Effects of Site-Directed Mutations on the Communicability between Local Segments and Binding Pocket Distortion of Engineered GH11 Xylanases Visualized through Network Topology Analysis
Abstract
1. Introduction
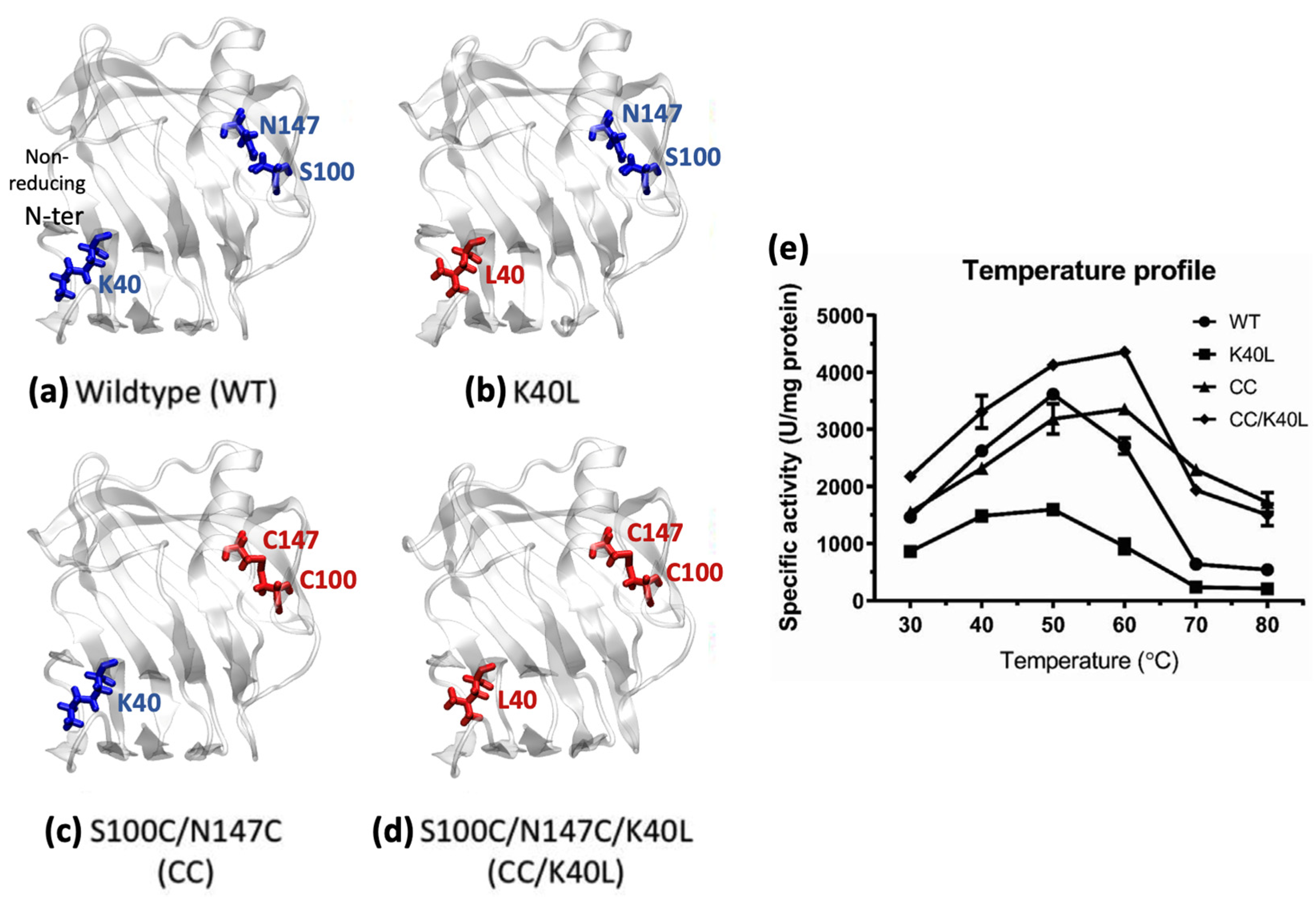
2. Results and Discussion
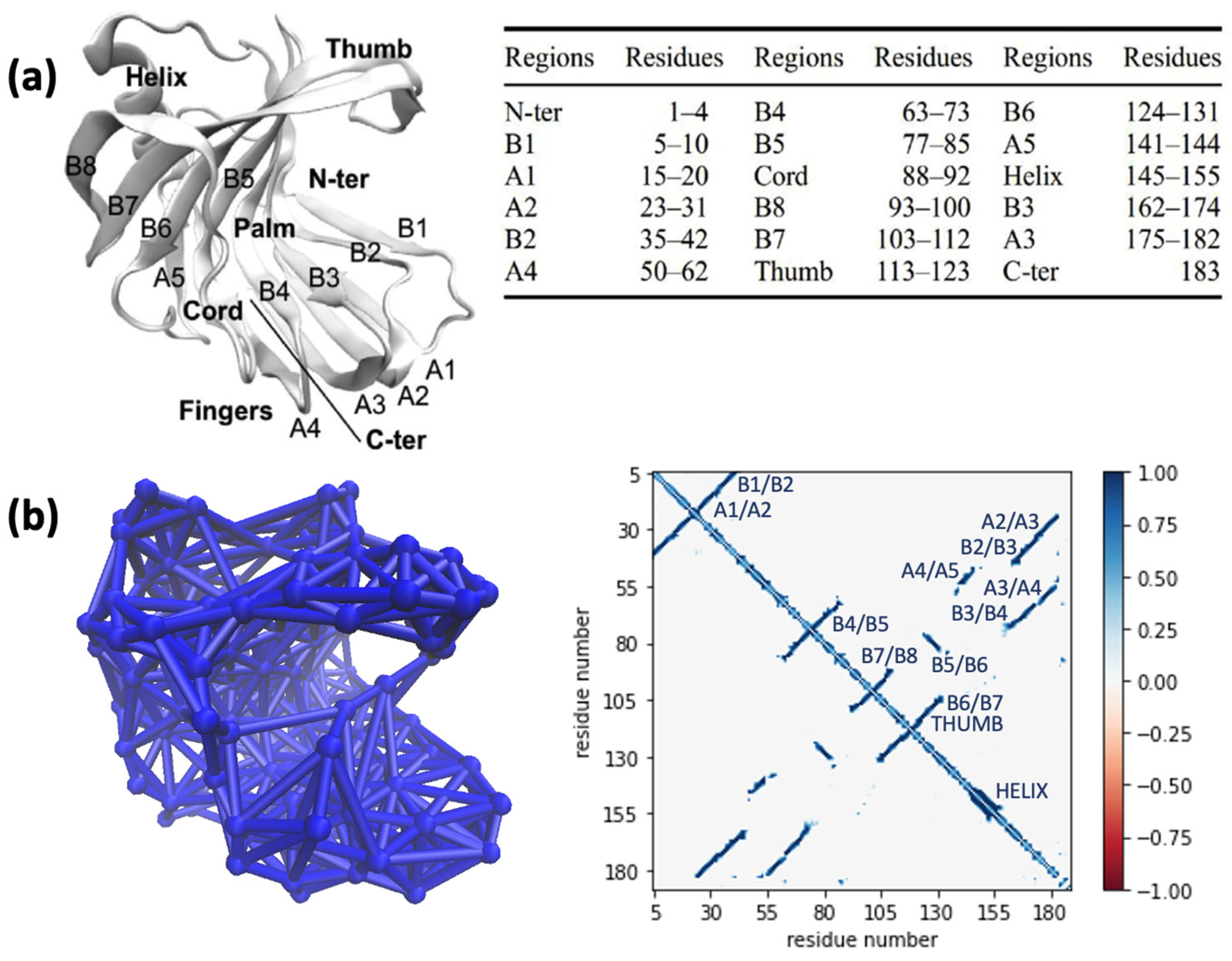
2.1. Characterization of Structural Network of Wildtype Xyn11A through the Adjacency Matrix
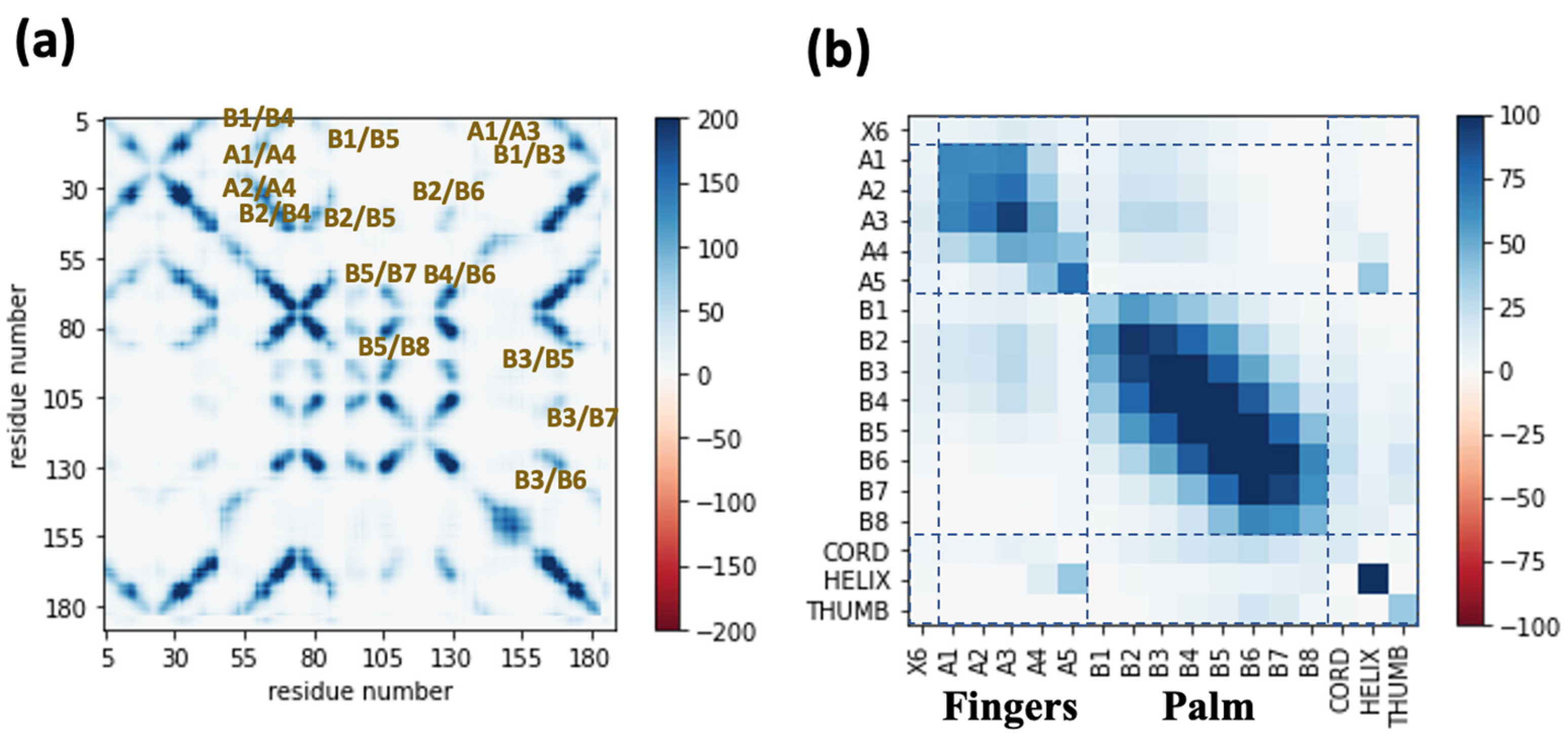
2.2. Characterization of Structural Network of Wildtype Xyn11A through the Communicability Matrix
2.3. Effects of Mutation to Binding Pocket Distortion Visualized through the Communicability Matrix
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, C.; Zhang, R.; Wang, J.; Wilson, L.M.; Yan, Y. Protein Engineering for Improving and Diversifying Natural Product Biosynthesis. Trends Biotechnol. 2020, 38, 729–744. [Google Scholar] [CrossRef] [PubMed]
- Modarres, H.P.; Mofrad, M.R.; Sanati-Nezhad, A. Protein Thermostability Engineering. RSC Adv. 2016, 6, 115252–115270. [Google Scholar] [CrossRef]
- Mukhopadhyay, A. Tolerance Engineering in Bacteria for the Production of Advanced Biofuels and Chemicals. Trends Microbiol. 2015, 23, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Paës, G.; Berrin, J.-G.; Beaugrand, J. GH11 Xylanases: Structure/Function/Properties Relationships and Applications. Biotechnol. Adv. 2012, 30, 564–592. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Arantes, V.; Saddler, J.N. The Enhancement of Enzymatic Hydrolysis of Lignocellulosic Substrates by the Addition of Accessory Enzymes Such as Xylanase: Is It an Additive or Synergistic Effect? Biotechnol. Biofuels 2011, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, N.; Kumar, B.; Verma, P. A Detailed Overview of Xylanases: An Emerging Biomolecule for Current and Future Prospective. Bioresour. Bioprocess. 2019, 6, 40. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, S.; Shang, W.; Yan, Z.; Wu, X.; Li, Y.; Chen, G.; Liu, X.; Wang, L. Synergistic Mechanism of GH11 Xylanases with Different Action Modes from Aspergillus Niger An76. Biotechnol. Biofuels 2021, 14, 118. [Google Scholar] [CrossRef]
- Nordberg Karlsson, E.; Schmitz, E.; Linares-Pastén, J.A.; Adlercreutz, P. Endo-Xylanases as Tools for Production of Substituted Xylooligosaccharides with Prebiotic Properties. Appl. Microbiol. Biotechnol. 2018, 102, 9081–9088. [Google Scholar] [CrossRef]
- Stephens, D.E.; Rumbold, K.; Permaul, K.; Prior, B.A.; Singh, S. Directed Evolution of the Thermostable Xylanase from Thermomyces Lanuginosus. J. Biotechnol. 2007, 127, 348–354. [Google Scholar] [CrossRef]
- Xu, X.; Liu, M.; Huo, W.; Dai, X. Obtaining a Mutant of Bacillus Amyloliquefaciens Xylanase A with Improved Catalytic Activity by Directed Evolution. Enzyme Microb. Technol. 2016, 86, 59–66. [Google Scholar] [CrossRef]
- Prajapati, A.S.; Panchal, K.J.; Pawar, V.A.; Noronha, M.J.; Patel, D.H.; Subramanian, R.B. Review on Cellulase and Xylanase Engineering for Biofuel Production. Ind. Biotechnol. 2018, 14, 38–44. [Google Scholar] [CrossRef]
- Takita, T.; Nakatani, K.; Katano, Y.; Suzuki, M.; Kojima, K.; Saka, N.; Mikami, B.; Yatsunami, R.; Nakamura, S.; Yasukawa, K. Increase in the Thermostability of GH11 Xylanase XynJ from Bacillus Sp. Strain 41M-1 Using Site Saturation Mutagenesis. Enzyme Microb. Technol. 2019, 130, 109363. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-S.; Chen, C.-C.; Huang, C.-H.; Ko, T.-P.; Luo, W.; Huang, J.-W.; Liu, J.-R.; Guo, R.-T. Structural Analysis of a Glycoside Hydrolase Family 11 Xylanase from Neocallimastix Patriciarum. J. Biol. Chem. 2014, 289, 11020–11028. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Miao, H.; Ding, J.; Li, J.; Mu, Y.; Zhou, J.; Huang, Z. Improving the Thermostability of a Fungal GH11 Xylanase via Site-Directed Mutagenesis Guided by Sequence and Structural Analysis. Biotechnol. Biofuels 2017, 10, 133. [Google Scholar] [CrossRef]
- Wu, X.; Tian, Z.; Jiang, X.; Zhang, Q.; Wang, L. Enhancement in Catalytic Activity of Aspergillus Niger XynB by Selective Site-Directed Mutagenesis of Active Site Amino Acids. Appl. Microbiol. Biotechnol. 2018, 102, 249–260. [Google Scholar] [CrossRef]
- Zhang, S.; He, Y.; Yu, H.; Dong, Z. Seven N-Terminal Residues of a Thermophilic Xylanase Are Sufficient to Confer Hyperthermostability on Its Mesophilic Counterpart. PLoS ONE 2014, 9, e87632. [Google Scholar] [CrossRef]
- Boonyaputthikul, H.; Muhammad, A.; Roekring, S.; Rattanarojpong, T.; Khunrae, P.; Sutthibutpong, T. Synergistic Effects between the Additions of a Disulphide Bridge and an N-Terminal Hydrophobic Sidechain on the Binding Pocket Tilting and Enhanced Xyn11A Activity. Arch. Biochem. Biophys. 2019, 672, 108068. [Google Scholar] [CrossRef]
- Ngenyoung, A.; Muhammad, A.; Rattanarojpong, T.; Sutthibutpong, T.; Khunrae, P. Effect of N-Terminal Modification on the Mode of Action between the Xyn11A and Xylotetraose. Int. J. Biol. Macromol. 2021, 170, 240–247. [Google Scholar] [CrossRef]
- Grewal, R.; Roy, S. Modeling Proteins as Residue Interaction Networks. Protein Pept. Lett. 2015, 22, 923–933. [Google Scholar] [CrossRef]
- O’Rourke, K.F.; Gorman, S.D.; Boehr, D.D. Biophysical and Computational Methods to Analyze Amino Acid Interaction Networks in Proteins. Comput. Struct. Biotechnol. J. 2016, 14, 245–251. [Google Scholar] [CrossRef]
- Stetz, G.; Verkhivker, G.M. Computational Analysis of Residue Interaction Networks and Coevolutionary Relationships in the Hsp70 Chaperones: A Community-Hopping Model of Allosteric Regulation and Communication. PLoS Comput. Biol. 2017, 13, e1005299. [Google Scholar] [CrossRef] [PubMed]
- Michaud-Agrawal, N.; Denning, E.J.; Woolf, T.B.; Beckstein, O. MDAnalysis: A Toolkit for the Analysis of Molecular Dynamics Simulations. J. Comput. Chem. 2011, 32, 2319–2327. [Google Scholar] [CrossRef] [PubMed]
- Eargle, J.; Luthey-Schulten, Z. NetworkView: 3D Display and Analysis of Protein{middle Dot}RNA Interaction Networks. Bioinformatics 2012, 28, 3000–3001. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual Molecular Dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Estrada, E. Topological Analysis of SARS CoV-2 Main Protease. Chaos Interdiscip. J. Nonlinear Sci. 2020, 30, 061102. [Google Scholar] [CrossRef]
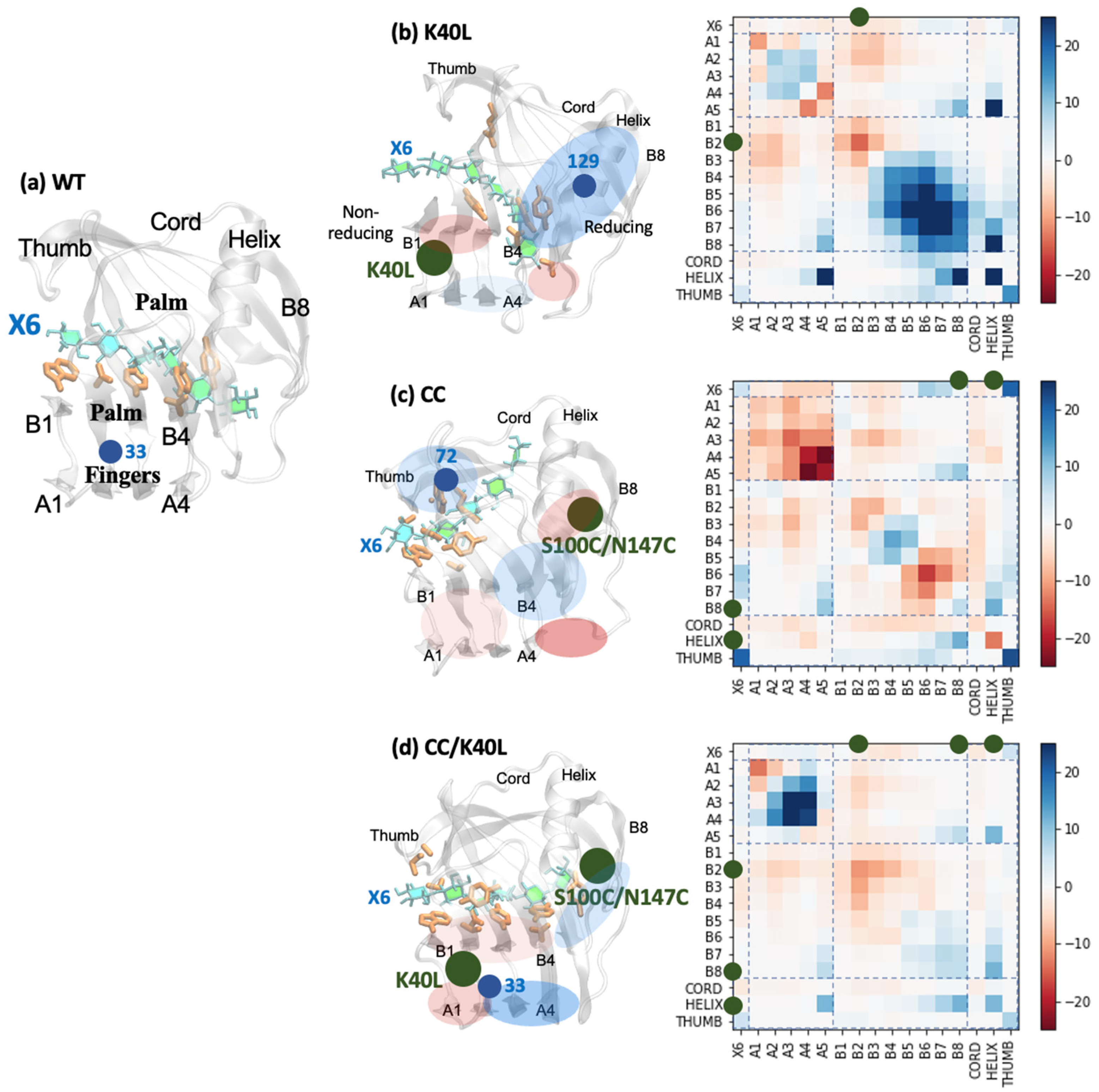
| WT | K40L | CC | CC/K40L | |
|---|---|---|---|---|
| max. subgraph centrality | 458.59 | 399.14 | 419.09 | 366.75 |
| residue with max. subgraph centrality | 33 | 129 | 72 | 33 |
| region with max. subgraph centrality | Fingers | reducing | nonreducing | Fingers |
| average subgraph centrality [protein] | 128.90 | 139.97 | 122.81 | 133.11 |
| average subgraph centrality [catalytic] | 177.30 | 184.02 | 213.05 | 186.32 |
| communicability [enzyme-enzyme] | 22.66 | 24.99 | 21.25 | 23.15 |
| communicability [enzyme-X6] | 5.99 | 5.35 | 5.88 | 5.86 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutthibutpong, T.; Muhammad, A.; Sawang, N.; Khunrae, P. Effects of Site-Directed Mutations on the Communicability between Local Segments and Binding Pocket Distortion of Engineered GH11 Xylanases Visualized through Network Topology Analysis. Catalysts 2022, 12, 1165. https://doi.org/10.3390/catal12101165
Sutthibutpong T, Muhammad A, Sawang N, Khunrae P. Effects of Site-Directed Mutations on the Communicability between Local Segments and Binding Pocket Distortion of Engineered GH11 Xylanases Visualized through Network Topology Analysis. Catalysts. 2022; 12(10):1165. https://doi.org/10.3390/catal12101165
Chicago/Turabian StyleSutthibutpong, Thana, Auwal Muhammad, Nuttawat Sawang, and Pongsak Khunrae. 2022. "Effects of Site-Directed Mutations on the Communicability between Local Segments and Binding Pocket Distortion of Engineered GH11 Xylanases Visualized through Network Topology Analysis" Catalysts 12, no. 10: 1165. https://doi.org/10.3390/catal12101165
APA StyleSutthibutpong, T., Muhammad, A., Sawang, N., & Khunrae, P. (2022). Effects of Site-Directed Mutations on the Communicability between Local Segments and Binding Pocket Distortion of Engineered GH11 Xylanases Visualized through Network Topology Analysis. Catalysts, 12(10), 1165. https://doi.org/10.3390/catal12101165






