Assembly of Smart Microgels and Hybrid Microgels on Graphene Sheets for Catalytic Reduction of Nitroarenes
Abstract
1. Introduction
2. Results and Discussion
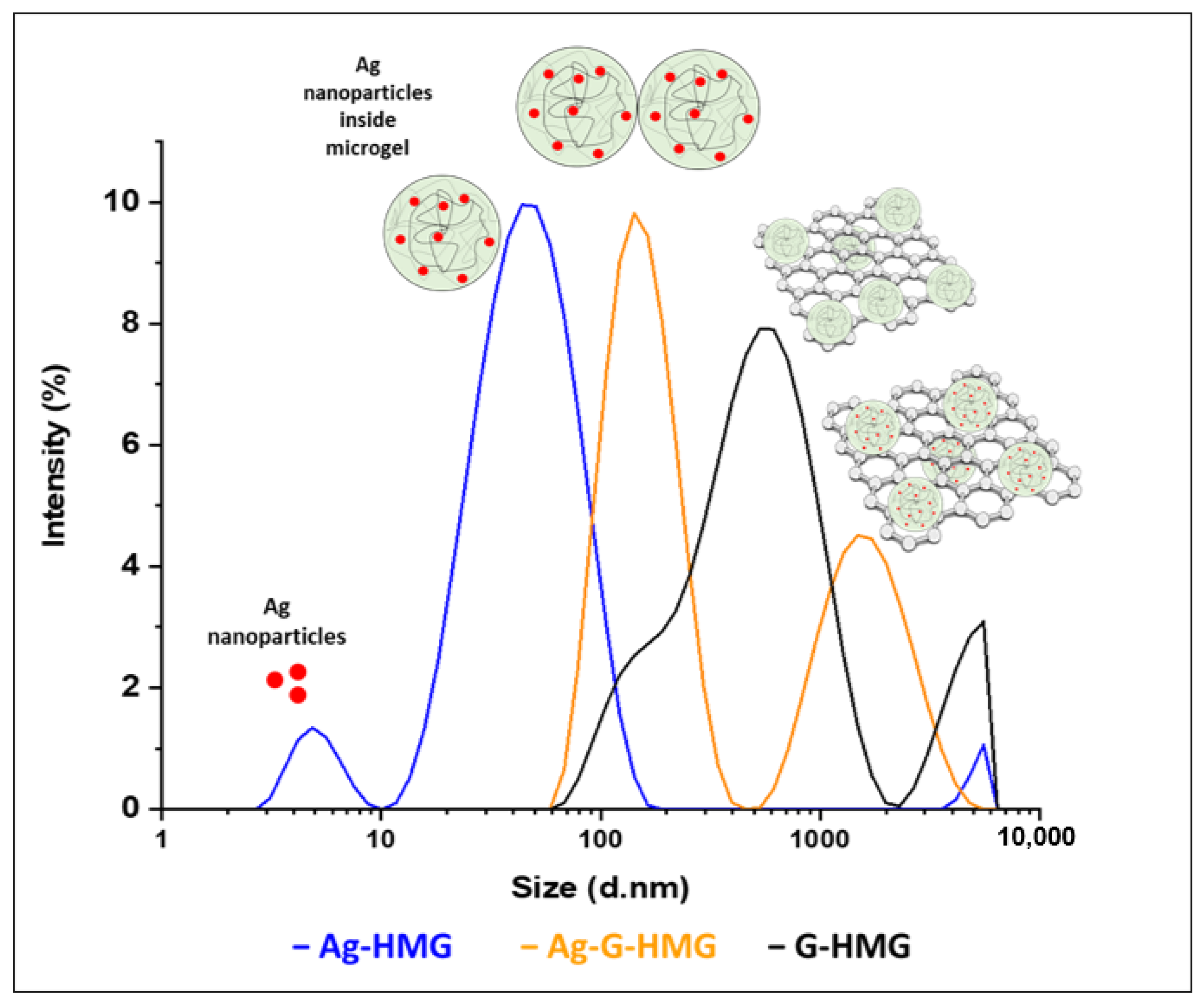
2.1. Zeta Potential Analysis of Hybrid Microgels
2.2. Catalytic Applications of Hybrid Microgels
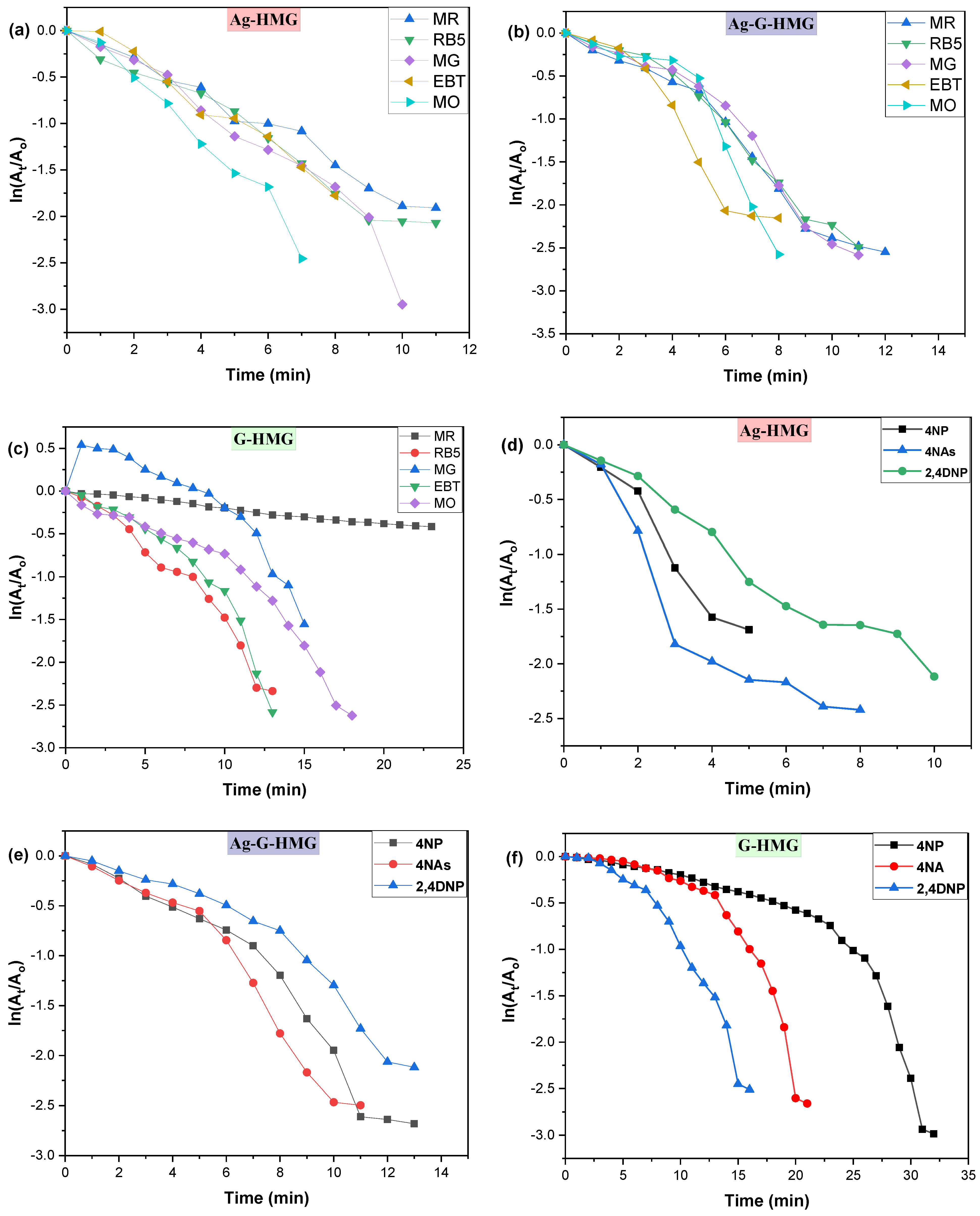
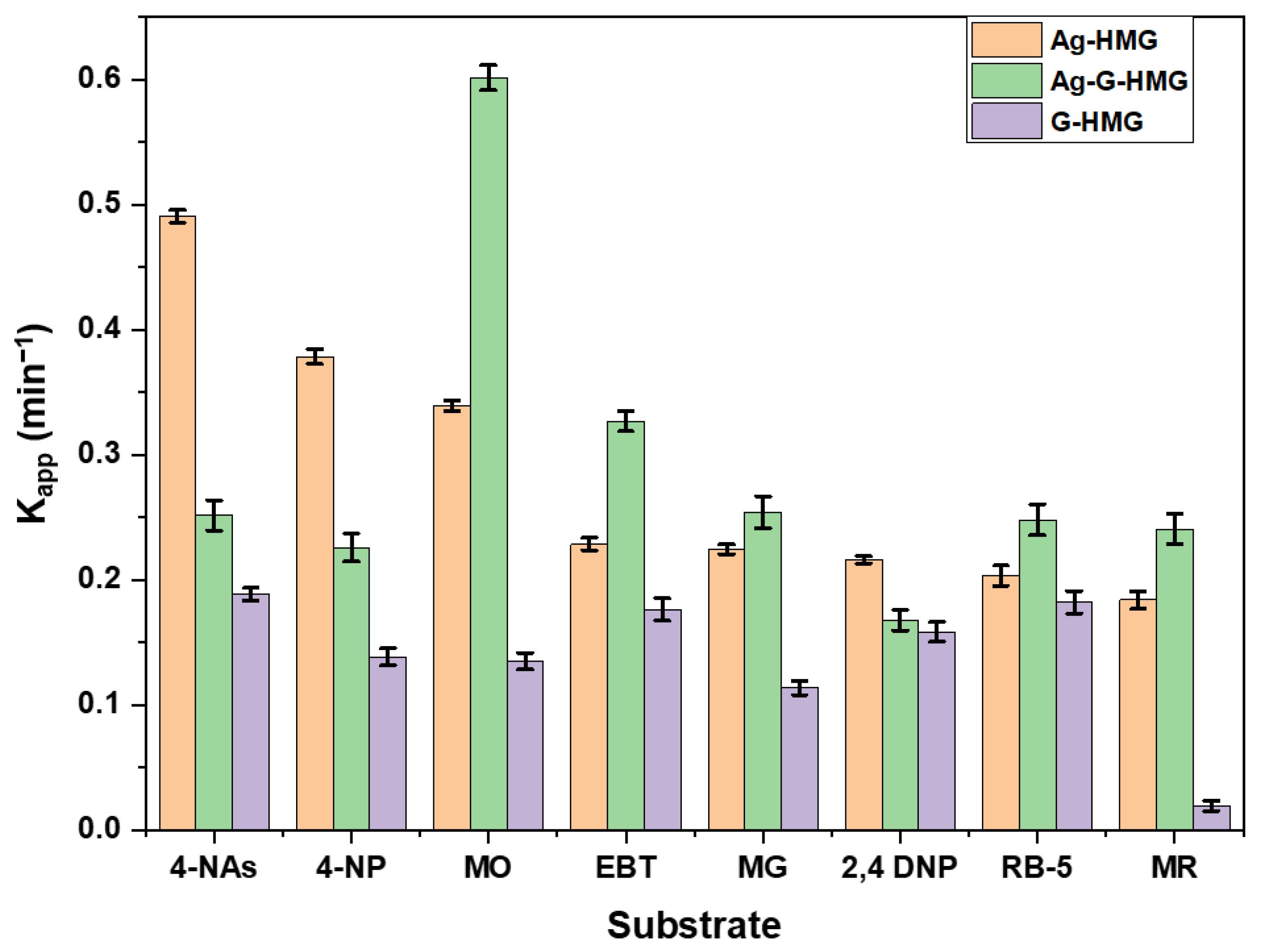
2.2.1. Comparison of kapp of Dyes and Nitro Compounds
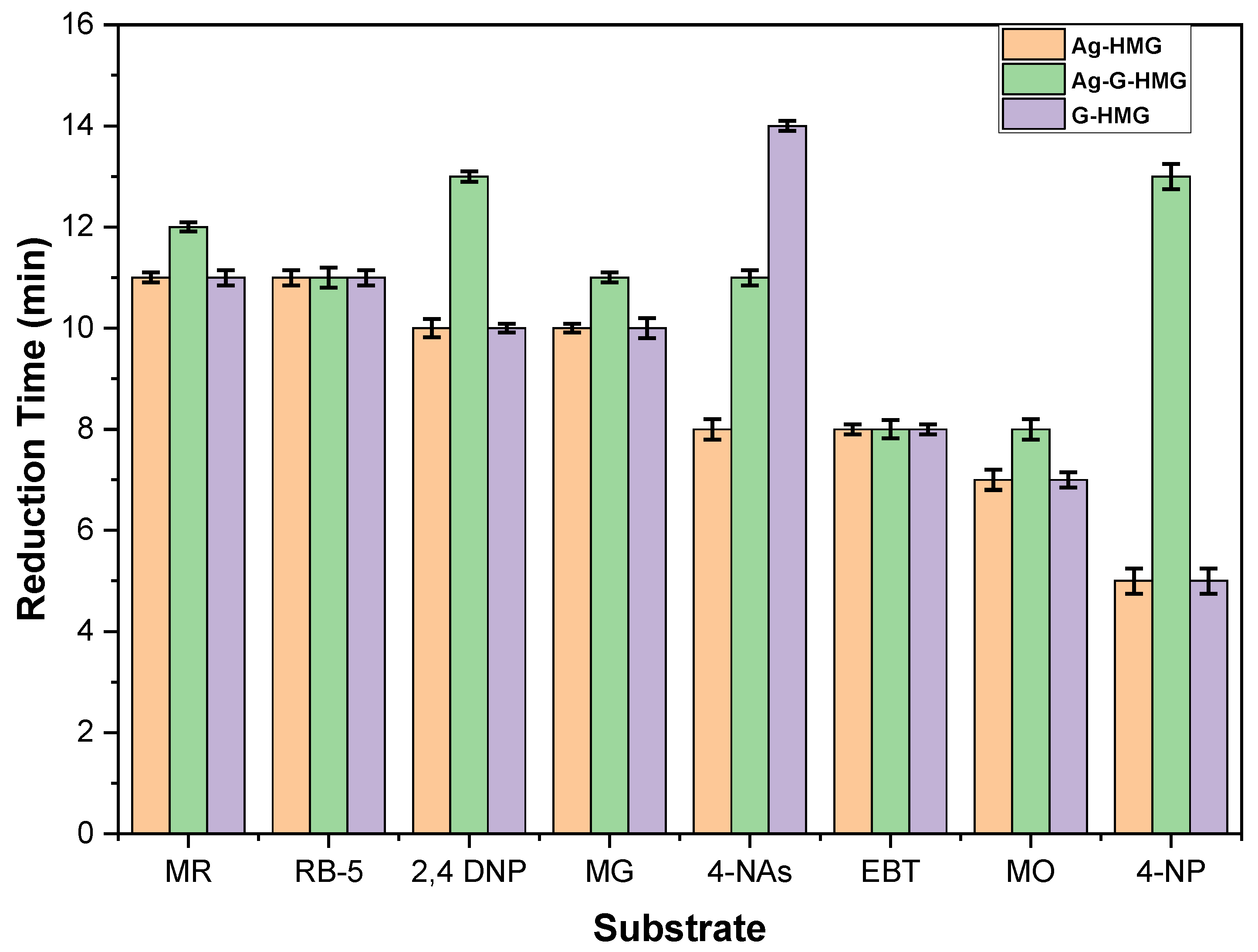
2.2.2. Comparison of Reduction Time
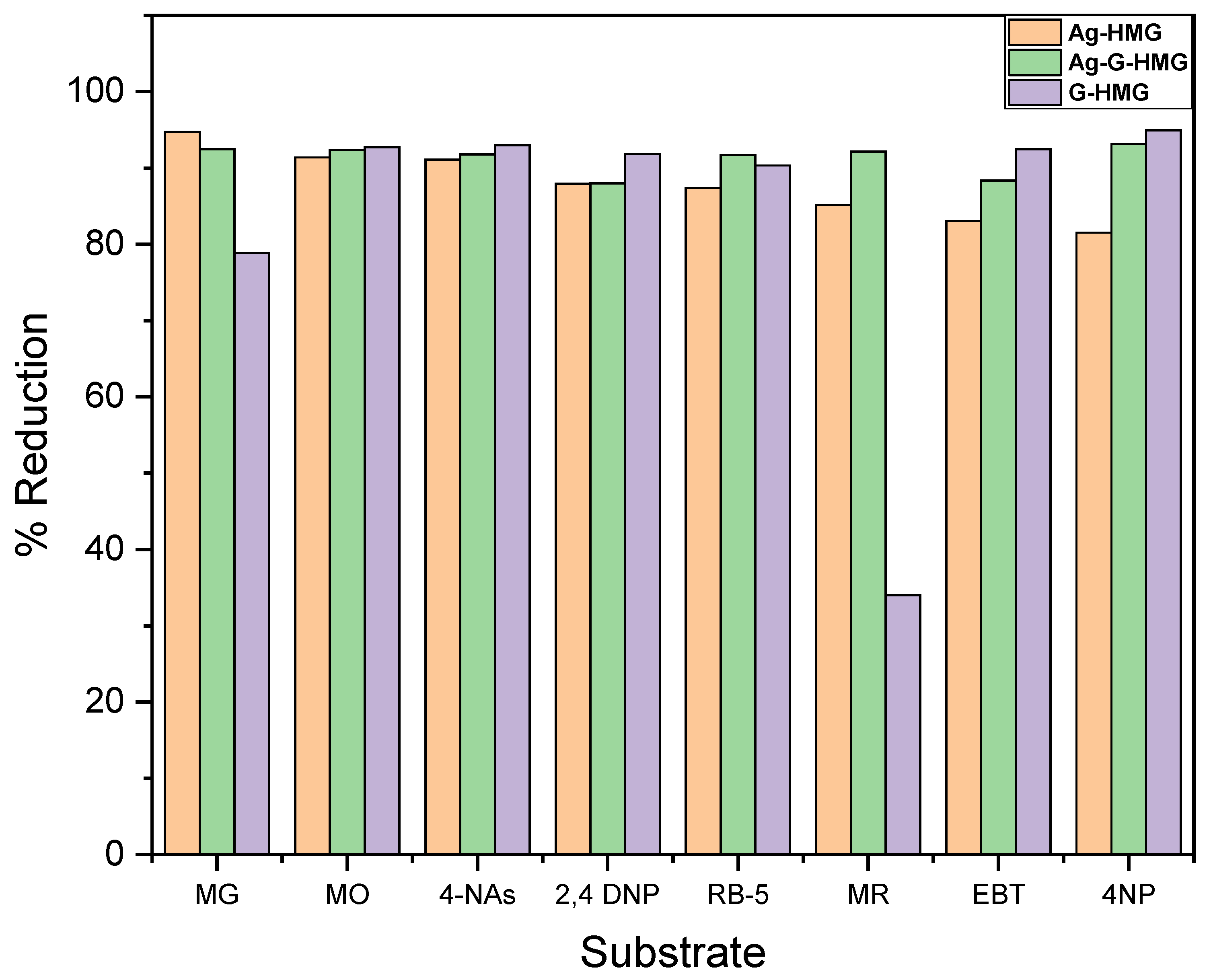
2.2.3. Comparison of Percentage Reduction
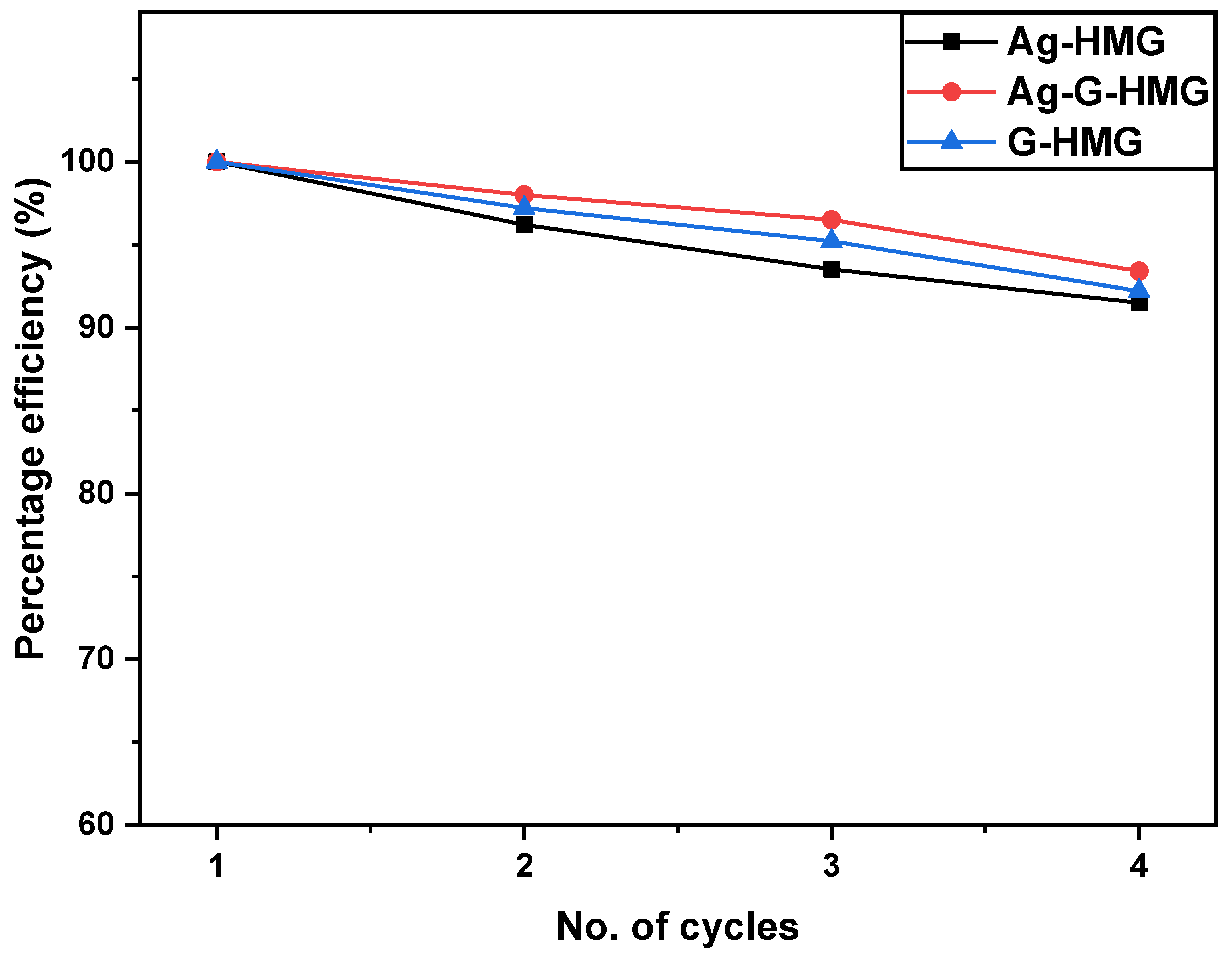
2.2.4. Comparison of Stability
3. Experimental
3.1. Materials
3.2. Synthesis of p(NIPAM-Acrylic Acid) Microgel
3.3. Synthesis of Graphene
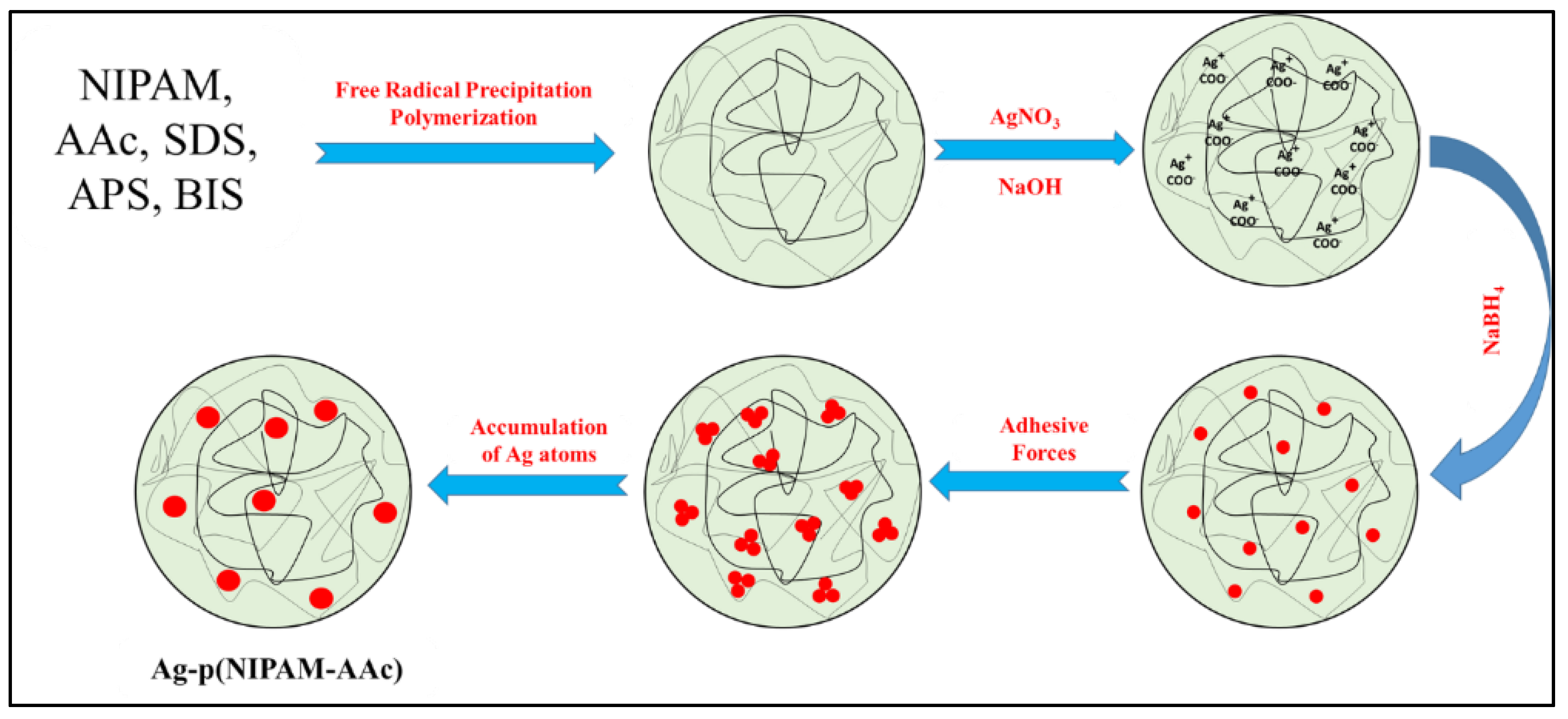
3.4. Synthesis of Hybrid Microgels
3.5. Catalytic Application
3.6. Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prashant, P.; Seo, S.S. Organophosphate Nerve Agent Sensor Based on Polystyrene Core and Zirconium Shell Colloidal Crystal Array. Int. J. Polym. Anal. Charact. 2009, 14, 481–492. [Google Scholar] [CrossRef]
- Karg, M.; Hellweg, T. Smart inorganic/organic hybrid microgels: Synthesis and characterisation. J. Mater. Chem. 2009, 19, 8714–8727. [Google Scholar] [CrossRef]
- Brugger, B.; Richtering, W. Emulsions stabilized by stimuli-sensitive poly (N-isopropylacrylamide)-co-methacrylic acid polymers: Microgels versus low molecular weight polymers. Langmuir 2008, 24, 7769–7777. [Google Scholar] [CrossRef]
- Khan, A.; El-Toni, A.M.; Alrokayan, S.; Alsalhi, M.; Alhoshan, M.; Aldwayyan, A.S. Microwave-assisted synthesis of silver nanoparticles using poly-N-isopropylacrylamide/acrylic acid microgel particles. Colloids Surf. A 2011, 377, 356–360. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Liu, X.-Y.; Yang, J.-M.; Lin, D.-L.; Chen, X.; Zha, L.-S. Investigation of Ag nanoparticles loading temperature responsive hybrid microgels and their temperature controlled catalytic activity. Colloids Surf. A Physicochem. Eng. Asp. 2012, 393, 105–110. [Google Scholar] [CrossRef]
- Plamper, F.A.; Richtering, W. Functional microgels and microgel systems. Acc. Chem. Res. 2017, 50, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, C.; Fernandes, S.N.; Godinho, M.H.; Borges, J.P.; Soares, P.I. Functional stimuli-responsive gels: Hydrogels and microgels. Gels 2018, 4, 54. [Google Scholar] [CrossRef]
- Das, T.K.; Das, N.C. Advances on catalytic reduction of 4-nitrophenol by nanostructured materials as benchmark reaction. Int. Nano Lett. 2022, 12, 223–242. [Google Scholar] [CrossRef]
- Singh, S.; Rao, C.; Nandi, C.K.; Mukherjee, T.K. Quantum Dot-Embedded Hybrid Photocatalytic Nanoreactors for Visible Light Photocatalysis and Dye Degradation. ACS Appl. Nano Mater. 2022, 5, 7427–7439. [Google Scholar] [CrossRef]
- Naz, M.; Rafiq, A.; Ikram, M.; Haider, A.; Ahmad, S.O.A.; Haider, J.; Naz, S. Elimination of dyes by catalytic reduction in the absence of light: A review. J. Mater. Sci. 2021, 56, 15572–15608. [Google Scholar] [CrossRef]
- Mejía, Y.R.; Bogireddy, N.K.R. Reduction of 4-nitrophenol using green-fabricated metal nanoparticles. RSC Adv. 2022, 12, 18661–18675. [Google Scholar] [CrossRef]
- Das, T.K.; Remanan, S.; Ghosh, S.; Ghosh, S.K.; Das, N.C. Efficient synthesis of catalytic active silver nanoparticles illuminated cerium oxide nanotube: A mussel inspired approach, Environmental Nanotechnology. Monit. Manag. 2021, 15, 100411. [Google Scholar] [CrossRef]
- Kanti Das, T.; Ganguly, S.; Remanan, S.; Das, N.C. Temperature-Dependent Study of Catalytic Ag Nanoparticles Entrapped Resin Nanocomposite towards Reduction of 4-Nitrophenol. ChemistrySelect 2019, 4, 3665–3671. [Google Scholar] [CrossRef]
- Jamil, S.; Ahmad, Z.; Ali, M.; Khan, S.R.; Ali, S.; Hammami, M.A.; Haroon, M.; Saleh, T.A. Synthesis and characterization of polyaniline/nickel oxide composites for fuel additive and dyes reduction. Chem. Phys. Lett. 2021, 776, 138713. [Google Scholar] [CrossRef]
- Shahid, M.; Farooqi, Z.H.; Begum, R.; Arif, M.; Wu, W.; Irfan, A. Hybrid microgels for catalytic and photocatalytic removal of nitroarenes and organic dyes from aqueous medium: A review. Crit. Rev. Anal. Chem. 2020, 50, 513–537. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Concepción, P.; Serna, P. A different reaction pathway for the reduction of aromatic nitro compounds on gold catalysts. Angew. Chem. Int. Ed. 2007, 46, 7266–7269. [Google Scholar] [CrossRef]
- Gupta, V.; Jain, R.; Mittal, A.; Salah și, T.; Nayak, A. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. 2012, 32, 12–17. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When i’m calling you, will you answer too? Curr. Biol. 2017, 27, R713–R715. [Google Scholar] [CrossRef]
- Zhang, J.T.; Wei, G.; Keller, T.F.; Gallagher, H.; Stötzel, C.; Müller, F.A.; Gottschaldt, M.U.; Schubert, K.D. Jandt, Responsive hybrid polymeric/metallic nanoparticles for catalytic applications. Macromol. Mater. Eng. 2010, 295, 1049–1057. [Google Scholar] [CrossRef]
- Bharech, S.; Kumar, R. A review on the properties and applications of graphene. J. Mater. Sci. Mech. Eng. 2015, 2, 70. [Google Scholar]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- John, R.; Merlin, B. Theoretical investigation of structural, electronic, and mechanical properties of two dimensional C, Si, Ge, Sn. Cryst. Struct. Theor. Appl. 2016, 5, 43–55. [Google Scholar] [CrossRef]
- Abbott’s, I.E. Graphene: Exploring carbon flatland. Phys. Today 2007, 60, 35. [Google Scholar]
- Liu, S.; Liu, C.; Guo, J.; Yan, W. Microstructure and superior electrochemical activity of Cu3P/reduced graphene oxide composite for an anode in lithium-ion batteries. J. Electrochem. Soc. 2017, 164, A2390. [Google Scholar] [CrossRef]
- Abdi, G.; Alizadeh, A.; Zinadini, S.; Moradi, G. Removal of dye and heavy metal ion using a novel synthetic polyethersulfone nanofiltration membrane modified by magnetic graphene oxide/metformin hybrid. J. Membr. Sci. 2018, 552, 326–335. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Upadhyay, R.K.; Soin, N.; Roy, S.S. Role of graphene/metal oxide composites as photocatalysts, adsorbents and disinfectants in water treatment: A review. RSC Adv. 2014, 4, 3823–3851. [Google Scholar] [CrossRef]
- Gong, Z.; Li, S.; Han, W.; Wang, J.; Ma, J.; Zhang, X. Recyclable graphene oxide grafted with poly (N-isopropylacrylamide) and its enhanced selective adsorption for phenols. Appl. Surf. Sci. 2016, 362, 459–468. [Google Scholar] [CrossRef]
- Ali, S.; Shah, S.J.U.H.; Jamil, S.; Bibi, S.; Shah, M.U.; Aqib, A.I.; Zaheer, T.; Khan, S.R.; Janjua, M.R.S.A. Zirconium nanoparticles-poly (N-isopropylacrylamide-methacrylic acid) hybrid microgels decorated graphene sheets for catalytic reduction of organic pollutants. Chem. Phys. Lett. 2021, 780, 138915. [Google Scholar] [CrossRef]
- Ashraf, S.; Begum, R.; Rehan, R.; Wu, W.; Farooqi, Z.H. Synthesis and characterization of pH-responsive organic–inorganic hybrid material with excellent catalytic activity. J. Inorg. Organomet. Polym. Mater. 2018, 28, 1872–1884. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Khalid, R.; Begum, R.; Farooq, U.; Wu, Q.; Wu, W.; Ajmal, M.; Irfan, A.; Naseem, K. Facile synthesis of silver nanoparticles in a crosslinked polymeric system by in situ reduction method for catalytic reduction of 4-nitroaniline. Environ. Technol. 2019, 40, 2027–2036. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Ijaz, A.; Begum, R.; Naseem, K.; Usman, M.; Ajmal, M.; Saeed, U. Synthesis and characterization of inorganic–organic polymer microgels for catalytic reduction of 4-nitroaniline in aqueous medium. Polym. Compos. 2018, 39, 645–653. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Naseem, K.; Ijaz, A.; Begum, R. Engineering of silver nanoparticle fabricated poly (N-isopropylacrylamide-co-acrylic acid) microgels for rapid catalytic reduction of nitrobenzene. J. Polym. Eng. 2016, 36, 87–96. [Google Scholar] [CrossRef]
- Zhang, K.; Suh, J.M.; Choi, J.-W.; Jang, H.W.; Shokouhimehr, M.; Varma, R.S. Recent advances in the nanocatalyst-assisted NaBH4 reduction of nitroaromatics in water. ACS Omega 2019, 4, 483–495. [Google Scholar] [CrossRef]
- Lu, Y.; Mei, Y.; Ballauff, M.; Drechsler, M. Thermosensitive core−shell particles as carrier systems for metallic nanoparticles. J. Phys. Chem. B 2006, 110, 3930–3937. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Ali, F.; Shahid, M.; Begum, R.; Irfan, A.; Wu, W.; Shaukat, S.; Farooqi, Z.H. Silver nanoparticles supported on smart polymer microgel system for highly proficient catalytic reduction of Cr+ 6 to Cr+ 3 with formic acid. Appl. Organomet. Chem. 2021, 35, e6405. [Google Scholar] [CrossRef]
- Shah, L.A.; Sayed, M.; Fayaz, M.; Bibi, I.; Nawaz, M.; Siddiq, M. Ag-loaded thermo-sensitive composite microgels for enhanced catalytic reduction of methylene blue. Nanotechnol. Environ. Eng. 2017, 2, 14. [Google Scholar] [CrossRef]
- Khan, S.R.; Farooqi, Z.H.; Ali, A.; Begum, R.; Kanwal, F.; Siddiq, M. Kinetics and mechanism of reduction of nitrobenzene catalyzed by silver-poly (N-isopropylacryl amide-co-allylacetic acid) hybrid microgels. Mater. Chem. Phys. 2016, 171, 318–327. [Google Scholar] [CrossRef]
- Sargin, I.; Baran, T.; Arslan, G. Environmental remediation by chitosan-carbon nanotube supported palladium nanoparticles: Conversion of toxic nitroarenes into aromatic amines, degradation of dye pollutants and green synthesis of biaryls. Sep. Purif. Technol. 2020, 247, 116987. [Google Scholar] [CrossRef]
- Albukhari, S.M.; Ismail, M.; Akhtar, K.; Danish, E.Y. Catalytic reduction of nitrophenols and dyes using silver nanoparticles@ cellulose polymer paper for the resolution of waste water treatment challenges. Colloids Surf. A Physicochem. Eng. Asp. 2019, 577, 548–561. [Google Scholar] [CrossRef]
- Ferraz, E.; de Oliveira, G.; de Oliveira, D.P. The impact of aromatic amines on the environment: Risks and damages. Front. Biosci. 2019, 4, 914–923. [Google Scholar] [CrossRef]
- Chen, K.; Chen, W.; Yi, X.; Chen, W.; Liu, M.; Wu, H. Sterically hindered N-heterocyclic carbene/palladium (ii) catalyzed Suzuki–Miyaura coupling of nitrobenzenes. Chem. Commun. 2019, 55, 9287–9290. [Google Scholar] [CrossRef]
- Chequer, F.D.; De Oliveira, G.R.; Ferraz, E.A.; Cardoso, J.C.; Zanoni, M.B.; de Oliveira, D.P. Textile dyes: Dyeing process and environmental impact. Eco-Friendly Text. Dyeing Finish. 2013, 6, 151–176. [Google Scholar]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Gürses, A.; Açıkyıldız, M.; Güneş, K.; Gürses, M.S. Dyes and Pigments: Their Structure and Properties. In Dyes and Pigments; Springer: Berlin/Heidelberg, Germany, 2016; pp. 13–29. [Google Scholar]
- Lin, Y.; Cao, Y.; Yao, Q.; Chai, O.J.H.; Xie, J. Engineering noble metal nanomaterials for pollutant decomposition. Ind. Eng. Chem. Res. 2020, 59, 20561–20581. [Google Scholar] [CrossRef]
- Din, M.I.; Khalid, R.; Hussain, Z.; Hussain, T.; Mujahid, A.; Najeeb, J.; Izhar, F. Nanocatalytic assemblies for catalytic reduction of nitrophenols: A critical review. Crit. Rev. Anal. Chem. 2020, 50, 322–338. [Google Scholar] [CrossRef]
- Bibi, G.; Khan, S.R.; Ali, S.; Jamil, S.; Bibi, S.; Shehroz, H.; Janjua, M.R.S.A. Role of capping agent in the synthesis of zinc–cobalt bimetallic nanoparticles and its application as catalyst and fuel additive. Appl. Nanosci. 2022, 12, 2169–2181. [Google Scholar] [CrossRef]
- UlAin, Q.; Ali, S.; Jamil, S.; Bibi, S.; Khan, S.R.; UrRehman, S.; Bibi, G.; Khan, T.; Shehroz, H.; Hashaam, M. Comparison of catalytic and fuel additive properties of bimetallic nanoparticles and its composite: FeMnO3 and PANI-FeMnO3. Mater. Sci. Semicond. Proc. 2022, 144, 106630. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Sakhawat, T.; Khan, S.R.; Kanwal, F.; Usman, M.; Begum, R. Synthesis, characterization and fabrication of copper nanoparticles in N-isopropylacrylamide based co-polymer microgels for degradation of p-nitrophenol. Mater. Sci. Pol. 2015, 33, 185–192. [Google Scholar] [CrossRef][Green Version]
- Farooqi, Z.H.; Tariq, N.; Begum, R.; Khan, S.R.; Iqbal, Z.; Khan, A. Fabrication of silver nanoparticles in poly (N-isopropylacrylamide-co-allylacetic acid) microgels for catalytic reduction of nitroarenes. Turk. J. Chem. 2015, 39, 576–588. [Google Scholar] [CrossRef]
- Zubair, N.F.; Jamil, S.; Fatima, S.; Khan, S.R.; Khan, M.U.; Janjua, M.R.S.A. Synthesis of needle like nano composite of rGO-Mn2O and their applications as photo-catalyst. Chem. Phys. Lett. 2020, 757, 137874. [Google Scholar] [CrossRef]
- Farooqi, Z.H.; Khan, S.R.; Hussain, T.; Begum, R.; Ejaz, K.; Majeed, S.; Ajmal, M.; Kanwal, F.; Siddiq, M. Effect of crosslinker feed content on catalaytic activity of silver nanoparticles fabricated in multiresponsive microgels. Korean J. Chem. Eng. 2014, 31, 1674–1680. [Google Scholar] [CrossRef]


| Ag-HMG | Ag-G-HMG | G-HMG | ||||||
|---|---|---|---|---|---|---|---|---|
| Substrate | Initial Concentration (mM) | Final Concentration (mM) | Reduced Concentration (mM) | Final Concentration (mM) | Reduced Concentration (mM) | Final Concentration (mM) | Reduced Concentration (mM) | |
| 4-NP | 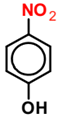 | 0.1 | 0.019 | 0.081 | 0.007 | 0.093 | 0.005 | 0.095 |
| 4-NAs |  | 0.1 | 0.009 | 0.091 | 0.008 | 0.092 | 0.007 | 0.093 |
| 2,4 DNP | 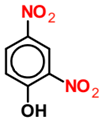 | 0.1 | 0.012 | 0.088 | 0.012 | 0.088 | 0.008 | 0.092 |
| MR | 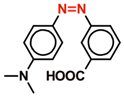 | 0.1 | 0.0155 | 0.0845 | 0.008 | 0.092 | 0.067 | 0.033 |
| RB-5 | 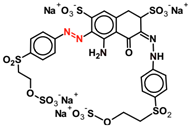 | 0.1 | 0.013 | 0.087 | 0.009 | 0.091 | 0.009 | 0.091 |
| MG |  | 0.1 | 0.005 | 0.095 | 0.008 | 0.092 | 0.021 | 0.079 |
| EBT | 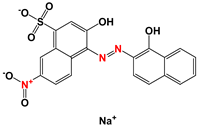 | 0.1 | 0.017 | 0.083 | 0.012 | 0.088 | 0.007 | 0.093 |
| MO | 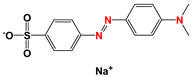 | 0.1 | 0.009 | 0.091 | 0.008 | 0.092 | 0.007 | 0.093 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashaam, M.; Ali, S.; Khan, T.; Salman, M.; Khan, S.R.; Aqib, A.I.; Zaheer, T.; Bibi, S.; Jamil, S.; Al-Sharif, M.S.; et al. Assembly of Smart Microgels and Hybrid Microgels on Graphene Sheets for Catalytic Reduction of Nitroarenes. Catalysts 2022, 12, 1172. https://doi.org/10.3390/catal12101172
Hashaam M, Ali S, Khan T, Salman M, Khan SR, Aqib AI, Zaheer T, Bibi S, Jamil S, Al-Sharif MS, et al. Assembly of Smart Microgels and Hybrid Microgels on Graphene Sheets for Catalytic Reduction of Nitroarenes. Catalysts. 2022; 12(10):1172. https://doi.org/10.3390/catal12101172
Chicago/Turabian StyleHashaam, Muhammad, Sarmed Ali, Tahreem Khan, Muhammad Salman, Shanza Rauf Khan, Amjad Islam Aqib, Tean Zaheer, Shamsa Bibi, Saba Jamil, Merfat S. Al-Sharif, and et al. 2022. "Assembly of Smart Microgels and Hybrid Microgels on Graphene Sheets for Catalytic Reduction of Nitroarenes" Catalysts 12, no. 10: 1172. https://doi.org/10.3390/catal12101172
APA StyleHashaam, M., Ali, S., Khan, T., Salman, M., Khan, S. R., Aqib, A. I., Zaheer, T., Bibi, S., Jamil, S., Al-Sharif, M. S., Mahmoud, S. F., & Yao, W. (2022). Assembly of Smart Microgels and Hybrid Microgels on Graphene Sheets for Catalytic Reduction of Nitroarenes. Catalysts, 12(10), 1172. https://doi.org/10.3390/catal12101172







