Ceramic Papers as Structured Catalysts: Preparation and Application for Particulate Removal
Abstract
1. Introduction
2. Results and Discussion
2.1. Optimization of the Mechanical Properties of Ceramic Papers
2.1.1. Polyelectrolyte Titrations by Charge-Flow Potential Measurements
2.1.2. Retention of Inorganic Solids
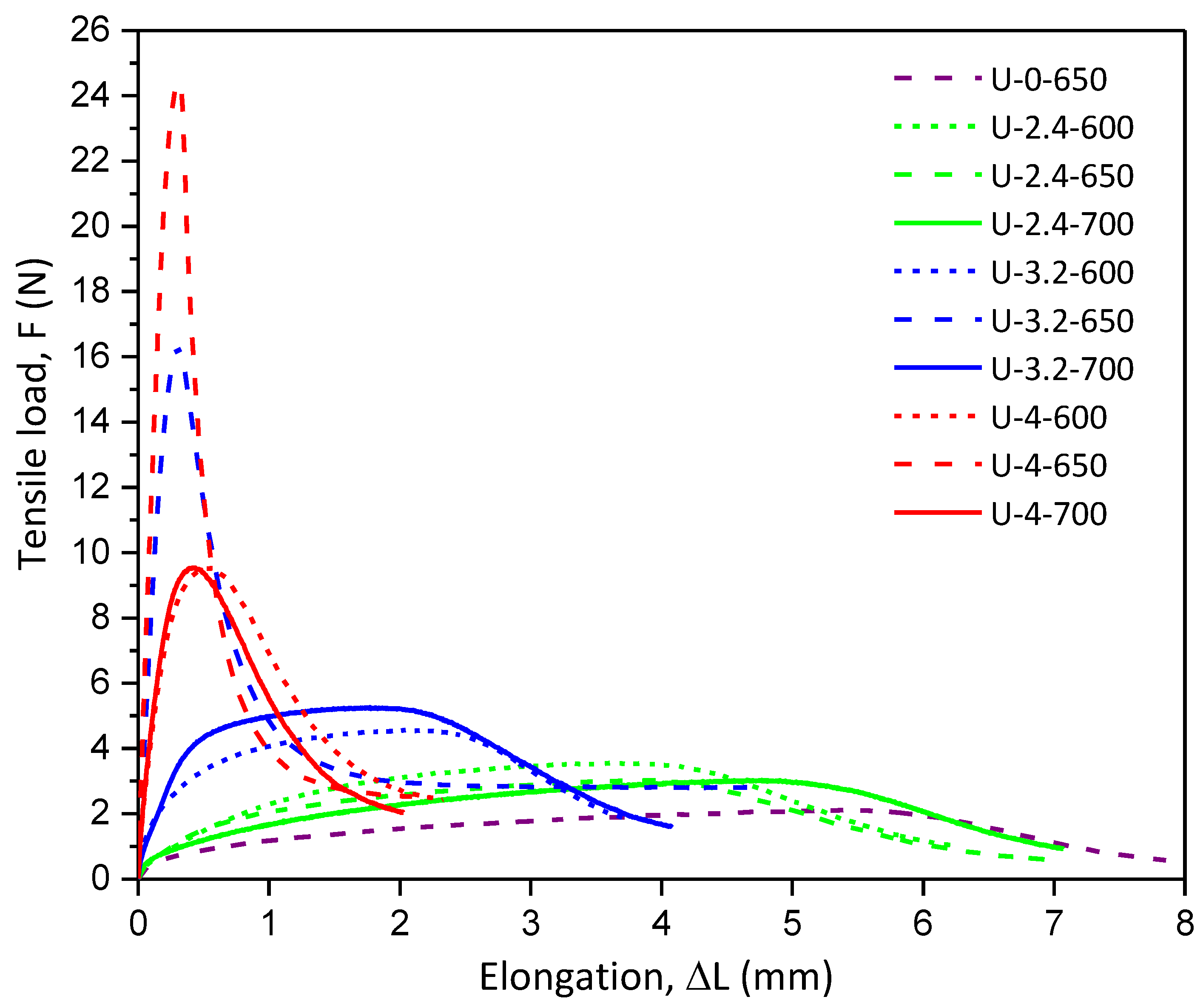
2.1.3. Mechanical Testing of Ceramic Papers with Different Binder Quantities and Calcination Temperatures
2.2. Characterization
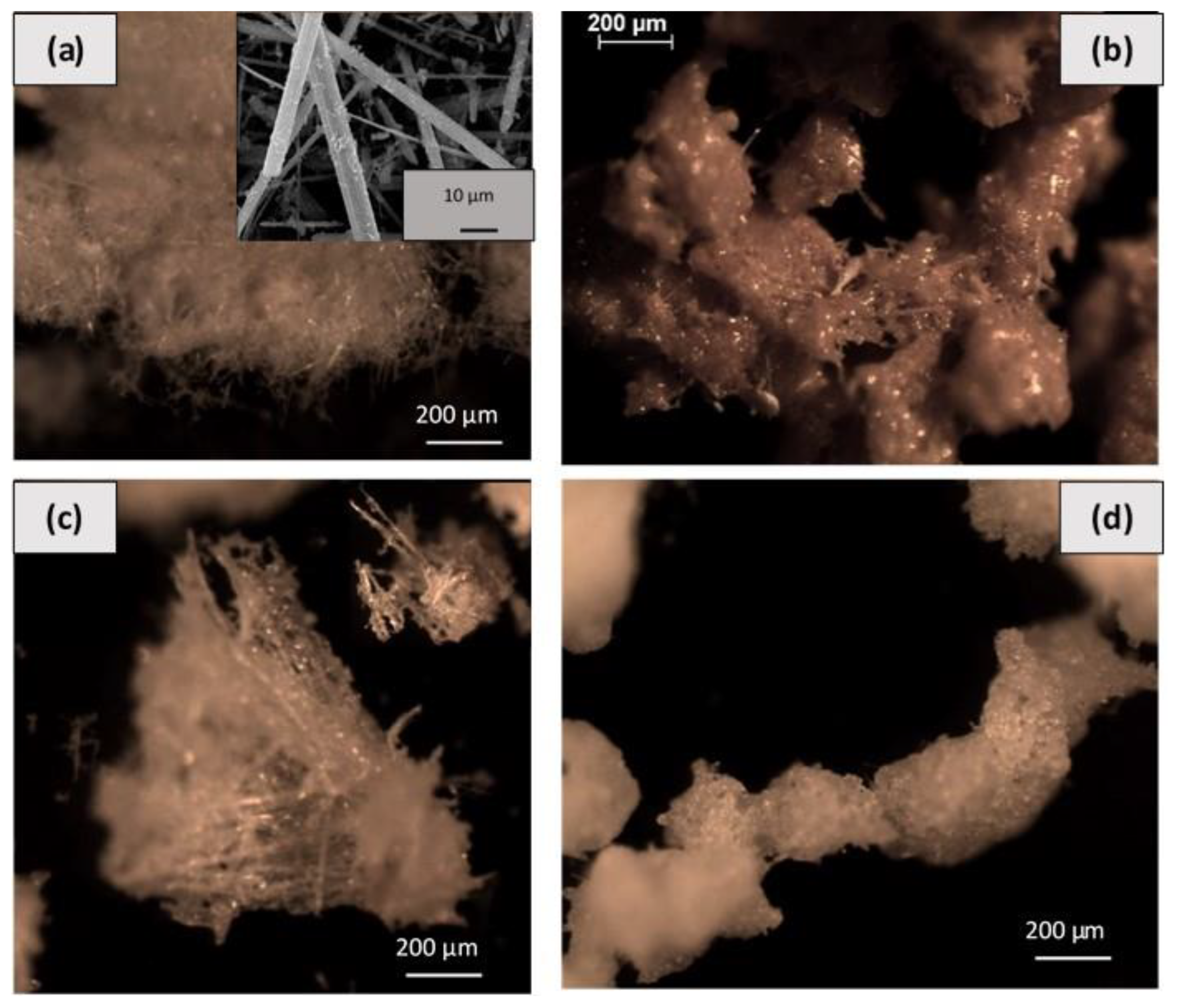
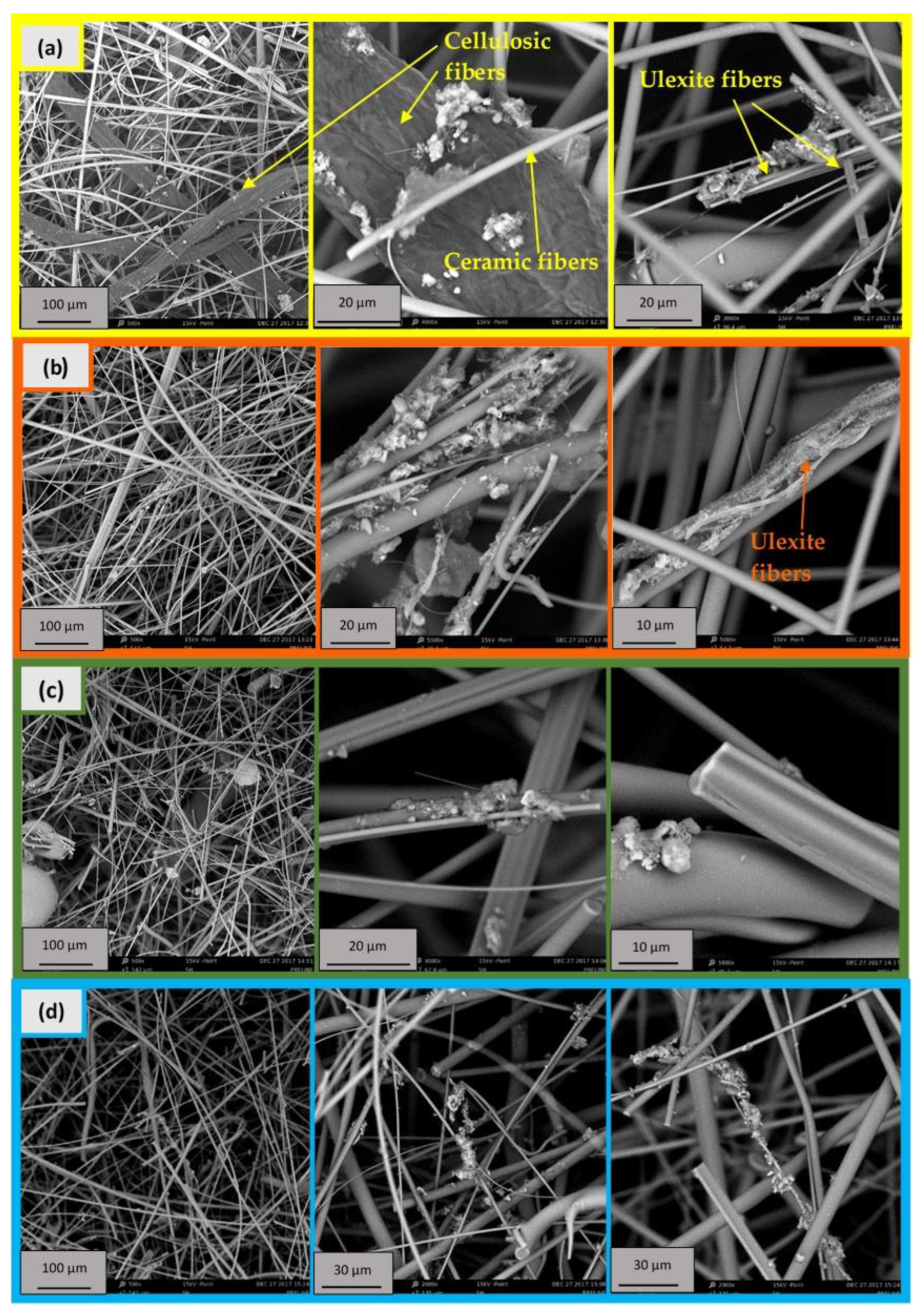
2.2.1. Ceramic Paper
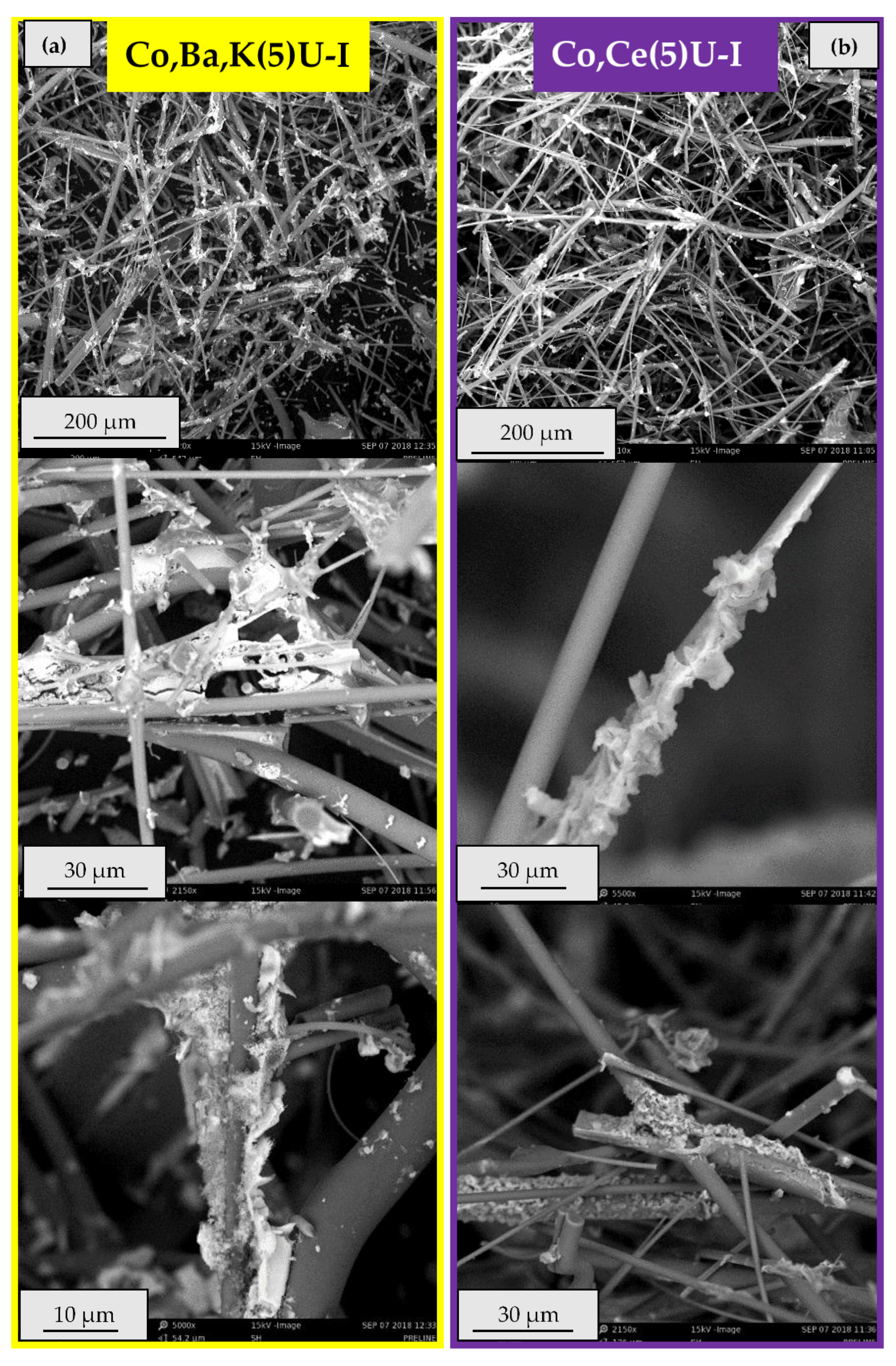
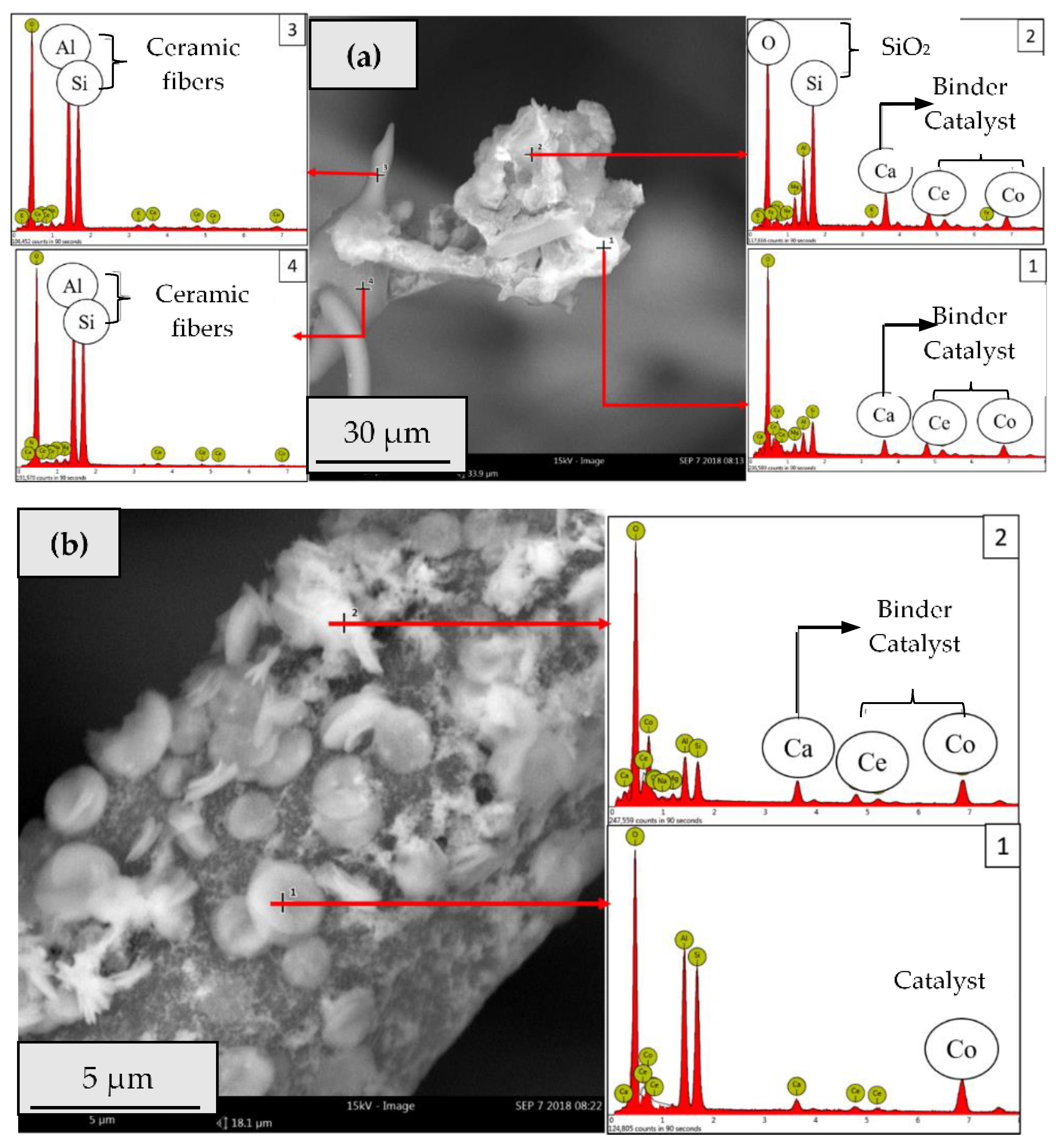
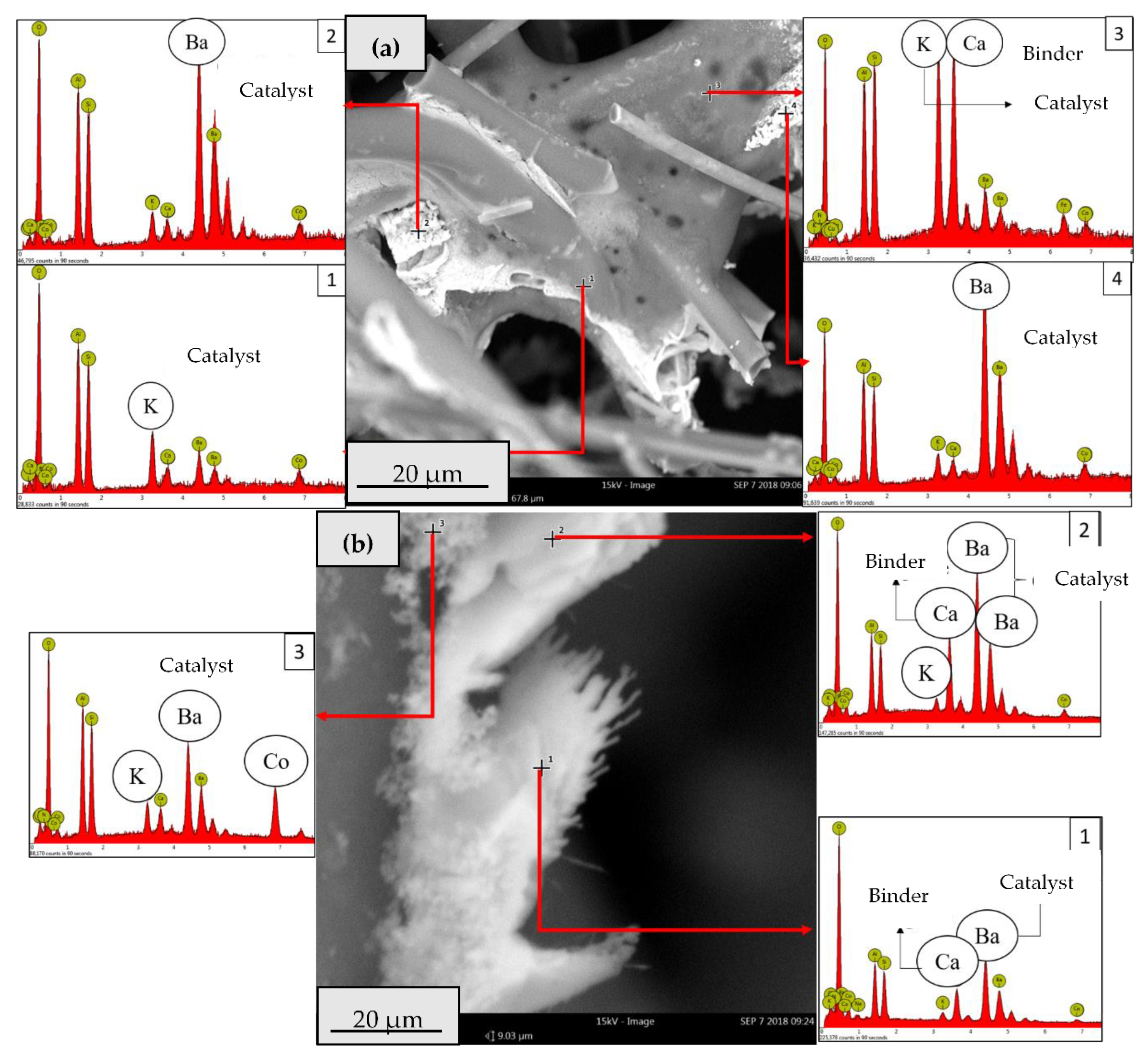
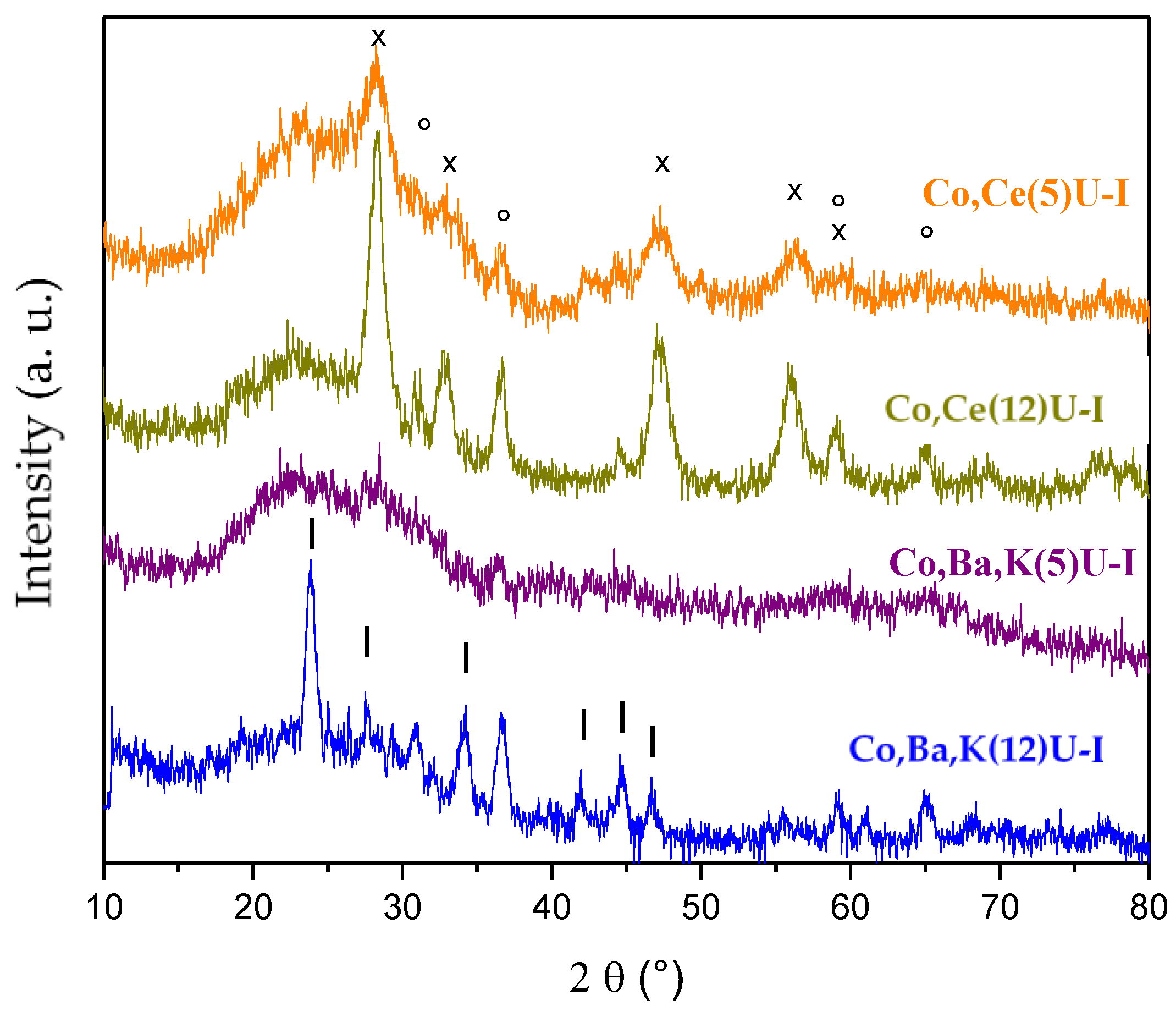
2.2.2. Catalytic Ceramic Paper
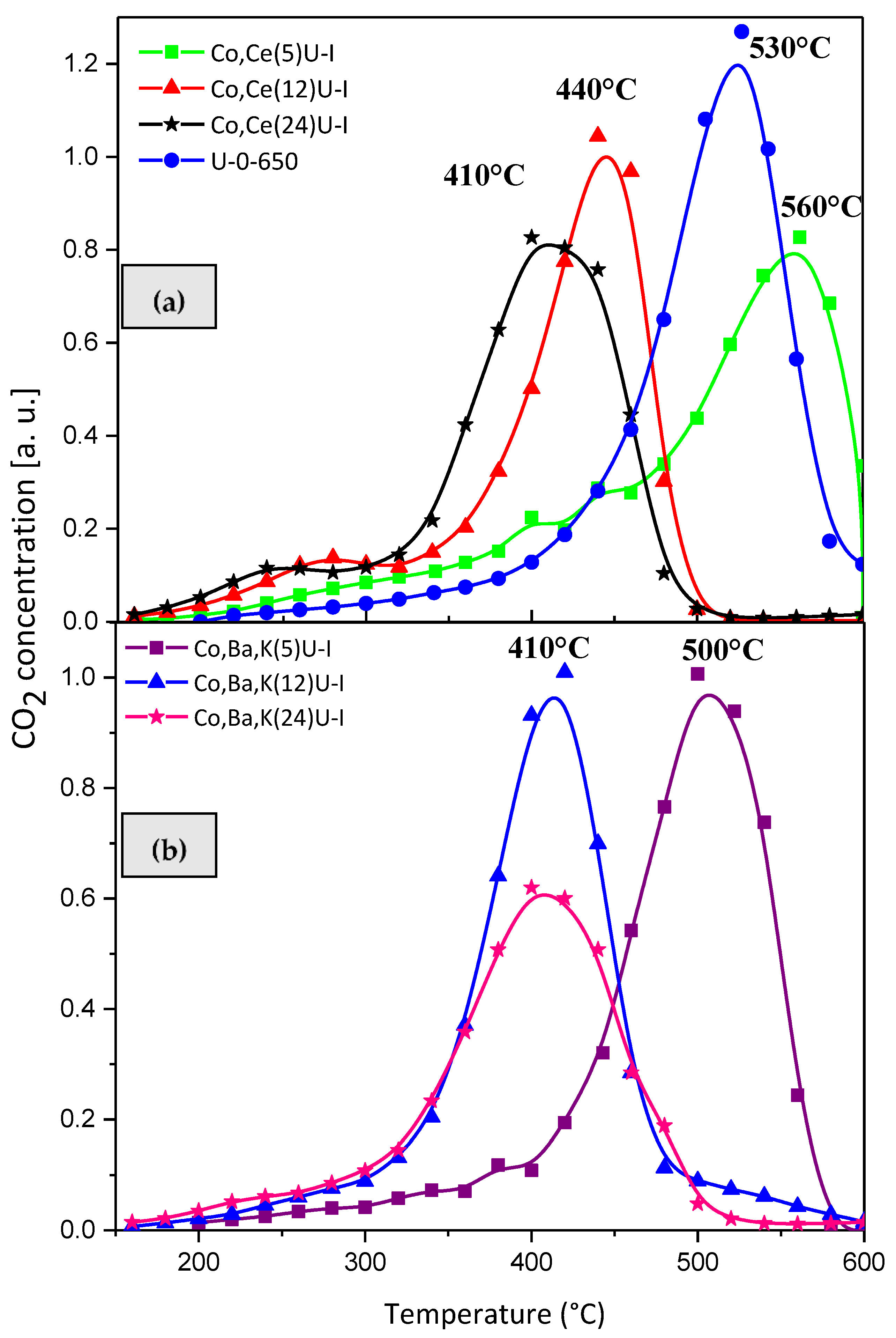
2.3. Catalytic Activity Relationship with Composition/Morphology of Catalytic Ceramic Paper
3. Materials and Methods
3.1. Conditioning of Materials to Be Used in the Preparation of Ceramic Papers
3.1.1. Binder Conditioning
3.1.2. Conditioning of Ceramic Fibers
3.1.3. Conditioning of Cellulosic Fibers
3.2. Obtaining Catalytic Ceramic Papers
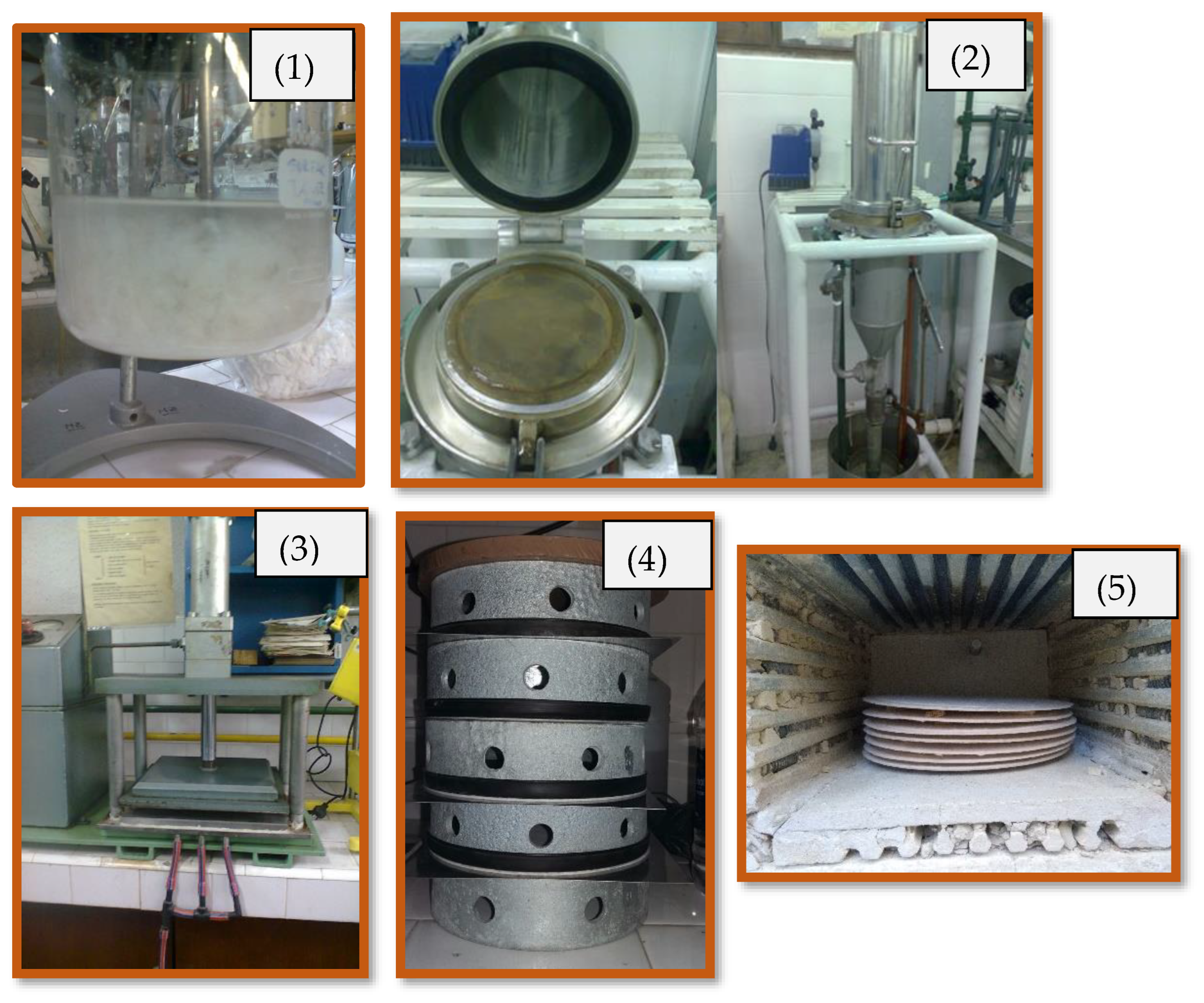
3.2.1. Papermaking Technique
3.2.2. Polyelectrolyte Titration by Charge-Flow Potential Measurements
- Sample 1: NaCl + PVAm;
- Sample 2: NaCl + PVAm + Ceramic Fibers;
- Sample 3: NaCl + PVAm + Ceramic fibers + Natural Ulexite;
- Sample 4: NaCl + PVAm + Ceramic Fibers + Natural Ulexite + Cellulosic Fibers;
- Sample 5: NaCl + PVAm + Ceramic Fibers + Natural Ulexite + Cellulosic Fibers + A-PAM.
3.2.3. Incorporation of the Active Phases
3.3. Characterization of Catalytic Ceramic Paper
3.4. Catalytic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padhi, A.; Bansal, M.; Habib, G.; Samiksha, S.; Raman, R.S. Physical, chemical and optical properties of PM2.5 and gaseous emissions from cooking with biomass fuel in the Indo-Gangetic Plain. Sci. Total Environ. 2022, 841, 156730. [Google Scholar] [CrossRef] [PubMed]
- Hama, S.; Ouchen, I.; Wyche, K.P.; Cordell, R.L.; Monks, P.S. Carbonaceous aerosols in five European cities: Insights into primary emissions and secondary particle formation. Atmos. Res. 2022, 274, 106180. [Google Scholar] [CrossRef]
- Lee, S.; Lim, S.; Lee, H.; Park, S. Probing the oxidation reactivity of ultra-low-sulfur diesel soot with controlled particle size and organic mass fraction. J Anal. Appl. Pyrolysis 2019, 140, 264–273. [Google Scholar] [CrossRef]
- Pérez, V.R.; Bueno-López, A. Catalytic regeneration of diesel particulate filters: Comparison of Pt and CePr active phases. Chem. Eng. J. 2015, 279, 79–85. [Google Scholar] [CrossRef]
- Millo, F.; Andreata, M.; Ra, M.; Mercuri, D.; Pozzi, C. Impact on vehicle fuel economy of the soot loading on diesel particulate filters made of different substrate materials. Energy 2015, 86, 19–30. [Google Scholar] [CrossRef]
- Godoy, M.L.; Banús, E.D.; Miró, E.E.; Milt, V.G. Single and double bed stacked wire mesh cartridges for the catalytic treatment of diésel exhaust. J. Environ. Chem. Eng. 2019, 7, 103290. [Google Scholar] [CrossRef]
- Sacco, N.A.; Banús, E.D.; Milt, V.G.; Miró, E.E.; Bortolozzi, J.P. Catalytic Paper Filters for Diesel Soot Abatement: Studies at Laboratory and Bench Scales. Emiss. Control Sci. Technol. 2020, 6, 450–461. [Google Scholar] [CrossRef]
- Leonardi, S.A.; Zanuttini, M.A.; Miró, E.E.; Milt, V.G. Catalytic paper made from ceramic fibres and natural ulexite. Application to diesel particulate removal. Chem. Eng. J. 2017, 317, 394–403. [Google Scholar] [CrossRef]
- Leonardi, S.A.; Tuler, F.E.; Gaigneaux, E.; Debecker, D.P.; Miró, E.E.; Milt, V.G. Novel ceramic paper structures for diesel exhaust purification. Environ. Sci. Pollut. Res. 2018, 25, 35276–35286. [Google Scholar] [CrossRef]
- Schnell, C.N.; Tarrés, Q.; Galván, M.V.; Mocchiutti, P.; Delgado-Aguilar, M.; Zanuttini, M.A.; Mutjé, P. Polyelectrolyte complexes for assisting the application of lignocellulosic micro/nanofibers in papermaking. Cellulose 2018, 25, 6083–6092. [Google Scholar] [CrossRef]
- Raunio, J.; Asikainen, T.; Wilo, M.; Kallio, E.; Csóka, L. Affecting the bonding between PLA fibrils and kraft pulp for improving paper dry-strength. Nord. Pulp Pap. Res. J. 2020, 35, 185–194. [Google Scholar] [CrossRef]
- Salmi, J.; Österberg, M.; Stenius, P.; Laine, J. Surface forces between cellulose surfaces in cationic polyelectrolyte solutions: The effect of polymer molecular weight and charge density. Nord. Pulp Pap. Res. J. 2007, 22, 249–257. [Google Scholar] [CrossRef]
- Leonardi, S.A.; Miró, E.E.; Milt, V.G. Activity of Catalytic Ceramic Papers to Remove Soot Particles—A Study of Different Types of Soot. Catalysts 2022, 12, 855. [Google Scholar] [CrossRef]
- Cecchini, J.P.; Serra, R.M.; Ulla, M.A.; Zanuttini, M.A.; Milt, V.G. Enhancing mechanical properties of ceramic papers loaded with zeolites using borates compounds as binders. Bioresources 2013, 8, 313–326. [Google Scholar] [CrossRef][Green Version]
- Cecchini, J.P.; Banús, E.D.; Leonardi, S.A.; Zanuttini, M.A.; Ulla, M.A.; Milt, V.G. Flexible-Structured Systems Made of Ceramic Fibers Containing Pt-NaY Zeolite Used as CO Oxidation Catalysts. J. Mater. Sci. 2014, 50, 755–768. [Google Scholar] [CrossRef]
- Milt, V.G.; Querini, C.A.; Miró, E.E.; Ulla, M.A. Abatement of Diesel Exhaust Pollutants: NOx Adsorption on Co, Ba, K/CeO2. Catalysts. J. Catal. 2003, 220, 424–432. [Google Scholar] [CrossRef]
- Sui, L.; Yu, L. Diesel soot oxidation catalyzed by Co-Ba-K catalysts: Evaluation of the performance of the catalysts. Chem. Eng. J. 2008, 142, 327–330. [Google Scholar] [CrossRef]
- Banús, E.D.; Milt, V.G.; Miró, E.E.; Ulla, M.A. Co,Ba,K/ZrO2 coated onto metallic foam (AISI 314) as a structured catalyst for soot combustion: Coating preparation and characterization. Appl. Catal. A Gen. 2010, 379, 95–104. [Google Scholar] [CrossRef]
- Banús, E.D.; Milt, V.G.; Miró, E.E.; Ulla, M.A. Catalytic coating synthesized onto cordierite monolith walls. Its application to diesel soot combustion. Appl. Catal. B Environ. 2013, 132–133, 479–486. [Google Scholar] [CrossRef]
- Xing, L.; Yang, Y.; Ren, W.; Zhao, D.; Tian, Y.; Ding, T.; Zhang, J.; Zheng, L.; Lia, X. Highly efficient catalytic soot combustión performance of hierarchically meso-macroporous Co3O4/CeO2 nanosheet monolithic catalysts. Catal. Today 2020, 351, 83–93. [Google Scholar] [CrossRef]
- Zheng, C.; Bao, S.; Mao, D.; Xu, Z.; Zheng, S. Insight into phase structure-dependent soot oxidation activity of K/MnO2 catalyst. J. Environ. Sci. 2022, 126, 668–682. [Google Scholar] [CrossRef]
- Xu, H.; Zeng, L.; Cui, L.; Guo, W.; Gong, C.; Xue, G. In-situ generation of platinum nanoparticles on LaCoO3 matrix for soot oxidation. J. Rare Earths 2022, 40, 888–896. [Google Scholar] [CrossRef]
- Soares, S.; Camino, G.; Levinchik, S. Comparative study of the thermal decomposition of pure cellulose and pulp paper. Polym. Degrad. Stab. 1995, 49, 275–283. [Google Scholar] [CrossRef]
- Bhardwaj, N.K.; Duong, T.D.; Nguyen, K.L. Pulp charge determination by different methods: Effect of beating/refining. Colloids Surf. A Physicochem. Eng. Asp. 2004, 236, 39–44. [Google Scholar] [CrossRef]
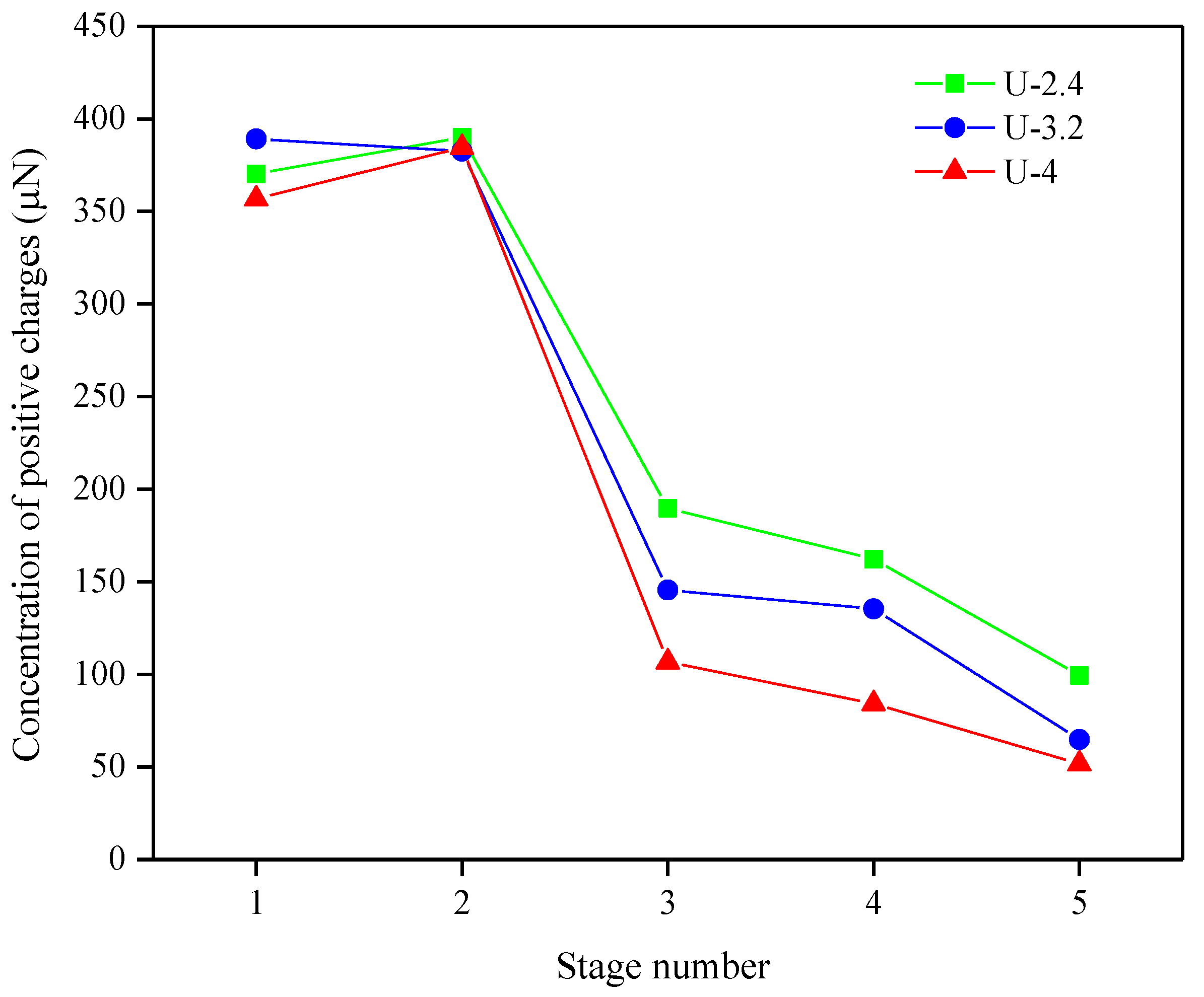


| Stage Number | Components | pH | Concentration of Positive Charges (µN = µeq.L−1) | ||
|---|---|---|---|---|---|
| U-4 * | U-3.2 * | U-2.4 * | |||
| 1 | NaCl + PVAm | 7 | 357 | 389 | 370 |
| 2 | +Ceramic fiber | 7 | 385 | 382 | 390 |
| 3 | +Natural ulexite | 9.5 | 107 | 146 | 190 |
| 4 | +Cellulosic fiber | 9.5 | 84 | 135 | 162 |
| 5 | +A-PAM | 9.5 | 63 | 65 | 99 |
| Sample | Mass in Suspension [g] | Mass of Ceramic Paper [g] | Solids Retained [%] | Average of Solid Retained [%] |
|---|---|---|---|---|
| U-2.4-600 | 11.81 | 9.76 | 82.64 | 82.79 |
| U-2.4-650 | 11.81 | 9.83 | 83.24 | |
| U-2.4-700 | 11.81 | 9.74 | 82.50 | |
| U-3.2-600 | 12.41 | 10.36 | 83.44 | 82.87 |
| U-3.2-650 | 12.41 | 10.40 | 83.81 | |
| U-3.2-700 | 12.41 | 10.10 | 81.37 | |
| U-4-600 | 13.02 | 10.57 | 81.21 | 81.57 |
| U-4-650 | 13.02 | 10.75 | 82.62 | |
| U-4-700 | 13.02 | 10.53 | 80.90 | |
| U-0-650 | 10 | 9.62 | 96.17 | 96.17 |
| Nomenclature | TI (N.m.g−1) | EM (MPa) |
|---|---|---|
| U-0-650 | 0.09 ± 0.02 | 0.76 ± 0.19 |
| U-2.4-600 | 0.15 ± 0.01 | 1.38 ± 0.23 |
| U-2.4-650 | 0.13 ±0.01 | 0.75 ± 0.24 |
| U-2.4-700 | 0.13 ± 0.02 | 0.53 ± 0.18 |
| U-3.2-600 | 0.18 ± 0.03 | 2.46 ± 0.41 |
| U-3.2-650 | 0.67 ± 0.04 | 27.43 ± 3.47 |
| U-3.2-700 | 0.22 ± 0.03 | 2.76 ± 0.96 |
| U-4-600 | 0.38 ±0.04 | 11.37 ± 2.42 |
| U-4-650 | 0.96 ± 0.03 | 36.52 ± 2.67 |
| U-4-700 | 0.39 ± 0.01 | 17.23 ± 3.45 |
| Element | Sample | |||||||
|---|---|---|---|---|---|---|---|---|
| Uncalcined | Calcined at 600 °C | Calcined at 650 °C | Calcined at 700 °C | |||||
| Point 1 | Point 2 | Point 1 | Point 2 | Point 1 | Point 2 | Point 1 | Point 2 | |
| O | 75.7 | 77.2 | 70.6 | 74.7 | 72.0 | 73.4 | 69.9 | 66.7 |
| Si | 10.4 | 9.7 | 7.3 | 10.6 | 9.3 | 6.7 | 13.0 | 11.9 |
| Al | 3.3 | 9.1 | 11.3 | 10.6 | 8.5 | 8.2 | 7.0 | 9.3 |
| Ca | 0.9 | - | 5.2 | 5.5 | 4.7 | 8.0 | 0.6 | 2.4 |
| Na | - | - | 0.3 | - | 1.1 | - | 1.1 | 1.1 |
| Mg | 7.9 | 2.3 | 0.6 | - | 3.6 | 2.0 | 7.1 | 3.7 |
| B | - | 1.1 | 2.3 | - | 0.9 | - | 1.0 | |
| N | - | - | 3.7 | - | - | - | - | - |
| Fe | 1.7 | 0.3 | - | - | 0.4 | 0.4 | 0.9 | 0.9 |
| K | - | 1.3 | - | - | 0.3 | 0.2 | 0.3 | 0.3 |
| Catalyst/Support | TM (°C) | Oxidizing Flow | References |
|---|---|---|---|
| Co,Ba,K/CeO2 | 370 | 4% NO + 18% O2 | [16] |
| Co,Ba,K/α-Al2O3 | 450 | Air | [17] |
| Co,Ba,K/ZrO2/Metallic foam | 380 | 0.1% NO + 18% O2 | [18] |
| Co,Ba,K/ceramic paper a | 390 | 0.1% NO + 18% O2 | [9] |
| Co,Ba,K/ZrO2/Cordierite monolith | 400 | 0.1% NO + 18% O2 | [19] |
| Co,Ce/ceramic paper a | 480 | 0.1% NO + 18% O2 | [9] |
| Co,Ce/Ni nanosheet | 430 | 600 ppm NO + 10% O2 | [20] |
| K/MnO2 | 490 | 10% O2 | [21] |
| Pt/LaCoO3 | 393 | Air | [22] |
| Co,Ba,K/ceramic paper b | 410 | 0.1% NO + 18% O2 | [this work] |
| Co,Ce/ceramic paper b | 440 | 0.1% NO + 18% O2 | [this work] |
| Nomenclature | Natural Ulexite (g) | Calcination Temperature (°C) |
|---|---|---|
| U-2.4-600 | 2.4 | 600 |
| U-2.4-650 | 2.4 | 650 |
| U-2.4-700 | 2.4 | 700 |
| U-3.2-600 | 3.2 | 600 |
| U-3.2-650 | 3.2 | 650 |
| U-3.2-700 | 3.2 | 700 |
| U-4-600 | 4 | 600 |
| U-4-650 | 4 | 650 |
| U-4-700 | 4 | 700 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, S.A.; Miró, E.E.; Milt, V.G. Ceramic Papers as Structured Catalysts: Preparation and Application for Particulate Removal. Catalysts 2022, 12, 1153. https://doi.org/10.3390/catal12101153
Leonardi SA, Miró EE, Milt VG. Ceramic Papers as Structured Catalysts: Preparation and Application for Particulate Removal. Catalysts. 2022; 12(10):1153. https://doi.org/10.3390/catal12101153
Chicago/Turabian StyleLeonardi, Sabrina A., Eduardo E. Miró, and Viviana G. Milt. 2022. "Ceramic Papers as Structured Catalysts: Preparation and Application for Particulate Removal" Catalysts 12, no. 10: 1153. https://doi.org/10.3390/catal12101153
APA StyleLeonardi, S. A., Miró, E. E., & Milt, V. G. (2022). Ceramic Papers as Structured Catalysts: Preparation and Application for Particulate Removal. Catalysts, 12(10), 1153. https://doi.org/10.3390/catal12101153







