A Mechanistic Study of Methanol Steam Reforming on Ni2P Catalyst
Abstract
1. Introduction
2. Results and Discussion
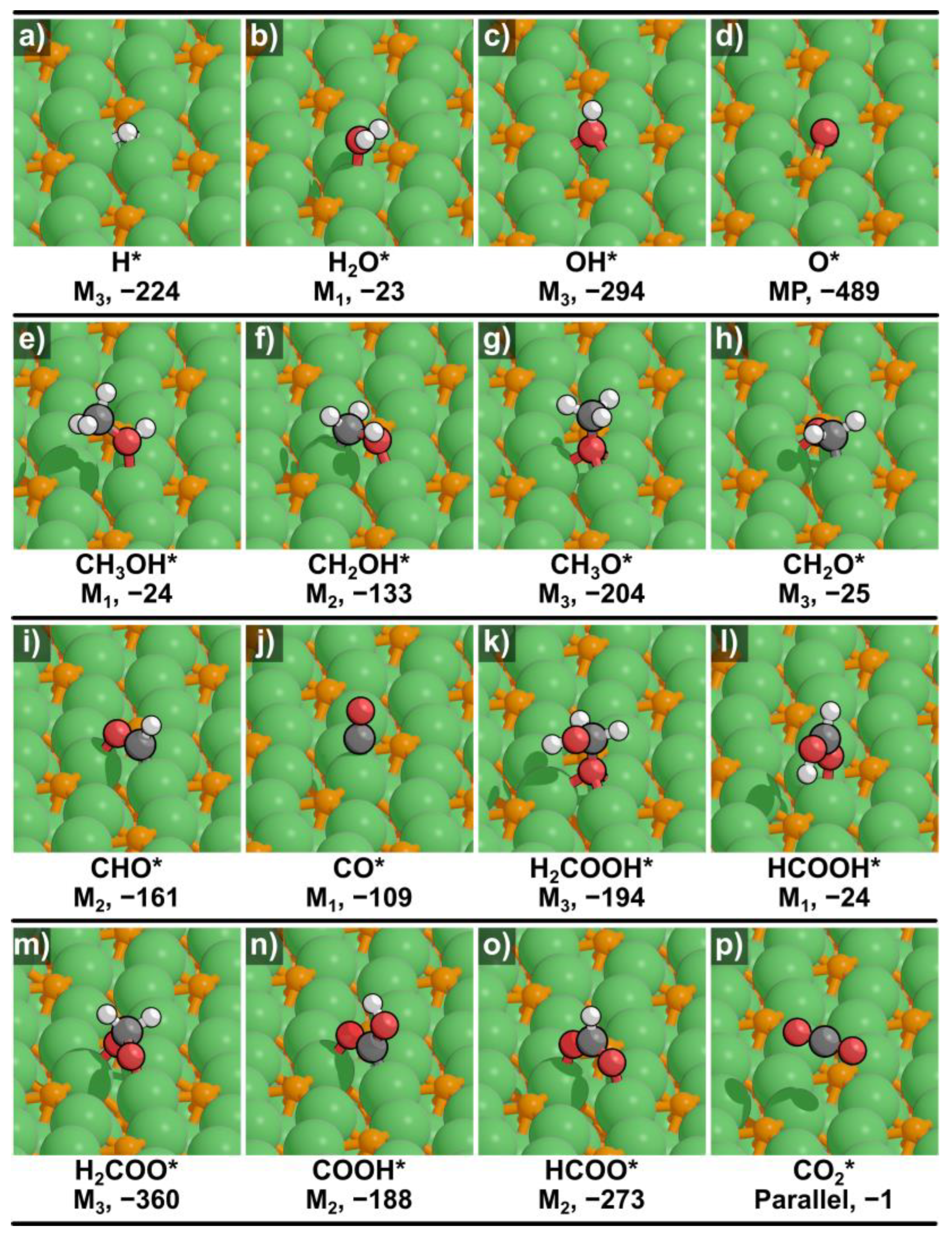
2.1. Structures and Energetics of Adsorbed Intermediates
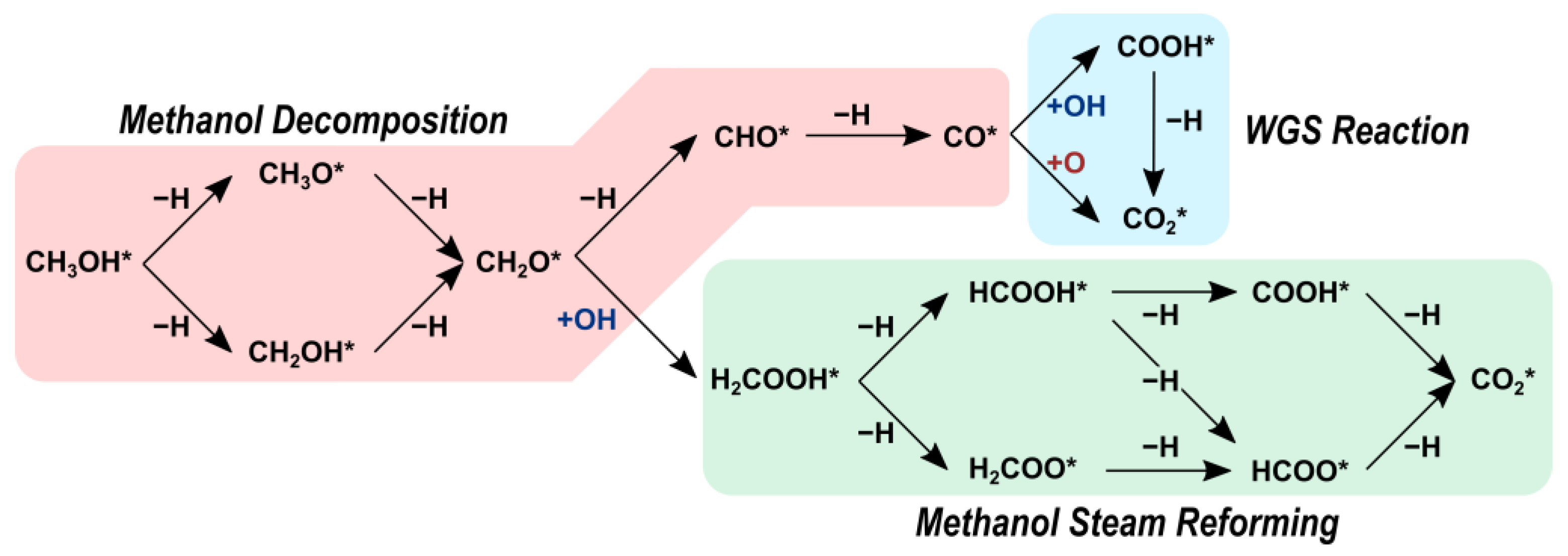
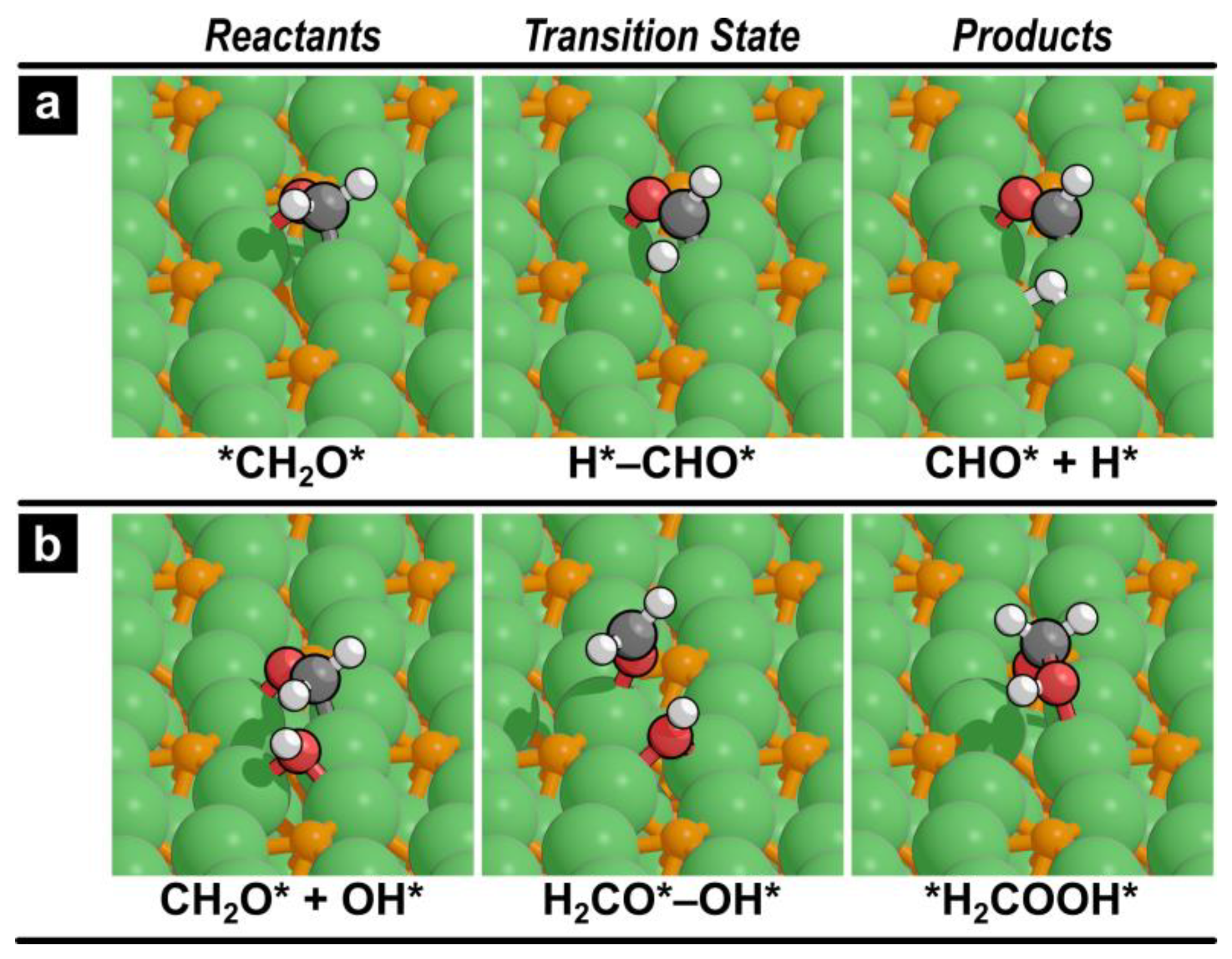
2.2. Reaction Pathways
2.2.1. Water Dissociation
2.2.2. Methanol Decomposition
2.2.3. Methanol Steam Reforming
2.2.4. Water-Gas Shift Reaction
3. Computational Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef]
- Kurnia, J.C.; Chaedir, B.A.; Sasmito, A.P.; Shamim, T. Progress on Open Cathode Proton Exchange Membrane Fuel Cell: Performance, Designs, Challenges and Future Directions. Appl. Energy 2021, 283, 116359. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shuai, K.; Xu, B. Review on Copper and Palladium Based Catalysts for Methanol Steam Reforming to Produce Hydrogen. Catalysts 2017, 7, 183. [Google Scholar] [CrossRef]
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for Methanol Steam Reforming—A Review. Appl. Catal. B 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. [Google Scholar] [CrossRef] [PubMed]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef]
- Schlapbach, L.; Züttel, A. Hydrogen-Storage Materials for Mobile Applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Agrell, J.; Birgersson, H.; Boutonnet, M. Steam Reforming of Methanol over a Cu/ZnO/Al2O3 Catalyst: A Kinetic Analysis and Strategies for Suppression of CO Formation. J. Power Sources 2002, 106, 249–257. [Google Scholar] [CrossRef]
- Agrell, J. Production of Hydrogen from Methanol over Cu/ZnO Catalysts Promoted by ZrO2 and Al2O3. J. Catal. 2003, 219, 389–403. [Google Scholar] [CrossRef]
- Levitan, D.; Rozenblit, A.; Laborde, M.; Giunta, P. Self-Sustained Oscillations in the Potential of a CO-Poisoned PEM Fuel Cell: A Model Based on Physical Principles. J. Electroanal. Chem. 2021, 880, 114924. [Google Scholar] [CrossRef]
- Bellows, R.J.; Marucchi-Soos, E.P.; Buckley, D.T. Analysis of Reaction Kinetics for Carbon Monoxide and Carbon Dioxide on Polycrystalline Platinum Relative to Fuel Cell Operation. Ind. Eng. Chem. Res. 1996, 35, 1235–1242. [Google Scholar] [CrossRef]
- Denkwitz, Y.; Karpenko, A.; Plzak, V.; Leppelt, R.; Schumacher, B.; Behm, R. Influence of CO2 and H2 on the Low-Temperature Water–Gas Shift Reaction on Au/CeO2 Catalysts in Idealized and Realistic Reformate. J. Catal. 2007, 246, 74–90. [Google Scholar] [CrossRef]
- Gu, X.-K.; Li, W.-X. First-Principles Study on the Origin of the Different Selectivities for Methanol Steam Reforming on Cu(111) and Pd(111). J. Phys. Chem. C 2010, 114, 21539–21547. [Google Scholar] [CrossRef]
- Luo, W.; Asthagiri, A. Density Functional Theory Study of Methanol Steam Reforming on Co(0001) and Co(111) Surfaces. J. Phys. Chem. C 2014, 118, 15274–15285. [Google Scholar] [CrossRef]
- Lin, S.; Johnson, R.S.; Smith, G.K.; Xie, D.; Guo, H. Pathways for Methanol Steam Reforming Involving Adsorbed Formaldehyde and Hydroxyl Intermediates on Cu(111): Density Functional Theory Studies. Phys. Chem. Chem. Phys. 2011, 13, 9622–9631. [Google Scholar] [CrossRef]
- Sutton, J.E.; Panagiotopoulou, P.; Verykios, X.E.; Vlachos, D.G. Combined DFT, Microkinetic, and Experimental Study of Ethanol Steam Reforming on Pt. J. Phys. Chem. C 2013, 117, 4691–4706. [Google Scholar] [CrossRef]
- Smith, G.K.; Lin, S.; Lai, W.; Datye, A.; Xie, D.; Guo, H. Initial Steps in Methanol Steam Reforming on PdZn and ZnO Surfaces: Density Functional Theory Studies. Surf. Sci. 2011, 605, 750–759. [Google Scholar] [CrossRef]
- Lin, S.; Xie, D.; Guo, H. Pathways of Methanol Steam Reforming on Pdzn and Comparison with Cu. J. Phys. Chem. C 2011, 115, 20583–20589. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Paxinou, A.; Słowik, G.; Neophytides, S.; Avgouropoulos, G. Steam Reforming of Methanol over Nanostructured Pt/TiO2 and Pt/CeO2 Catalysts for Fuel Cell Applications. Catalysts 2018, 8, 544. [Google Scholar] [CrossRef]
- Wu, H.; la Parola, V.; Pantaleo, G.; Puleo, F.; Venezia, A.; Liotta, L. Ni-Based Catalysts for Low Temperature Methane Steam Reforming: Recent Results on Ni-Au and Comparison with Other Bi-Metallic Systems. Catalysts 2013, 3, 563–583. [Google Scholar] [CrossRef]
- Köpfle, N.; Mayr, L.; Schmidmair, D.; Bernardi, J.; Knop-Gericke, A.; Hävecker, M.; Klötzer, B.; Penner, S. A Comparative Discussion of the Catalytic Activity and CO2-Selectivity of Cu-Zr and Pd-Zr (Intermetallic) Compounds in Methanol Steam Reforming. Catalysts 2017, 7, 53. [Google Scholar] [CrossRef]
- Twigg, M.V. Deactivation of Copper Metal Catalysts for Methanol Decomposition, Methanol Steam Reforming and Methanol Synthesis. Top. Catal. 2003, 22, 191–203. [Google Scholar] [CrossRef]
- Kurtz, M.; Wilmer, H.; Genger, T.; Hinrichsen, O.; Muhler, M. Deactivation of Supported Copper Catalysts for Methanol Synthesis. Catal. Lett. 2003, 86, 77–80. [Google Scholar] [CrossRef]
- Takezawa, N.; Iwasa, N. Steam Reforming and Dehydrogenation of Methanol: Difference in the Catalytic Functions of Copper and Group VIII Metals. Catal. Today 1997, 36, 45–56. [Google Scholar] [CrossRef]
- Iwasa, N.; Masuda, S.; Ogawa, N.; Takezawa, N. Steam Reforming of Methanol over Pd/ZnO: Effect of the Formation of PdZn Alloys upon the Reaction. Appl. Catal. A Gen. 1995, 125, 145–157. [Google Scholar] [CrossRef]
- Iwasa, N.; Mayanagi, T.; Nomura, W.; Arai, M.; Takezawa, N. Effect of Zn Addition to Supported Pd Catalysts in the Steam Reforming of Methanol. Appl. Catal. A Gen. 2003, 248, 153–160. [Google Scholar] [CrossRef]
- Iwasa, N. New Supported Pd and Pt Alloy Catalysts for Steam Reforming and Dehydrogenation of Methanol. Top. Catal. 2003, 22, 215–224. [Google Scholar] [CrossRef]
- Al-Ali, L.I.; Elmutasim, O.; al Ali, K.; Singh, N.; Polychronopoulou, K. Transition Metal Phosphides (TMP) as a Versatile Class of Catalysts for the Hydrodeoxygenation Reaction (HDO) of Oil-Derived Compounds. Nanomaterials 2022, 12, 1435. [Google Scholar] [CrossRef]
- Oyama, S. Effect of Phosphorus Content in Nickel Phosphide Catalysts Studied by XAFS and Other Techniques. J. Catal. 2002, 210, 207–217. [Google Scholar] [CrossRef]
- Li, W.; Dhandapani, B.; Oyama, S.T. Molybdenum Phosphide: A Novel Catalyst for Hydrodenitrogenation. Chem. Lett. 1998, 27, 207–208. [Google Scholar] [CrossRef]
- Bui, P.; Cecilia, J.A.; Oyama, S.T.; Takagaki, A.; Infantes-Molina, A.; Zhao, H.; Li, D.; Rodríguez-Castellón, E.; Jiménez López, A. Studies of the Synthesis of Transition Metal Phosphides and Their Activity in the Hydrodeoxygenation of a Biofuel Model Compound. J. Catal. 2012, 294, 184–198. [Google Scholar] [CrossRef]
- Liu, P.; Rodriguez, J.A.; Takahashi, Y.; Nakamura, K. Water–Gas-Shift Reaction on a Ni2P(001) Catalyst: Formation of Oxy-Phosphides and Highly Active Reaction Sites. J. Catal. 2009, 262, 294–303. [Google Scholar] [CrossRef]
- Yin, P.; Yang, Y.-S.; Chen, L.-F.; Xu, M.; Chen, C.-Y.; Zhao, X.-J.; Zhang, X.; Yan, H.; Wei, M. DFT Study on the Mechanism of the Water Gas Shift Reaction Over NixPy Catalysts: The Role of P. J. Phys. Chem. C 2020, 124, 6598–6610. [Google Scholar] [CrossRef]
- Witzke, M.E.; Almithn, A.; Conrad, C.L.; Hibbitts, D.D.; Flaherty, D.W. Mechanisms and Active Sites for C–O Bond Rupture within 2-Methyltetrahydrofuran over Ni, Ni12P5, and Ni2P Catalysts. ACS Catal. 2018, 8, 7141–7157. [Google Scholar] [CrossRef]
- Witzke, M.E.; Almithn, A.; Conrad, C.L.; Triezenberg, M.D.; Hibbitts, D.D.; Flaherty, D.W. In Situ Methods for Identifying Reactive Surface Intermediates during Hydrogenolysis Reactions: C–O Bond Cleavage on Nanoparticles of Nickel and Nickel Phosphides. J. Am. Chem. Soc. 2019, 141, 16671–16684. [Google Scholar] [CrossRef]
- Jiang, C.J.; Trimm, D.L.; Wainwright, M.S.; Cant, N.W. Kinetic Study of Steam Reforming of Methanol over Copper-Based Catalysts. Appl. Catal. A Gen. 1993, 93, 245–255. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–Steam Reforming on Cu/ZnO/Al2O3 Catalysts. Part 2. A Comprehensive Kinetic Model. Appl. Catal. A Gen. 1999, 179, 31–49. [Google Scholar] [CrossRef]
- Błoński, P.; López, N. On the Adsorption of Formaldehyde and Methanol on a Water-Covered Pt(111): A DFT-D Study. J. Phys. Chem. C 2012, 116, 15484–15492. [Google Scholar] [CrossRef]
- Li, J.; Wan, Q.; Lin, G.; Lin, S. DFT Study on the Catalytic Role of α-MoC(100) in Methanol Steam Reforming. Chin. J. Chem. Phys. 2022, 35, 639–646. [Google Scholar] [CrossRef]
- Gokhale, A.A.; Dumesic, J.A.; Mavrikakis, M. On the Mechanism of Low-Temperature Water Gas Shift Reaction on Copper. J. Am. Chem. Soc. 2008, 130, 1402–1414. [Google Scholar] [CrossRef] [PubMed]
- Michel, C.; Auneau, F.; Delbecq, F.; Sautet, P. C–H versus O–H Bond Dissociation for Alcohols on a Rh(111) Surface: A Strong Assistance from Hydrogen Bonded Neighbors. ACS Catal. 2011, 1, 1430–1440. [Google Scholar] [CrossRef]
- Greeley, J.; Mavrikakis, M. Methanol Decomposition on Cu(111): A DFT Study. J. Catal. 2002, 208, 291–300. [Google Scholar] [CrossRef]
- Jiang, R.; Guo, W.; Li, M.; Fu, D.; Shan, H. Density Functional Investigation of Methanol Dehydrogenation on Pd(111). J. Phys. Chem. C 2009, 113, 4188–4197. [Google Scholar] [CrossRef]
- Peppley, B.A.; Amphlett, J.C.; Kearns, L.M.; Mann, R.F. Methanol–Steam Reforming on Cu/ZnO/Al2O3. Part 1: The Reaction Network. Appl. Catal. A Gen. 1999, 179, 21–29. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab Initio Molecular Dynamics for Liquid Metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab Initio Molecular-Dynamics Simulation of the Liquid-Metal–Amorphous-Semiconductor Transition in Germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kravchenko, P.; Plaisance, C.; Hibbitts, D. A New Computational Interface for Catalysis. ChemRxiv 2019. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved Adsorption Energetics within Density-Functional Theory Using Revised Perdew-Burke-Ernzerhof Functionals. Phys. Rev. B 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, W. Comment on “Generalized Gradient Approximation Made Simple”. Phys. Rev. Lett. 1998, 80, 890. [Google Scholar] [CrossRef]
- Larsson, E. An X-ray Investigation of Ni-P System and Crystal Structures of NiP and NiP2. Ark. Kemi 1965, 23, 335. [Google Scholar]
- Ren, J.; Wang, J.; Li, J.; Li, Y. Density Functional Theory Study on Crystal Nickel Phosphides. J. Fuel Chem. Technol. 2007, 35, 458–464. [Google Scholar] [CrossRef]
- Rundqvist, S. X-ray Investigations of Mn3P, Mn2P, and Ni2P. Acta Chem. Scand 1962, 16, 992–998. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special Points for Brillouin-Zone Integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Pack, J.D.; Monkhorst, H.J. “Special Points for Brillouin-Zone Integrations”—a Reply. Phys. Rev. B 1977, 16, 1748–1749. [Google Scholar] [CrossRef]
- Henkelman, G.; Jónsson, H. Improved Tangent Estimate in the Nudged Elastic Band Method for Finding Minimum Energy Paths and Saddle Points. J. Chem. Phys. 2000, 113, 9978–9985. [Google Scholar] [CrossRef]
- Jónsson, H.; Mills, G.; Jacobsen, K.W. Nudged Elastic Band Method for Finding Minimum Energy Paths of Transitions. In Classical and Quantum Dynamics in Condensed Phase Simulations; World Scientific: Singapore, 1998; pp. 385–404. [Google Scholar]
- Henkelman, G.; Jónsson, H. A Dimer Method for Finding Saddle Points on High Dimensional Potential Surfaces Using Only First Derivatives. J. Chem. Phys. 1999, 111, 7010–7022. [Google Scholar] [CrossRef]


| Species | Adsorption Mode | ΔEads |
|---|---|---|
| kJ mol−1 | ||
| H* | M3 | −224 |
| H2O* | M1 | −23 |
| OH* | M3 | −294 |
| O* | MP | −489 |
| CH3OH* | M1 | −24 |
| CH2OH* | M2 | −133 |
| CH3O* | M3 | −204 |
| CH2O* | M3 | −25 |
| CHO* | M2 | −161 |
| CO* | M1 | −109 |
| H2COOH* | M3 | −194 |
| HCOOH* | M1 | −24 |
| H2COO* | M3 | −360 |
| COOH* | M2 | −188 |
| HCOO* | M2 | −273 |
| CO2* | Parallel | −1 |
| No. | Reaction | ΔHact | ΔHrxn |
|---|---|---|---|
| kJ mol−1 | kJ mol−1 | ||
| 1 | CH3OH → CH2OH + H | 114 | 63 |
| 2 | CH3OH → CH3O + H | 98 | 9 |
| 3 | CH2OH → CH2O + H | 82 | −8 |
| 4 | CH3O → CH2O + H | 73 | 41 |
| 5 | CH2O → CHO + H | 17 | −3 |
| 6 | CHO → CO + H | 19 | −60 |
| 7 | CH2O + OH → H2COOH | 5 | −40 |
| 8 | H2COOH → HCOOH + H | 33 | −38 |
| 9 | H2COOH → H2COO + H | 87 | 43 |
| 10 | HCOOH → COOH + H | 82 | 11 |
| 11 | HCOOH → HCOO + H | 61 | 20 |
| 12 | H2COO → HCOO + H | 13 | −64 |
| 13 | HCOO → CO2 + H | 39 | −33 |
| 14 | COOH → CO2 + H | 115 | −25 |
| 15 | CO + OH → COOH | 69 | 53 |
| 16 | CO + O → CO2 | 128 | 3 |
| 17 | H2O → OH + H | 91 | 5 |
| 18 | OH → O + H | 161 | 81 |
| 19 | OH + OH → H2O + O | 63 | 55 |
| 20 | 2H2O → H2O + OH + H | 68 | 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Almithn, A.; Alhulaybi, Z. A Mechanistic Study of Methanol Steam Reforming on Ni2P Catalyst. Catalysts 2022, 12, 1174. https://doi.org/10.3390/catal12101174
Almithn A, Alhulaybi Z. A Mechanistic Study of Methanol Steam Reforming on Ni2P Catalyst. Catalysts. 2022; 12(10):1174. https://doi.org/10.3390/catal12101174
Chicago/Turabian StyleAlmithn, Abdulrahman, and Zaid Alhulaybi. 2022. "A Mechanistic Study of Methanol Steam Reforming on Ni2P Catalyst" Catalysts 12, no. 10: 1174. https://doi.org/10.3390/catal12101174
APA StyleAlmithn, A., & Alhulaybi, Z. (2022). A Mechanistic Study of Methanol Steam Reforming on Ni2P Catalyst. Catalysts, 12(10), 1174. https://doi.org/10.3390/catal12101174






