Tailoring the Biochar Physicochemical Properties Using a Friendly Eco-Method and Its Application on the Oxidation of the Drug Losartan through Persulfate Activation
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reagents Used
3.2. Biochar Preparation
3.3. Physicochemical Characterization
3.4. Oxidation Experiments and Losartan Measurement
4. Conclusions
- -
- A mild treatment of biochars (i.e., low temperature and dilute acid or base) is capable of significantly altering its physicochemical properties. The effect is more pronounced on the surface area, the pzc, and the concentration of minerals.
- -
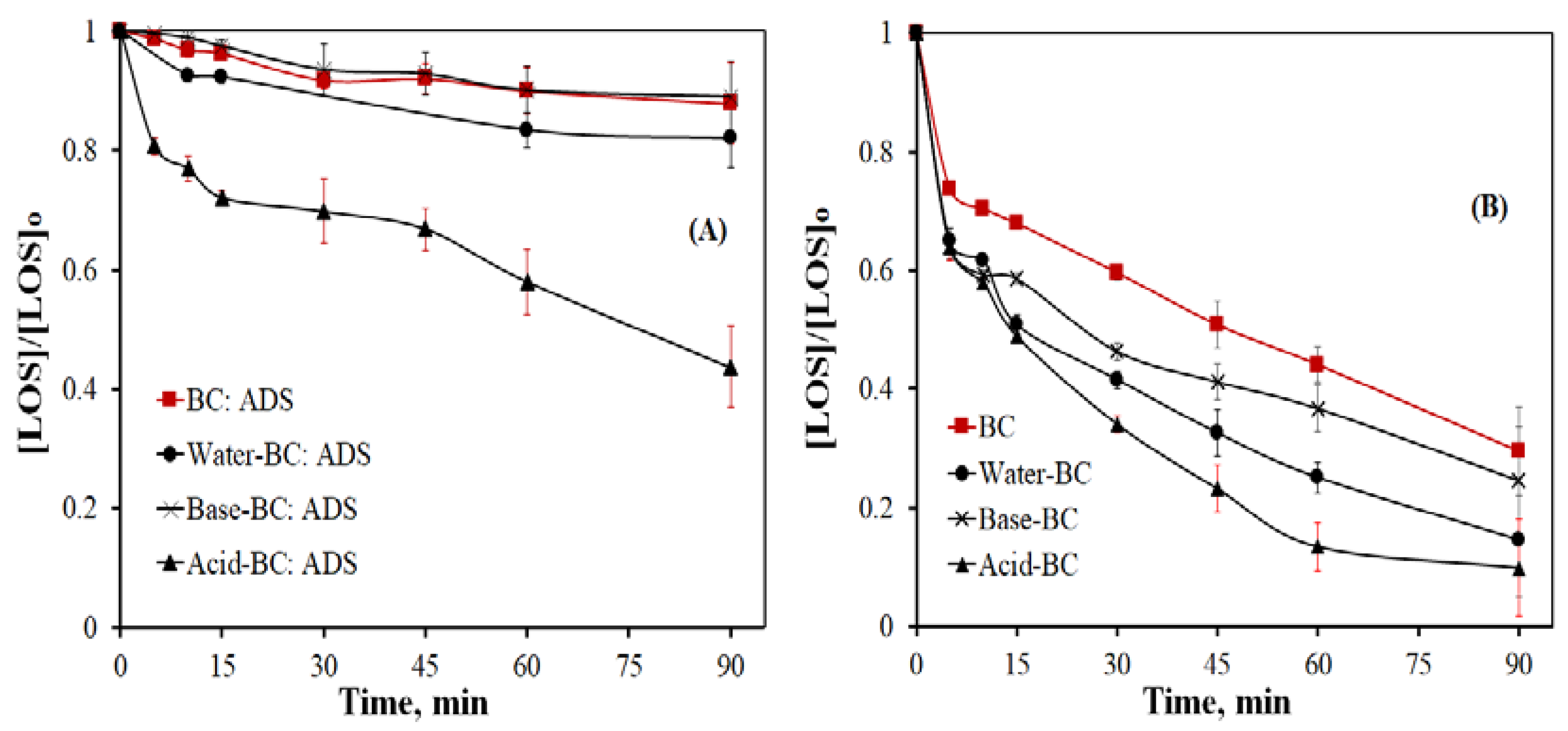
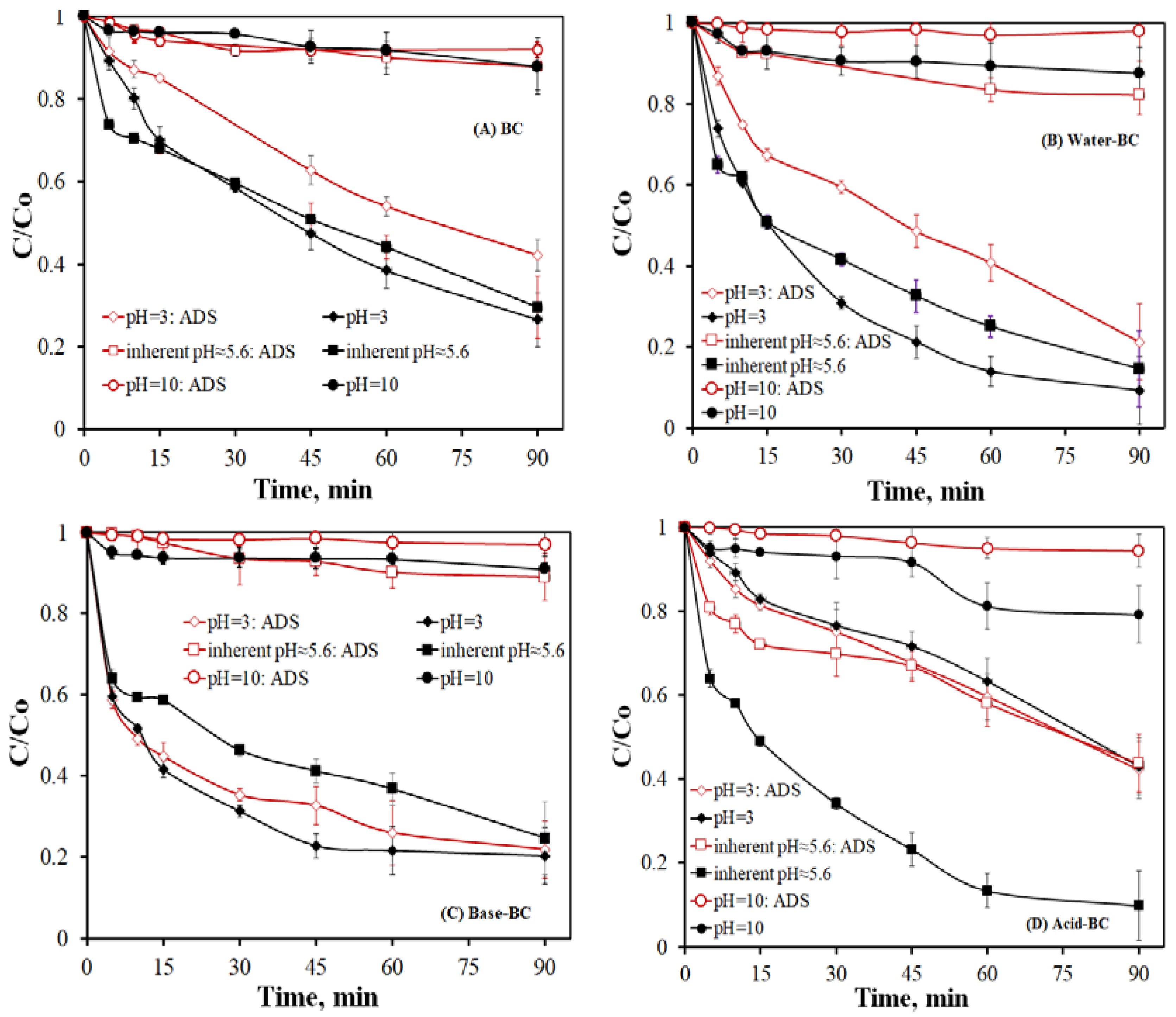
- From the various samples tested, the acid-treated BC generally exhibits the highest efficiency. Conversely, oxidation is significantly delayed at alkaline conditions and/or in a complex secondary effluent. This material behaves predominantly as an adsorbent in acidic conditions and becomes a persulfate activator at near-neutral and alkaline environments.
- -
- Indirect scavenging experiments confirm the contribution of a non-radical mechanism (singlet oxygen) in addition to the radical pathways induced by sulfate and hydroxyl radicals.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauvé, S.; Bernard, S.; Sloan, P. Environmental sciences, sustainable development and circular economy: Alternative concepts for trans-disciplinary research. Environ. Dev. 2016, 17, 48–56. [Google Scholar] [CrossRef]
- Romanovski, V. Chapter 25- Agricultural waste based-nanomaterials: Green technology for water purification. In Micro and Nano Technologies; Abd-Elsalam, K.A., Zahid, M., Eds.; Aquananotechnology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 577–595. ISBN 9780128211410. [Google Scholar] [CrossRef]
- Ganesh, K.S.; Sridhar, A.; Vishali, S. Utilization of fruit and vegetable waste to produce value-added products: Conventional utilization and emerging opportunities—A review. Chemosphere 2022, 287, 132221. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. Preparation, modification and environmental application of biochar: A review. J. Clean. Prod. 2019, 227, 1002–1022. [Google Scholar] [CrossRef]
- Zhang, A.; Li, X.; Xing, J.; Xu, G. Adsorption of potentially toxic elements in water by modified biochar: A review. J. Environ. Chem. Eng. 2020, 8, 104196. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, B.; Wu, P.; Lee, X.; Xing, Y.; Chen, M.; Gao, B. Adsorption of emerging contaminants from water and wastewater by modified biochar: A review. Environ. Pollut. 2021, 273, 116448. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Gu, W.; Zhou, L.; Wang, L.; Zhang, J.; Liu, Y.; Lei, J. Recent advances in MOF-derived carbon-based nanomaterials for environmental applications in adsorption and catalytic degradation. Chem. Eng. J. 2022, 427, 131503. [Google Scholar] [CrossRef]
- Guo, D.; You, S.; Li, F.; Liu, Y. Engineering carbon nanocatalysts towards efficient degradation of emerging organic contaminants via persulfate activation: A review. Chinese Chem. Lett. 2022, 33, 1–10. [Google Scholar] [CrossRef]
- Dimitriadou, S.; Frontistis, Z.; Petala, A.; Bampos, G.; Mantzavinos, D. Carbocatalytic activation of persulfate for the removal of drug diclofenac from aqueous matrices. Catal. Today 2020, 355, 937–944. [Google Scholar] [CrossRef]
- Bekris, L.; Frontistis, Z.; Trakakis, G.; Sygellou, L.; Galiotis, C.; Mantzavinos, D. Graphene: A new activator of sodium persulfate for the advanced oxidation of parabens in water. Water Res. 2017, 126, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kemmou, L.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of antibiotic sulfamethoxazole by biochar-activated persulfate: Factors affecting the activation and degradation processes. Catal. Today 2018, 313, 128–133. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Li, X.; Jiang, L.; Wang, H. Burgeoning prospects of biochar and its composite in persulfate-advanced oxidation process. J. Hazard. Mater. 2021, 409, 124893. [Google Scholar] [CrossRef]
- Magioglou, E.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Activation of Persulfate by Biochars from Valorized Olive Stones for the Degradation of Sulfamethoxazole. Catalysts 2019, 9, 419. [Google Scholar] [CrossRef]
- Huang, B.C.; Jiang, J.; Huang, G.X.; Yu, H.Q. Sludge biochar-based catalysts for improved pollutant degradation by activating peroxymonosulfate. J. Mater. Chem. A 2018, 6, 8978–8985. [Google Scholar] [CrossRef]
- Lykoudi, A.; Frontistis, Z.; Vakros, J.; Manariotis, I.D.; Mantzavinos, D. Degradation of sulfamethoxazole with persulfate using spent coffee grounds biochar as activator. J. Environ. Manage. 2020, 271, 111022. [Google Scholar] [CrossRef]
- Song, G.; Qin, F.; Yu, J.; Tang, L.; Pang, Y.; Zhang, C.; Wang, J.; Deng, L. Tailoring biochar for persulfate-based environmental catalysis: Impact of biomass feedstocks. J. Hazard. Mater. 2022, 424, 127663. [Google Scholar] [CrossRef] [PubMed]
- Grilla, E.; Vakros, J.; Konstantinou, I.; Manariotis, I.D.; Mantzavinos, D. Activation of persulfate by biochar from spent malt rootlets for the degradation of trimethoprim in the presence of inorganic ions. J. Chem. Technol. Biotechnol. 2020, 95, 2348–2358. [Google Scholar]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. On the Performance of a Sustainable Rice Husk Biochar for the Activation of Persulfate and the Degradation of Antibiotics. Catalysts 2021, 11, 1303. [Google Scholar] [CrossRef]
- Azargohar, R.; Dalai, A.K. Steam and KOH activation of biochar: Experimental and modeling studies. Microporous Mesoporous Mater. 2008, 110, 413–421. [Google Scholar] [CrossRef]
- Xia, D.; Tan, F.; Zhang, C.; Jiang, X.; Chen, Z.; Li, H.; Zheng, Y.; Li, Q.; Wang, Y. ZnCl2-activated biochar from biogas residue facilitates aqueous As(III) removal. Appl. Surf. Sci. 2016, 377, 361–369. [Google Scholar] [CrossRef]
- Zhu, L.; Zhao, N.; Tong, L.; Lv, Y. Structural and adsorption characteristics of potassium carbonate activated biochar. RSC Adv. 2018, 8, 21012–21019. [Google Scholar] [CrossRef]
- Ntaflou, M.; Vakros, J. Transesterification activity of modified biochars from spent malt rootlets using triacetin. J. Clean. Prod. 2020, 259, 120931. [Google Scholar] [CrossRef]
- Ioannidi, A.; Arvaniti, O.S.; Nika, M.-C.; Aalizadeh, R.; Thomaidis, N.S.; Mantzavinos, D.; Frontistis, Z. Removal of drug losartan in environmental aquatic matrices by heat-activated persulfate: Kinetics, transformation products and synergistic effects. Chemosphere 2022, 287, 131952. [Google Scholar] [CrossRef] [PubMed]
- Avramiotis, E.; Frontistis, Z.; Manariotis, I.D.; Vakros, J.; Mantzavinos, D. Oxidation of Sulfamethoxazole by Rice Husk Biochar-Activated Persulfate. Catalysts 2021, 11, 850. [Google Scholar] [CrossRef]
- Tang, H.; Zhao, Y.; Shan, S.; Yang, X.; Liu, D.; Cui, F.; Xing, B. Theoretical insight into the adsorption of aromatic compounds on graphene oxide. Environ. Sci. Nano 2018, 5, 2357–2367. [Google Scholar] [CrossRef]
- Mrozik, W.; Minofar, B.; Thongsamer, T.; Wiriyaphong, N.; Khawkomol, S.; Plaimart, J.; Vakros, J.; Karapanagioti, H.; Vinitnantharat, S.; Werner, D. Valorisation of agricultural waste derived biochars in aquaculture to remove organic micropollutants from water—experimental study and molecular dynamics simulations. J. Environ. Manag. 2021, 300, 113717. [Google Scholar] [CrossRef]
- Ejsmont, A.; Stasiłowicz-Krzemień, A.; Ludowicz, D.; Cielecka-Piontek, J.; Goscianska, J. Synthesis and Characterization of Nanoporous Carbon Carriers for Losartan Potassium Delivery. Materials 2021, 14, 7345. [Google Scholar] [CrossRef]
- Ren, W.; Xiong, L.; Yuan, X.; Yu, Z.; Zhang, H.; Duan, X.; Wang, S. Activation of Peroxydisulfate on Carbon Nanotubes: Electron-Transfer Mechanism. Environ. Sci. Technol. 2019, 53, 14595–14603. [Google Scholar] [CrossRef]
- Ioannidi, A.; Frontistis, Z.; Mantzavinos, D. Destruction of propyl paraben by persulfate activated with UV-A light emitting diodes. J. Environ. Chem. Eng. 2018, 6, 2992–2997. [Google Scholar] [CrossRef]
- Li, F.; Xie, Y.; Wang, Y.; Fan, X.; Cai, Y.; Mei, Y. Improvement of dyes degradation using hydrofluoric acid modified biochar as persulfate activator. Environ. Pollut. Bioavailab. 2019, 31, 32–37. [Google Scholar] [CrossRef]
- Xue, Y.; Guo, Y.; Zhang, X.; Kamali, M.; Aminabhavi, T.M.; Appels, L.; Dewil, R. Efficient adsorptive removal of ciprofloxacin and carbamazepine using modified pinewood biochar—A kinetic, mechanistic study. Chem. Eng. J. 2022, 450, 137896. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Liu, D. Plasma regulates active sites on biochar to boost peroxomonosulfate activation for phenol degradation. J. Environ. Chem. Eng. 2022, 10, 107833. [Google Scholar] [CrossRef]
- Chen, K.; Zhou, L.; Xu, W.; Hu, Z.; Jia, M.; Liu, L. A novel way of activating peroxysulfate by zero-valent copper and ferroferric oxide co-modified biochar to remove bisphenol A in aqueous solution: Performance, mechanism and potential toxicity. Appl. Catal. A Gen. 2022, 636, 118575. [Google Scholar] [CrossRef]
- Qian, L.; Guo, F.; Jia, X.; Zhan, Y.; Zhou, H.; Jiang, X.; Tao, C. Recent development in the synthesis of agricultural and forestry biomass-derived porous carbons for supercapacitor applications: A review. Ionics 2020, 26, 3705–3723. [Google Scholar] [CrossRef]
- Gao, G.; Cheong, L.Z.; Wang, D.; Shen, C. Pyrolytic carbon derived from spent coffee grounds as anode for sodium-ion batteries. Carbon Resour. Convers. 2018, 1, 104–108. [Google Scholar] [CrossRef]
- Kaczmarczyk, B. FTIR study of conjugation in selected aromatic polyazomethines. J. Mol. Struct. 2013, 1048, 179–184. [Google Scholar] [CrossRef]
- Suganuma, S.; Nakajima, K.; Kitano, M.; Yamaguchi, D.; Kato, H.; Hayashi, S.; Hara, M. Hydrolysis of Cellulose by Amorphous Carbon Bearing SO3H, COOH, and OH Groups. J. Am. Chem. Soc. 2008, 130, 12787–12793. [Google Scholar] [CrossRef]
- Tala, W.; Chantara, S. Use of spent coffee ground biochar as ambient PAHs sorbent and novel extraction method for GC–MS analysis Environ. Sci. Poll. Res. 2019, 26, 13025–13040. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef]
- McElroy, W.J. A laser photolysis study of the reaction of sulfate (1-) with chloride and the subsequent decay of chlorine (1-) in aqueous solution. J. Phys. Chem. 1990, 94, 2435–2441. [Google Scholar] [CrossRef]
- Jayson, G.G.; Parsons, B.J.; Swallow, A.J. Some simple, highly reactive, inorganic chlorine derivatives in aqueous solution. Their formation using pulses of radiation and their role in the mechanism of the Fricke dosimeter. J. Chem. Soc. Faraday Trans. 1 1973, 69, 1597. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- de Andrade, J.R.; Vieira, M.G.A.; da Silva, M.G.C.; Wang, S. Oxidative degradation of pharmaceutical losartan potassium with N-doped hierarchical porous carbon and peroxymonosulfate. Chem. Eng. J. 2020, 382, 122971. [Google Scholar] [CrossRef]
- Bourikas, K.; Vakros, J.; Kordulis, C.; Lycourghiotis, A. Potentiometric Mass Titrations: Experimental and Theoretical Establishment of a New Technique for Determining the Point of Zero Charge (PZC) of Metal (Hydr)Oxides. J. Phys. Chem. B 2003, 107, 9441–9451. [Google Scholar] [CrossRef]
- Papatheodorou, G.; Ntzoufra, P.; Hapeshi, E.; Vakros, J.; Mantzavinos, D. Hybrid Biochar/Ceria Nanomaterials: Synthesis, Characterization and Activity Assessment for the Persulfate-Induced Degradation of Antibiotic Sulfamethoxazole. Nanomaterials 2022, 12, 194. [Google Scholar] [CrossRef] [PubMed]
- Ntzoufra, P.; Vakros, J.; Frontistis, Z.; Tsatsos, S.; Kyriakou, G.; Kennou, S.; Manariotis, I.D.; Mantzavinos, D. Effect of Sodium Persulfate Treatment on the Physicochemical Properties and Catalytic Activity of Biochar Prepared from Spent Malt Rootlets. J. Environ. Chem. Eng. 2021, 9, 105071. [Google Scholar] [CrossRef]
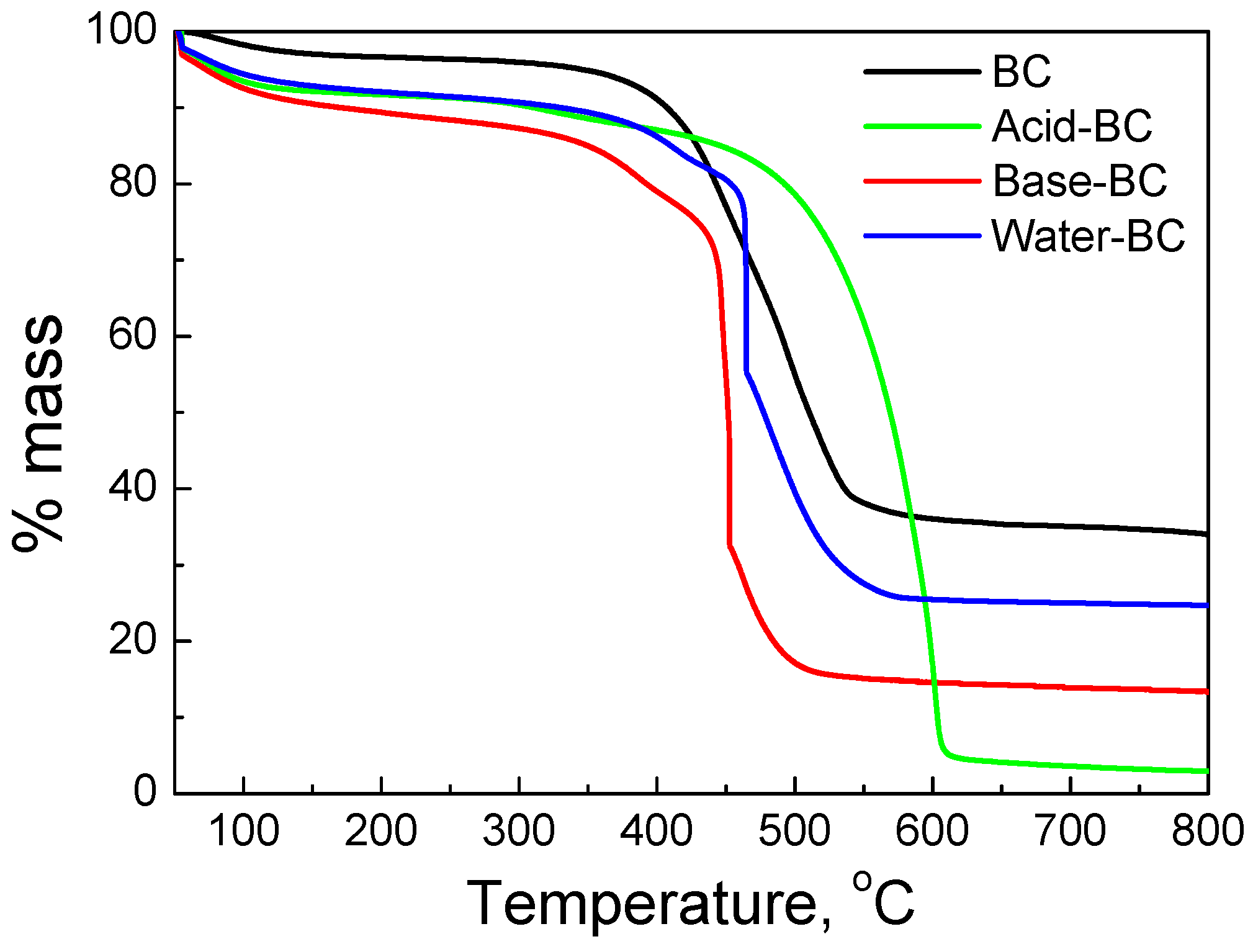
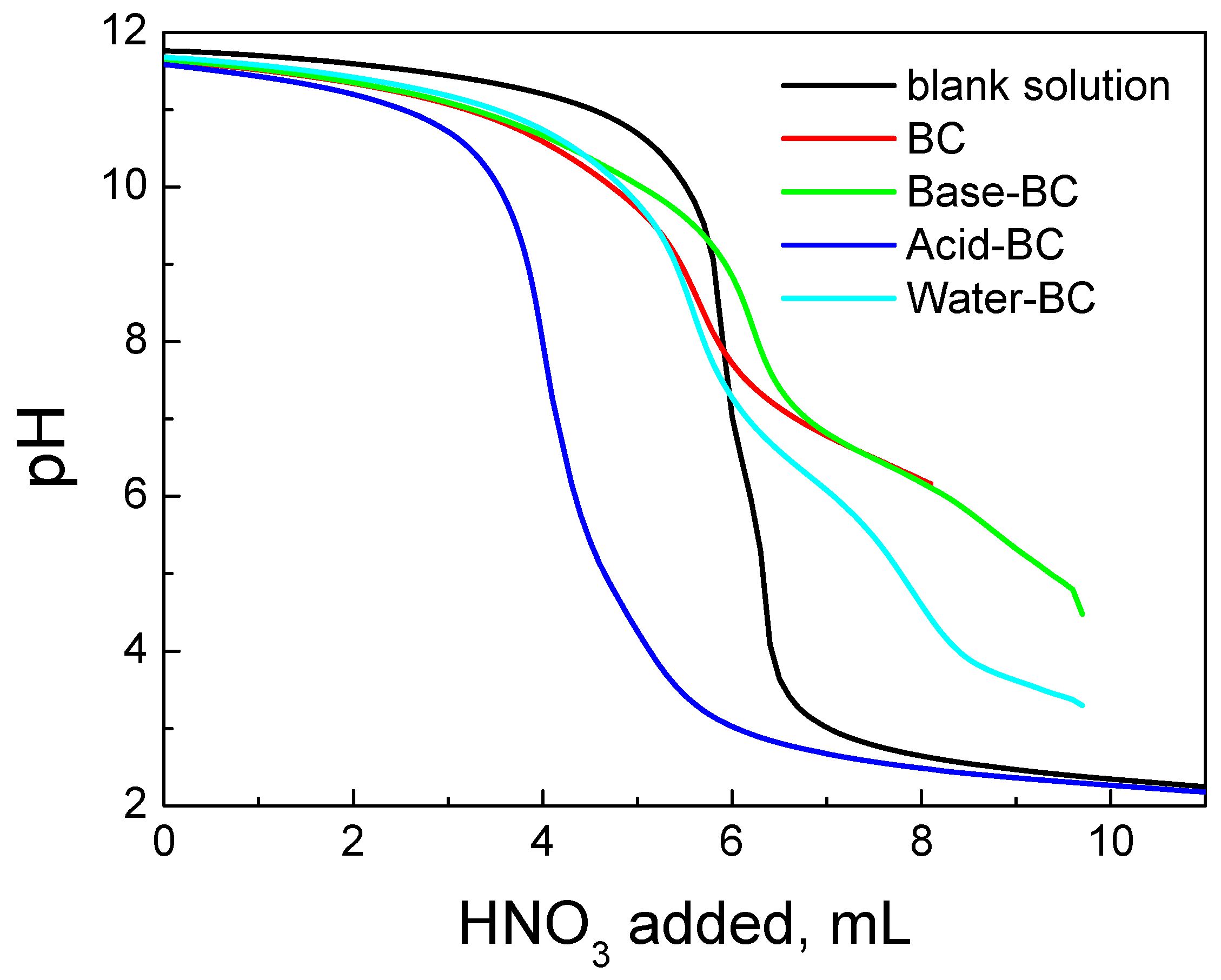
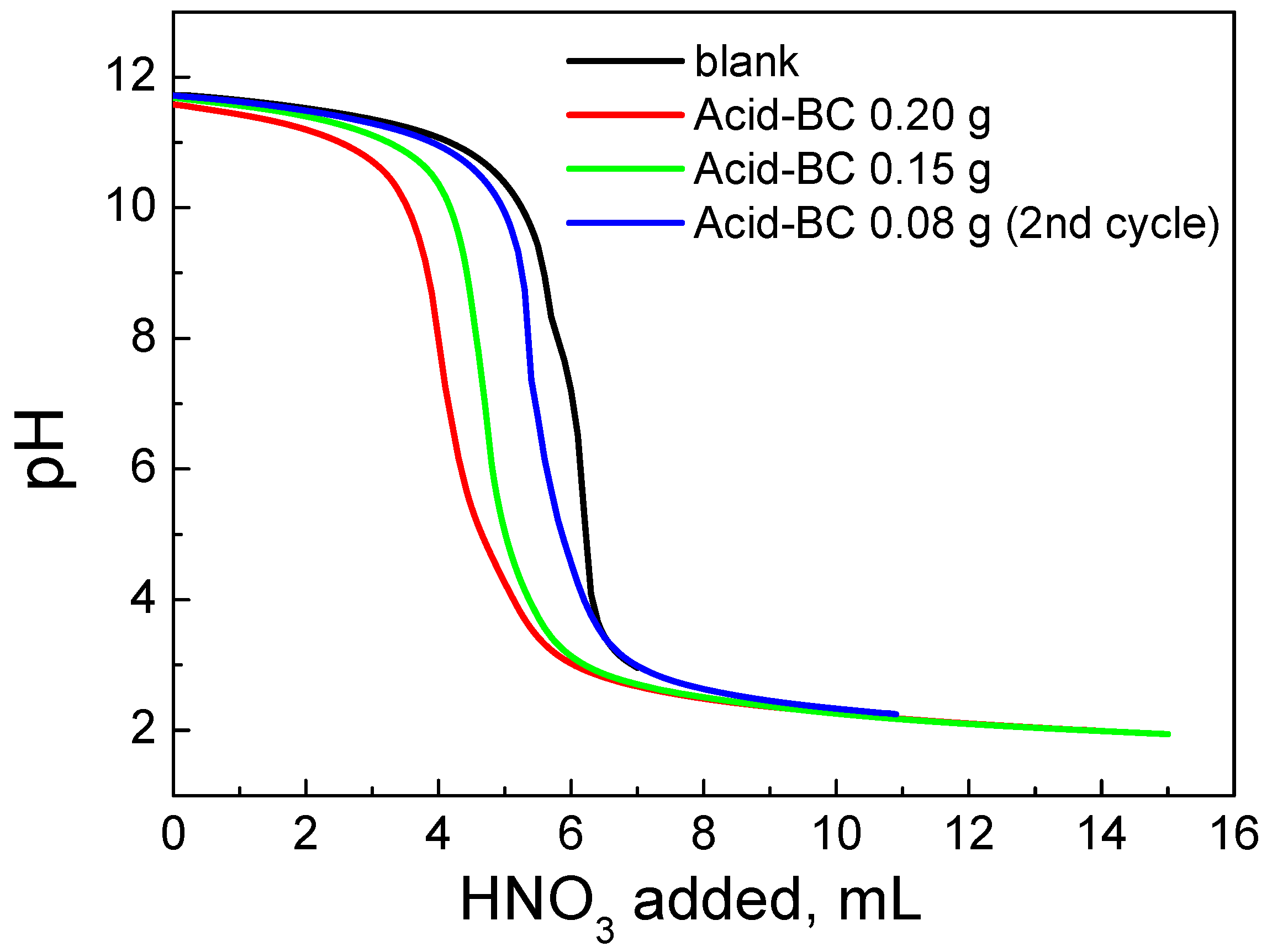
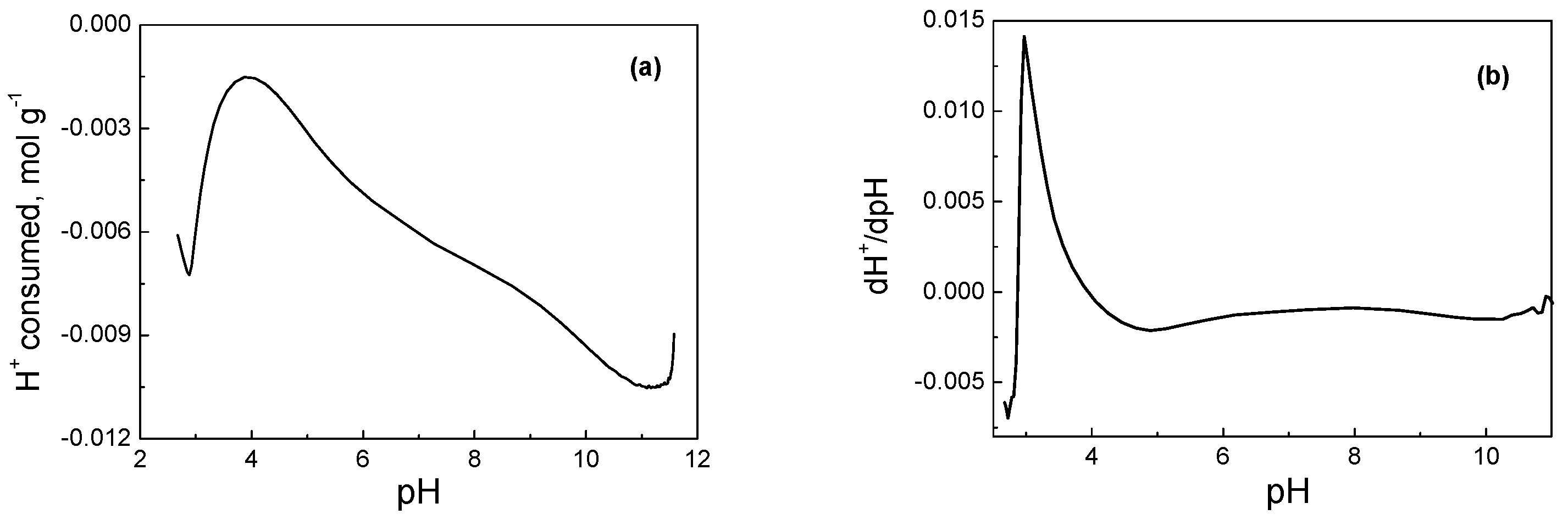

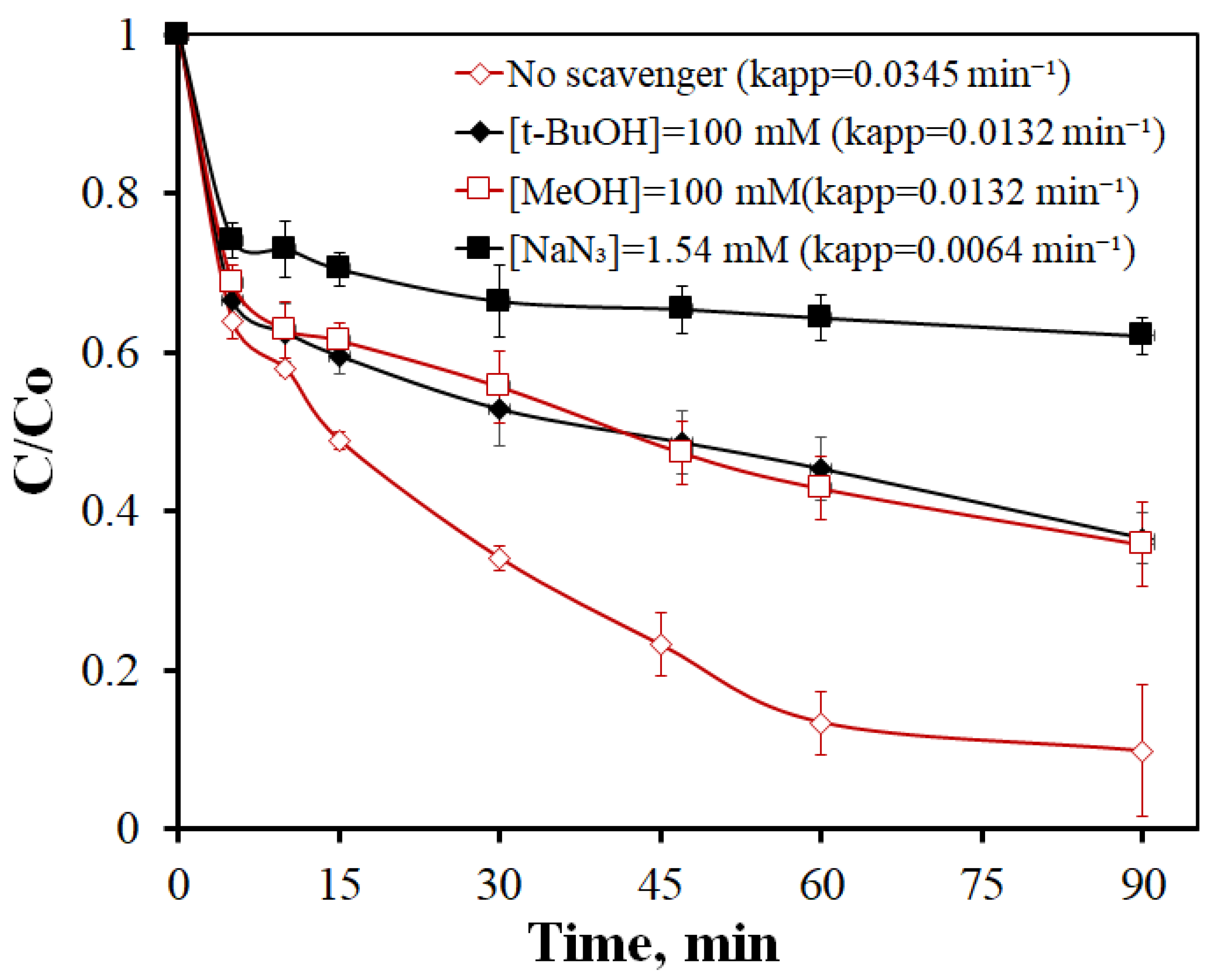
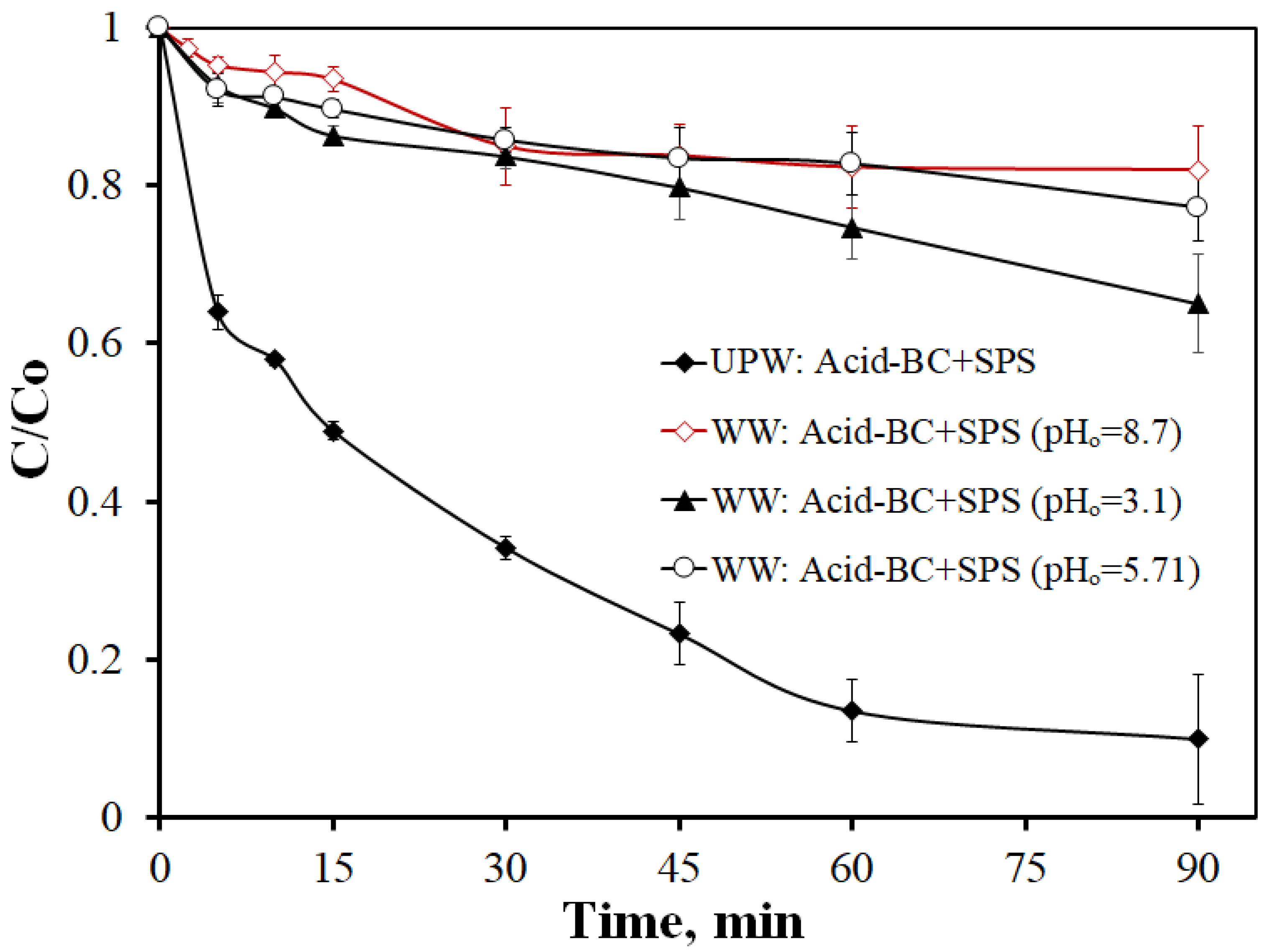
| Sample | SSA (m2g−1) | Micropores SSA (m2g−1) | pzc | Minerals (%) | TGA Temp. (°C) |
|---|---|---|---|---|---|
| BC | 100 | 58 | 8.2 | 32 | 476 |
| Acid–BC | 428 | 190 | <2.5 | 3 | 570 |
| Base–BC | 362 | 175 | 9.5 | 13 | 451 |
| Water–BC | 308 | 142 | 7.2 | 24 | 462 |
| Biochar | pHinitial |
pHfinal Oxid/Ads |
koxidation,
min−1 |
kadsorption,
min−1 | koxidation/kadsorption |
|---|---|---|---|---|---|
| BC | pHinherent ≈ 5.6 | 4.2/6.0 | 0.0142 | 0.0016 | 8.875 |
| Water–BC | pHinherent ≈ 5.6 | 3.9/5.7 | 0.0230 | 0.0025 | 9.200 |
| Base–BC | pHinherent ≈ 5.6 | 4.6/6.2 | 0.0170 | 0.0014 | 12.143 |
| Acid–BC | pHinherent ≈ 5.6 | 3.8/4.5 | 0.035 | 0.0093 | 3.763 |
| BC | 3.0 | 3.0/3.0 | 0.0160 | 0.0099 | 1.616 |
| Water–BC | 3.0 | 3.0/3.0 | 0.0350 | 0.0166 | 2.108 |
| Base–BC | 3.0 | 3.0/3.0 | 0.0310 | 0.0196 | 1.582 |
| Acid–BC | 3.0 | 3.0/3.0 | 0.0090 | 0.0093 | 0.968 |
| BC | 10.0 | 9.4/9.9 | 0.0011 | 0.0009 | 1.222 |
| Water–BC | 10.0 | 9.8/97 | 0.0017 | 0.0006 | 2.833 |
| Base–BC | 10.0 | 10.0/9.7 | 0.0012 | 0.0003 | 4.000 |
| Acid–BC | 10.0 | 9.5/9.6 | 0.0026 | 0.0007 | 3.714 |
| Biomass Source | Modification Treatment | BC Concentration, mg/L | Oxidant, mg/L | Target Pollutant, mg/L | Removal, % | Ref. |
|---|---|---|---|---|---|---|
| Rice-hull | Hydrofluoric acid | 10000 | Persulfate, 1359 | Acid orange 7, 20 | 70% in deionized water at 120 min | [30] |
| Pinewood | KOH | 1000 | Persulfate, 238 | Carbamazepine, 5 | 40% in UPW at 60 min | [31] |
| Pine needle leaves | Plasma (250 W) | 200 | Peroxymonosulfate, 456.6 | Phenol, 10 | 98% in deionized water at 120 min | [32] |
| Citrus peels | Cu0Fe3O4@biochar (65%) | 600 | Peroxymonosulfate, 1000 | Bisphenol A, 20 | 95% in deionized water at 120 min | [33] |
| Spent malt rootlet | H2SO4 NaOH Water | 90 | Persulfate, 250 | Losartan, 0.25 | 91%, 76%, 84% in UPW at 90 min | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ioannidi, A.A.; Vakros, J.; Frontistis, Z.; Mantzavinos, D. Tailoring the Biochar Physicochemical Properties Using a Friendly Eco-Method and Its Application on the Oxidation of the Drug Losartan through Persulfate Activation. Catalysts 2022, 12, 1245. https://doi.org/10.3390/catal12101245
Ioannidi AA, Vakros J, Frontistis Z, Mantzavinos D. Tailoring the Biochar Physicochemical Properties Using a Friendly Eco-Method and Its Application on the Oxidation of the Drug Losartan through Persulfate Activation. Catalysts. 2022; 12(10):1245. https://doi.org/10.3390/catal12101245
Chicago/Turabian StyleIoannidi, Alexandra A., John Vakros, Zacharias Frontistis, and Dionissios Mantzavinos. 2022. "Tailoring the Biochar Physicochemical Properties Using a Friendly Eco-Method and Its Application on the Oxidation of the Drug Losartan through Persulfate Activation" Catalysts 12, no. 10: 1245. https://doi.org/10.3390/catal12101245
APA StyleIoannidi, A. A., Vakros, J., Frontistis, Z., & Mantzavinos, D. (2022). Tailoring the Biochar Physicochemical Properties Using a Friendly Eco-Method and Its Application on the Oxidation of the Drug Losartan through Persulfate Activation. Catalysts, 12(10), 1245. https://doi.org/10.3390/catal12101245









