconductor materials. Due to their stability, resistance to photo-corrosion, non-toxicity, and inexpensive preparation, and the metal oxides such as TiO2, ZnO, and SnO2 are commonly applied as photocatalysts [1,2,3,4]. Despite these favorable characteristics, a major drawback of using these metal oxides as photocatalysts is their large band gap energies (≥3 eV) [5]. Such wide band gaps only enable them to absorb the ultraviolet portion of the solar spectrum (~4% of the total solar energy), which restricts widespread practical applications [2,3,4]. Many strategies have been devised to prepare metal oxides with narrower band gaps for improved harvesting of visible light [4,6,7]. However, the scope for band gap tuning by doping and other strategies is limited, and much larger decreases in band gap can be achieved by moving outside the oxide compositional space.
Chalcogenides, compounds comprising one or more electropositive elements and at least one chalcogen ion (S2−, Se2−, or Te2−) [8], are well known for their narrow band gap energies [9,10]. These compounds continue to attract attention due to their numerous desirable properties, including narrow band gap energy, low toxicity, biocompatibility, low cost, and facile synthesis [11,12]. The utilization of chalcogenides and chalcogenide-based semiconductor materials in photocatalytic applications has been reported widely, mainly because of their narrow band gap energies that enable efficient harvesting of visible light [13].
Owing to the devastating impact of conventional fossil fuel use on the environment and the growing demand for energy, an increasing number of countries, companies, and researchers are considering hydrogen (H2) energy as a potential solution to the pressing environmental and sustainability problems associated with the current global energy system [14]. H2 is an alternative source of energy because they are clean, non-toxic, eco-friendly, and renewable. Unlike other alternative energy sources, H2 has the advantage of producing only water as a by-product upon consumption, which does not cause any pollution or release greenhouse gases [15]. Moreover, the potential clean and green energy source H2 as a replacement for fossil fuels can be produced in large quantities using simple and low-cost methods [16]. However, H2 in nature exists in combination with other elements, such as oxygen in water, thus, methods to obtain pure hydrogen should be considered. The production of H2 employing visible light is one of the most promising approaches for sustainable energy and environmental problems.
Water splitting is a process whereby water molecules are separated into oxygen and hydrogen. The equation for the chemical reaction is as follows:
2H2O → 2H2 + O2
Since pure water in nature does not absorb solar energy readily, a photocatalyst is required for the water-splitting process [17]. The reaction takes place in three steps as shown in Figure 1: step (1) to generate photoexcited electron-hole (e−/h+) pairs, the photocatalyst absorbs incoming photons with energies greater than the band gap of the material, step (2) the photogenerated carriers separate and migrate to the catalyst surface, and step (3) surface-adsorbed species (protons, H2O molecules, and intermediates) are reduced to form hydrogen and oxidized to form oxygen by photogenerated electrons and holes, respectively [18]. According to Maeda and co-workers, the first and second steps depend on the electronic structure and properties of the semiconductor used. In general, high crystallinity promotes water-splitting activity because the density of defects acting as recombination centers is low [19]. In contrast, the third step occurs in the presence of a solid co-catalyst, which is usually a noble metal such as platinum or rhodium, or a metal oxide such as nickel oxide. The loading of the co-catalyst onto the photocatalyst surface provides active sites and lowers the activation energy required for H2/O2 gas evolution.
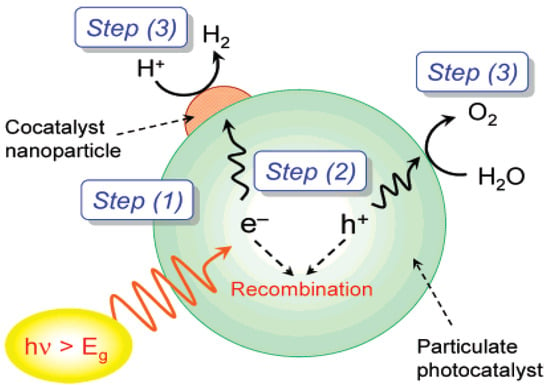
Figure 1.
Overall steps involved in the photocatalytic water-splitting process [18].
There are several factors affecting the photocatalytic H2O splitting as shown in Figure 2. The solar-to-hydrogen conversion efficiency of a material is mainly based on (1) light absorption, (2) e−/h+ photogeneration, and (3) separation and migration of e−/h+. There are a few principles to keep in mind, including the band gap energy, stability, the use of sacrificial reagents, solution pH, band structure and alignment, effective mass, carrier transport, crystallinity, and surface area of the material used.
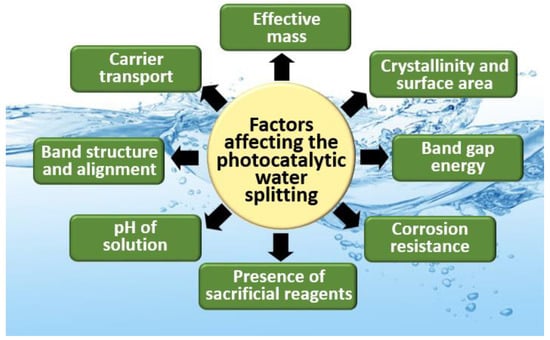
Figure 2.
Factors affecting the photocatalytic water-splitting process.
According to Maeda and co-workers, photocatalysts for water splitting under visible-light irradiation must satisfy two requirements; the band gap energy should fall in the range of 1.23 eV (the thermodynamic minimum) to 3.0 eV (the edge of the visible spectrum), and the conduction and valence band edges should align with the H+/H2 and H2O/O2 redox potentials [19]. In practice, the lower end of the band gap range must be considerably larger than 1.23 eV, perhaps as high as ~2.4 eV, to account for entropic losses in the photocatalyst as well as concentration and kinetic over potential losses. Apart from that, the electron and hole-effective masses are among the key factors for efficient H2O splitting. For a given mean free time between collisions, a smaller effective mass translates into larger carrier mobility and thus faster charge transport to the semiconductor–liquid interface. In general, the effective mass depends on the crystallographic direction, and therefore certain directions are more favorable for charge transport.
When selecting the material for H2O splitting, the band edge position is crucial. Their VB should be more positive than the O2 generation potential, whereas the CB should be more negative than the H2 generation potential [20]. In addition to possessing a suitable band gap and band edge alignment, photocatalytic materials must have adequate light absorption in the visible region, which accounts for ~43% of the solar energy incident on the earth. The crystallinity and surface-active sites of the selected materials are also important for e−/h+ transfer and oxygen evolution reaction (OER)/hydrogen evolution reaction (HER), respectively. Materials with high crystallinity and few defects can reduce the e−/h+ recombination. The rate of OER/HER reactions should be larger than backward reactions, which is mostly depending on the number of active sites. Moreover, the material chosen should be stable either in acidic or basic electrolytes.
The present review provides an overview of recent literature on chalcogenides and chalcogenide-based heterostructures, with an emphasis on their synthesis and application for H2 production by photocatalytic water splitting and the associated mechanisms. The main developments in binary and ternary chalcogenides of different metals including cadmium, copper, gallium, molybdenum, tin, titanium, vanadium, and zinc as well as their heterostructures are summarized. The relevant geometric structures and band structures of these chalcogenides as well as the key optical, electrical, and photocatalytic properties are also highlighted. Finally, the prospects of chalcogenide-based photocatalysts are discussed, and some general conclusions are drawn. The following sections cover the attributes of various chalcogenides and chalcogenide-based heterostructures that have recently been applied to photocatalytic water splitting [19,21].
2. General Synthesis Approaches of Chalcogenides
Although various synthesis methods of chalcogenides have been reported, synthesizing chalcogenides with high stability and to be photocatalytically active simultaneously is very challenging. Appropriate temperature, pressure, metal precursors, source of sulfur, and solvent combinations are important to synthesize these materials. Researchers have developed various synthesis routes for the fabrication of chalcogenides including hydrothermal [22], hot injection [23], solvothermal [24], and sol-gel [25]. Table 1 shows the different synthesis methods of chalcogenides. Gu et al. have synthesized AgInS2 using a low-temperature liquid method and doped it with Mn2+ for H2O splitting [26]. They yielded flower-like AgInS2 spheres with sizes in the range of 200−800 nm. While the particles of Mn-doped AgInS2 (1:100) are spherical with uniform particle sizes of about 300–400 nm. The calculated band gap energies of AgInS2 and Mn-doped AgInS2 (1:100) were found to be 1.63 and 1.52 eV, respectively. The H2 production rate of AgInS2 is 53 μmol h−1g−1. After Mn doping, the H2 production rate of Mn-doped AgInS2 (1:100) increases slightly, reaching 73 μmol h−1g−1. The hydrogen generation of Mn-doped AgInS2 (1:100) was enhanced by loading multi-walled carbon nanotubes, reaching 105 μmol h−1g−1, which is ~2 times of AgInS2 and ~1.4 times of Mn-doped AgInS2 (1:100).
Hydrothermal involves a chemical reaction in an aqueous solution at elevated temperature and high pressure. For instance, Kannan et al. have hydrothermally synthesized Cd0.5Zn0.5S using Cu(NO3)2·3H2O, Na2WO4·2H2O and L-Cysteine as the starting materials at different temperatures (120, 140, 160, 180, and 200 °C) for 24 h [22]. Different morphologies, including flake-like and sponge-like examples of Cd0.5Zn0.5S, were observed. Moreover, they found that Cu2WS4 synthesized at the high temperature of 180 °C and exhibited excellent antibacterial activity against different bacterial strains. This shows that precise control over the reaction conditions, including pressure, time, pH value, concentration, and temperature are necessary for the successful synthesis of chalcogenides. In another study, Yang et al. have successfully fabricated NiS2 using the hydrothermal method and Ni(NO3)3·6H2O as Ni source and thioacetamide as the source of sulfur.
Solvothermal is similar to the hydrothermal method, but it uses a non-aqueous solvent (solvent other than water). For example, Wang et al. have used different Cd(CH3COO)2·2H2O and sulfur powder ratios (1:1, 4:5, 2:3, 4:7, 1:2, and 1:3), precursor concentrations (1.5 and 6 mmol), temperatures (100, 140, 180, and 220 °C), and reaction times (4, 24, and 60 h) to yield CdS with different sizes and morphologies using solvothermal method [27]. The authors reported that the morphology, phase composition, and crystallinity of CdS were highly influenced by the precursor ratio, precursor concentration, temperature, and reaction time. The Cd concentration regulated the morphology of CdS morphology due to its effect on the formation of shape-determinant CdS nuclei. Nanorods, multipods, and triangular-like shape CdS nanocrystals were obtained with 1.5, 3, and 6 mmol of Cd, respectively. The arm diameter in CdS multipods increases from 10 to 60 nm with the sulfur concentration increasing when the cadmium concentration is maintained at 3 mmol.

Table 1.
Different preparation methods of chalcogenides.
Table 1.
Different preparation methods of chalcogenides.
| Synthesized Materials | Synthesis Method | Metal Precursor Used | Source of Sulfur | Solvent Used | Ref. |
|---|---|---|---|---|---|
| AgInS2 | Low-temperature liquid method | AgNO3 In(NO3)3·xH2O | Thioglycollic acid Thioacetamide | Water | [26] |
| AgInS2 | Microwave | AgNO3 In(NO3)3∙ 4.5H2O | Sulfur powder | Glycerol, Oleic acid, Oleylamine, 1-dodecanethiol and 1-octadecene | [28] |
| Cd0.5Zn0.5S | Hydrothermal | Cd(CH3COO)2·2H2O Zn(CH3COO)2·2H2O | Na2S·9H2O | Water | [29] |
| CdS | Solvothermal | Cd(CH3COO)2·2H2O | Sulfur powder | Dodecylamine | [27] |
| Cu2WS4 | Hydrothermal | Cu(NO3)2· 3H2O Na2WO4 2H2O | L-Cysteine | Water | [22] |
| Cu2WSe4 | Hot injection | CuCl2·2H2O WCl4 | Se powder | Oleylamine | [23] |
| Cu2ZnSnS4 | Hot injection | Cu(acac)2 Zn(OAc)2 ·2H2O Sn(OAc)4 | 1-dodecylthiol and tert-dodecylthiol | 1-octadecene | [30] |
| CuCdS2 | Solvothermal | Copper nitrate Cadmium acetate | Sodium thiosulphate pentahydrate | Ethylene glycol | [24] |
| CuS | Hydrothermal | Copper acetate dihydrate | Thiourea | Water | [31] |
| MoS2 | One-pot liquid-phase reaction | (NH4)6Mo7O24 | Na2S | Water | [32] |
| NiS2 | Hydrothermal | Ni(NO3)3·6H2O | Thioacetamide | Water | [33] |
| VS2 | Single-step chemical vapor deposition | VCl3 | Sulphur powder | - | [34] |
| Zn0.5Cd0.5S | Combustion method | Zn(NO3)2⋅4H2O, Cd(NO3)2⋅6H2O | Thioacetamide | Water | [35] |
| ZnS | Co- precipitation | Zn(NO3)2 | Na2S | Water | [36] |
Microwave-assisted synthesis is reported to be a clean, fast, and convenient synthetic route of chalcogenides. It works by applying microwave irradiation to chemical reactions which is based on efficient heating [5]. For example, Hu et al. have successfully synthesized AgInS2 with tunable composition and optical properties using microwave-assisted synthesis [28]. In another study, the co-precipitation reaction between Na2S solution and Zn(NO3)2 at room temperature produced ZnS [36]. The combustion method carried out by Tang et al. to produce Zn0.5Cd0.5S using Zn(NO3)2·4H2O, Cd(NO3)2·6H2O and thioacetamide [35]. Using water as the solvent, they heated the starting materials on the heating jacket until the gel-like precursor was formed, then the precursor was transferred into a Muffle furnace and heated at 300 °C for 15 min which resulted in the formation of yellow Zn0.5Cd0.5S solid. Chemical vapor deposition (CVD) is a technique that uses thermally induced chemical reactions at the surface of a heated substrate. For example, Gopalakrishnan et al. have deposited VS2 on the surface of vertically aligned Si nanowires using the CVD method [34]. In the typical synthesis, they used VCl3 and sulfur powder as the precursors of V and S, respectively.
Based on the findings, it can be observed that the properties of chalcogenides can be tuned by controlling the reaction parameters during synthesis. Therefore, the optimization of these parameters is of crucial importance to fabricate the desired properties of chalcogenides with enhanced H2O splitting ability.
3. Binary Chalcogenides and Their Photocatalytic Water-Splitting Activities
H2 is a clean fuel, and its usage can address many of the issues caused by using fossil fuels. It has several uses, as shown in Figure 3; it is widely used as a feedstock in the chemical industry to produce ammonia, methanol, and various fuels like diesel, gasoline, etc. It is also used as a transport fuel. It has several other applications in the production of metals and agricultural industries. A cost-effective and long-lasting chalcogenide-based photocatalysts can make the H2 generating process more economical and suitable. The use of binary chalcogenides and their modifications (compounds consisting of only one chalcogen and one electropositive atom [10]) for photocatalytic water splitting will be discussed in the following subsections.
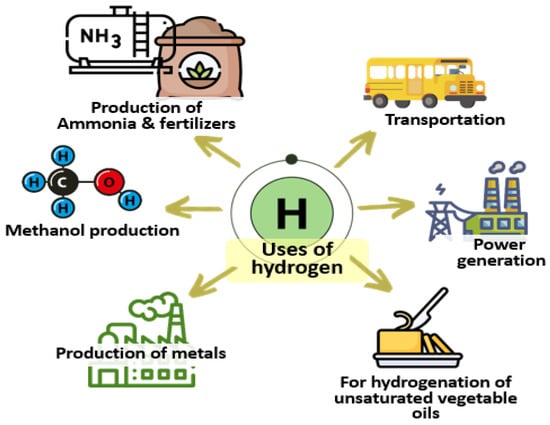
Figure 3.
Several applications of H2.
3.1. Cd-Based Chalcogenides
CdS and CdSe have both been used extensively in the fields of photocatalysis and photovoltaics, particularly in photoelectrochemical cells. Despite having a favorable bandgap for water splitting (~2.42 eV) [37,38] CdS is unstable in an aqueous solution under light irradiation due to photocorrosion. Therefore, in order to improve its photostability and photocatalytic activity, many studies have focused on synthesizing different morphologies of CdS together with the addition of co-catalysts and sacrificial agents [38]. Some of the different morphologies of CdS nanostructures that have been studied include hollow spheres [27], quasi-nanospheres [39], nanowires [39], and nanotubes [39], all of which can be synthesized by hydrothermal methods. Among these, CdS nanospheres were found to exhibit the highest H2 evolution rate during photocatalytic testing in the presence of Na2SO3 and Na2S hole scavengers.
In a recent study, one-dimensional CdS nanotubes displayed a remarkably high valence band edge compared with bulk CdS due to quantum confinement effects, and it was found that the photocatalytic efficiency of CdS nanotubes is higher than that of bulk CdS. Furthermore, the stabilized valence band, tubular structure, and strong p-d orbital hybridization led to a significant enhancement in photostability, making applications in aqueous solutions feasible [40].
In addition to the aforementioned studies focused on enhancing CdS through the preparation of different morphologies and sizes, another strategy to impart anti-photocorrosion properties to CdS is surface-modification with non-noble co-catalysts such as Ni, Ni2P, and CuS-NixP [41,42,43] as well as noble metals such as Pt and Au [44,45].
Another strategy to improve the photostability of CdS in an aqueous solution is to coat it with a thin layer of ZnO. Tso and co-workers have synthesized composite nanowires comprising CdS cores covered with thin ZnO shells, yielding CdS/ZnO core–shell nanowires [46]. These heterostructures provided improved stability compared with bare CdS nanowires and also led to reduced electron-hole recombination, causing an increase in H2 evolution activity of over two orders of magnitude as shown in Figure 4. Investigations also revealed that nanowires with 10–30 nm-thick shells absorbed more incident light than nanowires with thinner ZnO shells and had a lower probability of electron-hole recombination, the combination of which led to higher H2 evolution activity.
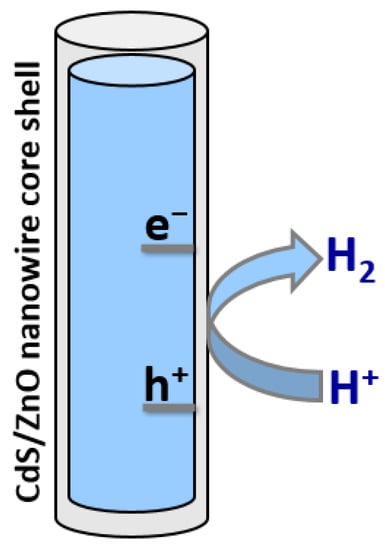
Figure 4.
Schematic diagram of photocatalytic H2 production using CdS/ZnO core–shell nanowire.
Several recent studies have examined photogenerated electron-hole pair separation in CdS/CuS nanocomposites [46]. Pan and co-workers found a high H2 evolution rate of 561.7 μmol h−1 during photocatalytic water splitting using a CdS/CuS nanocomposite [47]. In another work, a CdS/CuS nanostructured heterojunction was synthesized [48]. The prepared material was applied as a water-splitting photocatalyst and showed that the nanocomposites exhibit an enhanced rate of photocatalytic H2 evolution under visible-light irradiation. It can be concluded that CuS as a non-noble catalyst can improve the stability of CdS for photocatalytic H2 production in an aqueous solution. The enhancement in stability found in the CdS/CuS system can be explained by the transfer of holes from CdS to CuS, which inhibits the photocorrosion of CdS (Figure 5) [49].
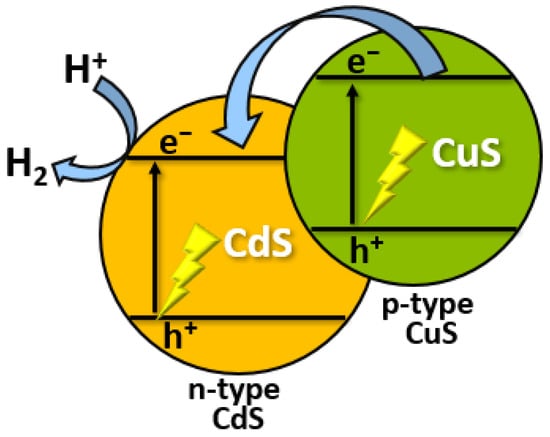
Figure 5.
Mechanism of photocatalytic H2 evolution using CdS/CuS nanocomposite.
Several authors have studied the factors affecting the performance of nanocrystalline CdSe quantum dots (QD) in photocatalytic water-splitting applications. Osterloh and co-workers showed a clear relationship between the extent of quantum size confinement and the photocatalytic water-splitting activity of suspended CdSe QDs. They found that the rate of H2 evolution increased with QD size in nm as follows: 2.20 > 2.00 > 2.48 > 1.75 > 2.90 > 2.61 > 2.75 > 3.05 > 3.49 > 4.03 > 4.81. These results confirm that photocatalytic activity can be controlled by the extent of quantum confinement (Figure 6) [50].
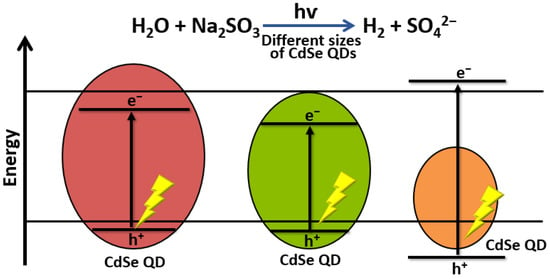
Figure 6.
Schematic illustration of the effect of CdSe QD size in photocatalytic H2 production.
Covalent organic frameworks (COFs) have drawn research interest owing to their stability, porous structure, large surface area, and tunable band structure [51]. It is an organic polymer arranged periodically with high crystallinity and high porosity, that is built from the integration of selected organic blocks via covalent bond linkage [52]. For instance, You et al. have synthesized COFs-p-phenylenediamine@CdS (COFs-Ph@CdS) using the hydrothermal method [53]. The authors reported that of the H2 generation of bare CdS, COFs-Ph@CdS-x (x = 1, 2, 3, 4) photocatalysts were 16.4, 139.1,17.5, 37.0, and 15.2 μmol, respectively. The photocatalytic H2 production activity of solid COFs-Ph@CdS-1 exhibited 29 and eight times higher compared to those of pristine COFs and CdS, respectively. The enhanced activity may be attributed to a stable coordination bond between Cd2+ ions and N atoms in COFs-Ph@CdS was formed, which served as a migrated channel for photo-generated carriers and their unique hollow structure that enables the production of more photo-generated electrons and holes and absorb light more efficiently.
High rates of photocatalytic H2 production were demonstrated by Das and co-workers using a surface ligand that binds strongly to CdSe QDs via tridentate coordination [54]. These authors also reported the effects of surface stabilizing and solubilizing agent dynamics. In more recent work, the influence of ligand exchange on the photocatalytic activity of CdSe QDs in solar water-splitting cells was studied [55]. It was found that CdSe QDs bearing shorter ligands showed better photocatalytic activity when compared to pristine CdSe QDs in a photoelectrochemical cell. These results should assist with the design and optimization of photochemical energy conversion systems employing nanocrystalline CdSe QD photocatalysts.
In another study, Zhou et al. have successfully prepared peanut-chocolate-ball-like CdS/Cd for H2 evolution [56]. They reported that CdS/Cd exhibited a high photocatalytic H2 production of 95.40 lmol h−1 which is about 32.3 times higher than bare CdS and displays exceptional photocatalytic stability over 205 h in comparison to CdS-based photocatalyst reported in previous studies. In recent studies, Li et al. 2% NiS/CdS present a high H2 evolution rate of 18.9 mmol g−1h−1 in comparison to pristine CdS [57]. The improved activity may be attributed to the presence of an interface between NiS and CdS that efficiently promote the charge separation and NiS nanoparticles serve as highly active H2 evolution sites.
3.2. Cu-Based Chalcogenides
Visible-light-active CuS/Au nanostructures were obtained by decorating CuS particles with Au nanoparticles, which were prepared by reducing HAuCl4 on the CuS surface [58]. The as-synthesized nanostructures displayed excellent catalytic performance in electrochemical H2 evolution tests, with good durability under acidic conditions. It was found that the CuS/Au nanostructured catalyst could produce a current density of 10 mA/cm2 upon the application of only 0.179 V versus the reversible hydrogen electrode (RHE). The CuS/Au catalyst also proved to be an efficient photocatalyst for the degradation of organic pollutants under visible-light irradiation.
The coupling of CuS with metal oxide has been shown to enhance its photocatalytic activities. For instance, Chandra and co-workers have synthesized CuS/TiO2 heterostructures with varying TiO2 contents using a simple hydrothermal method for the photocatalytic evolution of H2 [59]. The highest rate of visible-light photocatalytic H2 production was 1262 μmol h−1 g−1, which is around 9.7 and 9.3 times faster in comparison to pristine TiO2 nanospindles or CuS nanoflakes, respectively. The enhanced rate of H2 evolution is attributable to increased light harvesting and more efficient charge separation when an optimum amount of CuS is deposited on TiO2. Growing CuS nanoflakes on TiO2 nanoparticles to form a CuS/TiO2 heterostructure facilitates charge carrier separation at the heterostructure interface and thus enhances photocatalytic performance.
In another study, Dubale et al. fabricated nanocomposites comprising CuS and Cu2O/CuO heterostructures with and without decoration by Pt nanoparticles using an in-situ growth method [60]. Cu2O/CuO was prepared by straightforward anodization of Cu film, and a sputtering technique was used to deposit Pt nanoparticles onto the prepared Cu2O/CuO/CuS photocathodes. The prepared heterostructures were found to be very promising and highly stable photocathodes for H2 evolution under visible-light irradiation, with optimized Cu2O/CuO/CuS photocathodes reaching photocurrent densities of up to 5.4 mA cm2, some 2.5 times higher than that achieved by bare Cu2O/CuO. Upon decorating the Cu2O/CuO with both CuS and Pt, a further increase in photocurrent density to 5.7 mA/cm2 was achieved due to the suppression of the charge carrier recombination.
Metal loading on the surface of Cu-based chalcogenides has been reported to improve the photocatalytic evolution of H2. For instance, Ma and co-workers have reported that hydrothermally synthesized Au/CuSe/Pt nanoplates showed strong dual-plasmonic resonance and have been employed for photocatalytic H2 generation under irradiation of visible and near-infrared [61]. The as-prepared Au/CuSe/Pt nanoplates exhibited outstanding H2 generation activities of around 7.8 and 9.7 times those of Au/CuSe and Pt/CuSe composites, respectively. In the Au/CuSe/Pt system, light harvesting is enhanced by the plasmonic units (CuSe and Au), while Pt is mainly present as a co-catalyst promoting the H2 evolution reaction as shown in Figure 7.
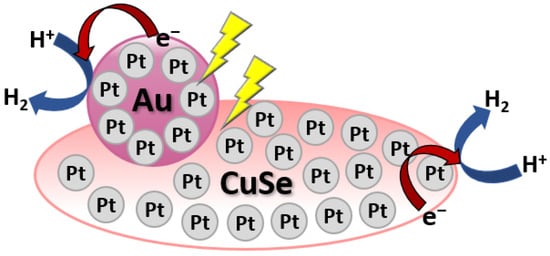
Figure 7.
Schematic diagram of Au/CuSe/Pt ultrathin nanoplate hybrid for H2 evolution under visible and near-infrared irradiation.
In a study conducted by Liang et al. CuS was deposited on the surface of CdS nanorods using the cation exchange method for generation of H2 under the irradiation of light [62].
In a study by Hou et al., CuS has been used as a co-sensitizer along with CdS to improve photocatalytic H2 production [63]. They decorated CdS–CuS on TiO2 nanotube arrays using the hydrothermal method. The as-prepared material exhibited remarkably high photocatalytic H2 evolution ability, and the photocatalytic H2 production rate was about 62.02 μmol cm−2h−1. In a different study, Mandari and co-workers synthesized a stable CuS/Ag2O/g-C3N4 material using hydrothermal and precipitation methods [31]. The as-synthesized material displayed a remarkable photocatalytic H2 production of 1752 μmol h−1g−1 which is considerably superior to those of the pristine CuS and g-C3N4. The enhanced H2 production activity may be ascribed to the formation of heterojunctions which resulted in the increase in visible light harvested and suppressed the photogenerated charge carrier recombination.
3.3. Ga-Based Chalcogenides
Monolayers of GaS and GaSe have been studied as photocatalysts by several authors but their performance is limited by low optical absorption and inefficient electron-hole pair separation [64,65]. One strategy to overcome these limitations is the construction of van der Waals (vdW) heterostructures by pairing GaX (GaX, X = S, Se) with arsenene (two-dimensional arsenic), as demonstrated by Peng and co-workers using first-principle calculations [66]. Such GaX/As heterostructures possess band gaps and band alignment that satisfy the requirements for photocatalytic water splitting. In contrast to pristine GaX monolayers, the type of band gap in Se0.5GaS0.5/As and S0.5GaSe0.5/As changes favorably from indirect to direct as the interlayer distance is varied. Furthermore, these heterostructures exhibit transport anisotropy with high electron mobilities of up to ∼2000 cm2 V−1 s−1, which facilitates photogenerated electron-hole pair separation and migration. All of the GaX/As heterostructures studied also show enhanced visible-light absorption beyond that of pristine GaX monolayers. It seems likely that the exceptional properties of GaX/As heterostructures will render them competitive with existing photocatalysts for solar-driven water splitting [66].
3.4. Mo-Based Chalcogenides
Molybdenum disulfide (MoS2) is a two-dimensional (2D) material, and it tends to exhibit more remarkable photocatalytic properties than its bulk counterparts [67,68]. Li and co-workers have investigated the photocatalytic properties of pristine MoS2 nanosheets and Ag-modified MoS2 nanosheets. They found that the rate of photocatalytic H2 production by Ag-modified MoS2 nanosheets could reach 2695 μmol h−1 g−1. They also found that Ag-doped MoS2 nanosheets exhibit superior stability and higher H2 evolution efficiency compared with pristine MoS2 nanosheets, which is mainly due to increased visible-light absorption with increasing Ag content (Figure 8) [69].
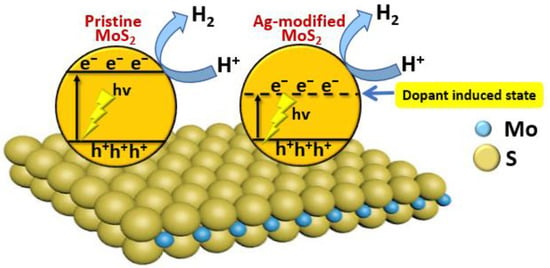
Figure 8.
The mechanism of photocatalytic H2 production using Ag-modified MoS2 nanosheets.
Photocorrosion-resistant material, MoS2/Zn0.5Cd0.5S/g-C3N4, has been successfully synthesized by Tang et al. to produce H2 under irradiation of visible light [35]. The authors reported that the as-prepared materials exhibited a maximum H2 production rate of 4914 μmol g−1h−1 in Na2S-Na2SO3 solutions which served as the sacrificial agent. The high evolution rate of H2 was owing to the improved charge separation between the interfaces.
In another study, Yuan et al. designed 2D-2D MoS2@Cu-ZnIn2S4 using the solvothermal method for photocatalytic production of H2 [70]. The highest H2 evolution rate of 5463 μmol g−1h−1 was observed for 6% MoS2@Cu-ZnIn2S4 under irradiation of visible light which is 72 times higher than that of pristine Cu-ZnIn2S4. The excellent activity may be ascribed to the high visible light absorption property and abundant active sites for the H2 evolution reaction to take place. Moreover, the production rate of H2 remain unchanged after three cycles which suggested the high durability of the synthesized MoS2@Cu-ZnIn2S4 for photocatalytic H2 production.
An enhanced photocatalytic H2 evolution was performed by Yin and co-workers with the maximum evolution rate of 872.3 mol h−1 using MoS2/C composites sensitized with Erythrosin B [32]. The enhanced performance was attributed to the efficient photo-generated charge transfer and separation between Erythrosin B and MoS2 or C as well as the presence of more active sites for H2 evolution. The photocatalytic evolution of H2 could also be achieved through photoelectrochemical catalytic activities. For instance, Hassan and co-workers have reported the optimized MoS2/GaN2 exhibited significantly higher photoelectrochemical performance in comparison to the bare materials under the same visible conditions [71]. The as-prepared photoanode achieved efficient light harvesting with a photocurrent density of 5.2 mA cm−2 which is 2.6 times higher than bare GaN. Moreover, MoS2/GaN2 exhibited enhanced applied-bias-photon-to-current conversion efficiency of 0.91%, while the reference GaN yielded an efficiency of 0.32%. The authors reported that the decrease in charge transfer resistance between the electrolyte and semiconductor interface and the improved separation charge carriers in the MoS2/GaN heterostructures were all attributed to significant improvements in photocurrent density and efficiency.
MoS2-based nanomaterials have been found to be an efficient visible light-responsive material for H2 production. Based on the reported work, revealed that the surface modification of the MoS2 could lead to an improved photocatalytic activity owing to the effective charge transfer and separation of photo-generated e−/h+ pairs.
New strategies have been developed to use MoSe2-based nanosheets as photocatalysts for the H2 evolution reaction. Zhao and co-workers achieved an enhancement in the photocatalytic activity of MoSe2 by surface modification and doping with Ni and Co [72]. Their study found that Ni0.15Mo0.85Se2 nanosheets exhibited the highest photocatalytic activity in both alkaline and acidic media. These results provide an attractive alternative to Pt-based catalysts.
3.5. Sn-Based Chalcogenides
Recently, it has been discovered that monolayers of the Sn-based mono- and dichalcogenides SnX and SnX2 (X = S or Se) are promising candidates for photocatalytic water splitting. These materials exhibit very good visible light absorption, low exciton binding energies, and high carrier mobility. However, in their pure and strain-free state, the valence band edges of Sn-monochalcogenide monolayers are predicted to be too high in energy for effective water oxidation. This issue can potentially be overcome by crystal engineering to induce a moderate tensile strain, which is predicted to lower the valence band edge to a level suitable for water oxidation [21]. Li and co-workers reported that monolayers of both SnX and SnX2 are expected to exhibit good optical absorption in the visible region of the solar spectrum, and the predicted sequence of visible light absorption strength is as follows: SnSe > SnSe2 > SnS > SnS2. Moreover, SnX and SnX2 monolayers also have excellent carrier mobility, which results in the fast migration of photogenerated carriers to the surface. Hence, the possibility of rapid redox reactions on the surface of the photocatalyst.
In another study, Lei et al. successfully prepared SnS/g-C3N4 nanosheets using a facile ultrasonic and microwave heating approach, which formed intimate interfacial contact and a suitable energy band structure [73]. The optimized sample displayed enhanced photocatalytic H2 evolution from H2O with the aid of Pt as a co-catalyst in comparison to pure g-C3N4. The stability of the photocatalysts was significantly improved after being loaded with MoO3 particles due to the formation of a Z-scheme heterojunction. Among all as-prepared samples, the 10% SnS/g-C3N4 exhibits the highest photocatalytic rate of 818.93 mmol h−1g−1 under AM1.5G irradiation, 2.90 times to pure g-C3N4, due to the matched energy band structure between g-C3N4 and SnS, which improves the separation efficiency of photo-generated carriers and hinders the recombination of hole-electron pairs. Additionally, SnS nanosheets have improved the light absorption efficiency of the prepared material and generated more catalytic active sites as well as shortening the carriers’ transport path as well.
Liu and co-workers have synthesized flower-like nanostructure SnS2 with generated sulfur vacancies using a new simple strategy of ion exchange [74]. The morphology of the flower-like structure provides shorter carrier diffusion lengths and more surface-active sites. While, the presence of S vacancies helps introduce defect levels in SnS2, which leads to a reduction in work function, eventually improving carrier density and separation efficiency. The well-engineered sample 5%Cu/SnS2-x shows the highest photocatalytic activity with an H2 yield of 1.37 mmol h−1, which is about six times higher than that of pure SnS2.
SnS2/ZnIn2S4 composites were successfully fabricated by decorating SnS2 nanosheets with ZnIn2S4 microspheres [75]. Geng and co-workers reported that the SnS2/ZnIn2S4 composites showed superior photocatalytic properties for H2 generation in comparison to pristine ZnIn2S4. Furthermore, a notable impact was observed on the photocatalytic activity of ZnIn2S4 when varying the mass ratio of SnS2. The 2.5% SnS2/ZnIn2S4 in particular exhibited the highest photocatalytic H2 generation rate of 769 μmol g−1h−1, which was about 10.5 times the photocatalytic activity of pristine ZnIn2S4. The enhanced photocatalytic activity may be due to the formation of SnS2/ZnIn2S4 heterojunction, which enabled highly active charge separation and transfers on the interface of SnS2 and ZnIn2S4.
Li and co-workers have synthesized SnO2/SnS2 with excellent photocatalytic H2 evolution performance under simulated light irradiation [76]. The material exhibited a high H2 production rate of 50 μmol h−1 which is 4.2 times higher than that of pure SnO2 under the same condition. A different study by Mangiri et al. has successfully synthesized a stable CdS/MoS2-SnS2 for water splitting via the solvothermal method [77]. The as-prepared CdS/MoS2-SnS2 (6 wt%) exhibited the highest H2 production rate of 185.36 mmol h−1g−1 which is much higher than pristine CdS (2.5 mmol h−1g−1) and 6 wt% of MoS2-loaded CdS nanorods (123 mmol h−1g−1). The effective photocatalytic performance of MoS2-SnS2-loaded CdS may be ascribed to the ability of the material to harvest light effectively, better separation of e−/h+ pairs, formation of trapping sites, high active catalytic zones, migration of charge carriers towards the surface of a semiconductor, and suitable energy levels.
3.6. Ti-Based Chalcogenides
The tri-chalcogenide TiS3 was found to be an effective photoanode for use in photoelectrochemical cells. Photocatalytic H2 evolution rates of up to 1.80 ± 0.05 μmol min−1 were obtained, corresponding to a photoconversion efficiency of ~7% [78]. Furthermore, the ternary compounds TixNb1-xS3 (Nb-rich) and NbxTi1−xS3 (Ti-rich) were tested as photoanodes and compared with the corresponding binary trisulfides. The binary compounds were polycrystalline with a nanoribbon morphology and adopted the monoclinic TiS3 (P21/m) and triclinic NbS3 (P2) crystal structures, respectively. H2 generation experiments revealed that the Ti-rich phase was a superior photocatalyst compared with the Nb-rich phase, exhibiting H2 production rates of up to 2.2 ± 0.1 mol/min cm2 [79]. Ti-based chalcogenides are not widely explored for their photocatalytic H2 production activity. Therefore, further studies and optimizations are required.
3.7. V-Based Chalcogenides
Certain transition metals located on the first row of the periodic table can participate in the formation of tetra-chalcogenides thanks to their ability to adopt +4 oxidation states (Figure 9). Among these metals is vanadium, which can form the binary tetra-chalcogenide VS4. Nanocomposites comprising VS4 and graphene were evaluated for solar-driven water splitting by Guo and co-workers. Excellent performance was obtained due to the formation of C–S bonds with a π-conjugated structure, which assists with transferring electrons from the S 2p orbital to graphene [80]. The ternary tetra-chalcogenides Cu3Nb1−xVxS4 and Cu3Ta1−xVxS4, which exist as solid solutions and adopt the sulvanite structure, were successfully synthesized in a solid-state reaction by Ikeda and co-workers [81]. The band gaps of the synthesized materials were estimated to fall in the range of 1.6–1.7 eV, and the band structure of the Cu3M1−xVxS4 system varies with composition. It was found that for most compositions of solid solution, superior photocatalytic activity was achieved compared with the ternary Cu3MS4 (M = V, Nb, Ta) compounds [81].
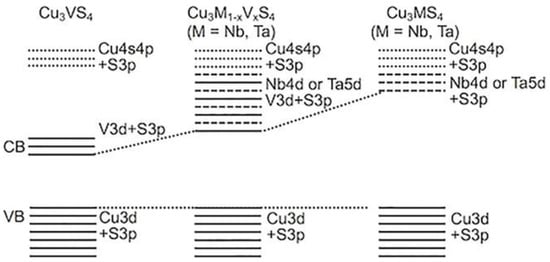
Figure 9.
Proposed band structures for Cu3M1−xVxS4 (M = Nb and Ta) solid solutions.
Li et al. synthesized the VS2@C3N4 heterojunction with S vacancies via the in situ supramolecular self-assembly method [82]. The as-synthesized material shows a remarkable synergistic H2 production rate (9628 μmol g−1h−1) and wastewater degradation efficiency as well as stability, which is 16.0 times that of pristine C3N4. Based on the theoretical calculations and experiment findings, it shows that S vacancies have resulted in the formation of compact heterostructure and the reduction in the work function, which promotes interfacial carriers’ transfer and surface properties. The core–shell structure improves the stability of S vacancies.
In another study, Gopalakrishnan et al. synthesized a core–shell heterostructures photocathode consisting of VS2 deposited on a silicon nanowire surface via single-step chemical vapor deposition for H2 evolution under solar irradiation [34]. The core–shell nanowire heterostructure photocathode displayed an outstanding solar-assisted water reduction performance at pH∼7 over a pristine photocathode. The as-prepared material produces about 23 μmol cm−2 h−1 (at 0 V vs. RHE) of H2 gas. Recently, Zhong et al. have doped Ni3S2 with nitrogen and coupled it with VS2 using the hydrothermal method [83]. Based on their results, Ni3S2/VS2 without nitrogen doping exhibited improved oxygen evolution reaction (OER) performance with an extremely low overpotential of 227 mV at 10 mA/cm2 which may be attributed to the presence of high number of active sites and excellent interfaces. Moreover, Ni3S2/VS2 with nitrogen doping shows high electrocatalytic hydrogen evolution reaction (HER) performance with a low HER overpotential (151 mV at 10 mA/cm2) and this is due to the presence of nitrogen doping that significantly improves the conductivity and increases the number of catalytic active sites.
3.8. Zn-Based Chalcogenides
Kurnia and co-workers have investigated the performance of ZnS thin films during photoelectrochemical H2 generation [84]. They found that ZnS is an inexpensive photocatalyst that exhibits activity under visible-light irradiation without the addition of a co-catalyst. The ZnS thin films were estimated to have band gaps of ~2.4 eV, and photocurrent densities exceeding 1.5 mA/cm2 were achieved under visible-light irradiation (λ ≥ 435 nm). These photocurrents are remarkably high for H2 generation by undoped ZnS under visible-light irradiation.
A modification of ZnO nanosheets, using a thin layer of ZnS, was developed to enhance visible light absorption, leading to improved photoelectrochemical activity in water-splitting experiments [85,86]. Sánchez-Tovar and co-workers have prepared ZnO/ZnS heterostructures using anodization of Zn in glycerol/water/NH4F electrolytes under controlled hydrodynamic conditions in the presence of various concentrations of Na2S [85]. In another work, Zhang et al. synthesized ZnS/ZnO/ZnS sandwich nanosheets with an effective band gap of ~2.72 eV using thermal evaporation, and these materials resulted in potent visible-light photocatalytic activity [86]. These works show that coating ZnO with an ultrathin surface layer of ZnS is an interesting approach for enhancing its water-splitting activity under solar irradiation.
Arai and co-workers have synthesized hollow Cu-doped ZnS particles as visible-light photocatalysts for the decomposition of hydrogen sulfide under solar irradiation to generate H2. The Cu-doped ZnS particles were synthesized starting from a ZnO precursor that was co-precipitated on Cu by exploiting the difference in reduction potential between Zn and Cu. The visible-light photoactivity of Cu-doped ZnS was six- and 130-times higher than the Cu-free “ZnS-shell” and pristine ZnS particles prepared using co-precipitation, respectively. A comparative study found that the “Cu-ZnS-shell” particles were highly active under visible-light irradiation (λ > 440 nm), and the rate of H2 evolution was optimized with a Cu (2 wt%)/ZnS photocatalyst [36]. In another recent study, Cu/ZnS microspheres were prepared using a template-free microwave irradiation method for H2 generation from an aqueous Na2S solution under visible-light irradiation. The optimized H2 evolution rate of 973.1 μmol h−1 g−1 was obtained for 2.0 mol% Cu-doped ZnS [87].
4. Ternary Chalcogenide Heterostructures for Water Splitting
Recently, ternary mixed-metal chalcogenides have shown excellent photocatalytic H2 production activities, as shown in Table 2. For instance, ZnxIn2S3+x (x = 1–5) has attracted the attention of researchers as a new member of the semiconductor family. These compounds possess unique electronic structures, tunable optical properties, and, for certain compositions, band gaps suitable for visible-light absorption and energy bands that straddle the water redox potentials, making them ideal for use in photocatalysis and energy conversion reactions [88]. Wu and co-workers have successfully synthesized ZnxIn2S3+x with x ranging from 1 to 5 [88]. ZnxIn2S3+x samples display strong absorption of visible light due to excitation of the fundamental band gap transition, and the absorption edges of the samples move to a shorter wavelength as x is increased from 1 to 5 due to the widening of the band gap. ZnxIn2S3+x samples have band gap energies ranging from 2.65 eV to 2.84 eV, depending on chemical composition. ZnIn2S4 exhibited the lowest electron-hole recombination rate among all the compositions studied. One of the contributing factors leading to this finding is the presence of fewer composition faults in the ZnIn2S4 structure compared with the other samples. The existence of composition faults results in additional energy barriers to be overcome by charge carriers during transit to the particle surface, which leads to increased recombination and lower photocatalytic reaction rate. Based on the photoelectrochemical experiments conducted, it was found that ZnIn2S4 can generate more photocurrent than the other compositions. Electrochemical impedance spectroscopy results also suggest that ZnIn2S4 has the lowest resistance to interfacial electron transfer among the compositions studied, suggesting that ZnIn2S4 is the most efficient at interfacial charge transfer and consistent with the photocurrent trend. ZnIn2S4 shows the highest photocatalytic activity, followed by Zn2In2S5, Zn3In2S6, Zn4In2S7, and, lastly, Zn4In2S8, with H2 production rates of 2.93, 2.86, 2.32, 2.15, and 2.05 mmol h−1g−1, respectively. Overall, it can be concluded that ternary metal chalcogenides with the formula ZnxIn2S3+x (x = 1–5) exhibit appropriate properties for use as photocatalysts, especially for water-splitting applications. In a different study, Fan et al. fabricated CuS@ZnIn2S4 hierarchical nanocages for H2 evolution under visible light [89]. The presence of an interface between CuS and ZnIn2S4 improved the solar energy utilization and separation and transfer efficiency of photogenerated carriers. The as-prepared materials exhibited a photocatalytic H2 evolution rate as high as 7910 μmol h−1g−1.

Table 2.
Photocatalytic H2 production activities using different ternary chalcogenides.
In another report by Hojamberdiev and co-workers, they have synthesized layered crystals of trigonal ZnIn2S4 by a flux method using a range of binary fluxes, including CaCl2:InCl3, SrCl2:InCl3, BaCl2:InCl3, NaCl:InCl3, KCl:InCl3, and CsCl:InCl3, from waste containing ZnS from the mining industry [7]. Among them, KCl:InCl3 was the most favorable for synthesizing phase-pure trigonal ZnIn2S4. The trigonal ZnIn2S4 crystals grown in this study using KCl:InCl3 flux exhibited higher photocatalytic H2 evolution activity (132 μmol h−1) in comparison to previously reported hexagonal ZnIn2S4 crystals prepared using the hydrothermal method. The superior performance obtained using the binary flux method can be ascribed to higher crystallinity and decreased defect density. The existence of secondary phases (namely ZnS and In2S3) in ZnIn2S4 crystals grown with CaCl2:InCl3 and NaCl:InCl3 fluxes were found to have a positive impact on the photocatalytic activity, leading to H2 evolution rates of 232 and 188 μmol h−1, respectively. The enhanced H2 production activity may be ascribed to efficient charge separation and interfacial charge transfer. Moreover, Mn0.5Cd0.5S/carbon black/CuS have been fabricated for H2 production using sonochemical loading, and the subsequent in-situ deposition routes [96]. Lv et al. reported that the optimized Mn0.5Cd0.5S/carbon black/CuS exhibited the highest H2 production of 819.9 μmol h−1, which is 4.79-, 2.08-, and 1.47-fold increments more than pristine Mn0.5Cd0.5S, Mn0.5Cd0.5S/0.5 carbon black and Mn0.5Cd0.5S/CuS, respectively.
Group-III chalcogenide monolayers adopting two-faced ‘Janus’ structures are reported to be efficient photocatalysts for solar-driven water splitting. Several research groups have theoretically investigated the geometric structure, chemical stability, and electronic and optical properties of Janus group-III chalcogenide monolayers with layered ternary (XMMX′, where X, X′ = S, Se, or Te and M = Ga or In) [97,98] or quaternary composition (XMM′X′, where M and M′ are Ga and In) [99,100]. Among the ternary Janus monolayers, SGa2Te, SeGa2Te, SIn2Te, and SeIn2Te are found to possess direct band gaps. Since direct gap semiconductors generally possess higher absorption coefficients than indirect gap semiconductors, these ternary group-III chalcogenide monolayers can potentially exhibit good light harvesting and efficient electron-hole pair generation [97]. However, most of these materials have not yet been synthesized, which will be an important next step in their photocatalytic evaluation. As reported by Bai and co-workers, group-III chalcogenide monolayers adopt a honeycomb-like geometric structure with a double M m (M = Ga or In) layer sandwiched between two chalcogen layers.
Depending on the combination of metal and chalcogen, both direct and indirect band gaps occur, with gap energies ranging from 1.54 eV to 2.91 eV. Although all these band gaps surpass the free energy change of the water-splitting reaction (1.23 eV per electron), photocatalytic water splitting is not guaranteed because the band edges must also straddle the water redox potentials. Figure 10 shows the calculated alignment between the band edges of the Janus XMMX′ monolayers and the water redox potentials. According to these calculations, all of the monolayers are capable of complete water splitting with the exception of SGa2Te, which cannot drive the oxygen evolution reaction due to its valence band edge laying above the O2/H2O redox potential [97].
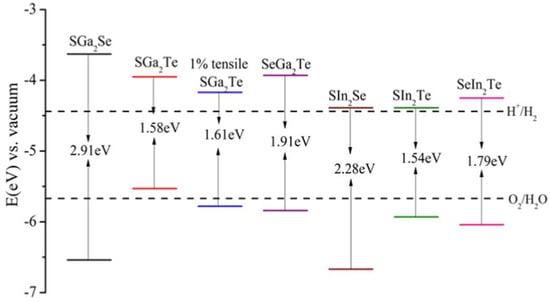
Figure 10.
Calculated alignment of the band edges of the Janus XMMX′ monolayer with respect to the redox potentials of the water.
Although the pristine GaTe and InTe monolayers exhibit strong absorption at short wavelengths, they are inferior to the corresponding Janus monolayers at longer wavelengths. Among the Ga-based Janus chalcogenide monolayers, SeGa2Te and SGa2Te possess the strongest optical absorption, while SeIn2Te and SIn2Te exhibit the strongest absorption of all the Janus-type monolayers studied.
5. Prospects of Chalcogenides and Chalcogenide-Based Heterostructures
Even though steady progress has been made in utilizing chalcogenides as photocatalysts, a range of challenges still need to be addressed to promote the field, gain interest from other researchers, and achieve commercially exploitable results:
- In order to further boost the activity and stability of chalcogenides for water splitting, it is important to create precise methods for obtaining pure phase, active interface, exposed active surface, optimized electronic structure, and enhanced electronic conductivity.
- Recent research has shown that chalcogenides are very promising materials for H2 evolution by photocatalytic water splitting. It is expected that, with further knowledge, controlled doping, surface engineering, and development of their performance can be further improved [101].
- Binary metal chalcogenides such as CdS and CdSe are unstable in acidic media and are also susceptible to photocorrosion. As such, potential replacements that are more stable in acidic media and that do not exhibit photocorrosion should be explored.
- Despite many recent studies on the use of ternary and quaternary chalcogenides as photocatalysts for H2 production, the exact cause of photocorrosion in these materials is yet to be explored in detail and should be researched thoroughly in order to synthesize highly stable, multifunctional chalcogenides.
- Preparation of chalcogenide using low-cost methods while can produce large quantities of products also require more attention. Optimization of different parameters in the synthetic reactions such as precursors, temperature, pH, and reaction time should be studied for optimal yield to facilitate the production of these materials at a commercial scale.
- Detailed studies on extending the lifetime of photo-generated carriers and suppressing recombination are required to improve the photocatalytic activity of these materials for broader applications. Several approaches that could be employed include coupling with other semiconductors, loading of noble metals, and doping with metal or non-metal ions.
6. Conclusions
In summary, this review provides an overview of chalcogenide and chalcogenide-based heterostructures that have been extensively used for photocatalytic H2O-splitting activities. This article summarizes the different modifications of chalcogenide materials that can improve their absorption of visible light ability, enhancing charge separation and reducing the recombination rate for water splitting. Moreover, this review also includes the mechanisms involved in the water splitting of binary, ternary, and chalcogenide-based heterostructures. Furthermore, combining different metal-based chalcogenides with other semiconductors has the potential to improve photocatalytic efficiency for generating H2 and O2 by water splitting.
Author Contributions
M.M.K.: Supervision, Conceptualization, Funding acquisition, Writing—review and editing. A.R.: Methodology, Data curation, Writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Universiti Brunei Darussalam, grant number UBD/RSCH/1.4/FICBF(b)/2021/035.
Acknowledgments
The authors would like to acknowledge the FIC block grant UBD/RSCH/1.4/FICBF(b)/2021/035 received from Universiti Brunei Darussalam, Brunei Darussalam.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khan, M.M.; Ansari, S.A.; Pradhan, D.; Ansari, M.O.; Han, D.H.; Lee, J.; Cho, M.H. Band gap engineered TiO2 nanoparticles for visible light induced photoelectrochemical and photocatalytic studies. J. Mater. Chem. A 2013, 2, 637–644. [Google Scholar] [CrossRef]
- Rahman, A.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Zinc oxide and zinc oxide-based nanostructures: Biogenic and phytogenic synthesis, properties and applications. Bioprocess Biosyst. Eng. 2021, 44, 1333–1372. [Google Scholar] [CrossRef]
- Matussin, S.; Harunsani, M.H.; Tan, A.L.; Khan, M.M. Plant-Extract-Mediated SnO2 Nanoparticles: Synthesis and Applications. ACS Sustain. Chem. Eng. 2020, 8, 3040–3054. [Google Scholar] [CrossRef]
- Khan, M.M.; Adil, S.F.; Al-Mayouf, A. Metal oxides as photocatalysts. J. Saudi Chem. Soc. 2015, 19, 462–464. [Google Scholar] [CrossRef]
- Khan, M.M.; Rahman, A.; Matussin, S.N. Recent Progress of Metal-Organic Frameworks and Metal-Organic Frameworks-Based Heterostructures as Photocatalysts. Nanomaterials 2022, 12, 2820. [Google Scholar] [CrossRef]
- Khan, M.M.; Pradhan, D.; Sohn, Y. Nanocomposites for Visible Light-Induced Photocatalysis; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Cai, Y.; Vequizo, J.J.M.; Khan, M.M.; Vargas, R.; Yubuta, K.; Yamakata, A.; Teshima, K.; Hasegawa, M. Binary flux-promoted formation of trigonal ZnIn2S4 layered crystals using ZnS-containing industrial waste and their photocatalytic performance for H2 production. Green Chem. 2018, 20, 3845–3856. [Google Scholar] [CrossRef]
- Matussin, S.N.; Rahman, A.; Khan, M.M. Role of Anions in the Synthesis and Crystal Growth of Selected Semiconductors. Front. Chem. 2022, 10, 881518. [Google Scholar] [CrossRef]
- Bouroushian, M. Chalcogens and Metal Chalcogenides. In Electrochemistry of Metal Chalcogenides; Monographs in Electrochemistry; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–56. [Google Scholar] [CrossRef]
- Rahman, A.; Khan, M.M. Chalcogenides as photocatalysts. New J. Chem. 2021, 45, 19622–19635. [Google Scholar] [CrossRef]
- Choi, Y.I.; Lee, S.; Kim, S.K.; Kim, Y.-I.; Cho, D.W.; Khan, M.M.; Sohn, Y. Fabrication of ZnO, ZnS, Ag-ZnS, and Au-ZnS microspheres for photocatalytic activities, CO oxidation and 2-hydroxyterephthalic acid synthesis. J. Alloys Compd. 2016, 675, 46–56. [Google Scholar] [CrossRef]
- Khan, M.E.; Cho, M.H. CdS-graphene Nanocomposite for Efficient Visible-light-driven Photocatalytic and Photoelectrochemical Applications. J. Colloid Interface Sci. 2016, 482, 221–232. [Google Scholar] [CrossRef]
- Khan, M.M. Chalcogenide-Based Nanomaterials as Photocatalysts; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Tian, M.-W.; Yuen, H.-C.; Yan, S.-R.; Huang, W.-L. The multiple selections of fostering applications of hydrogen energy by integrating economic and industrial evaluation of different regions. Int. J. Hydrogen Energy 2019, 44, 29390–29398. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X.; Liu, M.; Su, J.; Shen, S. Towards efficient solar-to-hydrogen conversion: Fundamentals and recent progress in copper-based chalcogenide photocathodes. Nanophotonics 2016, 5, 524–547. [Google Scholar] [CrossRef]
- Revankar, S.T. Nuclear hydrogen production. In Storage and Hybridization of Nuclear Energy: Techno-Economic Integration of Renewable and Nuclear Energy; Elsevier: Amsterdam, The Netherlands, 2018; pp. 49–117. [Google Scholar] [CrossRef]
- Yerga, R.M.N.; Alvarez-Galvan, M.C.; Del Valle, F.; De La Mano, J.A.V.; Fierro, J.L.G. Water Splitting on Semiconductor Catalysts under Visible-Light Irradiation. ChemSusChem 2009, 2, 471–485. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Domen, K. New Non-Oxide Photocatalysts Designed for Overall Water Splitting under Visible Light. J. Phys. Chem. C 2007, 111, 7851–7861. [Google Scholar] [CrossRef]
- Maeda, K.; Lu, D.; Domen, K. Direct Water Splitting into Hydrogen and Oxygen under Visible Light by using Modified TaON Photocatalysts with d0Electronic Configuration. Chem. A Eur. J. 2013, 19, 4986–4991. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.G.; Warren, E.L.; McKone, J.R.; Boettcher, S.W.; Mi, Q.; Santori, E.A.; Lewis, N.S. Solar Water Splitting Cells. Chem. Rev. 2010, 110, 6446–6473. [Google Scholar] [CrossRef]
- Li, X.; Zuo, X.; Jiang, X.; Li, D.; Cui, B.; Liu, D. Enhanced photocatalysis for water splitting in layered tin chalcogenides with high carrier mobility. Phys. Chem. Chem. Phys. 2019, 21, 7559–7566. [Google Scholar] [CrossRef]
- Kannan, S.; Vinitha, P.; Mohanraj, K.; Sivakumar, G. Antibacterial studies of novel Cu2WS4 ternary chalcogenide synthesized by hydrothermal process. J. Solid State Chem. 2018, 258, 376–382. [Google Scholar] [CrossRef]
- Sarilmaz, A.; Can, M.; Ozel, F. Ternary copper tungsten selenide nanosheets synthesized by a facile hot-injection method. J. Alloys Compd. 2017, 699, 479–483. [Google Scholar] [CrossRef]
- Saravanan, K.; Selladurai, S.; Ananthakumar, S.; Suriakarthick, R. Solvothermal synthesis of copper cadmium sulphide (CuCdS2) nanoparticles and its structural, optical and morphological properties. Mater. Sci. Semicond. Process. 2019, 93, 345–356. [Google Scholar] [CrossRef]
- Khalil, A.A.I.; El-Gawad, A.-S.H.A.; Gadallah, A.-S. Impact of silver dopants on structural, morphological, optical, and electrical properties of copper-zinc sulfide thin films prepared via sol-gel spin coating method. Opt. Mater. 2020, 109, 110250. [Google Scholar] [CrossRef]
- Gu, X.; Tan, C.; He, L.; Guo, J.; Zhao, X.; Qi, K.; Yan, Y. Mn2+ doped AgInS2 photocatalyst for formaldehyde degradation and hydrogen production from water splitting by carbon tube enhancement. Chemosphere 2022, 304, 135292. [Google Scholar] [CrossRef]
- Wang, X.; Feng, Z.; Fan, D.; Fan, F.; Li, C. Shape-Controlled Synthesis of CdS Nanostructures via a Solvothermal Method. Cryst. Growth Des. 2010, 10, 5312–5318. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, T.; Xie, Z.; Guo, C.; Jiang, W.; Chen, Y.; Xu, Y. Emission tunable AgInS2 quantum dots synthesized via microwave method for white light-emitting diodes application. Opt. Mater. 2022, 124, 111975. [Google Scholar] [CrossRef]
- Li, S.; Cai, M.; Liu, Y.; Wang, C.; Yan, R.; Chen, X. Constructing Cd0.5Zn0.5S/Bi2WO6 S-scheme heterojunction for boosted photocatalytic antibiotic oxidation and Cr(VI) reduction. Adv. Powder Mater. 2023, 2, 100073. [Google Scholar] [CrossRef]
- Thompson, M.J.; Ruberu, T.P.A.; Blakeney, K.J.; Torres, K.V.; Dilsaver, P.S.; Vela, J. Axial Composition Gradients and Phase Segregation Regulate the Aspect Ratio of Cu2ZnSnS4 Nanorods. J. Phys. Chem. Lett. 2013, 4, 3918–3923. [Google Scholar] [CrossRef][Green Version]
- Mandari, K.K.; Son, N.; Kang, M. CuS/Ag2O nanoparticles on ultrathin g-C3N4 nanosheets to achieve high performance solar hydrogen evolution. J. Colloid Interface Sci. 2022, 615, 740–751. [Google Scholar] [CrossRef]
- Yin, M.; Sun, J.; Li, Y.; Ye, Y.; Liang, K.; Fan, Y.; Li, Z. Efficient photocatalytic hydrogen evolution over MoS2/activated carbon composite sensitized by Erythrosin B under LED light irradiation. Catal. Commun. 2020, 142, 106029. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, X.; Zhang, Z.; Yang, J.; Yu, C.; Dong, S.; Xiang, M.; Qin, H. NiS2@V2O5/VS2 ternary heterojunction for a high-performance electrocatalyst in overall water splitting. Int. J. Hydrogen Energy 2022, 47, 27338–27346. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Paulraj, G.; Eswaran, M.K.; Ray, A.; Singh, N.; Jeganathan, K. VS2 wrapped Si nanowires as core-shell heterostructure photocathode for highly efficient photoelectrochemical water reduction performance. Chemosphere 2022, 302, 134708. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Zhang, D.; Pu, X.; Ge, B.; Huang, Y. Noble metal-free ternary MoS2/Zn0.5Cd0.5S/g-C3N4 heterojunction composite for highly efficient photocatalytic H2 production. Mater. Res. Bull. 2018, 110, 214–222. [Google Scholar] [CrossRef]
- Arai, T.; Senda, S.-I.; Sato, Y.; Takahashi, H.; Shinoda, K.; Jeyadevan, B.; Tohji, K. Cu-Doped ZnS Hollow Particle with High Activity for Hydrogen Generation from Alkaline Sulfide Solution under Visible Light. Chem. Mater. 2008, 20, 1997–2000. [Google Scholar] [CrossRef]
- Oliva, A. Formation of the band gap energy on CdS thin films growth by two different techniques. Thin Solid Films 2001, 391, 28–35. [Google Scholar] [CrossRef]
- Cheng, L.; Xiang, Q.; Liao, Y.; Zhang, H. CdS-Based photocatalysts. Energy Environ. Sci. 2018, 11, 1362–1391. [Google Scholar] [CrossRef]
- Li, C.; Yuan, J.; Han, B.; Shangguan, W. Synthesis and photochemical performance of morphology-controlled CdS photocatalysts for hydrogen evolution under visible light. Int. J. Hydrogen Energy 2011, 36, 4271–4279. [Google Scholar] [CrossRef]
- Garg, P.; Bhauriyal, P.; Mahata, A.; Rawat, K.S.; Pathak, B. Role of Dimensionality for Photocatalytic Water Splitting: CdS Nanotube versus Bulk Structure. ChemPhysChem 2018, 20, 383–391. [Google Scholar] [CrossRef]
- Luo, M.; Liu, Y.; Hu, J.; Liu, H.; Li, J. One-Pot Synthesis of CdS and Ni-Doped CdS Hollow Spheres with Enhanced Photocatalytic Activity and Durability. ACS Appl. Mater. Interfaces 2012, 4, 1813–1821. [Google Scholar] [CrossRef]
- Zhen, W.; Ning, X.; Yang, B.; Wu, Y.; Li, Z.; Lu, G. The enhancement of CdS photocatalytic activity for water splitting via anti-photocorrosion by coating Ni2P shell and removing nascent formed oxygen with artificial gill. Appl. Catal. B Environ. 2018, 221, 243–257. [Google Scholar] [CrossRef]
- Li, Y.-H.; Qi, M.-Y.; Li, J.-Y.; Tang, Z.-R.; Xu, Y.-J. Noble metal free CdS@CuS-NixP hybrid with modulated charge transfer for enhanced photocatalytic performance. Appl. Catal. B Environ. 2019, 257, 117934. [Google Scholar] [CrossRef]
- Yang, J.; Yan, H.; Wang, X.; Wen, F.; Wang, Z.; Fan, D.; Shi, J.; Li, C. Roles of cocatalysts in Pt–PdS/CdS with exceptionally high quantum efficiency for photocatalytic hydrogen production. J. Catal. 2012, 290, 151–157. [Google Scholar] [CrossRef]
- Yang, T.-T.; Chen, W.-T.; Hsu, Y.-J.; Wei, K.-H.; Lin, T.-Y. Interfacial Charge Carrier Dynamics in Core−Shell Au-CdS Nanocrystals. J. Phys. Chem. C 2010, 114, 11414–11420. [Google Scholar] [CrossRef]
- Tso, S.; Li, W.-S.; Wu, B.-H.; Chen, L.-J. Enhanced H2 production in water splitting with CdS-ZnO core-shell nanowires. Nano Energy 2017, 43, 270–277. [Google Scholar] [CrossRef]
- Zhang, F.; Zhuang, H.-Q.; Zhang, W.; Yin, J.; Cao, F.-H.; Pan, Y.-X. Noble-metal-free CuS/CdS photocatalyst for efficient visible-light-driven photocatalytic H2 production from water. Catal. Today 2018, 330, 203–208. [Google Scholar] [CrossRef]
- Cheng, F.; Xiang, Q. A solid-state approach to fabricate a CdS/CuS nano-heterojunction with promoted visible-light photocatalytic H2-evolution activity. RSC Adv. 2016, 6, 76269–76272. [Google Scholar] [CrossRef]
- Wu, Z.; Zhao, G.; Zhang, Y.-N.; Tian, H.; Li, D. Enhanced Photocurrent Responses and Antiphotocorrosion Performance of CdS Hybrid Derived from Triple Heterojunction. J. Phys. Chem. C 2012, 116, 12829–12835. [Google Scholar] [CrossRef]
- Holmes, M.A.; Townsend, T.K.; Osterloh, F.E. Quantum confinement controlled photocatalytic water splitting by suspended CdSe nanocrystals. Chem. Commun. 2011, 48, 371–373. [Google Scholar] [CrossRef]
- Yang, Q.; Luo, M.; Liu, K.; Cao, H.; Yan, H. Covalent organic frameworks for photocatalytic applications. Appl. Catal. B Environ. 2020, 276, 119174. [Google Scholar] [CrossRef]
- You, J.; Zhao, Y.; Wang, L.; Bao, W. Recent developments in the photocatalytic applications of covalent organic frameworks: A review. J. Clean. Prod. 2021, 291, 125822. [Google Scholar] [CrossRef]
- You, D.; Pan, Z.; Cheng, Q. COFs-Ph@CdS S-scheme heterojunctions with photocatalytic hydrogen evolution and efficient degradation properties. J. Alloys Compd. 2023, 930, 167069. [Google Scholar] [CrossRef]
- Das, A.; Han, Z.; Haghighi, M.G.; Eisenberg, R. Photogeneration of hydrogen from water using CdSe nanocrystals demonstrating the importance of surface exchange. Proc. Natl. Acad. Sci. USA 2013, 110, 16716–16723. [Google Scholar] [CrossRef]
- Park, Y.; Park, B. Effect of ligand exchange on photocurrent enhancement in cadmium selenide (CdSe) quantum dot water splitting cells. Results Phys. 2018, 11, 162–165. [Google Scholar] [CrossRef]
- Zhou, W.; Li, F.; Yang, X.; Yang, W.; Wang, C.; Cao, R.; Zhou, C.; Tian, M. Peanut-chocolate-ball-inspired construction of the interface engineering between CdS and intergrown Cd: Boosting both the photocatalytic activity and photocorrosion resistance. J. Energy Chem. 2023, 76, 75–89. [Google Scholar] [CrossRef]
- Li, K.; Pan, H.; Wang, F.; Zhang, Z.; Min, S. In-situ exsolved NiS nanoparticle-socketed CdS with strongly coupled interfaces as a superior visible-light-driven photocatalyst for hydrogen evolution. Appl. Catal. B Environ. 2023, 321, 122028. [Google Scholar] [CrossRef]
- Basu, M.; Nazir, R.; Fageria, P.; Pande, S. Construction of CuS/Au Heterostructure through a Simple Photoreduction Route for Enhanced Electrochemical Hydrogen Evolution and Photocatalysis. Sci. Rep. 2016, 6, 34738. [Google Scholar] [CrossRef]
- Chandra, M.; Bhunia, K.; Pradhan, D. Controlled Synthesis of CuS/TiO2 Heterostructured Nanocomposites for Enhanced Photocatalytic Hydrogen Generation through Water Splitting. Inorg. Chem. 2018, 57, 4524–4533. [Google Scholar] [CrossRef]
- Dubale, A.A.; Tamirat, A.G.; Chen, H.-M.; Berhe, T.A.; Pan, C.-J.; Su, W.-N.; Hwang, B.-J. A highly stable CuS and CuS–Pt modified Cu2O/CuO heterostructure as an efficient photocathode for the hydrogen evolution reaction. J. Mater. Chem. A 2015, 4, 2205–2216. [Google Scholar] [CrossRef]
- Ma, L.; Yang, D.-J.; Song, X.-P.; Li, H.-X.; Ding, S.-J.; Xiong, L.; Qin, P.-L.; Chen, X.-B. Pt Decorated (Au Nanosphere)/(CuSe Ultrathin Nanoplate) Tangential Hybrids for Efficient Photocatalytic Hydrogen Generation via Dual-Plasmon-Induced Strong VIS–NIR Light Absorption and Interfacial Electric Field Coupling. Sol. RRL 2019, 4, 1900376. [Google Scholar] [CrossRef]
- Liang, H.; Mei, J.; Sun, H.; Cao, L. Enhanced photocatalytic hydrogen evolution of CdS@CuS core-shell nanorods under visible light. Mater. Sci. Semicond. Process. 2023, 153, 107105. [Google Scholar] [CrossRef]
- Hou, J.; Huang, B.; Kong, L.; Xie, Y.; Liu, Y.; Chen, M.; Wang, Q. One-pot hydrothermal synthesis of CdS–CuS decorated TiO2 NTs for improved photocatalytic dye degradation and hydrogen production. Ceram. Int. 2021, 47, 30860–30868. [Google Scholar] [CrossRef]
- Zhuang, H.L.; Hennig, R.G. Single-Layer Group-III Monochalcogenide Photocatalysts for Water Splitting. Chem. Mater. 2013, 25, 3232–3238. [Google Scholar] [CrossRef]
- Peng, Q.; Xiong, R.; Sa, B.; Zhou, J.; Wen, C.; Wu, B.; Anpo, M.; Sun, Z. Computational mining of photocatalysts for water splitting hydrogen production: Two-dimensional InSe-family monolayers. Catal. Sci. Technol. 2017, 7, 2744–2752. [Google Scholar] [CrossRef]
- Peng, Q.; Guo, Z.; Sa, B.; Zhou, J.; Sun, Z. New gallium chalcogenides/arsenene van der Waals heterostructures promising for photocatalytic water splitting. Int. J. Hydrogen Energy 2018, 43, 15995–16004. [Google Scholar] [CrossRef]
- Gupta, U.; Rao, C. Hydrogen generation by water splitting using MoS2 and other transition metal dichalcogenides. Nano Energy 2017, 41, 49–65. [Google Scholar] [CrossRef]
- Rahman, A.; Jennings, J.R.; Tan, A.L.; Khan, M.M. Molybdenum Disulfide-Based Nanomaterials for Visible-Light-Induced Photocatalysis. ACS Omega 2022, 7, 22089–22110. [Google Scholar] [CrossRef]
- Li, M.; Cui, Z.; Li, E. Silver-modified MoS2 nanosheets as a high-efficiency visible-light photocatalyst for water splitting. Ceram. Int. 2019, 45, 14449–14456. [Google Scholar] [CrossRef]
- Yuan, Y.-J.; Chen, D.; Zhong, J.; Yang, L.-X.; Wang, J.; Liu, M.-J.; Tu, W.-G.; Yu, Z.-T.; Zou, Z.-G. Interface engineering of a noble-metal-free 2D–2D MoS2/Cu-ZnIn2S4 photocatalyst for enhanced photocatalytic H2 production. J. Mater. Chem. A 2017, 5, 15771–15779. [Google Scholar] [CrossRef]
- Hassan, M.A.; Kim, M.-W.; Johar, M.A.; Waseem, A.; Kwon, M.-K.; Ryu, S.-W. Transferred monolayer MoS2 onto GaN for heterostructure photoanode: Toward stable and efficient photoelectrochemical water splitting. Sci. Rep. 2019, 9, 20141. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, X.; Wang, S.; Rui, K.; Chen, Y.; Yu, H.; Ma, J.; Dou, S.X.; Sun, W. Heteroatom-doped MoSe 2 Nanosheets with Enhanced Hydrogen Evolution Kinetics for Alkaline Water Splitting. Chem. Asian J. 2018, 14, 301–306. [Google Scholar] [CrossRef]
- Lei, W.; Wang, F.; Pan, X.; Ye, Z.; Lu, B. Z-scheme MoO3-2D SnS nanosheets heterojunction assisted g-C3N4 composite for enhanced photocatalytic hydrogen evolutions. Int. J. Hydrogen Energy 2022, 47, 10877–10890. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, Y.; Zhou, X.; Jin, X.; Li, B.; Liu, J.; Chen, G. Cu doped SnS2 nanostructure induced sulfur vacancy towards boosted photocatalytic hydrogen evolution. Chem. Eng. J. 2020, 407, 127180. [Google Scholar] [CrossRef]
- Geng, Y.; Zou, X.; Lu, Y.; Wang, L. Fabrication of the SnS2/ZnIn2S4 heterojunction for highly efficient visible light photocatalytic H2 evolution. Int. J. Hydrogen Energy 2022, 47, 11520–11527. [Google Scholar] [CrossRef]
- Li, Y.-Y.; Wang, J.-G.; Sun, H.-H.; Hua, W.; Liu, X.-R. Heterostructured SnS2/SnO2 nanotubes with enhanced charge separation and excellent photocatalytic hydrogen production. Int. J. Hydrogen Energy 2018, 43, 14121–14129. [Google Scholar] [CrossRef]
- Mangiri, R.; Kumar, K.S.; Subramanyam, K.; Ratnakaram, Y.; Sudharani, A.; Reddy, D.A.; Vijayalakshmi, R. Boosting solar driven hydrogen evolution rate of CdS nanorods adorned with MoS2 and SnS2 nanostructures. Colloids Interface Sci. Commun. 2021, 43, 100437. [Google Scholar] [CrossRef]
- Barawi, M.; Flores, E.; Ferrer, I.J.; Ares, J.R.; Sánchez, C. Titanium trisulphide (TiS3) nanoribbons for easy hydrogen photogeneration under visible light. J. Mater. Chem. A 2015, 3, 7959–7965. [Google Scholar] [CrossRef]
- Flores, E.; Ares, J.; Sánchez, C.; Ferrer, I. Ternary transition titanium-niobium trisulfide as photoanode for assisted water splitting. Catal. Today 2018, 321–322, 107–112. [Google Scholar] [CrossRef]
- Guo, W.; Wu, D. Facile synthesis of VS4/graphene nanocomposites and their visible-light-driven photocatalytic water splitting activities. Int. J. Hydrogen Energy 2014, 39, 16832–16840. [Google Scholar] [CrossRef]
- Ikeda, S.; Aono, N.; Iwase, A.; Kobayashi, H.; Kudo, A. Cu3MS4 (M=V, Nb, Ta) and its Solid Solutions with Sulvanite Structure for Photocatalytic and Photoelectrochemical H2 Evolution under Visible-Light Irradiation. ChemSusChem 2018, 12, 1977–1983. [Google Scholar] [CrossRef]
- Li, G.; Deng, X.; Chen, P.; Wang, X.; Ma, J.; Liu, F.; Yin, S.-F. Sulphur vacancies-VS2@C3N4 drived by in situ supramolecular self-assembly for synergistic photocatalytic degradation of real wastewater and H2 production: Vacancies taming interfacial compact heterojunction and carriers transfer. Chem. Eng. J. 2022, 433, 134505. [Google Scholar] [CrossRef]
- Zhong, X.; Tang, J.; Wang, J.; Shao, M.; Chai, J.; Wang, S.; Yang, M.; Yang, Y.; Wang, N.; Wang, S.; et al. 3D heterostructured pure and N-Doped Ni3S2/VS2 nanosheets for high efficient overall water splitting. Electrochim. Acta 2018, 269, 55–61. [Google Scholar] [CrossRef]
- Kurnia, F.; Ng, Y.H.; Amal, R.; Valanoor, N.; Hart, J.N. Defect engineering of ZnS thin films for photoelectrochemical water-splitting under visible light. Sol. Energy Mater. Sol. Cells 2016, 153, 179–185. [Google Scholar] [CrossRef]
- Sánchez-Tovar, R.; Fernández-Domene, R.M.; Montañés, M.T.; Sanz-Marco, A.; Garcia-Antón, J. ZnO/ZnS heterostructures for hydrogen production by photoelectrochemical water splitting. RSC Adv. 2016, 6, 30425–30435. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.-Z.; Wu, D.-Y.; Liu, X.-H.; Zhang, R.; Liu, H.; Dong, C.-K.; Yang, J.; Kulinich, S.A.; Du, X.-W. ZnO nanosheets with atomically thin ZnS overlayers for photocatalytic water splitting. J. Mater. Chem. A 2018, 6, 9057–9063. [Google Scholar] [CrossRef]
- Lee, G.-J.; Anandan, S.; Masten, S.J.; Wu, J.J. Photocatalytic hydrogen evolution from water splitting using Cu doped ZnS microspheres under visible light irradiation. Renew. Energy 2016, 89, 18–26. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, H.; Tu, W.; Wu, S.; Chew, J.W. Effects of composition faults in ternary metal chalcogenides (Zn In2S3+, x = 1 − 5) layered crystals for visible-light-driven catalytic hydrogen generation and carbon dioxide reduction. Appl. Catal. B Environ. 2019, 256, 117810. [Google Scholar] [CrossRef]
- Fan, H.-T.; Wu, Z.; Liu, K.-C.; Liu, W.-S. Fabrication of 3D CuS@ZnIn2S4 hierarchical nanocages with 2D/2D nanosheet subunits p-n heterojunctions for improved photocatalytic hydrogen evolution. Chem. Eng. J. 2022, 433, 134474. [Google Scholar] [CrossRef]
- Ni, T.; Yang, Z.; Cao, Y.; Lv, H.; Liu, Y. Rational design of MoS2/g-C3N4/ZnIn2S4 hierarchical heterostructures with efficient charge transfer for significantly enhanced photocatalytic H2 production. Ceram. Int. 2021, 47, 22985–22993. [Google Scholar] [CrossRef]
- Guo, X.; Peng, Y.; Liu, G.; Xie, G.; Guo, Y.; Zhang, Y.; Yu, J. An Efficient ZnIn2S4@CuInS2 Core–Shell p–n Heterojunction to Boost Visible-Light Photocatalytic Hydrogen Evolution. J. Phys. Chem. C 2020, 124, 5934–5943. [Google Scholar] [CrossRef]
- Yang, W.; Ma, G.; Fu, Y.; Peng, K.; Yang, H.; Zhan, X.; Yang, W.; Wang, L.; Hou, H. Rationally designed Ti3C2 MXene@TiO2/CuInS2 Schottky/S-scheme integrated heterojunction for enhanced photocatalytic hydrogen evolution. Chem. Eng. J. 2021, 429, 132381. [Google Scholar] [CrossRef]
- Raja, A.; Son, N.; Swaminathan, M.; Kang, M. Facile synthesis of sphere-like structured ZnIn2S4-rGO-CuInS2 ternary heterojunction catalyst for efficient visible-active photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2021, 602, 669–679. [Google Scholar] [CrossRef]
- Li, Q.; Qiao, X.-Q.; Jia, Y.; Hou, D.; Li, D.-S. Amorphous CoMoS4 Nanostructure for Photocatalytic H2 Generation, Nitrophenol Reduction, and Methylene Blue Adsorption. ACS Appl. Nano Mater. 2019, 3, 68–76. [Google Scholar] [CrossRef]
- Yao, Z.; Wang, L.; Zhang, Y.; Yu, Z.; Jiang, Z. Carbon nanotube modified Zn0.83Cd0.17S nanocomposite photocatalyst and its hydrogen production under visible-light. Int. J. Hydrogen Energy 2014, 39, 15380–15386. [Google Scholar] [CrossRef]
- Lv, H.; Kong, Y.; Gong, Z.; Zheng, J.; Liu, Y.; Wang, G. Engineering multifunctional carbon black interface over Mn0.5Cd0.5S nanoparticles/CuS nanotubes heterojunction for boosting photocatalytic hydrogen generation activity. Appl. Surf. Sci. 2022, 604, 154513. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, Q.; Xu, N.; Deng, K.; Kan, E. The Janus structures of group-III chalcogenide monolayers as promising photocatalysts for water splitting. Appl. Surf. Sci. 2019, 478, 522–531. [Google Scholar] [CrossRef]
- Hu, L.; Wei, D. Janus Group-III Chalcogenide Monolayers and Derivative Type-II Heterojunctions as Water-Splitting Photocatalysts with Strong Visible-Light Absorbance. J. Phys. Chem. C 2018, 122, 27795–27802. [Google Scholar] [CrossRef]
- Wang, P.; Zong, Y.; Liu, H.; Wen, H.; Wu, H.-B.; Xia, J.-B. Highly efficient photocatalytic water splitting and enhanced piezoelectric properties of 2D Janus group-III chalcogenides. J. Mater. Chem. C 2021, 9, 4989–4999. [Google Scholar] [CrossRef]
- Ahmad, I.; Shahid, I.; Ali, A.; Gao, L.; Cai, J. Electronic, mechanical, optical and photocatalytic properties of two-dimensional Janus XGaInY (X, Y;= S, Se and Te) monolayers. RSC Adv. 2021, 11, 17230–17239. [Google Scholar] [CrossRef]
- Chu, S.; Li, W.; Yan, Y.; Hamann, T.; Shih, I.; Wang, D.; Mi, Z. Roadmap on solar water splitting: Current status and future prospects. Nano Futur. 2017, 1, 022001. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).