N-Heterocyclic Molecules as Potential Liquid Organic Hydrogen Carriers: Reaction Routes and Dehydrogenation Efficacy
Abstract
1. Introduction
- −
- the hydrogen capacity must not be less than 5.5 wt.% and 1.5 kWh/kg [7];
- −
- be economically accessible and non-toxic;
- −
- the boiling point of the compound must ensure a good separation of hydrogen and the dehydrogenated form of a LOHC; thence, the LOHC system boiling temperature must be not less than 300 °C, and the melting point should also be quite low (less than −30 °C);
- −
- the compound must be stable in the process conditions;
- −
- the dehydrogenation enthalpy must be 40–70 kJ/mol to ensure the stability of a LOHC molecule.
2. Results and Discussion
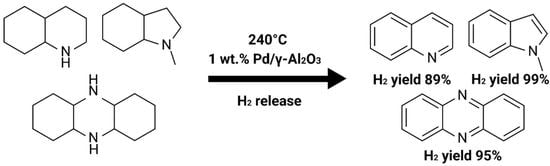
2.1. Dehydrogenation of Nitrogenic Heterocycles over Palladium-Containing Catalysts
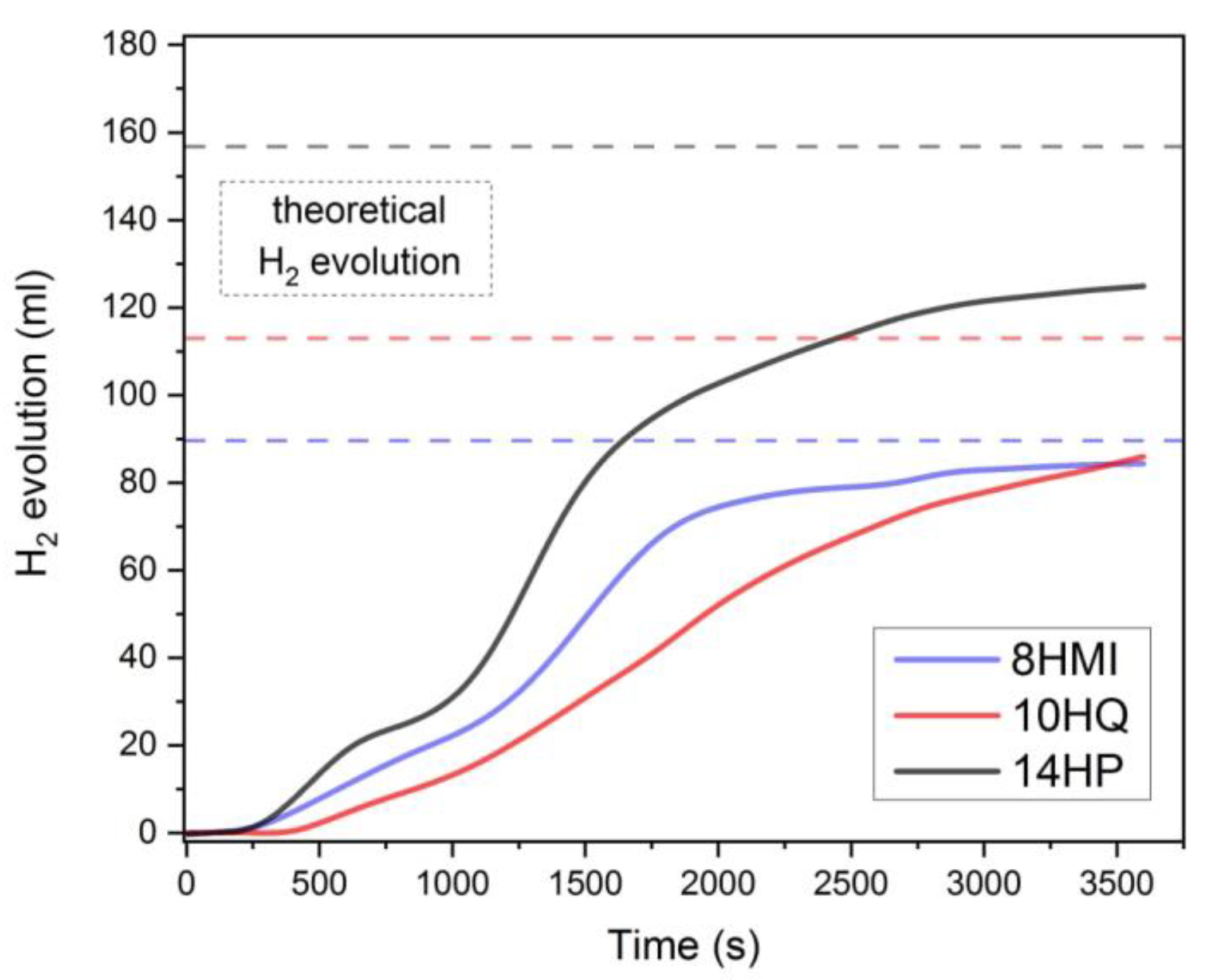
2.2. Comparative Testing of the Heterocycles
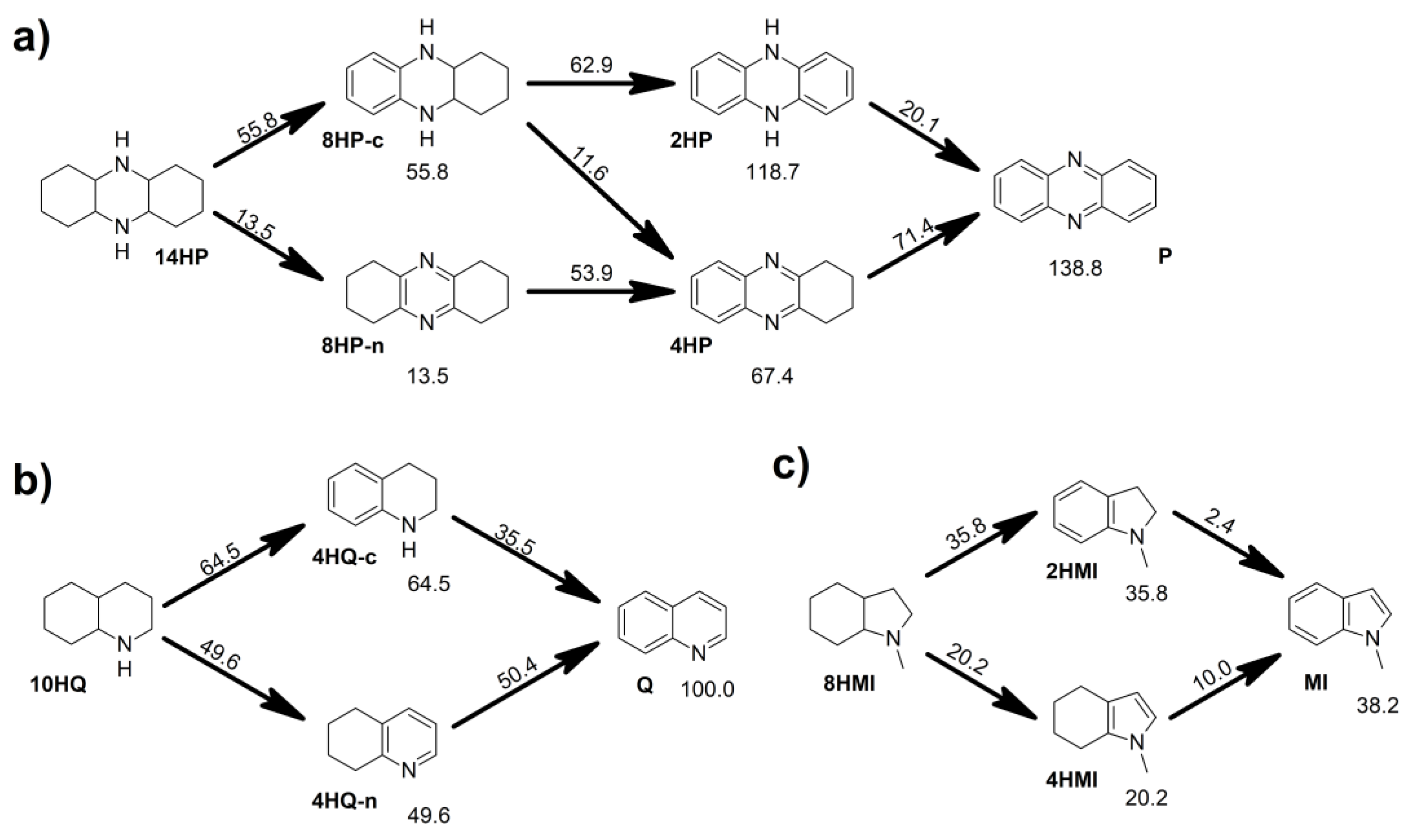
2.3. Thermodynamic Regularities of Dehydrogenation Mechanism of the Studied N-Heterocycles
3. Materials and Methods
3.1. Catalyst Preparation
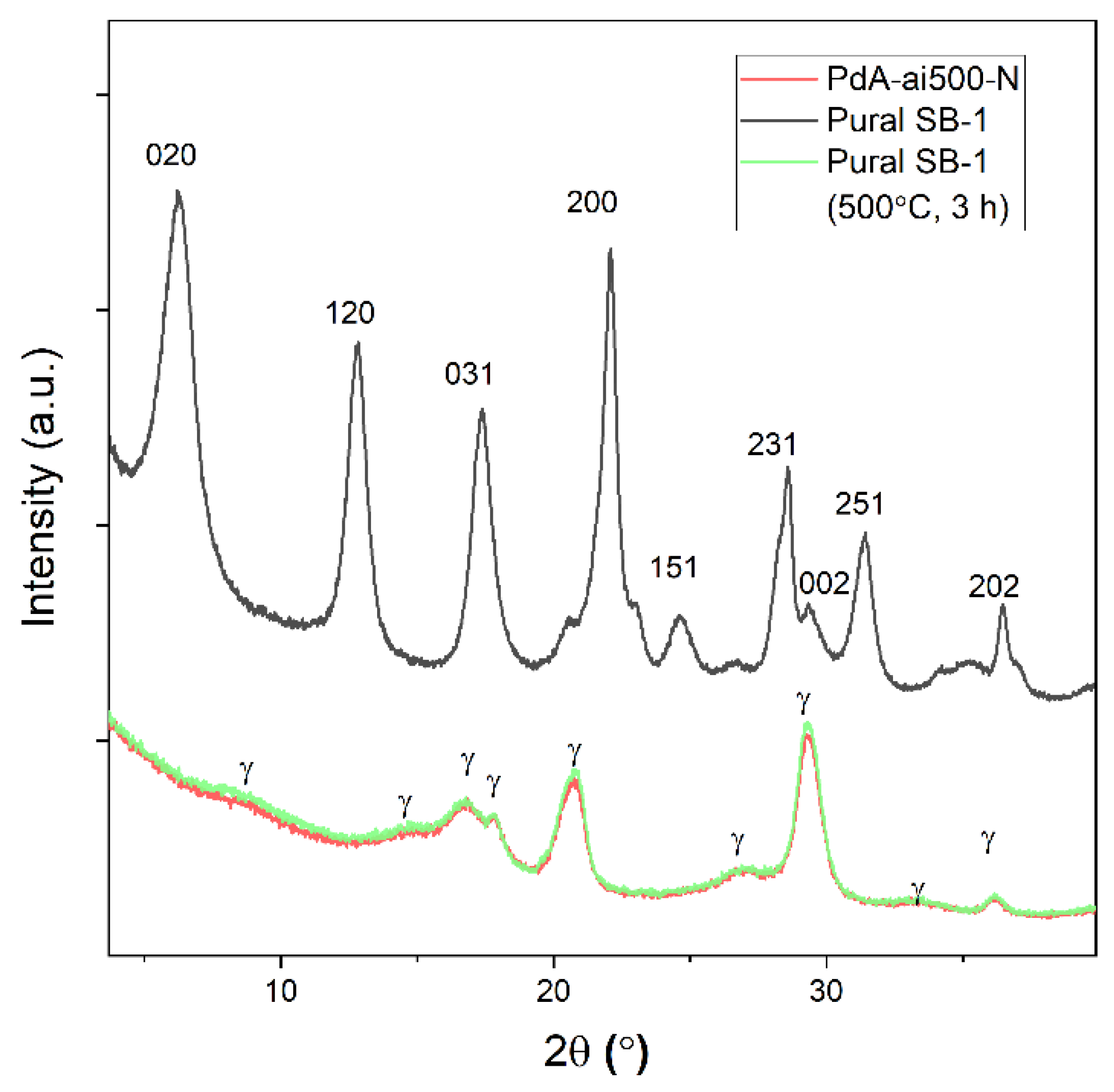
3.2. Catalyst Characterization
3.3. Thermostability Test of Heterocycles and Dehydrogenation Reaction
3.4. Computational Details
4. Summary
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid Organic Hydrogen Carriers for Transportation and Storing of Renewable Energy—Review and Discussion. J. Power Sources 2018, 396, 803–823. [Google Scholar] [CrossRef]
- Gianotti, E.; Taillades-Jacquin, M.; Rozière, J.; Jones, D.J. High-Purity Hydrogen Generation via Dehydrogenation of Organic Carriers: A Review on the Catalytic Process. ACS Catal. 2018, 8, 4660–4680. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-Free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef]
- Abdin, Z.; Tang, C.; Liu, Y.; Catchpole, K. Large-Scale Stationary Hydrogen Storage via Liquid Organic Hydrogen Carriers. iScience 2021, 24, 102966. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.C.; Yoon, M. Potential Liquid-Organic Hydrogen Carrier (LOCH) Systems: A Review on Recent Progress. Energies 2020, 13, 6040. [Google Scholar] [CrossRef]
- Niermann, M.; Beckendorff, A.; Kaltschmitt, M.; Bonhoff, K. Liquid Organic Hydrogen Carrier (LOHC)—Assessment Based on Chemical and Economic Properties. Int. J. Hydrogen Energy 2019, 44, 6631–6654. [Google Scholar] [CrossRef]
- US Department of Energy Target Explanation Document: Onboard Hydrogen Storage for Light-Duty Fuel Cell Vehicles. U.S Drive 2017, 1–19.
- Zhu, Q.L.; Xu, Q. Liquid Organic and Inorganic Chemical Hydrides for High-Capacity Hydrogen Storage. Energy Environ. Sci. 2015, 8, 478–512. [Google Scholar] [CrossRef]
- Hurskainen, M. Liquid Organic Hydrogen Carriers (LOHC)—Concept Evaluation and Techno-Economics; Research Report VTT-R-00057-19; VTT Technical Research Centre of Finland: Espoo, Finland, 2 December 2019. [Google Scholar]
- Sekine, Y.; Higo, T. Recent Trends on the Dehydrogenation Catalysis of Liquid Organic Hydrogen Carrier (LOHC): A Review. Top. Catal. 2021, 64, 470–480. [Google Scholar] [CrossRef]
- Emel’Yanenko, V.N.; Varfolomeev, M.A.; Verevkin, S.P.; Stark, K.; Müller, K.; Müller, M.; Bösmann, A.; Wasserscheid, P.; Arlt, W. Hydrogen Storage: Thermochemical Studies of N-Alkylcarbazoles and Their Derivatives as a Potential Liquid Organic Hydrogen Carriers. J. Phys. Chem. C 2015, 119, 26381–26389. [Google Scholar] [CrossRef]
- Yang, M.; Dong, Y.; Fei, S.; Ke, H.; Cheng, H. A Comparative Study of Catalytic Dehydrogenation of Perhydro-N-Ethylcarbazole over Noble Metal Catalysts. Int. J. Hydrogen Energy 2014, 39, 18976–18983. [Google Scholar] [CrossRef]
- Stark, K.; Emelyanenko, V.N.; Zhabina, A.A.; Varfolomeev, M.A.; Verevkin, S.P.; Müller, K.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Carbazole Partly and Fully Hydrogenated Derivatives. Ind. Eng. Chem. Res. 2015, 54, 7953–7966. [Google Scholar] [CrossRef]
- Emel’yanenko, V.N.; Zaitsau, D.H.; Pimerzin, A.A.; Verevkin, S.P. N-Phenyl-Carbazole as a Potential Liquid Organic Hydrogen Carrier: Thermochemical and Computational Study. J. Chem. Thermodyn. 2019, 132, 122–128. [Google Scholar] [CrossRef]
- Shin, B.S.; Yoon, C.W.; Kwak, S.K.; Kang, J.W. Thermodynamic Assessment of Carbazole-Based Organic Polycyclic Compounds for Hydrogen Storage Applications via a Computational Approach. Int. J. Hydrogen Energy 2018, 43, 12158–12167. [Google Scholar] [CrossRef]
- Stark, K.; Keil, P.; Schug, S.; Müller, K.; Wasserscheid, P.; Arlt, W. Melting Points of Potential Liquid Organic Hydrogen Carrier Systems Consisting of N-Alkylcarbazoles. J. Chem. Eng. Data 2016, 61, 1441–1448. [Google Scholar] [CrossRef]
- Yang, M.; Cheng, G.; Xie, D.; Zhu, T.; Dong, Y.; Ke, H.; Cheng, H. Study of Hydrogenation and Dehydrogenation of 1-Methylindole for Reversible Onboard Hydrogen Storage Application. Int. J. Hydrogen Energy 2018, 43, 8868–8876. [Google Scholar] [CrossRef]
- Dong, Y.; Yang, M.; Yang, Z.; Ke, H.; Cheng, H. Catalytic Hydrogenation and Dehydrogenation of N-Ethylindole as a New Heteroaromatic Liquid Organic Hydrogen Carrier. Int. J. Hydrogen Energy 2015, 40, 10918–10922. [Google Scholar] [CrossRef]
- Li, L.; Yang, M.; Dong, Y.; Mei, P.; Cheng, H. Hydrogen Storage and Release from a New Promising Liquid Organic Hydrogen Storage Carrier (LOHC): 2-Methylindole. Int. J. Hydrogen Energy 2016, 41, 16129–16134. [Google Scholar] [CrossRef]
- Forberg, D.; Schwob, T.; Zaheer, M.; Friedrich, M.; Miyajima, N.; Kempe, R. Single-Catalyst High-Weight% Hydrogen Storage in an N-Heterocycle Synthesized from Lignin Hydrogenolysis Products and Ammonia. Nat. Commun. 2016, 7, 1–6. [Google Scholar] [CrossRef]
- Li, J.; Tong, F.; Li, Y.; Liu, X.; Guo, Y.; Wang, Y. Dehydrogenation of Dodecahydro-N-Ethylcarbazole over Spinel Supporting Catalyst in a Continuous Flow Fixed Bed Reactor. Fuel 2022, 321, 124034. [Google Scholar] [CrossRef]
- Kulagina, M.A.; Simonov, P.A.; Gerasimov, E.Y.; Kvon, R.I.; Romanenko, A.V. To the Nature of the Support Effect in Palladium-Catalyzed Aqueous-Phase Hydrogenation of Maleic Acid. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 526, 29–39. [Google Scholar] [CrossRef]
- Simakova, O.A.; Simonov, P.A.; Romanenko, A.V.; Simakova, I.L. Preparation of Pd/C Catalysts via Deposition of Palladium Hydroxide onto Sibunit Carbon and Their Application to Partial Hydrogenation of Rapeseed Oil. React. Kinet. Catal. Lett. 2008, 95, 3–12. [Google Scholar] [CrossRef]
- Simonov, P.A.; Romanenko, A.V.; Likholobov, V.A. Hydrogenation of Ethyl P-Nitrobenzoate on Pd/Sibunit Catalysts. Solid Fuel Chem. 2014, 48, 364–370. [Google Scholar] [CrossRef]
- Lin, Q.; Ji, Y.; Jiang, Z.D.; Xiao, W. De Effects of Precursors on Preparation of Pd/α-Alumina Catalyst for Synthesis of Dimethyl Oxalate. Ind. Eng. Chem. Res. 2007, 46, 7950–7954. [Google Scholar] [CrossRef]
- Sasol Tech Data. Available online: http://www.sasoltechdata.com/tds/PURAL_CATAPAL.pdf (accessed on 22 September 2022).
- Zhang, J.W.; Li, D.D.; Lu, G.P.; Deng, T.; Cai, C. Reversible Dehydrogenation and Hydrogenation of N-Heterocycles Catalyzed by Bimetallic Nanoparticles Encapsulated in MIL-100(Fe). ChemCatChem 2018, 10, 4980–4986. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Huber, B.J.M.; Smith, K.J. Dehydrogenation Kinetics and Catalysis of Organic Heteroaromatics for Hydrogen Storage. Int. J. Hydrogen Energy 2012, 37, 2715–2722. [Google Scholar] [CrossRef]
- Oh, J.; Bathula, H.B.; Park, J.H.; Suh, Y.W. A Sustainable Mesoporous Palladium-Alumina Catalyst for Efficient Hydrogen Release from N-Heterocyclic Liquid Organic Hydrogen Carriers. Commun. Chem. 2019, 2. [Google Scholar] [CrossRef]
- Kim, Y.; Song, Y.; Choi, Y.; Jeong, K.; Park, J.H.; Ko, K.C.; Na, K. Catalytic Consequences of Supported Pd Catalysts on Dehydrogenative H2 Evolution from 2-[(n-Methylcyclohexyl)Methyl]Piperidine as the Liquid Organic Hydrogen Carrier. ACS Sustain. Chem. Eng. 2021, 9, 809–821. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 720–723. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the Damping Function in Dispersion Corrected Density Functional Theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]




| Catalysts | Synthesis Details | DCO 1, % | SBET, m2/g | YH2 2, % | |
|---|---|---|---|---|---|
| 20 °C | 100 °C | ||||
| Pd/C | [23] | 39 | 41 | 325 | 78 |
| A 3 | AlO(OH), 550 °C | - | - | 210 | - |
| PdA-wi-N | Al2O3 wi by H2[Pd(NO3)4], 500 °C | 27 | 26 | 205 | 79 |
| PdA-wi-Cl | Al2O3 wi by H2[PdCl4], 500 °C | 27 | 23 | - | 75 |
| PdA-ai120-N | Al2O3 ai by H2[Pd(NO3)4], 120 °C | 41 | 31 | 198 | 85 |
| PdA-ai120-Cl | Al2O3 ai by H2[PdCl4], 120 °C | 39 | 28 | - | 84 |
| PdA-wi500-N | Al2O3 ai by H2[Pd(NO3)4], 500 °C | 59 | 43 | - | 94 |
| PdA-wi500-Cl | Al2O3 ai by H2[PdCl4], 500 °C | 59 | 46 | - | 89 |
| LOHC Pairs | GC-MS Detected Compounds | Yi 1, mol.% | YH2, % | ||
|---|---|---|---|---|---|
| Pd/C | Pd/Al2O3 2 | Pd/C | Pd/Al2O3 2 | ||
| Q/10HQ | «10H»: (cis, trans)-decahydroquinoline (10HQ) | 8 | 2.3 | 75 | 89 |
| «4H»: 1,2,3,4-tetrahydroquinoline (4HQ-c); 5,6,7,8-tetrahydroquinoline (4HQ-n); 1-methyl-4HQ-c, 1-ethyl-4HQ-c | 7.4 26.6 trace | 3.7 12.5 trace | |||
| «0H»: quinoline (Q) | 57.9 | 81 | |||
| MI/8HMI | «8H»: (cis, trans)-1-methyl-octahydroindole (8HMI) | 0.8 | 0.4 | 94 | 99 |
| «4H»: 1-methyl-4,5,6,7-tetrahydroindole (4HMI) | 6 | 1.1 | |||
| «2H»: 1-methyl-indoline (2HMI) | 0.2 | 0.2 | |||
| «0H»: 1-methyl-indole (MI) | 91 | 98 | |||
| P/14HP | «14H»: tetradecahydrophenazine (14HP) | trace | trace | 78 | 94 |
| «8H»: 1,2,3,4,6,7,8,9-octahydrophenazine (8HP-n); (cis, trans)-1,2,3,4,4a,5,10,10a-octahydrophenazine (8HP-c) | 0.3 21 | 1.3 2.8 | |||
| «4H»: 1,2,3,4-tetrahydrophenazine (4HP) | 28.9 | 11.7 | |||
| «2H»: 5,10-dihydrophenazine (2HP); 5-methyl-2HP, 5,10-dimethyl-2HP, 5-ethyl-2HP | 1.8 trace | 1.5 trace | |||
| «0H»: phenazine (P) | 48.4 | 82.7 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stepanenko, S.A.; Shivtsov, D.M.; Koskin, A.P.; Koskin, I.P.; Kukushkin, R.G.; Yeletsky, P.M.; Yakovlev, V.A. N-Heterocyclic Molecules as Potential Liquid Organic Hydrogen Carriers: Reaction Routes and Dehydrogenation Efficacy. Catalysts 2022, 12, 1260. https://doi.org/10.3390/catal12101260
Stepanenko SA, Shivtsov DM, Koskin AP, Koskin IP, Kukushkin RG, Yeletsky PM, Yakovlev VA. N-Heterocyclic Molecules as Potential Liquid Organic Hydrogen Carriers: Reaction Routes and Dehydrogenation Efficacy. Catalysts. 2022; 12(10):1260. https://doi.org/10.3390/catal12101260
Chicago/Turabian StyleStepanenko, Sergey A., Danil M. Shivtsov, Anton P. Koskin, Igor P. Koskin, Roman G. Kukushkin, Petr M. Yeletsky, and Vadim A. Yakovlev. 2022. "N-Heterocyclic Molecules as Potential Liquid Organic Hydrogen Carriers: Reaction Routes and Dehydrogenation Efficacy" Catalysts 12, no. 10: 1260. https://doi.org/10.3390/catal12101260
APA StyleStepanenko, S. A., Shivtsov, D. M., Koskin, A. P., Koskin, I. P., Kukushkin, R. G., Yeletsky, P. M., & Yakovlev, V. A. (2022). N-Heterocyclic Molecules as Potential Liquid Organic Hydrogen Carriers: Reaction Routes and Dehydrogenation Efficacy. Catalysts, 12(10), 1260. https://doi.org/10.3390/catal12101260