Modified BaMnO3-Based Catalysts for Gasoline Particle Filters (GPF): A Preliminary Study
Abstract
1. Introduction
- *
- *
- *
- *
2. Results and Discussion
2.1. Characterization
2.1.1. Copper Content (ICP-OES) and Textural Properties
2.1.2. Crystal Structure: XRD
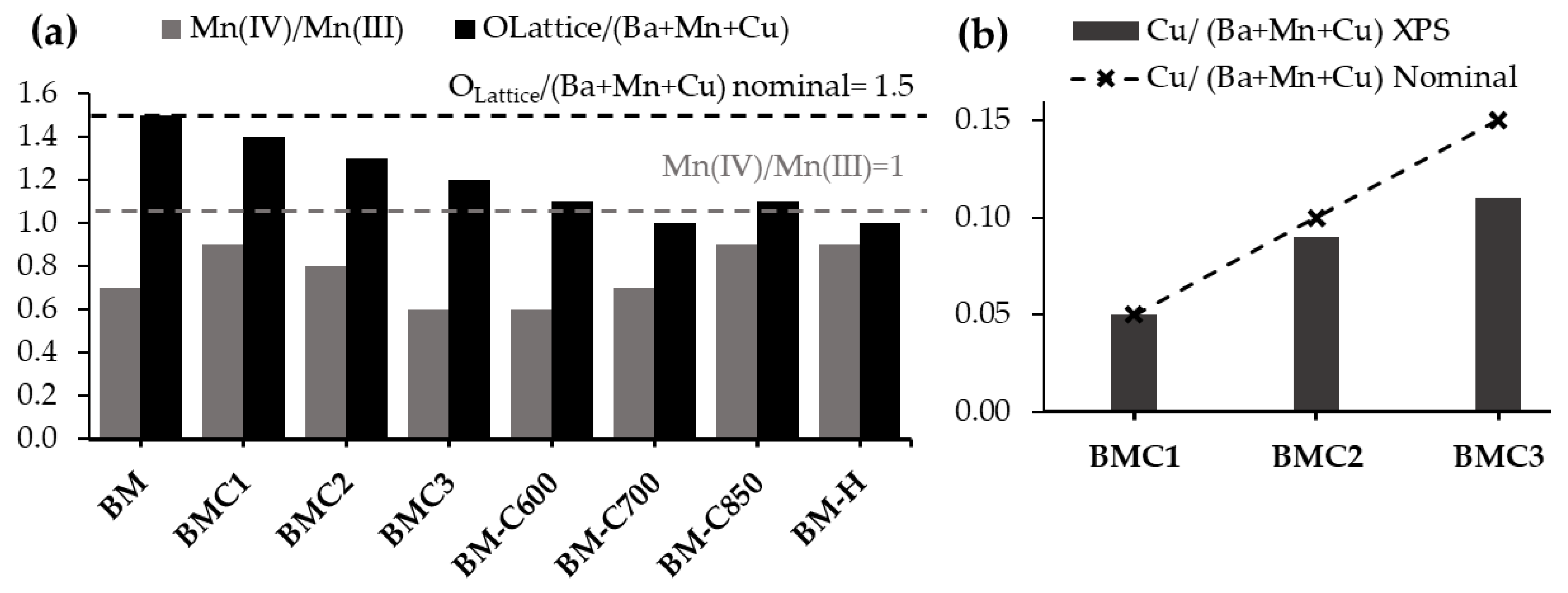
2.1.3. Surface Composition
2.1.4. Reducibility: H2-TPR
2.1.5. O2 Desorption: O2-TPD
2.2. Activity Tests
2.2.1. Preliminary Phase: TG-MS Experimental System
2.2.2. Second Phase: GC Experimental System
3. Materials and Methods
3.1. Synthesis of Catalysts
3.1.1. Conventional Sol-Gel Method for the Synthesis of BaMn1−xCuxO3 Series [45]
3.1.2. Modified Sol-Gel Method for The Synthesis of BM-CX Series [46]
3.1.3. Hydrothermal Method for The Synthesis of BM-H
3.2. Characterization Techniques
3.3. Activity Test
4. Conclusions
- All synthesis methods allow the formation of BaMnO3 solids with a perovskite-like structure, and only BMC3 presents the polytype structure as the majority phase due to the partial substitution of manganese by copper into the perovskite lattice.
- Mn (III) and Mn (IV) oxidation states coexist in all catalysts, with Mn (III) being the main oxidation state. The presence of copper causes an increase in the Mn (III) and in the amount of oxygen surface vacancies.
- Both strategies, the insertion of copper and using two different synthesis methods (hydrothermal and modified sol-gel synthesis), promote the formation of oxygen vacancies, the manganese reducibility and, hence, an improvement in the oxygen mobility.
- In the most favorable reaction conditions (preliminary study), a relationship was observed between the ability to catalyze the soot oxidation and the amount of β-O2 evolved, showing that BM-H and BMC3 samples show the best performances under the hardest GDI scenarios. In the absence of oxygen in the reaction atmosphere, the oxygen supplied by the perovskite (i.e., the oxygen evolved during the O2-TPD experiments) allows a high selectivity to CO2, since the passive oxidation of soot is carried out and BaMnO3 catalyzes the CO to CO2 oxidation. In the presence of a low amount of oxygen (1% O2 in He), the catalysts present a high activity to catalyze the CO to CO2 oxidation.
- In the reactions carried out in more realistic conditions (second phase), all BaMnO3-based catalysts, independently of the copper amount or the method used for synthesis, were active for soot oxidation in the absence of oxygen (He). In the presence of low amounts of oxygen (1% O2/He), the catalysts present a high CO2 selectivity.
- All BaMnO3 samples tested seem to be active for the soot oxidation in the regular operation conditions for GDI engines [41], i.e., in the absence of oxygen, due to their capacity to supply oxygen coming from the perovskite lattice. However, a revealing effect of the synthesis method was not observed.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A


References
- Pacella, M.; Garbujo, A.; Fabro, J.; Guiotto, M.; Xin, Q.; Natile, M.M.; Canu, P.; Cool, P.; Glisenti, A. PGM-Free CuO/LaCoO3 Nanocomposites: New Opportunities for TWC Application. Appl. Catal. B Environ. 2018, 227, 446–458. [Google Scholar] [CrossRef]
- Glisenti, A.; Pacella, M.; Guiotto, M.; Natile, M.M.M.; Canu, P. Largely Cu-Doped LaCo1−xCuxO3 Perovskites for TWC: Toward New PGM-Free Catalysts. Appl. Catal. B Environ. 2016, 180, 94–105. [Google Scholar] [CrossRef]
- Wu, J.; Dacquin, J.P.; Cordier, C.; Dujardin, C.; Granger, P. Optimization of the Composition of Perovskite Type Materials for Further Elaboration of Four-Way Catalysts for Gasoline Engine. Top. Catal. 2019, 62, 368–375. [Google Scholar] [CrossRef]
- Yi, Y.; Liu, H.; Chu, B.; Qin, Z.; Dong, L.; He, H.; Tang, C.; Fan, M.; Bin, L. Catalytic Removal NO by CO over LaNi0.5M0.5O3 (M = Co, Mn, Cu) Perovskite Oxide Catalysts: Tune Surface Chemical Composition to Improve N2 Selectivity. Chem. Eng. J. 2019, 369, 511–521. [Google Scholar] [CrossRef]
- Wu, J.; Dacquin, J.P.; Djelal, N.; Cordier, C.; Dujardin, C.; Granger, P. Calcium and Copper Substitution in Stoichiometric and La-Deficient LaFeO3 Compositions: A Starting Point in next Generation of Three-Way-Catalysts for Gasoline Engines. Appl. Catal. B Environ. 2021, 282, 119621. [Google Scholar] [CrossRef]
- Pinto, D.; Glisenti, A. Pulsed Reactivity on LaCoO3-Based Perovskites: A Comprehensive Approach to Elucidate the CO Oxidation Mechanism and the Effect of Dopants. Catal. Sci. Technol. 2019, 9, 2749–2757. [Google Scholar] [CrossRef]
- De Zanet, A.; Peron, G.; Natile, M.M.; Vittadini, A.; Glisenti, A. Synthesis and Development of Four Way Catalysts Starting from Critical Raw Material Free Perovskites: Influence of Doping and Synthesis Conditions. Top. Catal. 2019, 62, 237–243. [Google Scholar] [CrossRef]
- Bartel, C.J.; Sutton, C.; Goldsmith, B.R.; Ouyang, R.; Musgrave, C.B.; Ghiringhelli, L.M.; Scheffler, M. New Tolerance Factor to Predict the Stability of Perovskite Oxides and Halides. Sci. Adv. 2019, 5, eaav0693. [Google Scholar] [CrossRef] [PubMed]
- Peña, M.A.; Fierro, J.L.G. Chemical Structures and Performance of Perovskite Oxides. Chem. Rev. 2001, 101, 1981–2017. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Villanueva, A.; Alamdari, H.; Kaliaguine, S. Catalytic Reduction of NO by Propene over LaCo1−xCuxO3 Perovskites Synthesized by Reactive Grinding. Appl. Catal. B Environ. 2006, 64, 220–233. [Google Scholar] [CrossRef]
- Yamazoe, N.; Teraoka, Y. Oxidation Catalysis of Perovskites-Relationship to Bulk Structure and Composition (Valency, Defect, etc.). Catal. Today 1990, 8, 175–199. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Huang, C.; Zhu, Y.; Wang, X.; Liu, X.; Wang, J.; Zhang, T. Sn Promoted BaFeO3−Δ Catalysts for N2O Decomposition: Optimization of Fe Active Centers. J. Catal. 2017, 347, 9–20. [Google Scholar] [CrossRef]
- Giménez-Mañogil, J.; Bueno-López, A.; García-García, A. Preparation, Characterisation and Testing of CuO/Ce0.8Zr0.2O2 Catalysts for NO Oxidation to NO2 and Mild Temperature Diesel Soot Combustion. Appl. Catal. B Environ. 2014, 152–153, 99–107. [Google Scholar] [CrossRef]
- Wang, L.; Fang, S.; Feng, N.; Wan, H.; Guan, G. Efficient Catalytic Removal of Diesel Soot over Mg Substituted K/La0.8Ce0.2CoO3 Perovskites with Large Surface Areas. Chem. Eng. J. 2016, 293, 68–74. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Bueno-López, A.; Illán-Gómez, M.J.; Trawczynski, J. Potassium-Copper Perovskite Catalysts for Mild Temperature Diesel Soot Combustion. Appl. Catal. A Gen. 2014, 485, 214–221. [Google Scholar] [CrossRef]
- López-Suárez, F.E.; Parres-Esclapez, S.; Bueno-López, A.; Illán-Gómez, M.J.; Ura, B.; Trawczynski, J. Role of Surface and Lattice Copper Species in Copper-Containing (Mg/Sr)TiO3 Perovskite Catalysts for Soot Combustion. Appl. Catal. B Environ. 2009, 93, 82–89. [Google Scholar] [CrossRef]
- Onrubia-Calvo, J.A.; Pereda-Ayo, B.; De-La-Torre, U.; González-Velasco, J.R. Strontium Doping and Impregnation onto Alumina Improve the NOx Storage and Reduction Capacity of LaCoO3 Perovskites. Catal. Today 2019, 333, 208–218. [Google Scholar] [CrossRef]
- Schwickardi, M.; Johann, T.; Schmidt, W.; Schüth, F. High-Surface-Area Oxides Obtained by an Activated Carbon Route. Chem. Mater. 2002, 14, 3913–3919. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Liu, S.; Weng, D.; Zhang, H.; Ran, R. Formation of BaMnO3 in Ba/MnOx–CeO2 Catalyst upon the Hydrothermal Ageing and Its Effects on Oxide Sintering and Soot Oxidation Activity. Catal. Today 2015, 253, 83–88. [Google Scholar] [CrossRef]
- Nguyen, S.V.; Szabo, V.; Trong On, D.; Kaliaguine, S. Mesoporous Silica Supported LaCoO3 Perovskites as Catalysts for Methane Oxidation. Microporous Mesoporous Mater. 2002, 54, 51–61. [Google Scholar] [CrossRef]
- Yan, X.; Huang, Q.; Li, B.; Xu, X.; Chen, Y.; Zhu, S.; Shen, S. Catalytic Performance of LaCo0.5M0.5O3 (M=Mn, Cr, Fe, Ni, Cu) Perovskite-Type Oxides and LaCo0.5Mn0.5O3 pupported on Cordierite for CO Oxidation. J. Ind. Eng. Chem. 2013, 19, 561–565. [Google Scholar] [CrossRef]
- Schneider, R.; Kießling, D.; Wendt, G. Cordierite Monolith Supported Perovskite-Type Oxides—Catalysts for the Total Oxidation of Chlorinated Hydrocarbons. Appl. Catal. B Environ. 2000, 28, 187–195. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, X.; Tan, M.; Zou, X.; Ding, W.; Lu, X. Perovskite LaNiO3 Nanocrystals inside SBA-15 Silica: High Stability and Anti-Coking Performance in the Pre-Reforming of Liquefied Petroleum Gas at a Low Steam-to-Carbon Molar Ratio. ChemCatChem 2016, 8, 1055–1058. [Google Scholar] [CrossRef]
- Wu, Y.; Chu, B.; Zhang, M.; Yi, Y.; Dong, L.; Fan, M.; Jin, G.; Zhang, L.; Li, B. Influence of Calcination Temperature on the Catalytic Properties of LaCu0.25Co0.75O3 Catalysts in NOx Reduction. Appl. Surf. Sci. 2019, 481, 1277–1286. [Google Scholar] [CrossRef]
- Zhuang, S.; Liu, Y.; Zeng, S.; Lv, J.; Chen, X.; Zhang, J. A Modified Sol-Gel Method for Low-Temperature Synthesis of Homogeneous Nanoporous La1−xSrxMnO3 with Large Specific Surface Area. J. Sol-Gel Sci. Technol. 2016, 77, 109–118. [Google Scholar] [CrossRef]
- Milt, V.G.; Ulla, M.A.; Miró, E.E. NOx Trapping and Soot Combustion on BaCoO3-y Perovskite: LRS and FTIR Characterization. Appl. Catal. B Environ. 2005, 57, 13–21. [Google Scholar] [CrossRef]
- Zhu, H.; Zhang, P.; Dai, S. Recent Advances of Lanthanum-Based Perovskite Oxides for Catalysis. ACS Catal. 2015, 5, 6370–6385. [Google Scholar] [CrossRef]
- Tanaka, H.; Misono, M. Advances in Designing Perovskite Catalysts. Curr. Opin. Solid State Mater. Sci. 2001, 5, 381–387. [Google Scholar] [CrossRef]
- Gunasekaran, N.; Rajadurai, S.; Carberry, J.J.; Bakshi, N.; Alcock, C.B. Surface Characterization and Catalytic Properties of La1-XAxMO3 Perovskite Type Oxides. Part I. Studies on La0.95Ba0.05MO3 (M = Mn, Fe or Co) Oxides. Solid State Ionics 1994, 73, 289–295. [Google Scholar] [CrossRef]
- Rojas, M.L.; Fierro, J.L.G.; Tejuca, L.G.; Bell, T.A. Preparation and Characterization of LaMn1−xCuxO3+Λ; Perovskite Oxides. J. Catal. 1990, 124, 41–51. [Google Scholar] [CrossRef]
- Royer, S.; Bérubé, F.; Kaliaguine, S. Effect of the Synthesis Conditions on the Redox and Catalytic Properties in Oxidation Reactions of LaCo1-XFexO3. Appl. Catal. A Gen. 2005, 282, 273–284. [Google Scholar] [CrossRef]
- Roy, C.; Budhani, R.C. Raman, Infrared and X-Ray Diffraction Study of Phase Stability in La1−xBaxMnO3 Doped Manganites. J. Appl. Phys. 1999, 85, 3124–3131. [Google Scholar] [CrossRef]
- Caignaert, V.; Hervieu, M.; Domenges, B.; Nguyen, N. BaMn1−xFex03−γ, An Oxygen-Deficient 6H’ Oxide: Electron Microscopy, Powder Neutron Diffraction, and Mössbauer Study. J. Solid State Chem. 1988, 73, 107–117. [Google Scholar] [CrossRef]
- Patcas, F.; Buciuman, F.C.; Zsako, J. Oxygen Non-Stoichiometry and Reducibility of B-Site Substituted Lanthanum Manganites. Thermochim. Acta 2000, 360, 71–76. [Google Scholar] [CrossRef]
- Khaskheli, A.A.; Xu, L.; Liu, D. Manganese Oxide-Based Catalysts for Soot Oxidation: A Review on the Recent Advances and Future Directions. Energy Fuels 2022, 36, 7362–7381. [Google Scholar] [CrossRef]
- Zhang, R.; Villanueva, A.; Alamdari, H.; Kaliaguine, S. SCR of NO by Propene over Nanoscale LaMn1−xCuxO3 Perovskites. Appl. Catal. A Gen. 2006, 307, 85–97. [Google Scholar] [CrossRef]
- Dhakad, M.; Rayalu, S.S.; Kumar, R.; Doggali, P.; Bakardjieva, S.; Subrt, J.; Mitsuhashi, T.; Haneda, H.; Labhsetwar, N. Low Cost, Ceria Promoted Perovskite Type Catalysts for Diesel Soot Oxidation. Catal. Lett. 2008, 121, 137–143. [Google Scholar] [CrossRef]
- Shangguan, W.F.F.; Teraoka, Y.; Kagawa, S. Kinetics of Soot-O2, Soot-NO and Soot-O2-NO Reactions over Spinel-Type CuFe2O4 Catalyst. Appl. Catal. B Environ. 1997, 12, 237–247. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, L.; Fu, M.; Mao, L.; Zhao, X.; Zhang, X.; Xiao, Y.; Dong, G. Effect of A-Site Substitution on the Simultaneous Catalytic Removal of NOx and Soot by LaMnO3 Perovskites. New J. Chem. 2019, 43, 11684–11691. [Google Scholar] [CrossRef]
- Boger, T.; Rose, D.; Nicolin, P.; Gunasekaran, N.; Glasson, T. Oxidation of Soot (Printex®®U) in Particulate Filters Operated on Gasoline Engines. Emiss. Control Sci. Technol. 2015, 1, 49–63. [Google Scholar] [CrossRef]
- Martinovic, F.; Galletti, C.; Bensaid, S.; Pirone, R.; Deorsola, F.A. Soot Oxidation in Low-O2 and O2-Free Environments by Lanthanum-Based Perovskites: Structural Changes and the Effect of Ag Doping. Catal. Sci. Technol. 2022, 12, 5453–5464. [Google Scholar] [CrossRef]
- Matarrese, R. Catalytic Materials for Gasoline Particulate Filters Soot Oxidation. Catalysts 2021, 11, 890. [Google Scholar] [CrossRef]
- Hernández, W.Y.Y.; Tsampas, M.N.N.; Zhao, C.; Boreave, A.; Bosselet, F.; Vernoux, P. La/Sr-Based Perovskites as Soot Oxidation Catalysts for Gasoline Particulate Filters. Catal. Today 2015, 258, 525–534. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.-S.S.; Illán-Gómez, M.-J.J. Copper Doped BaMnO3 Perovskite Catalysts for NO Oxidation and NO2-Assisted Diesel Soot Removal. RSC Adv. 2017, 7, 35228–35238. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Improving the Performance of BaMnO3 Perovskite as Soot Oxidation Catalyst Using Carbon Black during Sol-Gel Synthesis. Nanomaterials 2022, 12, 219. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Analyzing the Role of Copper in the Soot Oxidation Performance of BaMnO3-Perovskite-Based Catalyst Obtained by Modified Sol-Gel Synthesis. Fuel 2022, 328, 125258. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Can, F.; Courtois, X.; Batiot-Dupeyrat, C.; Laassiri, S.; Alamdari, H. Perovskites as Substitutes of Noble Metals for Heterogeneous Catalysis: Dream or Reality. Chem. Rev. 2014, 114, 10292–10368. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, G.; Liu, Y. Perovskite-Type Oxides as the Catalyst Precursors for Preparing Supported Metallic Nanocatalysts: A Review. Ind. Eng. Chem. Res. 2018, 57, 1–17. [Google Scholar] [CrossRef]
- Kim, C.H.; Qi, G.; Dahlberg, K.; Li, W. Strontium-Doped Perovskites Rival Platinum Catalysts for Treating NOx in Simulated Diesel Exhaust. Science 2010, 327, 1624–1627. [Google Scholar] [CrossRef]
- Chen, J.; Shen, M.; Wang, X.; Qi, G.; Wang, J.; Li, W. The Influence of Nonstoichiometry on LaMnO3 Perovskite for Catalytic NO Oxidation. Appl. Catal. B Environ. 2013, 134–135, 251–257. [Google Scholar] [CrossRef]
- Niu, J.; Deng, J.; Liu, W.; Zhang, L.; Wang, G.; Dai, H.; He, H.; Zi, X. Nanosized Perovskite-Type Oxides La1−xSrxMO3−δ (M = Co, Mn; x = 0, 0.4) for the Catalytic Removal of Ethylacetate. Catal. Today 2007, 126, 420–429. [Google Scholar] [CrossRef]
- Di Castro, V.; Polzonetti, G. XPS Study of MnO Oxidation. J. Electron Spectros. Relat. Phenomena 1989, 48, 117–123. [Google Scholar] [CrossRef]
- Afzal, S.; Quan, X.; Zhang, J. High Surface Area Mesoporous Nanocast LaMO3 (M = Mn, Fe) Perovskites for Efficient Catalytic Ozonation and an Insight into Probable Catalytic Mechanism. Appl. Catal. B Environ. 2017, 206, 692–703. [Google Scholar] [CrossRef]
- Najjar, H.; Lamonier, J.F.; Mentré, O.; Giraudon, J.M.; Batis, H. Optimization of the Combustion Synthesis towards Efficient LaMnO3+y Catalysts in Methane Oxidation. Appl. Catal. B Environ. 2011, 106, 149–159. [Google Scholar] [CrossRef]
- Najjar, H.; Batis, H. La–Mn Perovskite-Type Oxide Prepared by Combustion Method: Catalytic Activity in Ethanol Oxidation. Appl. Catal. A Gen. 2010, 383, 192–201. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Hua, W.; Guo, Y.; Lu, G.; Gil, S.; Giroir-Fendler, A. Relationship between Catalytic Deactivation and Physicochemical Properties of LaMnO3 Perovskite Catalyst during Catalytic Oxidation of Vinyl Chloride. Appl. Catal. B Environ. 2016, 186, 173–183. [Google Scholar] [CrossRef]
- Merino, N.A.; Barbero, B.P.; Eloy, P.; Cadús, L.E. La1−xCaxCoO3 Perovskite-Type Oxides: Identification of the Surface Oxygen Species by XPS. Appl. Surf. Sci. 2006, 253, 1489–1493. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; López-Suárez, F.E.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. Tailoring the Properties of BaTi0.8Cu0.2O3 Catalyst Selecting the Synthesis Method. Appl. Catal. A Gen. 2016, 519, 7–15. [Google Scholar] [CrossRef]
- Albaladejo-Fuentes, V.; López-Suárez, F.E.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. BaTi1−xCuxO3 Perovskites: The Effect of Copper Content in the Properties and in the NOx Storage Capacity. Appl. Catal. A Gen. 2014, 488, 189–199. [Google Scholar] [CrossRef]
- Bnciuman, F.C.; Patcas, F.; Zsakó, J. TPR-Study of Substitution Effects on Reducibility and Oxidative Non-Stoichiometry of La0.8A’0.2MnO3+δ Perovskites. J. Therm. Anal. Calorim. 2000, 61, 819–825. [Google Scholar] [CrossRef]
- Irusta, S.; Pina, M.P.; Menéndez, M.; Santamaría, J. Catalytic Combustion of Volatile Organic Compounds over La-Based Perovskites. J. Catal. 1998, 179, 400–412. [Google Scholar] [CrossRef]
- Irusta, S.; Pina, M.P.; Menéndez, M.; Santamaría, J. Development and Application of Perovskite-Based Catalytic Membrane Reactors. Catal. Lett. 1998, 54, 69–78. [Google Scholar] [CrossRef]
- Peron, G.; Glisenti, A. Perovskites as Alternatives to Noble Metals in Automotive Exhaust Abatement: Activation of Oxygen on LaCrO3 and LaMnO3. Top. Catal. 2019, 62, 244–251. [Google Scholar] [CrossRef]
- Tien-Thao, N.; Alamdari, H.; Zahedi-Niaki, M.H.H.; Kaliaguine, S. LaCo1−xCuxO3−δ Perovskite Catalysts for Higher Alcohol Synthesis. Appl. Catal. A Gen. 2006, 311, 204–212. [Google Scholar] [CrossRef]
- Levasseur, B.; Kaliaguine, S. Effect of the Rare Earth in the Perovskite-Type Mixed Oxides AMnO3 (A = Y, La, Pr, Sm, Dy) as Catalysts in Methanol Oxidation. J. Solid State Chem. 2008, 181, 2953–2963. [Google Scholar] [CrossRef]
- Najjar, H.; Batis, H. Development of Mn-Based Perovskite Materials: Chemical Structure and Applications. Catal. Rev. 2016, 58, 371–438. [Google Scholar] [CrossRef]
- Kalogirou, M.; Samaras, Z. A Thermogravimetic Kinetic Study of Uncatalyzed Diesel Soot Oxidation. J. Therm. Anal. Calorim. 2009, 98, 215–224. [Google Scholar] [CrossRef]
- Hernández, W.Y.; Lopez-Gonzalez, D.; Ntais, S.; Zhao, C.; Boréave, A.; Vernoux, P. Silver-Modified Manganite and Ferrite Perovskites for Catalyzed Gasoline Particulate Filters. Appl. Catal. B Environ. 2018, 226, 202–212. [Google Scholar] [CrossRef]
- Torregrosa-Rivero, V.; Moreno-Marcos, C.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. BaFe1−xCuxO3 Perovskites as Active Phase for Diesel (DPF) and Gasoline Particle Filters (GPF). Nanomaterials 2019, 9, 1551. [Google Scholar] [CrossRef]
- Moreno-Marcos, C.; Torregrosa-Rivero, V.; Albaladejo-Fuentes, V.; Sánchez-Adsuar, M.S.; Illán-Gómez, M.J. BaFe1−xCuxO3 Perovskites as Soot Oxidation Catalysts for Gasoline Particulate Filters (GPF): A Preliminary Study. Top. Catal. 2019, 62, 413–418. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J.; Liu, Y.; Bai, B.; Wang, Y.; Li, X.; Xie, S.; Li, J. Three-Dimensionally Ordered Macroporous La0.6Sr0.4MnO3 with High Surface Areas: Active Catalysts for the Combustion of Methane. J. Catal. 2013, 307, 327–339. [Google Scholar] [CrossRef]
- Uppara, H.P.; Pasuparthy, J.S.; Pradhan, S.; Singh, S.K.; Labhsetwar, N.K.; Dasari, H. The Comparative Experimental Investigations of SrMn(Co3+/Co2+)O3±δ and SrMn(Cu2+)O3±δ Perovskites towards Soot Oxidation Activity. Mol. Catal. 2020, 482, 110665. [Google Scholar] [CrossRef]
- Gross, M.S.; Ulla, M.A.; Querini, C.A. Diesel Particulate Matter Combustion with CeO2 as Catalyst. Part I: System Characterization and Reaction Mechanism. J. Mol. Catal. A Chem. 2012, 352, 86–94. [Google Scholar] [CrossRef]









| Catalyst | Composition | Synthesis Procedure | Tcal (°C) | Nominal Cu (wt%) | Cu-ICP-OES (wt%) | SBET (m2·g−1) |
|---|---|---|---|---|---|---|
| BM | BaMnO3 | Conventional Sol-gel | 850 | - | - | 5 |
| BMC1 | BaMn0.9Cu0.1O3 | 2.6 | 2.4 | 4 | ||
| BMC2 | BaMn0.8Cu0.2O3 | 5.2 | 4.8 | 4 | ||
| BMC3 | BaMn0.7Cu0.3O3 | 7.8 | 7.6 | 4 | ||
| BM-C600 | BaMnO3 | Modified sol-gel | 600 | - | - | 20 |
| BM-C700 | BaMnO3 | 700 | - | - | 21 | |
| BM-C850 | BaMnO3 | 850 | - | - | 7 | |
| BM-H | Ba0.9MnO3 | Hydrothermal | 600 | - | - | 10 |
| Catalyst | Identified Crystal Phases (XRD) | Average Crystal Size (nm) a | Lattice Parameters b | |
|---|---|---|---|---|
| a (nm) | c (nm) | |||
| BM | BaMnO3 hexagonal Ba3Mn2O8 | 40 | 5.703 | 4.831 |
| BMC1 | BaMnO3 hexagonal BaMnO3 polytype | 39 (38) | 5.698 (5.774) | 4.808 (4.364) |
| BMC2 | BaMnO3 polytype BaMnO3 hexagonal, CuO | 43 (36) | 5.695 (5.784) | 4.811 (4.365) |
| BMC3 | BaMnO3 polytype, CuO | (33) | (5.792) | (4.368) |
| BM-C600 | BaMnO3 hexagonal Ba3Mn2O8 BaCO3 | 27 | 5.693 | 4.803 |
| BM-C700 | BaMnO3 hexagonal Ba3Mn2O8 BaCO3 | 31 | 5.693 | 4.798 |
| BM-C850 | BaMnO3 hexagonal Ba3Mn2O8 BaCO3 | 33 | 5.693 | 4.805 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torregrosa-Rivero, V.; Sánchez-Adsuar, M.-S.; Illán-Gómez, M.-J. Modified BaMnO3-Based Catalysts for Gasoline Particle Filters (GPF): A Preliminary Study. Catalysts 2022, 12, 1325. https://doi.org/10.3390/catal12111325
Torregrosa-Rivero V, Sánchez-Adsuar M-S, Illán-Gómez M-J. Modified BaMnO3-Based Catalysts for Gasoline Particle Filters (GPF): A Preliminary Study. Catalysts. 2022; 12(11):1325. https://doi.org/10.3390/catal12111325
Chicago/Turabian StyleTorregrosa-Rivero, Verónica, María-Salvadora Sánchez-Adsuar, and María-José Illán-Gómez. 2022. "Modified BaMnO3-Based Catalysts for Gasoline Particle Filters (GPF): A Preliminary Study" Catalysts 12, no. 11: 1325. https://doi.org/10.3390/catal12111325
APA StyleTorregrosa-Rivero, V., Sánchez-Adsuar, M.-S., & Illán-Gómez, M.-J. (2022). Modified BaMnO3-Based Catalysts for Gasoline Particle Filters (GPF): A Preliminary Study. Catalysts, 12(11), 1325. https://doi.org/10.3390/catal12111325






