Role of the Potential Range during Stress Testing of Platinum-Containing Electrocatalysts at Elevated Temperature
Abstract
1. Introduction
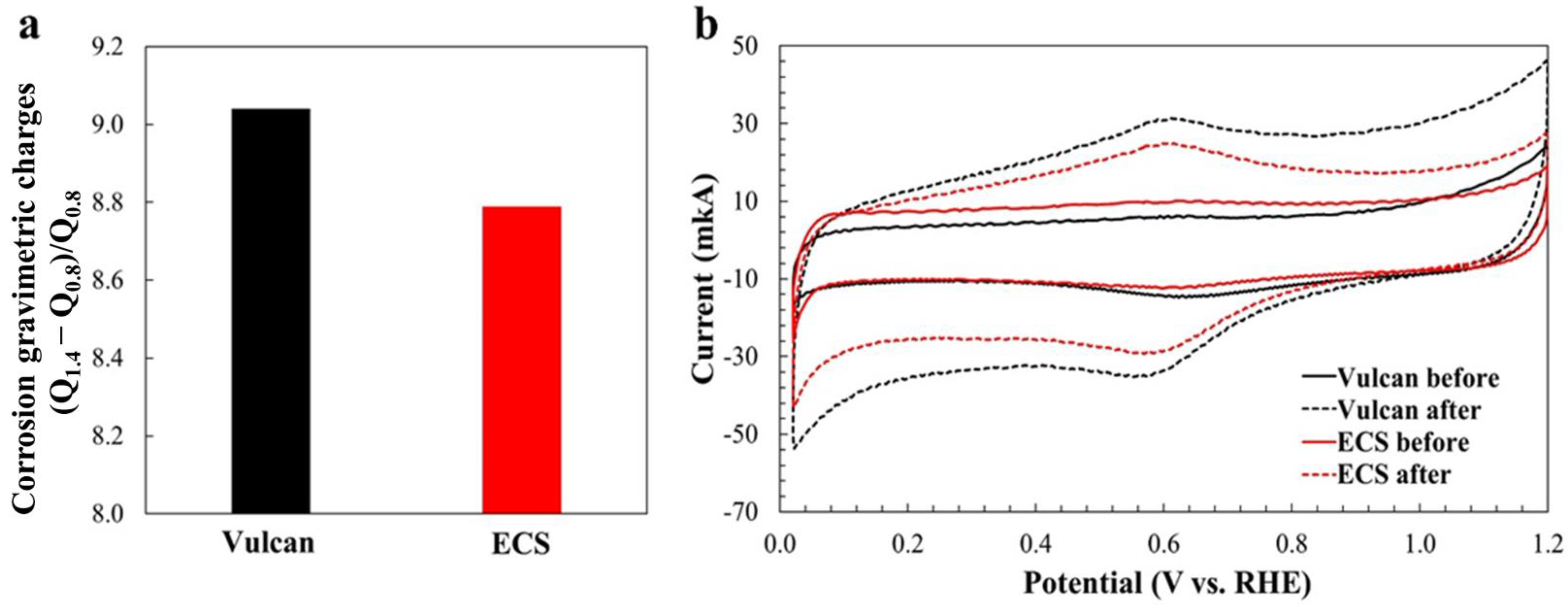
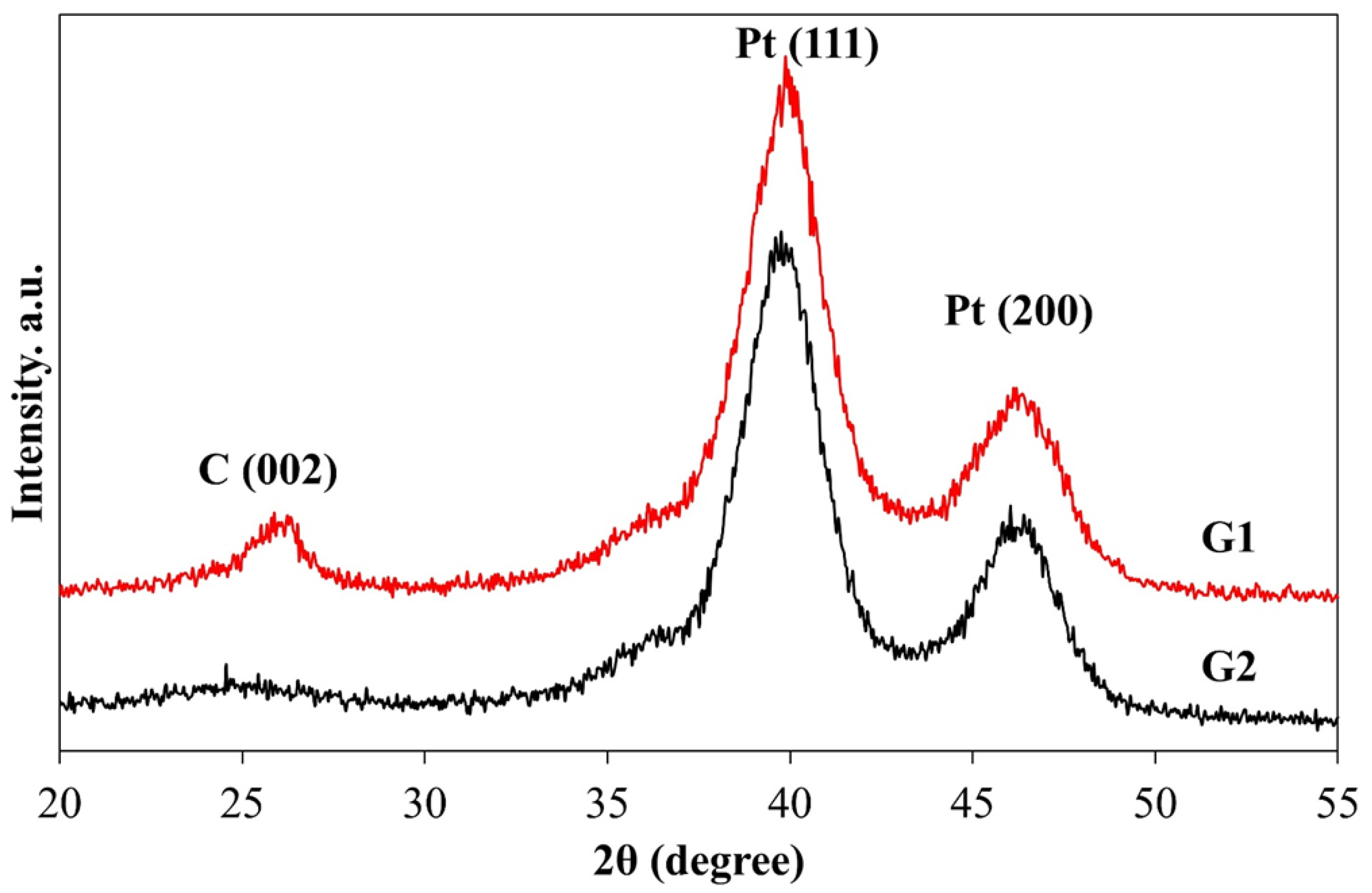
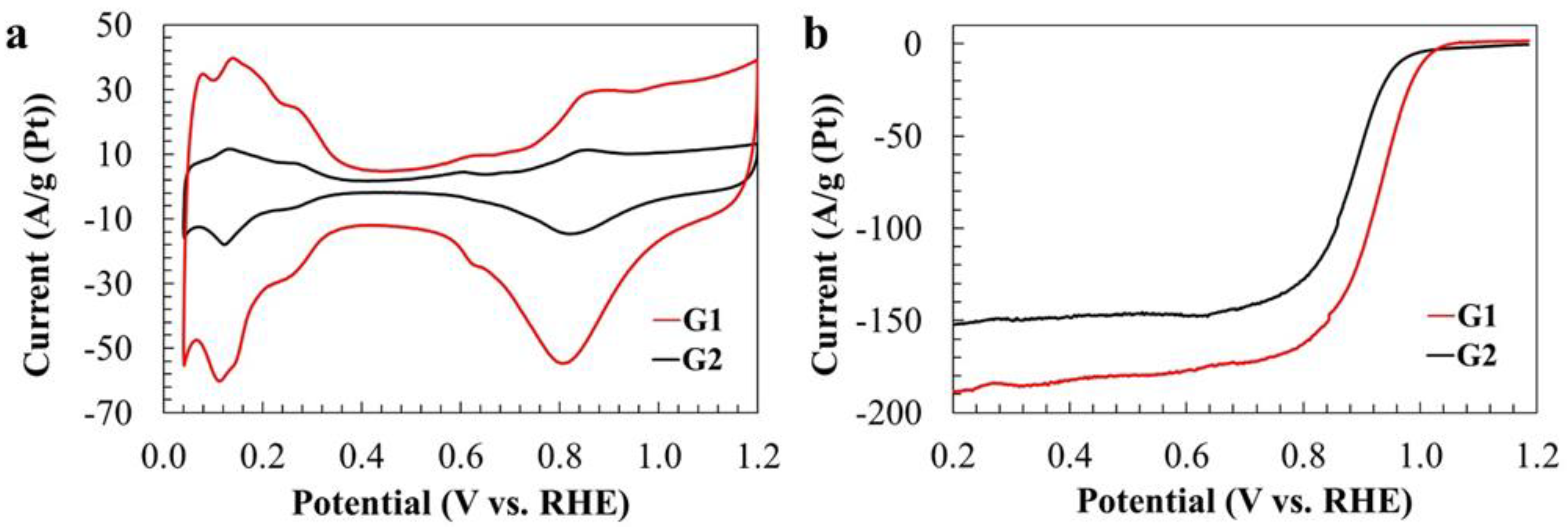
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of Catalysts
3.3. Attestation of Catalysts’ Structural and Morphological Characteristics
3.4. Attestation of the Electrochemical Characteristics of the Catalysts
3.5. Accelerated Degradation Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PEMFC | Proton exchange membrane fuel cells |
| ADT | Accelerated degradation testing |
| NPs | Nanoparticles |
| MEA | Membrane–electrode assembly |
| XRD | X-ray diffraction |
| TEM | Transmission electron microscopy |
| DSC | Differential scanning calorimetry |
| ESA | Electrochemically active surface area |
| CV | Cyclic voltammetry |
| ORR | Oxygen reduction reaction |
| TGA | Thermogravimetric analysis |
| RHE | Reversible hydrogen electrode |
| XRF | X-ray fluorescence analysis |
| LSV | Linear sweep voltammetry |
References
- Yaroslavtsev, A.B.; Dobrovolsckiy, Y.A.; Shaglaeve, N.S.; Frolova, L.A.; Gerasimova, E.V.; Sanginov, E.A. Nanostructured materials for low-temperature fuel cells. Russ. Chem. Rev. 2012, 81, 191–201. [Google Scholar] [CrossRef]
- Holton, O.T.; Stevenson, J.W. The Role of Platinum in Proton Exchange Membrane Fuel Cells. Platinum Met. Rev. 2013, 57, 259–271. [Google Scholar] [CrossRef]
- Bagotsky, V.S.; Skundin, A.M.; Volfkovich, Y.M. Electrochemical Power Sources: Batteries, Fuel Cells, and Supercapacitors; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; ISBN1 9781118942857. Available online: https://books.google.ru/books?hl=ru&lr=&id=ub3eBQAAQBAJ&oi=fnd&pg=PR15&ots=_H00vLANTe&sig=akODm2f5gWa8Ml1PQwI89orQwJ8&redir_esc=y#v=onepage&q&f=false (accessed on 1 May 2022)ISBN2 9781118942857.
- Lv, H.; Li, D.; Strmcnik, D.; Paulikas, A.P.; Markovic, N.M.; Stamenkovic, V.R. Recent advances in the design of tailored nanomaterials for efficient oxygen reduction reaction. Nano Energy 2016, 29, 149–165. [Google Scholar] [CrossRef]
- Yu, E.H.; Krewer, U.; Scott, K. Principles and Materials Aspects of Direct Alkaline Alcohol Fuel Cells. Energies 2010, 3, 1499–1528. [Google Scholar] [CrossRef]
- O’Hayre, R.; Cha, S.W.; Collela, W.; Prinz, F.B. Fuel Cell Fundamentals; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; p. 576. Available online: https://books.google.ru/books?hl=en&lr=&id=O2JYCwAAQBAJ&oi=fnd&pg=PR11&ots=RRDKUTO3lf&sig=wSEznU4TFJhk4YydjqA_wBUPC7A&redir_esc=y (accessed on 20 March 2022).
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Gao, K.; Pang, X.; Yang, H.; Volinsky, A.A. Electrochemical Oxidation of Methanol on Pt-SnOx/C Catalysts Characterized by Electrochemistry Methods. J. Electrochem. Soc. 2015, 162, 1540–1548. [Google Scholar] [CrossRef]
- Singh, R.N.; Awasthi, R.; Sharma, C.S. Review: An overview of recent development of platinum-based cathode materials for direct methanol fuel cells. Int. J. Electrochem. Sci. 2014, 9, 5607–5639. [Google Scholar]
- Chen, Y.; Yang, F.; Dai, Y.; Wang, W.; Chen, S. Ni@Pt Core-Shell Nanoparticles: Synthesis, Structural and Electrochemical Properties. J. Phys. Chem. C 2008, 112, 1645–1649. [Google Scholar] [CrossRef]
- Antolini, E. Carbon supports for low-temperature fuel cell catalysts. Appl. Catal. B Environ. 2009, 88, 1–24. [Google Scholar] [CrossRef]
- Dicks, A.L. The role of carbon in fuel cells. J. Power Sources 2006, 156, 128–141. [Google Scholar] [CrossRef]
- Kim, M.; Park, J.N.; Kim, H.; Song, S.; Lee, W.H. The Preparation of Pt/C Catalysts Using Various Carbon Materials for the Cathode of PEMFC. J. Power Sources 2006, 163, 93–97. [Google Scholar] [CrossRef]
- Gharibi, H.; Javaheri, M.; Mirzaie, R.A. The synergy between multi-wall carbon nanotubes and Vulcan XC-72R in microporous layers. Int. J. Hydrog. Energy 2010, 35, 9241–9251. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Nappor, T.W. Platinum Fuel Cell Nanoparticle Syntheses: Effect on Morphology, Structure and Electrocatalytic Behavior. In The Delivery of Nanoparticles; InTech Open: London, UK, 2012; pp. 403–431. [Google Scholar] [CrossRef]
- Meier, J.C.; Galeano, C.; Katsounaros, I.; Witte, J.; Bongard, H.J.; Topalov, A.A.; Baldizzone, C.; Mezzavilla, S.; Schüth, F.; Mayrhofer, K.J.J. Design criteria for stable Pt/C fuel cell catalysts. Beilstein J. Nanotechnol. 2014, 5, 44–67. [Google Scholar] [CrossRef]
- Yano, H.; Watanabea, M.; Iiyamaa, A.; Uchida, H. Particle-size effect of Pt cathode catalysts on durability in fuel cells. Nano Energy 2016, 29, 323–333. [Google Scholar] [CrossRef]
- Pavlov, V.I.; Gerasimova, E.V.; Zolotukhina, E.V.; Dobrovolsky, Y.A.; Don, G.M.; Yaroslavtsev, A.B. Degradation of Pt/C electrocatalysts having different morphology in low-temperature PEM fuel cells. Nanotechnol. Russ. 2016, 11, 743–750. [Google Scholar] [CrossRef]
- Katsounaros, I.; Cherevko, S.; Zeradjanin, A.R.; Mayrhofer, K.J.J.; Karl, J.J. Oxygen electrochemistry as a cornerstone for sustainable energy conversion. Angew. Chem. Int. Ed. Engl. 2014, 53, 102–121. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Guterman, V.E.; Volochaev, V.A.; Belenov, S.V. Effect of wet synthesis conditions on the microstructure and active surface area of Pt/C catalysts. Inorg. Mater. 2015, 51, 1258–1263. [Google Scholar] [CrossRef]
- Shao, M.H.; Chang, Q.W.; Dodelet, J.P.; Chenitz, R. Recent Advances in Electrocatalysts for Oxygen Reduction Reaction. Chem. Rev. 2016, 116, 3594–3657. [Google Scholar] [CrossRef]
- Antolini, E.; Salgado, J.R.C.; Gonzalez, E.R. The stability of Pt–M (M = first row transition metal) alloy catalysts and its effect on the activity in low temperature fuel cells: A literature review and tests on a Pt–Co catalyst. J. Power Sources 2006, 160, 957–968. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Guterman, V.E.; Belenov, S.V.; Menshikov, V.S.; Tabachkova, N.Y.; Safronenko, O.I.; Moguchikh, E.A. Pt/C electrocatalysts based on the nanoparticles with the gradient structure. Int. J. Hydrog. Energy 2018, 43, 3676–3687. [Google Scholar] [CrossRef]
- Guterman, V.E.; Belenov, S.V.; Alekseenko, A.A.; Tabachkova, N.Y.; Volochaev, V.A. The relationship between activity and stability of deposited platinum–carbon electrocatalysts. Russ. J. Electrochem. 2017, 53, 531–539. [Google Scholar] [CrossRef]
- Capelo, A.; Esteves, M.A.; Sa, A.I.; Silva, R.A.; Cangueiro, L.; Almeida, A.; Vilar, R.; Rangel, C.M. Stability and durability under r cycling of Pt/C catalyst with new surface-functionalized carbon support. Int. J. Hydrogen Energy 2016, 41, 12962–12975. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Wang, Y.; Ding, W.; Wu, R.; Li, L.; Qi, X.; Wei, Z. Study of the degradation mechanisms of carbon-supported platinum fuel cells catalyst via different accelerated stress test. J. Power Sources 2015, 273, 62–69. [Google Scholar] [CrossRef]
- Cui, C.; Gan, L.; Li, H.; Yu, S.; Heggen, M.; Strasser, P. Octahedral PtNi Nanoparticle Catalysts: Exceptional Oxygen Reduction Activity by Tuning the Alloy Particle Surface Composition. Nano Lett. 2012, 12, 5885–5889. [Google Scholar] [CrossRef]
- Paulus, U.A.; Wokaun, A.; Scherer, G.G.; Schmidt, T.J.; Stamenkovic, V.R.; Marković, N.M.; Ross, P.N. Oxygen reduction on high surface area Pt-based alloy catalysts in comparison to well defined smooth bulk alloy electrodes. Electrochim. Acta 2002, 47, 3787–3798. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Moguchikh, E.A.; Safronenko, O.I.; Guterman, V.E. Durability of de-alloyed PtCu/C electrocatalysts. Int. J. Hydrog. Energy 2018, 43, 22885–22895. [Google Scholar] [CrossRef]
- Leontyev, I.; Belenov, S.; Guterman, V.; Haghi-Ashtiani, P.; Shaganov, A.; Dkhil, B. Catalytic activity of carbon-supported Pt nanoelectrocatalysts. Why reducing the size of Pt nanoparticles is not always beneficial. J. Phys. Chem. C 2011, 115, 5429–5434. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, P.; Chen, H.; Pei, P. A review of automotive proton exchange membrane fuel cell degradation under start-stop operating condition. Appl. Energy 2018, 223, 249–262. [Google Scholar] [CrossRef]
- Wang, D.; Xin, H.; Hovden, R.; Wang, H.; Yu, Y.; Muller, D.; DiSalvo, F.; Abruña, H. Structurally ordered intermetallic platinum-cobalt core-shell nanoparticles with enhanced activity and stability as oxygen reduction electrocatalysts. Nat. Mater. 2013, 12, 81–87. [Google Scholar] [CrossRef]
- Ge, X.; Chen, L.; Kang, J.; Fujita, T.; Hirata, A.; Zhang, W.; Chen, M. A Core-shell nanoporous Pt-Cu catalyst with tunable composition and high catalytic activity. Adv. Funct. Mater. 2013, 23, 4156–4162. [Google Scholar] [CrossRef]
- Luo, M.; Wei, L.; Wang, F.; Han, K.; Zhu, H. Gram-Level Synthesis of Core–Shell Structured Catalysts for the Oxygen Reduction Reaction in Proton Exchange Membrane Fuel Cells. J. Power Sources 2014, 270, 34–41. [Google Scholar] [CrossRef]
- Cherevko, S.; Kulyk, N.; Mayrhofer, K.J.J. Durability of Platinum-Based Fuel Cell Electrocatalysts: Dissolution of Bulk and Nanoscale Platinum. Nano Energy 2016, 29, 275–298. [Google Scholar] [CrossRef]
- Stevens, D.A.; Hicks, M.T.; Haugen, G.M.; Dahn, J.R. Ex Situ and In Situ Stability Studies of PEMFC Catalysts: Effect of Carbon Type and Humidification on Degradation of the Carbon. J. Electrochem. Soc. 2005, 152, 2309–2315. [Google Scholar] [CrossRef]
- Đukić, T.; Moriau, L.J.; Pavko, L.; Kostelec, M.; Prokop, M.; Ruiz-Zepeda, F.; Šala, M.; Dražić, G.; Gatalo, M.; Hodnik, N. Understanding the Crucial Significance of the Temperature and Potential Window on the Stability of Carbon Supported Pt-Alloy Nanoparticles as Oxygen Reduction Reaction Electrocatalysts. ACS Catal. 2022, 12, 101–115. [Google Scholar] [CrossRef]
- Sadezky, H.A.; Muckenhuber, H.; Grothe, R.; Niessner, U. Pöschl Raman microspectroscopy of soot and related carbonaceous materials: Spectral analysis and structural information. Carbon 2005, 43, 1731–1742. [Google Scholar] [CrossRef]
- Serov, A.; Lubers, A.; McCool, G.; McKinney, S.; Romero, H.; Zulevi, B. VariporeTM: A Powerful Manufacturing Platform for Fuel Cell and Electrolyzer Applications. ECS Meet. Abstr. 2019, MA2019-02, 1734. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S.; et al. Raman Spectrum of Graphene and Graphene Layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Leontyev, I.N.; Leontyeva, D.V.; Kuriganova, A.B.; Popov, Y.V.; Maslova, O.A.; Glebova, N.V.; Nechitailov, A.A.; Zelenina, N.K.; Tomasov, A.A.; Hennet, L. Characterization of the Electrocatalytic Activity of Carbon-Supported Platinum-Based Catalysts by Thermal Gravimetric Analysis. Mendeleev Commun. 2015, 25, 468–469. [Google Scholar] [CrossRef]
- Baturina, O.A.; Aubuchon, S.R.; Wynne, K.J. Thermal Stability in Air of Pt/C Catalysts and PEM Fuel Cell Catalyst Layers. Chem. Mater. 2006, 18, 1498–1504. [Google Scholar] [CrossRef]
- Guterman, V.E.; Belenov, S.V.; Krikov, V.V.; Vysochina, L.L.; Yohannes, W.; Tabachkova, N.Y.; Balakshina, E.N. Reasons for the Differences in the Kinetics of Thermal Oxidation of the Support in Pt/C Electrocatalysts. J. Phys. Chem. C 2014, 118, 23835–23844. [Google Scholar] [CrossRef]
- Ortíz-Herrera, J.C.; Tellez-Cruz, M.M.; Solorza-Feria, O.; Medina, D.I. Effect of Different Carbon Supports on the Activity of PtNi Bimetallic Catalysts toward the Oxygen Reduction. Catalysts 2022, 12, 477. [Google Scholar] [CrossRef]
- Banham, D.; Feng, F.; Fürstenhaupt, T.; Pei, K.; Ye, S.; Birss, V. Novel Mesoporous Carbon Supports for PEMFC Catalysts. Catalysts 2015, 5, 1046–1067. [Google Scholar] [CrossRef]
- Ayyubov, I.; Tálas, E.; Salmanzade, K.; Kuncser, A.; Pászti, Z.; Neațu, Ș.; Mirea, A.G.; Florea, M.; Tompos, A.; Borbáth, I. Electrocatalytic Properties of Mixed-Oxide-Containing Composite-Supported Platinum for Polymer Electrolyte Membrane (PEM) Fuel Cells. Materials 2022, 15, 3671. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Mukerjee, S. Electrocatalysis of oxygen reduction on carbon-supported PtCo catalysts prepared by water-in-oil micro-emulsion. Electrochim. Acta 2010, 55, 1709–1719. [Google Scholar] [CrossRef]
- Huang, J.; Ding, C.; Yang, Y.; Liu, G.; Cai, W. bin An Alternate Aqueous Phase Synthesis of the Pt3Co/C Catalyst towards Efficient Oxygen Reduction Reaction. Chin. J. Catal. 2019, 12, 40. [Google Scholar] [CrossRef]
- Hernández-Fernández, P.; Rojas, S.; Ocón, P.; de la Fuente, J.L.G.; Terreros, P.; Peña, M.A.; García-Fierro, J.L. An Opening Route to the Design of Cathode Materials for Fuel Cells Based on PtCo Nanoparticles. Appl. Catal. B 2007, 77, 19–28. [Google Scholar] [CrossRef]
- Koffi, R.C.; Coutanceau, C.; Garnier, E.; Léger, J.M.; Lamy, C. Synthesis, Characterization and Electrocatalytic Behaviour of Non-Alloyed PtCr Methanol Tolerant Nanoelectrocatalysts for the Oxygen Reduction Reaction (ORR). Electrochim. Acta 2005, 50, 4117–4127. [Google Scholar] [CrossRef]
- Alekseenko, A.A.; Guterman, V.E.; Volochaev, V.A. Microstructure Optimization of Pt/C Catalysts for PEMFC. In Springer Proceedings in Physics; Springer: Berlin/Heidelberg, Germany, 2016; Volume 175, pp. 37–49. [Google Scholar] [CrossRef]
- Garsany, Y.; Atkinson, R.W.; Gould, B.D.; Martin, R.; Dubau, L.; Chatenet, M.; Swider-Lyons, K.E. Dual-Layer Catalyst Layers for Increased Proton Exchange Membrane Fuel Cell Performance. J. Power Sources 2021, 514, 230574. [Google Scholar] [CrossRef]
- Favilla, P.C.; Acosta, J.J.; Schvezov, C.E.; Sercovich, D.J.; Collet-Lacoste, J.R. Size Control of Carbon-Supported Platinum Nanoparticles Made Using Polyol Method for Low Temperature Fuel Cells. Chem. Eng. Sci. 2013, 101, 27–34. [Google Scholar] [CrossRef]
- Gražulis, S.; Daškevič, A.; Merkys, A.; Chateigner, D.; Lutterotti, L.; Quirós, M.; Serebryanaya, N.R.; Moeck, P.; Downs, R.T.; le Bail, A. Crystallography Open Database (COD): An Open-Access Collection of Crystal Structures and Platform for World-Wide Collaboration. Nucleic Acids Res. 2012, 40, 420–427. [Google Scholar] [CrossRef]
- Klug, H.P.; Alexander, L.E. X-ray Diffraction Procedures from 376 Polycrystalline and Amorphous Materials; John Wiley: New York, NY, USA, 1974; Available online: https://ui.adsabs.harvard.edu/abs/1974xdpf.book.....K/abstract (accessed on 25 August 2022).
- Gao, J.; Mao, M.; Li, P.; Liu, R.; Song, H.; Sun, K.; Zhang, S. Segmentation and Re-Encapsulation of Porous PtCu Nanoparticles by Generated Carbon Shell for Enhanced Ethylene Glycol Oxidation and Oxygen-Reduction Reaction. ACS Appl. Mater. Interfaces 2020, 12, 6298–6308. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Zack, J.W.; Pylypenko, S.; Pivovar, B.S.; Kocha, S.S. Oxygen reduction reaction measurements on platinum electrocatalysts utilizing rotating disk electrode technique: II.Influence of ink formulation, catalyst layer uniformity and thickness. J. Electrochem. Soc. 2015, 162, 1384–1396. [Google Scholar] [CrossRef]
- Hyun, K.; Lee, J.H.; Yoon, C.W.; Kwon, Y. The effect of platinum based bimetallic electrocatalysts on oxygen reduction reaction of proton exchange membrane fuel cells. Int. J. Electrochem. Sci. 2013, 8, 11752–11767. Available online: http://electrochemsci.org/papers/vol8/81011752.pdf (accessed on 25 August 2022).
- Forouzandeh, F.; Li, X.; Banham, D.W.; Feng, F.; Ye, S.; Birss, V. Understanding the Corrosion Resistance of Meso- and Micro-Porous Carbons for Application in PEM Fuel Cells. J. Electrochem. Soc. 2018, 165, 3230–3240. [Google Scholar] [CrossRef]



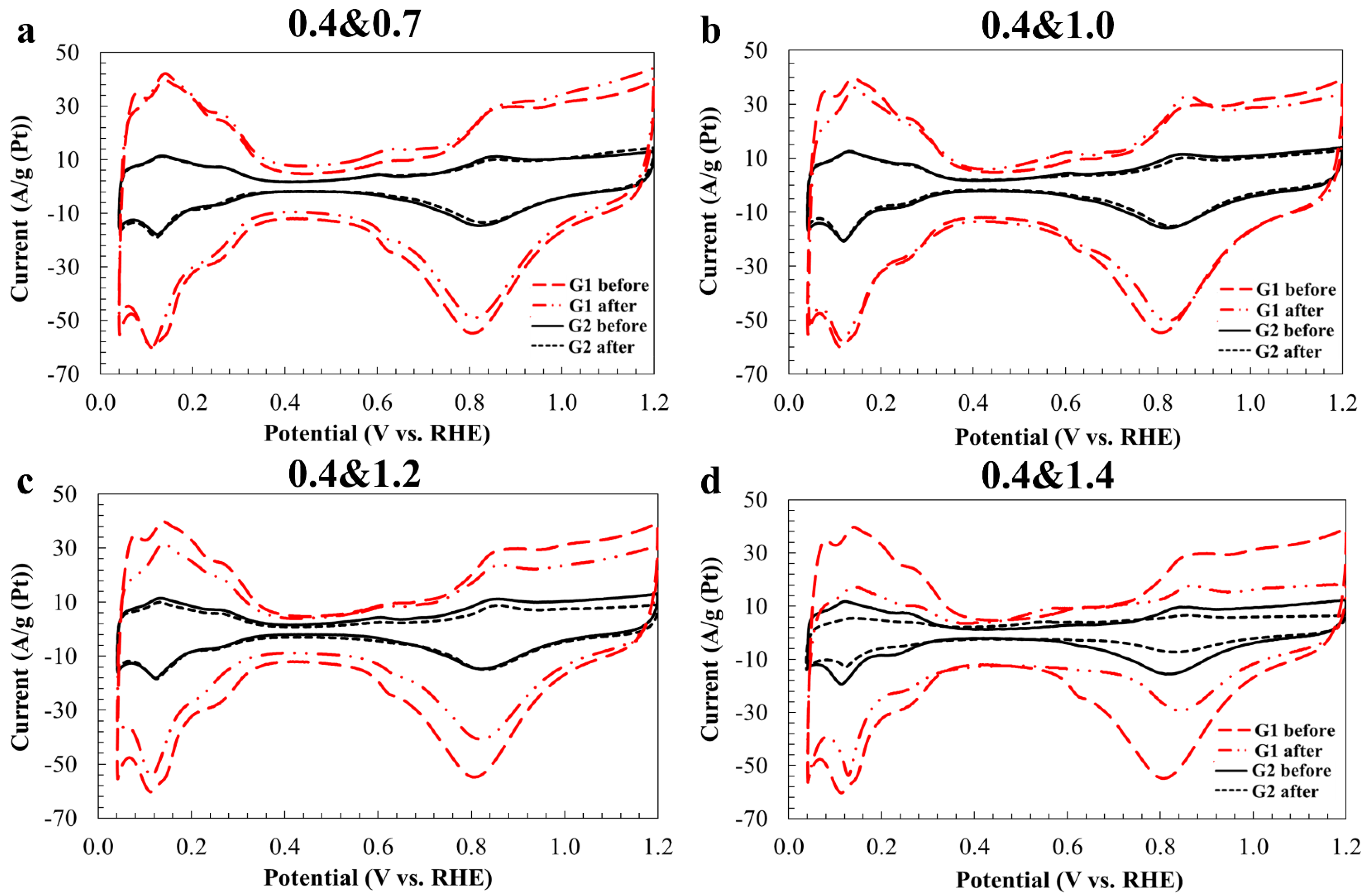
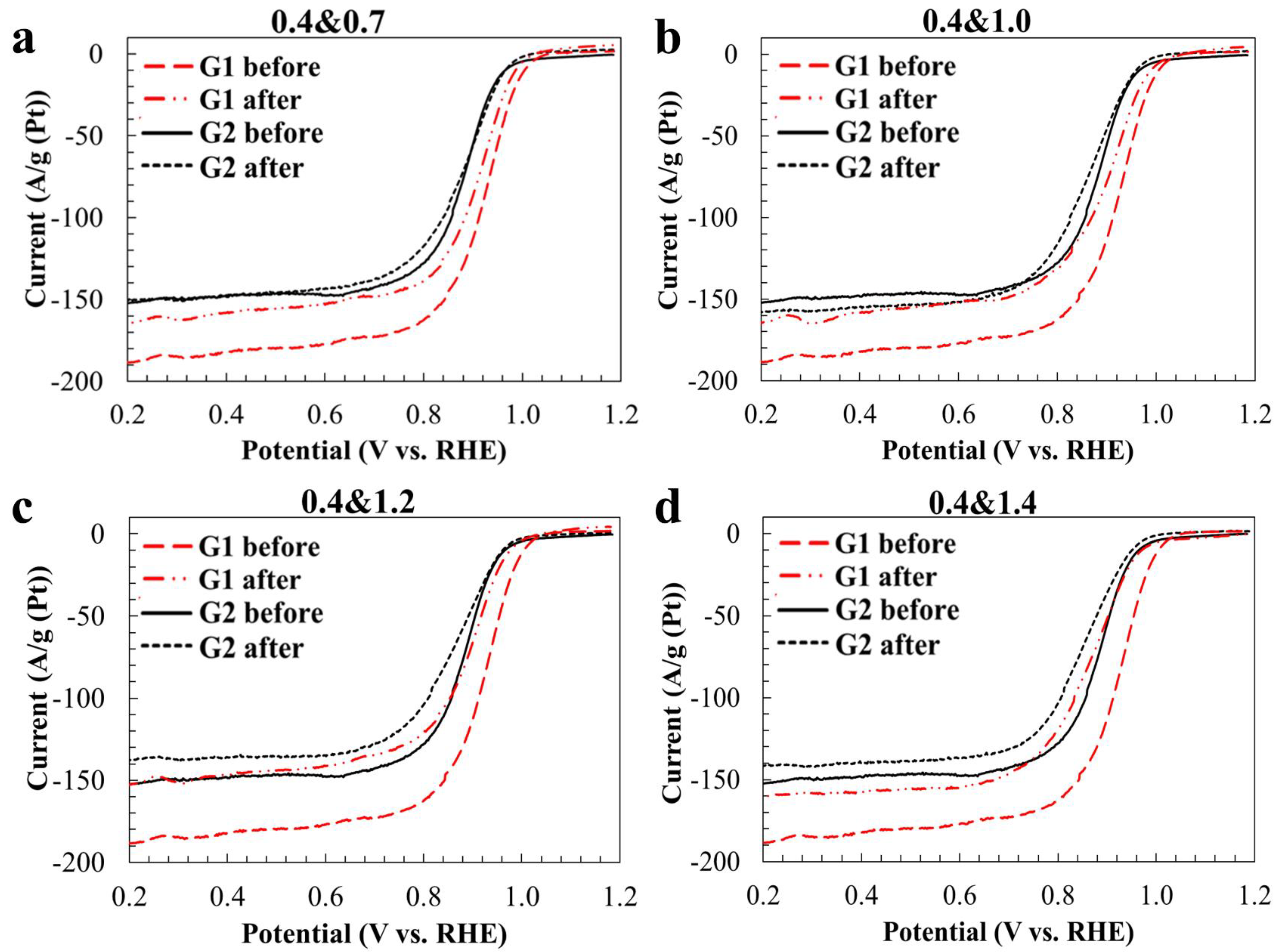

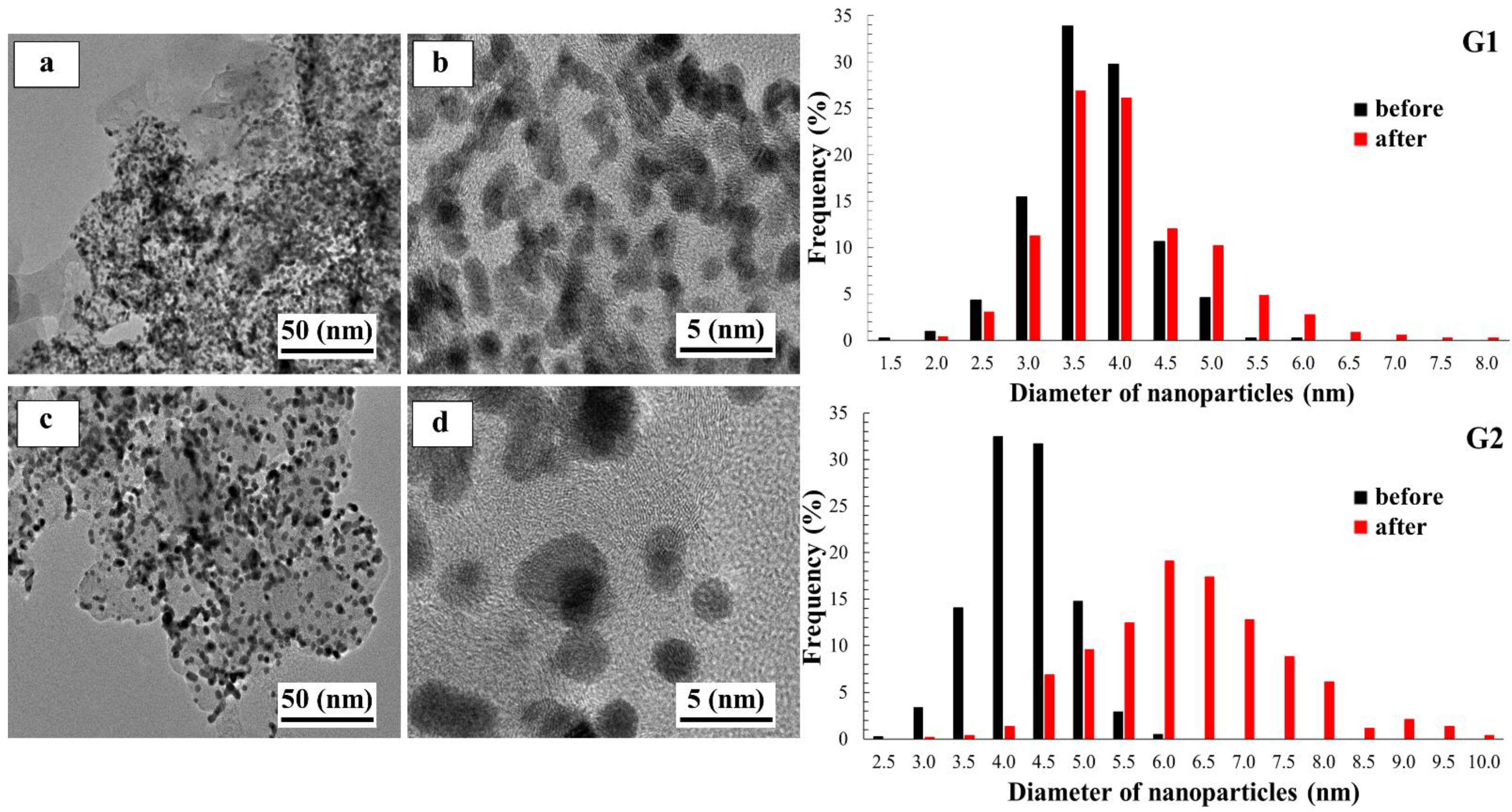
| Sample | Carbon Support | Pt-Loading, wt. % | Average Crystallites Diameter, DAv, nm (XRD) | Average NPs Size, DAv, nm (TEM) | ESA, m2 g−1 (Pt) | E1/2, V | Number of ē (E = 0.85 V) | Imass, Ag−1 (Pt) (E = 0.85 V) | Isp, Am−2 (Pt) (E = 0.85 V) | Tafel Slope, mV/dec |
|---|---|---|---|---|---|---|---|---|---|---|
| G1 | ECS | 39 | 3.2 | 3.7 | 130 | 0.90 | 4.0 | 479 | 3.7 | 63.6 |
| G2 | Vulcan XC-72 | 39 | 2.6 | 4.2 | 50 | 0.88 | 3.8 | 279 | 5.6 | 42.2 |
| Sample | ESA, m2g−1(Pt) | Durability, % | E1/2,V | Number of ē (E = 0.85 V) | Imass, Ag−1 (Pt) (E = 0.85 V) | Isp, A m−2 (Pt) (E = 0.85 V) | Tafel Slope, mV/dec |
|---|---|---|---|---|---|---|---|
| ADT potentials 0.4 and 0.7 V | |||||||
| G1 | 125 | 95 | 0.90 | 4.0 | 459 | 3.7 | 58.1 |
| G2 | 49 | 98 | 0.86 | 3.8 | 238 | 4.8 | 54.6 |
| ADT potentials 0.4 and 1.0 V | |||||||
| G1 | 118 | 91 | 0.90 | 3.9 | 389 | 3.3 | 66.2 |
| G2 | 45 | 96 | 0.83 | 3.7 | 188 | 4.2 | 52.0 |
| ADT potentials 0.4 and 1.2 V | |||||||
| G1 | 98 | 75 | 0.89 | 3.9 | 380 | 3.9 | 59.4 |
| G2 | 44 | 88 | 0.85 | 3.7 | 182 | 4.1 | 53.5 |
| ADT potentials 0.4 and 1.4 V | |||||||
| G1 | 73 | 56 | 0.87 | 3.7 | 165 | 2.3 | 54.2 |
| G2 | 30 | 60 | 0.82 | 3.6 | 145 | 4.8 | 48.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasimova, I.; Belenov, S.; Lyanguzov, N.; Pankov, I.; Tolstunov, M.; Pavlets, A. Role of the Potential Range during Stress Testing of Platinum-Containing Electrocatalysts at Elevated Temperature. Catalysts 2022, 12, 1179. https://doi.org/10.3390/catal12101179
Gerasimova I, Belenov S, Lyanguzov N, Pankov I, Tolstunov M, Pavlets A. Role of the Potential Range during Stress Testing of Platinum-Containing Electrocatalysts at Elevated Temperature. Catalysts. 2022; 12(10):1179. https://doi.org/10.3390/catal12101179
Chicago/Turabian StyleGerasimova, Irina, Sergey Belenov, Nikolai Lyanguzov, Ilya Pankov, Mikhail Tolstunov, and Angelina Pavlets. 2022. "Role of the Potential Range during Stress Testing of Platinum-Containing Electrocatalysts at Elevated Temperature" Catalysts 12, no. 10: 1179. https://doi.org/10.3390/catal12101179
APA StyleGerasimova, I., Belenov, S., Lyanguzov, N., Pankov, I., Tolstunov, M., & Pavlets, A. (2022). Role of the Potential Range during Stress Testing of Platinum-Containing Electrocatalysts at Elevated Temperature. Catalysts, 12(10), 1179. https://doi.org/10.3390/catal12101179