Environmentally Safe Magnetic Nanocatalyst for the Production of Biodiesel from Pongamia pinnata Oil
Abstract
1. Introduction
2. Results and Discussion
2.1. Effect of Catalysts Concentration on Biodiesel Yield
2.2. Effect of Oil to Methanol Ratio
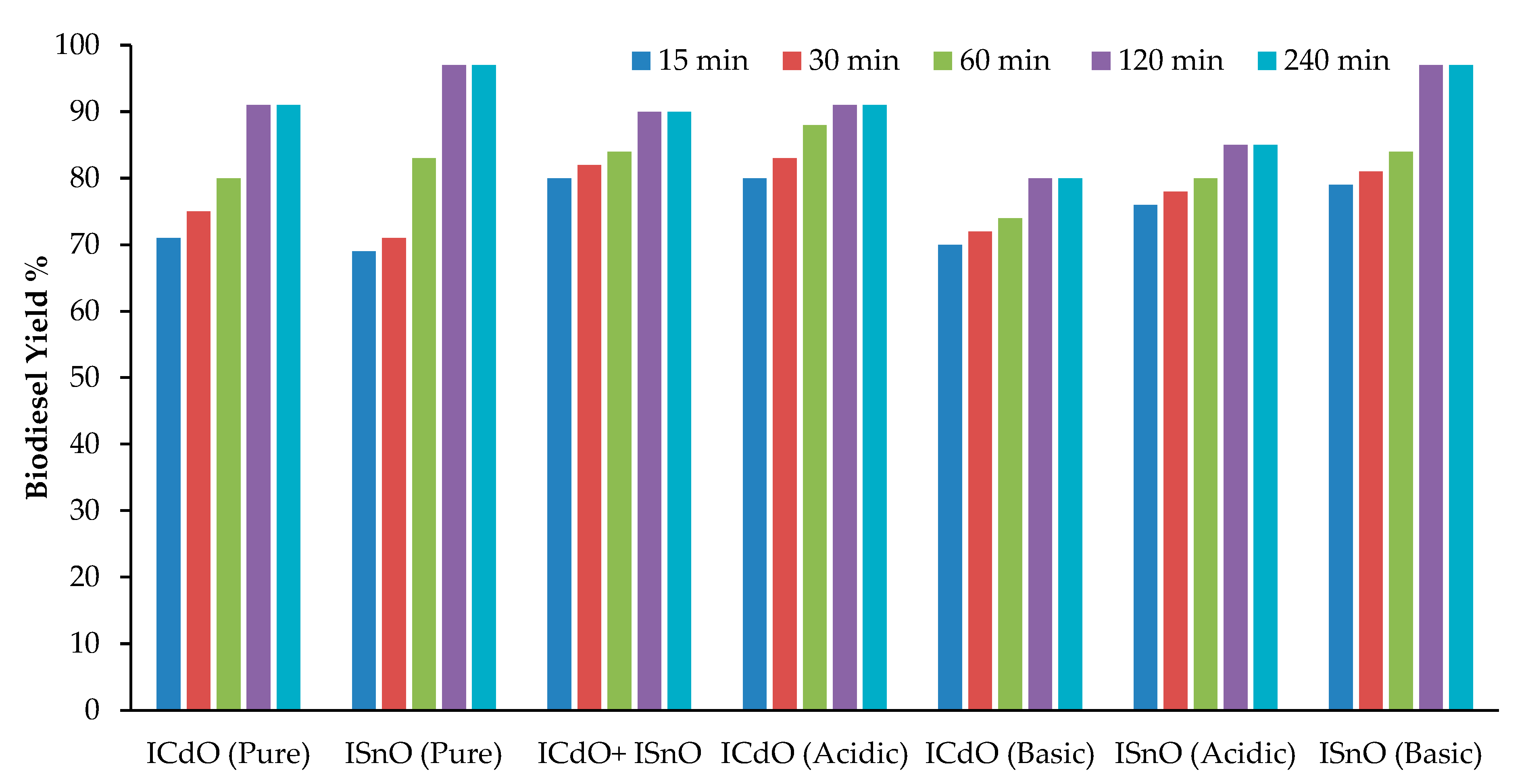
2.3. Effect of Reaction Time
2.4. Effect of Reaction Temperature
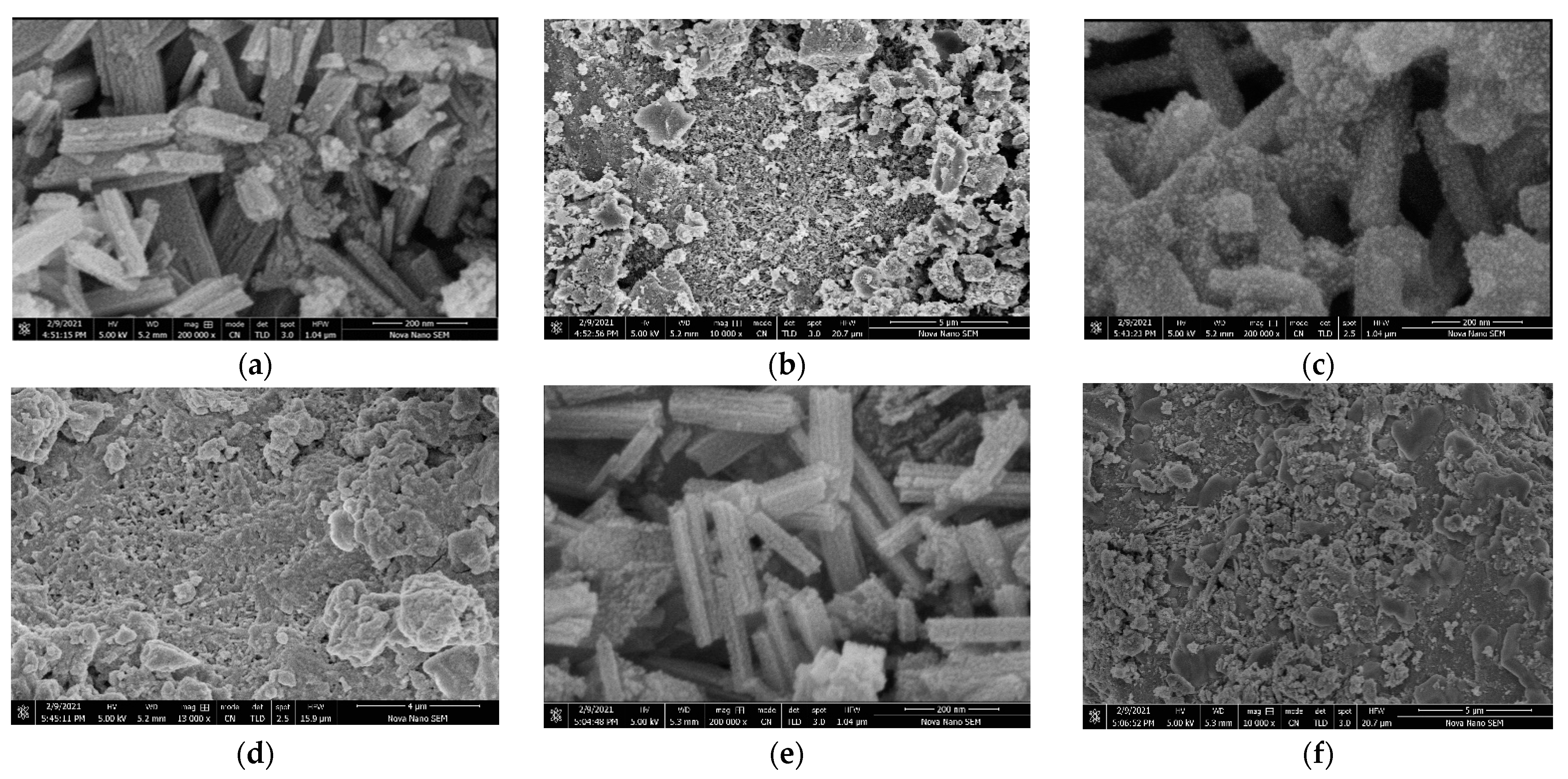
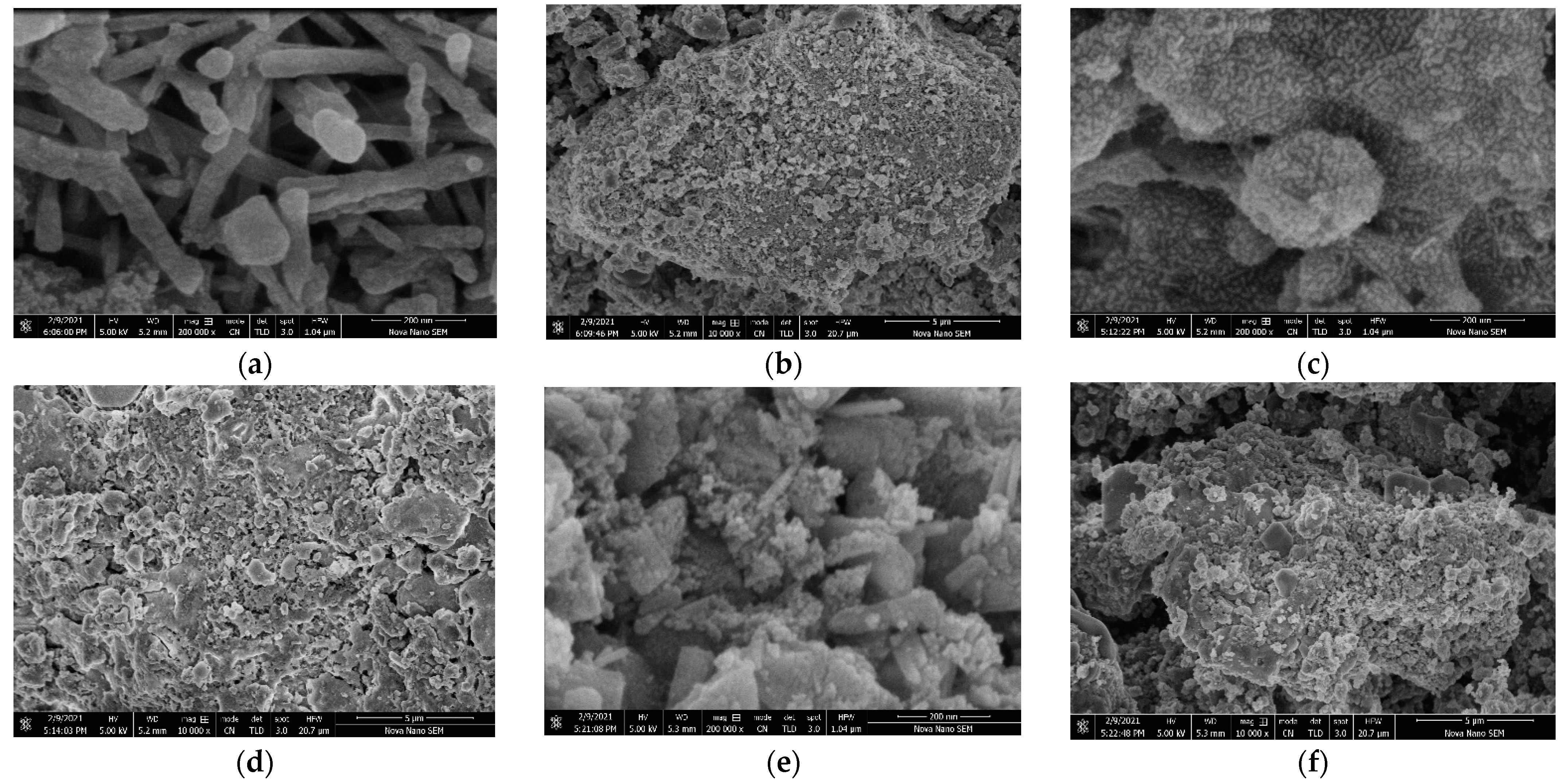
2.5. SEM Analysis of Catalysts
2.6. Recyclability and Leaching of the Catalysts
2.7. Gas Chromatographic-Mass Spectrometric (GC-MS) Analysis of Pongamia pinnata Seed Oil
2.8. Assessment of Fuel Quality Parameters
3. Materials and Methods
3.1. Materials
3.2. Extraction of Oil
3.3. Preparation of Cadmium and Tin Based Magnetic Nano-Catalysts
3.4. Modification of Nano-Catalysts
3.5. Transesterification
3.6. Surface Morphology of Catalysts
3.7. Measurement of Physiochemical Properties of Biodiesel
3.7.1. Determination of Iodine Value
3.7.2. Determination of Saponification Value
3.7.3. Determination of Free Fatty Acid Contents and Acid Value
3.7.4. Determination of Cetene Number
3.8. Stability and Durability Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thangaraj, B.; Solomon, P.R.; Muniyandi, B.; Ranganathan, S.; Lin, L. Catalysis in biodiesel production—A review. Clean Energy 2019, 3, 2–23. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Akhtar, M.N.; Nisar, N.; Rashid, N. Optimized production and advanced assessment of biodiesel: A review. Int. J. Energy Res. 2018, 42, 2070–2083. [Google Scholar] [CrossRef]
- Rengasamy, M.; Anbalagan, K.; Mohanraj, S.; Pugalenthi, V. Biodiesel production from Pongamia pinnata oil using synthesized iron nanocatalyst. Int. J. Chem. Tech. Res. 2014, 6, 4511–4516. [Google Scholar]
- Singh, D.; Sharma, D.; Soni, S.; Sharma, S.; Sharma, P.K.; Jhalani, A. A review on feedstocks, production processes, and yield for different generations of biodiesel. Fuel 2019, 262, 116553. [Google Scholar] [CrossRef]
- Cakirca, E.E.; Akin, A.N. Innovations in the biodiesel production. Press Acad. Procedia 2017, 5, 24–28. [Google Scholar] [CrossRef]
- Demirbas, A.; Bafail, A.; Ahmad, W.; Sheikh, M. Biodiesel production from non-edible plant oils. Energy Explor. Exploit. 2016, 34, 290–318. [Google Scholar] [CrossRef]
- Pinzi, S.; Leiva, D.; López-García, I.; Redel-Macías, M.D.; Dorado, M.P. Latest trends in feedstocks for biodiesel production. Biofuels Bioprod. Biorefin. 2014, 8, 126–143. [Google Scholar] [CrossRef]
- Nisar, J.; Waris, S.; Shah, A.; Anwar, F.; Ali, G.; Ahmad, A.; Muhammad, F. Production of bio-oil from de-oiled Karanja (Pongamia pinnata L.) seed press cake via pyrolysis: Kinetics and evaluation of anthill as the catalyst. Sustain. Chem. 2022, 3, 345–357. [Google Scholar] [CrossRef]
- Usharani, K.; Naik, D.; Manjunatha, R. Pongamia pinnata (L.): Composition and advantages in agriculture: A review. J. Pharmacogn. Phytochem. 2019, 8, 2181–2187. [Google Scholar]
- Bobade, S.; Khyade, V. Detail study on the properties of Pongamia Pinnata (Karanja) for the production of biofuel. Res. J. Chem. Sci. 2012, 2, 16–20. [Google Scholar]
- Fu, J.; Summers, S.; Morgan, T.J.; Turn, S.Q.; Kusch, W. Fuel properties of Pongamia (Milletia pinnata) seeds and pods grown in Hawaii. ACS Omega 2021, 6, 9222–9233. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Ishaq, A.; Sultana, F.A.; Shehzad, M.R. Reactions other than transesterification for biodiesel production. Int. J. Chem. Biochem. Sci. 2017, 12, 141–146. [Google Scholar]
- Tamjidi, S.; Esmaeili, H.; Moghadas, B.K. Performance of functionalized magnetic nanocatalysts and feedstocks on biodiesel production: A review study. J. Clean. Prod. 2021, 305, 127200. [Google Scholar] [CrossRef]
- Hanif, M.A.; Nisar, S.; Rashid, U. Supported solid and heteropoly acid catalysts for production of biodiesel. Catal. Rev. 2017, 59, 165–188. [Google Scholar] [CrossRef]
- Baskar, G.; Aiswarya, R. Trends in catalytic production of biodiesel from various feedstocks. Renew. Sustain. Energy Rev. 2016, 57, 496–504. [Google Scholar] [CrossRef]
- Hazmi, B.; Rashid, U.; Taufiq-Yap, Y.H.; Ibrahim, M.L.; Nehdi, I.A. Supermagnetic nano-bifunctional catalyst from rice husk: Synthesis, characterization and application for conversion of used cooking oil to biodiesel. Catalysts 2020, 10, 225. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Rashid, U.; Taufiq-Yap, Y.H.; Yaw, T.C.S.; Ismail, I. Synthesis of carbonaceous solid acid magnetic catalyst from empty fruit bunch for esterification of palm fatty acid distillate (PFAD). Energy Conver. Manag. 2019, 195, 480–491. [Google Scholar] [CrossRef]
- Wang, H.; Covarrubias, J.; Prock, H.; Wu, X.; Wang, D.; Bossmann, S.H. Acid-functionalized magnetic nanoparticle as heterogeneous catalysts for biodiesel synthesis. J. Phys. Chem. C 2015, 119, 26020–26028. [Google Scholar] [CrossRef]
- Ambat, I.; Srivastava, V.; Haapaniemi, E.; Sillanpää, M. Application of potassium ion impregnated titanium dioxide as nanocatalyst for transesterification of linseed oil. Energy Fuels 2018, 32, 11645–11655. [Google Scholar] [CrossRef]
- Junior, E.G.S.; Justo, O.R.; Perez, V.H.; Reyero, I.; Serrano-Lotina, A.; Ramirez, L.C.; dos Santos Dias, D.F. Extruded catalysts with magnetic properties for biodiesel production. Adv. Mater. Sci. Eng. 2018, 2018, 3980967. [Google Scholar]
- Salimi, Z.; Hosseini, S.A. Study and optimization of conditions of biodiesel production from edible oils using ZnO/BiFeO3 nano magnetic catalyst. Fuel 2019, 239, 1204–1212. [Google Scholar] [CrossRef]
- Ali, M.A.; Al-Hydary, I.A.; Al-Hattab, T.A. Nano-magnetic catalyst CaO-Fe3O4 for biodiesel production from date palm seed oil. Bull. Chem. React. Eng. Catal. 2017, 12, 460–468. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, P.; Fan, M.; Jiang, P. Biodiesel production from soybean oil catalyzed by magnetic nanoparticle MgFe2O4@ CaO. Fuel 2016, 164, 314–321. [Google Scholar] [CrossRef]
- Seffati, K.; Honarvar, B.; Esmaeili, H.; Esfandiari, N. Enhanced biodiesel production from chicken fat using CaO/CuFe2O4 nanocatalyst and its combination with diesel to improve fuel properties. Fuel 2019, 235, 1238–1244. [Google Scholar] [CrossRef]
- Kelarijani, A.F.; Zanjani, N.G.; Pirzaman, A.K. Ultrasonic assisted transesterification of rapeseed oil to biodiesel using nano magnetic catalysts. Waste Biomass Valorization 2020, 11, 2613–2621. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Alaei, S.; Haghighi, M.; Toghiani, J.; Vahid, B.R. Magnetic and reusable MgO/MgFe2O4 nanocatalyst for biodiesel production from sunflower oil: Influence of fuel ratio in combustion synthesis on catalytic properties and performance. Ind. Crops Prod. 2018, 117, 322–332. [Google Scholar] [CrossRef]
- Gardy, J.; Osatiashtiani, A.; Céspedes, O.; Hassanpour, A.; Lai, X.; Lee, A.F.; Wilson, K.; Rehan, M. A magnetically separable SO4/Fe-Al-TiO2 solid acid catalyst for biodiesel production from waste cooking oil. Appl. Catal. B Environ. 2018, 234, 268–278. [Google Scholar] [CrossRef]
- Feyzi, M.; Hassankhani, A.; Rafiee, H.R. Preparation and characterization of Cs/Al/Fe3O4 nanocatalysts for biodiesel production. Energy Convers. Manag. 2013, 71, 62–68. [Google Scholar] [CrossRef]
- dos Santos-Durndell, V.C.; Peruzzolo, T.M.; Ucoski, G.M.; Ramos, L.P.; Nakagaki, S. Magnetically recyclable nanocatalysts based on magnetite: An environmentally friendly and recyclable catalyst for esterification reactions. Biofuel Res. J. 2018, 5, 806. [Google Scholar] [CrossRef]
- Harreh, D.; Saleh, A.; Reddy, A.; Hamdan, S. An experimental investigation of Karanja biodiesel production in Sarawak, Malaysia. J. Eng. 2018, 2018, 4174205. [Google Scholar] [CrossRef]
- Thiruvengadaravi, K.; Nandagopal, J.; Baskaralingam, P.; Bala, V.S.S.; Sivanesan, S. Acid-catalyzed esterification of Karanja (Pongamia pinnata) oil with high free fatty acids for biodiesel production. Fuel 2012, 98, 1–4. [Google Scholar] [CrossRef]
- Alves, M.B.; Medeiros, F.; Sousa, M.H.; Rubim, J.C.; Suarez, P.A. Cadmium and tin magnetic nano-catalysts useful for biodiesel production. J. Braz. Chem. Soc. 2014, 25, 2304–2313. [Google Scholar]
- Rao, P.V.; Ramesh, S. Optimization of Biodiesel production parameters (Pongamia pinnata oil) by transesterification process. J. Adv. Appl. Sci. 2015, 3, 84–88. [Google Scholar]
- Bello, E.I.; Daniyan, I.A.; Akinola, A.O.; Ogedengbe, I.T. Development of a biodiesel processor. Res. J. Eng. Appl. Sci. 2013, 2, 182–186. [Google Scholar]
- Daniyan, I.; Adeodu, A.; Dada, O.; Adewumi, D. Effects of reaction time on biodiesel yield. J. Bioprocess. Chem. Eng. 2015, 3, 1–3. [Google Scholar]
- Joshi, R.M.; Pegg, M.J. Flow properties of biodiesel fuel blends at low temperatures. Fuel 2007, 86, 143–151. [Google Scholar] [CrossRef]
- Wang, L.; Yu, H. Biodiesel from Siberian apricot (Prunus sibirica L.) seed kernel oil. Bioresour. Technol. 2012, 112, 355–358. [Google Scholar] [CrossRef]
- Fadhil, A.B. Evaluation of apricot (Prunus armeniaca L.) seed kernel as a potential feedstock for the production of liquid biofuels and activated carbons. Energy Convers. Manag. 2017, 133, 307–317. [Google Scholar] [CrossRef]
- Sousa, M.H.; Tourinho, F.A.; Depeyrot, J.; da Silva, G.J.; Lara, M.C.F. New electric double-layered magnetic fluids based on copper, nickel, and zinc ferrite nanostructures. J. Phys. Chem. B 2001, 105, 1168–1175. [Google Scholar] [CrossRef]
- Nisar, S.; Farooq, U.; Idress, F.; Rashid, U.; Choong, T.S.Y.; Umar, A. Biodiesel by-product glycerol and its products. Int. J. Chem. Biochem. Sci. 2016, 9, 92–96. [Google Scholar]
- Tang, Z.-E.; Lim, S.; Pang, Y.-L.; Ong, H.-C.; Lee, K.-T. Synthesis of biomass as heterogeneous catalyst for application in biodiesel production: State of the art and fundamental review. Renew. Sustain. Energy Rev. 2018, 92, 235–253. [Google Scholar] [CrossRef]
- Nielsen, S.S. Study Questions Ultraviolet, Visible, and Fluorescence Spectroscopy. Instructor’s Manual for Food Analysis; Springer: Berlin/Heidelberg, Germany, 2003; pp. 111–114. [Google Scholar]
- Nadeem, F.; Hanif, M.A.; Shahzad, K.; Summan, A.S.A.; Ali, A.M. Cost-efficient biodiesel production from Pongamia pinatta by optimizing carbon chain length using condensation polymerization, catalytic breakdown, kink production, and double bond induction in the feedstock oil. Chem. Pap. 2022, 76, 6915–6929. [Google Scholar] [CrossRef]
- de Almeida, V.F.; García-Moreno, P.J.; Guadix, A.; Guadix, E.M. Biodiesel production from mixtures of waste fish oil, palm oil and waste frying oil: Optimization of fuel properties. Fuel Process. Technol. 2015, 133, 152–160. [Google Scholar] [CrossRef]
- Azeem, M.W.; Hanif, M.A.; Al-Sabahi, J.N.; Khan, A.A.; Naz, S.; Ijaz, A. Production of biodiesel from low priced, renewable and abundant date seed oil. Renew. Energy 2016, 86, 124–132. [Google Scholar] [CrossRef]

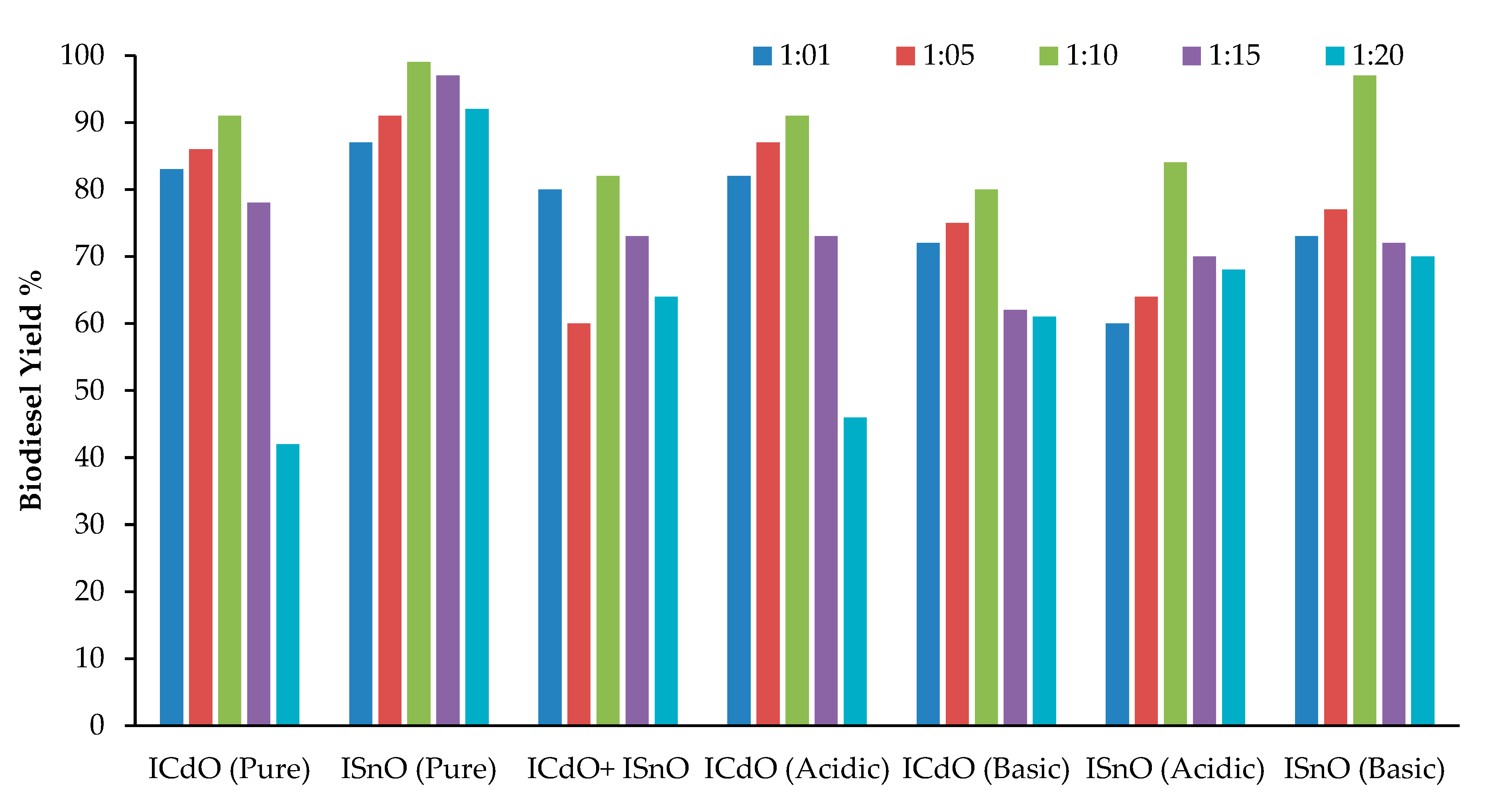


| Sr. No. | Fatty Acid | Molecular Formula | Percentage Composition |
|---|---|---|---|
| 1 | Oleic acid | C18H34O2 | 54.16 |
| 2 | Linoleic acid | C18H32O2 | 12.21 |
| 3 | Palmitic acid | C16H32O2 | 11.11 |
| 4 | Eicosanoic acid | C20H40O2 | 8.23 |
| 5 | Behenic acid | C22H44O2 | 3.46 |
| 6 | Lignoceric acid | C24H48O2 | 3.22 |
| 7 | Linolenic acid | C18H30O2 | 3.14 |
| 8 | Stearic acid | C18H36O2 | 2.66 |
| 9 | Myristic acid | C14H28O2 | 0.68 |
| 10 | Arachidic acid | C20H40O2 | 0.54 |
| 11 | Lauric acid | C12H24O2 | 0.23 |
| 12 | Capric acid | C10H20O2 | 0.14 |
| 13 | Margaric acid | C17H34O2 | 0.09 |
| 14 | Ricinoleic acid | C18H34O3 | 0.04 |
| 15 | Erucic acid | C22H42O2 | 0.02 |
| Type of Catalyst | Catalysts Conc. (Grams) | Oil:Methanol | Temperature (°C) | Time (h) | Shaking Speed (Rpm) | Saponification Value (mgKOH g−1) | Cetane Number | Iodine Value | Acid Value (mg KOH g−1) |
|---|---|---|---|---|---|---|---|---|---|
| ICdO (Pure) | 0.25 | 1:15 | 30 | 2 | 100 | 189.2 | 48 | 109.5 | 0.23 |
| ISnO (Pure) | 0.25 | 1:10 | 30 | 2 | 100 | 193.4 | 47 | 111.8 | 0.20 |
| ICdO + ISnO | 0.25 | 1:01 | 30 | 2 | 100 | 187.6 | 49 | 118.9 | 0.31 |
| ICdO (Acidic) | 0.25 | 1:15 | 30 | 2 | 100 | 187.4 | 49 | 107.6 | 0.17 |
| ISnO (Acidic) | 0.25 | 1:01 | 30 | 2 | 100 | 187.8 | 47 | 115.9 | 0.19 |
| ICdO (Basic) | 0.25 | 1:01 | 30 | 2 | 100 | 182.3 | 49 | 117.8 | 0.41 |
| ISnO (Basic) | 0.25 | 1:15 | 30 | 2 | 100 | 182.9 | 46 | 119.8 | 0.29 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sami, H.Q.u.A.; Hanif, M.A.; Rashid, U.; Nisar, S.; Bhatti, I.A.; Rokhum, S.L.; Tsubota, T.; Alsalme, A. Environmentally Safe Magnetic Nanocatalyst for the Production of Biodiesel from Pongamia pinnata Oil. Catalysts 2022, 12, 1266. https://doi.org/10.3390/catal12101266
Sami HQuA, Hanif MA, Rashid U, Nisar S, Bhatti IA, Rokhum SL, Tsubota T, Alsalme A. Environmentally Safe Magnetic Nanocatalyst for the Production of Biodiesel from Pongamia pinnata Oil. Catalysts. 2022; 12(10):1266. https://doi.org/10.3390/catal12101266
Chicago/Turabian StyleSami, Hafiza Qurat ul Ain, Muhammad Asif Hanif, Umer Rashid, Shafaq Nisar, Ijaz Ahmad Bhatti, Samuel Lalthazuala Rokhum, Toshiki Tsubota, and Ali Alsalme. 2022. "Environmentally Safe Magnetic Nanocatalyst for the Production of Biodiesel from Pongamia pinnata Oil" Catalysts 12, no. 10: 1266. https://doi.org/10.3390/catal12101266
APA StyleSami, H. Q. u. A., Hanif, M. A., Rashid, U., Nisar, S., Bhatti, I. A., Rokhum, S. L., Tsubota, T., & Alsalme, A. (2022). Environmentally Safe Magnetic Nanocatalyst for the Production of Biodiesel from Pongamia pinnata Oil. Catalysts, 12(10), 1266. https://doi.org/10.3390/catal12101266








