Single-Phase θ-Fe3C Derived from Prussian Blue and Its Catalytic Application in Fischer-Tropsch Synthesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Structure and Morphology of the Precursor
2.2. Synthesis of θ-Fe3C Catalyst
2.3. Structure and Morphology of the Prepared θ-Fe3C Sample
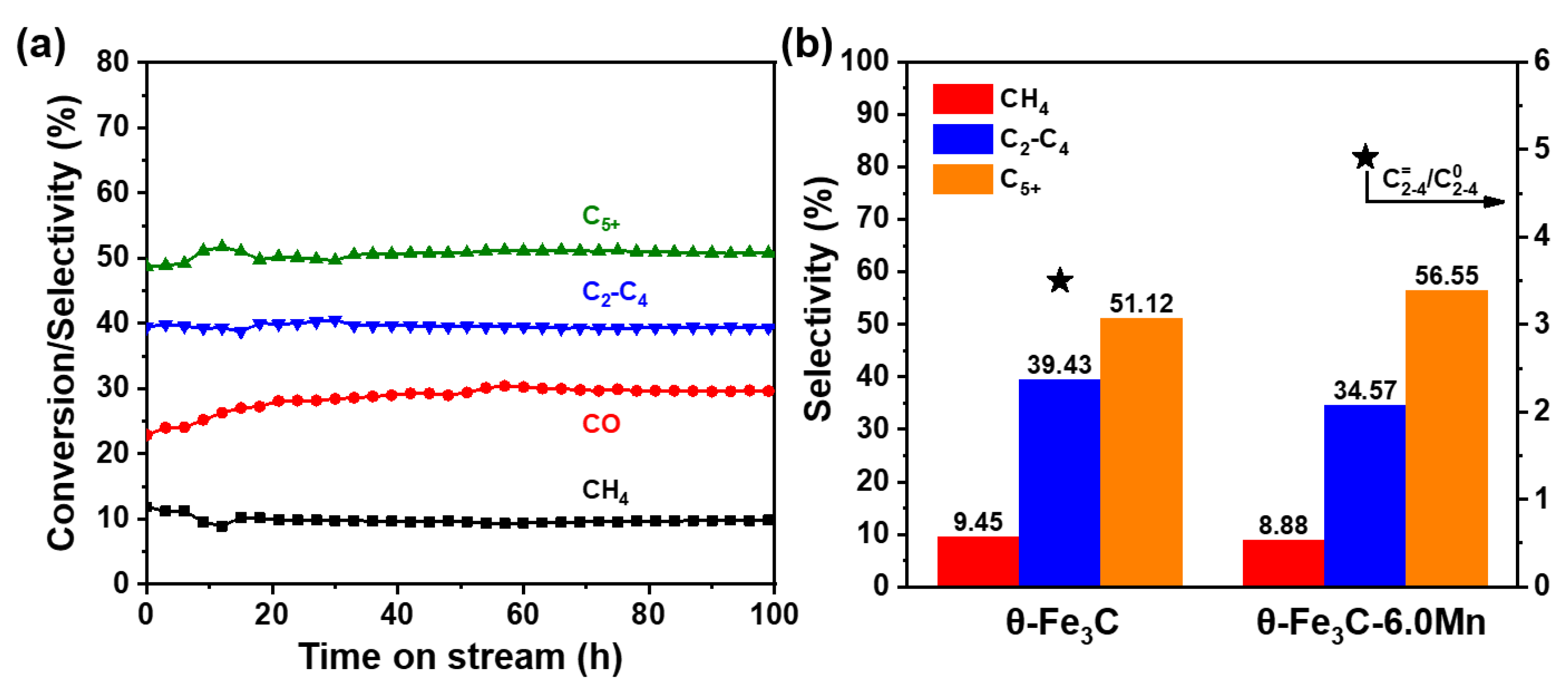
2.4. FTS Performances of the Prepared θ-Fe3C Sample
3. Materials and Methods
3.1. Sample Preparation
3.2. Sample Characterization
3.3. FTS Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Significant Advances in C1 Catalysis: Highly Efficient Catalysts and Catalytic Reactions. ACS Catal. 2019, 9, 3026–3053. [Google Scholar] [CrossRef]
- Abello, S.; Montane, D. Exploring iron-based multifunctional catalysts for Fischer-Tropsch synthesis: A review. ChemSusChem 2011, 4, 1538–1556. [Google Scholar] [CrossRef] [PubMed]
- Dry, M.E. The Fischer-Tropsch process: 1950–2000. Catal. Today 2002, 71, 227–241. [Google Scholar] [CrossRef]
- Dry, M.E. Present and future applications of the Fischer-Tropsch process. Appl. Catal. A Gen. 2004, 276, 1–3. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Gao, J.; Wang, J.; Ma, G.; Wen, X.; Yang, Y.; Li, Y.; Ding, M. A hydrophobic FeMn@Si catalyst increases olefins from syngas by suppressing C1 by-products. Science 2021, 371, 610–613. [Google Scholar] [CrossRef] [PubMed]
- Torres Galvis, H.M.; Bitter, J.H.; Khare, C.B.; Ruitenbeek, M.; Dugulan, A.I.; de Jong, K.P. Supported iron nanoparticles as catalysts for sustainable production of lower olefins. Science 2012, 335, 835–838. [Google Scholar] [CrossRef]
- Zhong, L.; Yu, F.; An, Y.; Zhao, Y.; Sun, Y.; Li, Z.; Lin, T.; Lin, Y.; Qi, X.; Dai, Y.; et al. Cobalt carbide nanoprisms for direct production of lower olefins from syngas. Nature 2016, 538, 84–87. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, X.; Li, L.; Qi, H.; Yang, C.; Liu, W.; Pan, X.; Liu, X.; Yang, X.; Huang, Y.; et al. Ru/TiO2 Catalysts with Size-Dependent Metal/Support Interaction for Tunable Reactivity in Fischer-Tropsch Synthesis. ACS Catal. 2020, 10, 12967–12975. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Yang, X.; Duan, H.; Qi, H.; Su, Y.; Liang, B.; Tao, H.; Liu, B.; Chen, D.; et al. Tuning reactivity of Fischer-Tropsch synthesis by regulating TiOx overlayer over Ru/TiO2 nanocatalysts. Nat. Commun. 2020, 11, 3185. [Google Scholar] [CrossRef]
- Wang, D.; Chen, B.; Duan, X.; Chen, D.; Zhou, X. Iron-based Fischer-Tropsch synthesis of lower olefins: The nature of χ-Fe5C2 catalyst and why and how to introduce promoters. J. Energy Chem. 2016, 25, 911–916. [Google Scholar] [CrossRef]
- Chang, Q.; Zhang, C.; Liu, C.; Wei, Y.; Cheruvathur, A.V.; Dugulan, A.I.; Niemantsverdriet, J.W.; Liu, X.; He, Y.; Qing, M.; et al. Relationship between Iron Carbide Phases (ε-Fe2C, Fe7C3, and χ-Fe5C2) and Catalytic Performances of Fe/SiO2 Fischer–Tropsch Catalysts. ACS Catal. 2018, 8, 3304–3316. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, W.; Chang, Q.; Wang, X.; Wang, H.; Chen, H.; Wei, Y.; Zhang, C.; Xiang, H.; Yang, Y.; et al. θ-Fe3C dominated Fe@C core–shell catalysts for Fischer-Tropsch synthesis: Roles of θ-Fe3C and carbon shell. J. Catal. 2021, 393, 238–246. [Google Scholar] [CrossRef]
- Yang, C.; Zhao, H.; Hou, Y.; Ma, D. Fe5C2 nanoparticles: A facile bromide-induced synthesis and as an active phase for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 2012, 134, 15814–15821. [Google Scholar] [CrossRef]
- Yin, J.; Liu, X.; Liu, X.-W.; Wang, H.; Wan, H.; Wang, S.; Zhang, W.; Zhou, X.; Teng, B.-T.; Yang, Y.; et al. Theoretical exploration of intrinsic facet-dependent CH4 and C2 formation on Fe5C2 particle. Appl. Catal. B Environ. 2020, 278, 119308. [Google Scholar] [CrossRef]
- Opeyemi Otun, K.; Yao, Y.; Liu, X.; Hildebrandt, D. Synthesis, structure, and performance of carbide phases in Fischer-Tropsch synthesis: A critical review. Fuel 2021, 296, 120689. [Google Scholar] [CrossRef]
- Reymond, J. Changes in the surface structure and composition of an iron catalyst of reduced or unreduced Fe2O3 during the reaction of carbon monoxide and hydrogen. J. Catal. 1982, 75, 39–48. [Google Scholar] [CrossRef]
- Kuivila, C. Compositional aspects of iron Fischer-Tropsch catalysts: An XPS/reaction study. J. Catal. 1989, 118, 299–311. [Google Scholar] [CrossRef]
- Butt, J.B. Carbide phases on iron-based Fischer-Tropsch synthesis catalysts part I: Characterization studies. Catal. Lett. 1991, 7, 61–81. [Google Scholar] [CrossRef]
- Butt, J.B. Carbide phases on iron-based Fischer-Tropsch synthesis catalysts part II: Some reaction studies. Catal. Lett. 1991, 7, 83–105. [Google Scholar] [CrossRef]
- Xu, K.; Sun, B.; Lin, J.; Wen, W.; Pei, Y.; Yan, S.; Qiao, M.; Zhang, X.; Zong, B. Epsilon-Iron carbide as a low-temperature Fischer-Tropsch synthesis catalyst. Nat. Commun. 2014, 5, 5783. [Google Scholar] [CrossRef]
- Wezendonk, T.A.; Sun, X.; Dugulan, A.I.; van Hoof, A.J.F.; Hensen, E.J.M.; Kapteijn, F.; Gascon, J. Controlled formation of iron carbides and their performance in Fischer-Tropsch synthesis. J. Catal. 2018, 362, 106–117. [Google Scholar] [CrossRef]
- Santos, V.P.; Wezendonk, T.A.; Jaen, J.J.; Dugulan, A.I.; Nasalevich, M.A.; Islam, H.U.; Chojecki, A.; Sartipi, S.; Sun, X.; Hakeem, A.A.; et al. Metal organic framework-mediated synthesis of highly active and stable Fischer-Tropsch catalysts. Nat. Commun. 2015, 6, 6451. [Google Scholar] [CrossRef] [PubMed]
- Smit, E.D.; Cinquini, F.; Beale, A.M.; Safonova, O.V.; Beek, W.V.; Sautet, P.; Weckhuysen, B.M. Stability and Reactivity of ε-χ-θ Iron Carbide Catalyst Phases in Fischer-Tropsch Synthesis: Controlling μc. J. Am. Chem. Soc. 2010, 132, 14928–14941. [Google Scholar] [CrossRef] [PubMed]
- Huo, C.F.; Li, Y.W.; Wang, J.; Jiao, H. Insight into CH4 formation in iron-catalyzed Fischer-Tropsch synthesis. J. Am. Chem. Soc. 2009, 131, 14713–14721. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, J.-F.; Bao, J.; Zhang, Y. Manganese-Modified Fe3O4 Microsphere Catalyst with Effective Active Phase of Forming Light Olefins from Syngas. ACS Catal. 2015, 5, 3905–3909. [Google Scholar] [CrossRef]
- Niu, L.; Liu, X.; Wen, X.; Yang, Y.; Xu, J.; Li, Y. Effect of potassium promoter on phase transformation during H2 pretreatment of a Fe2O3 Fischer Tropsch synthesis catalyst precursor. Catal. Today 2020, 343, 101–111. [Google Scholar] [CrossRef]
- An, B.; Cheng, K.; Wang, C.; Wang, Y.; Lin, W. Pyrolysis of Metal–Organic Frameworks to Fe3O4@Fe5C2 Core–Shell Nanoparticles for Fischer-Tropsch Synthesis. ACS Catal. 2016, 6, 3610–3618. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Wang, W.; Lei, X.; Yang, H. Fe3C and Mn doped Fe3C nanoparticles: Synthesis, morphology and magnetic properties. RSC Adv. 2015, 5, 57828–57832. [Google Scholar] [CrossRef]
- Barman, B.K.; Nanda, K.K. Prussian blue as a single precursor for synthesis of Fe/Fe3C encapsulated N-doped graphitic nanostructures as bi-functional catalysts. Green Chem. 2016, 18, 427–432. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Gao, J.; Chen, X.; Yang, H. Facile synthesis and magnetic properties of Fe3C/C nanoparticles via a sol-gel process. Dye. Pigment. 2015, 112, 305–310. [Google Scholar] [CrossRef]
- Niemantsverdriet, J.W.; Flipse, C.F.J.; Van Der Kraan, A.M.; Van Loef, J.J. A Mössbauer study of surface effects on iron Fischer-Tropsch catalysts. Appl. Surf. Sci. 1982, 10, 302–313. [Google Scholar] [CrossRef]
- Phillips, J. Application of M¨ossbauer spectroscopy for the characterization of iron-containing catalysts. Hyperfine Interact. 1998, 111, 3–16. [Google Scholar] [CrossRef]
- Liu, X.W.; Zhao, S.; Meng, Y.; Peng, Q.; Dearden, A.K.; Huo, C.F.; Yang, Y.; Li, Y.W.; Wen, X.D. Mossbauer Spectroscopy of Iron Carbides: From Prediction to Experimental Confirmation. Sci. Rep. 2016, 6, 26184. [Google Scholar] [CrossRef] [PubMed]
- Lyubutin, I.S.; Lin, C.R.; Korzhetskiy, Y.V.; Dmitrieva, T.V.; Chiang, R.K. Mössbauer spectroscopy and magnetic properties of hematite/magnetite nanocomposites. J. Appl. Phys. 2009, 106, 034311. [Google Scholar] [CrossRef]
- Bukur, D.B.; Okabe, K.; Rosynek, M.P.; Li, C.P.; Wang, D.J.; Rao, K.R.P.M.; Huffman, G.P. Activation Studies with a Precipitated Iron Catalyst for Fischer-Tropsch Synthesis. J. Catal. 1995, 155, 353–365. [Google Scholar] [CrossRef]
- Datye, A.K.; Jin, Y.; Mansker, L.; Motjope, R.T.; Dlamini, T.H.; Coville, N.J. The nature of the active phase in iron Fischer-Tropsch catalysts. Stud. Surf. Sci. Catal. 2000, 130, 1139–1144. [Google Scholar]
- Hong, S.Y.; Chun, D.H.; Yang, J.I.; Jung, H.; Lee, H.T.; Hong, S.; Jang, S.; Lim, J.T.; Kim, C.S.; Park, J.C. A new synthesis of carbon encapsulated Fe5C2 nanoparticles for high-temperature Fischer-Tropsch synthesis. Nanoscale 2015, 7, 16616–16620. [Google Scholar] [CrossRef]
- Wang, P.; Chen, W.; Chiang, F.K.; Dugulan, A.I.; Song, Y.; Pestman, R.; Zhang, K.; Yao, J.; Feng, B.; Miao, P.; et al. Synthesis of stable and low-CO2 selective epsilon-iron carbide Fischer-Tropsch catalysts. Sci. Adv. 2018, 4, eaau2947. [Google Scholar] [CrossRef]
- Yu, G.; Sun, B.; Pei, Y.; Xie, S.; Yan, S.; Qiao, M.; Fan, K.; Zhang, X.; Zong, B. FexOy@C spheres as an excellent catalyst for Fischer-Tropsch synthesis. J. Am. Chem. Soc. 2010, 132, 935–937. [Google Scholar] [CrossRef]
- Zhang, Q.; Gu, J.; Chen, J.; Qiu, S.; Wang, T. Facile fabrication of porous Fe@C nanohybrids from natural magnetite as excellent Fischer-Tropsch catalysts. Chem. Commun. 2020, 56, 4523–4526. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, F.; Tang, Y.; Liu, M.; Tao, F.F.; Zhang, Y. Effects of initial crystal structure of Fe2O3 and Mn promoter on effective active phase for syngas to light olefins. Appl. Catal. B Environ. 2020, 261, 118219. [Google Scholar] [CrossRef]
- Li, J.; Wu, L.; Zhang, S.; Wen, J.; Liu, M.; Wang, C.; Li, X. The promotional effect of Mn on Fe-based Fischer-Tropsch catalysts for the synthesis of C5+ hydrocarbons. Sustain. Energy Fuels 2019, 3, 219–226. [Google Scholar] [CrossRef]
- Zhang, Y.; Ma, L.; Wang, T.; Li, X. MnO2 coated Fe2O3 spindles designed for production of C5+ hydrocarbons in Fischer-Tropsch synthesis. Fuel 2016, 177, 197–205. [Google Scholar] [CrossRef]
- Dad, M.; Fredriksson, H.O.A.; Van de Loosdrecht, J.; Thüne, P.C.; Niemantsverdriet, J.W. Stabilization of iron by manganese promoters in uniform bimetallic FeMn Fischer–Tropsch model catalysts prepared from colloidal nanoparticles. Catal. Struct. React. 2015, 1, 101–109. [Google Scholar] [CrossRef]
- Das, D.; Ravichandran, G.; Chakrabarty, D.K. Conversion of syngas to light olefins over silicalite-1 supported iron and cobalt catalysts: Effect of manganese addition. Catal. Today 1997, 36, 285–293. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, Y.; Wan, H.; Li, T.; An, X.; Xiang, H.; Li, Y. Effect of manganese on a potassium-promoted iron-based Fischer-Tropsch synthesis catalyst. Catal. Lett. 2007, 114, 161–168. [Google Scholar] [CrossRef]
- Tao, Z.; Yang, Y.; Zhang, C.; Li, T.; Ding, M.; Xiang, H.; Li, Y. Study of Manganese Promoter on a Precipitated Iron-Based Catalyst for Fischer-Tropsch Synthesis. J. Nat. Gas Chem. 2007, 16, 278–285. [Google Scholar] [CrossRef]
- Campos, A.; Lohitharn, N.; Roy, A.; Lotero, E.; Goodwin, J.G.; Spivey, J.J. An activity and XANES study of Mn-promoted, Fe-based Fischer–Tropsch catalysts. Appl. Catal. A Gen. 2010, 375, 12–16. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Ma, L.; Shi, N.; Zhou, D.; Li, X. Promotional effects of Mn on SiO2-encapsulated iron-based spindles for catalytic production of liquid hydrocarbons. J. Catal. 2017, 350, 41–47. [Google Scholar] [CrossRef]
- Li, J.; Cheng, X.; Zhang, C.; Yang, Y.; Li, Y. Effects of alkali on iron-based catalysts for Fischer-Tropsch synthesis: CO chemisorptions study. J. Mol. Catal. A Chem. 2015, 396, 174–180. [Google Scholar] [CrossRef]
- Wang, S.; Yin, Q.; Guo, J.; Ru, B.; Zhu, L. Improved Fischer–Tropsch synthesis for gasoline over Ru, Ni promoted Co/HZSM-5 catalysts. Fuel 2013, 108, 597–603. [Google Scholar] [CrossRef]
- Govender, N.S.; Botes, F.G.; de Croon, M.H.J.M.; Schouten, J.C. Mechanistic pathway for methane formation over an iron-based catalyst. J. Catal. 2008, 260, 254–261. [Google Scholar] [CrossRef]
- Li, X.; Yuan, L.; Wang, J.; Jiang, L.; Rykov, A.I.; Nagy, D.L.; Bogdan, C.; Ahmed, M.A.; Zhu, K.; Sun, G.; et al. A “copolymer-co-morphology” conception for shape-controlled synthesis of Prussian blue analogues and as-derived spinel oxides. Nanoscale 2016, 8, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
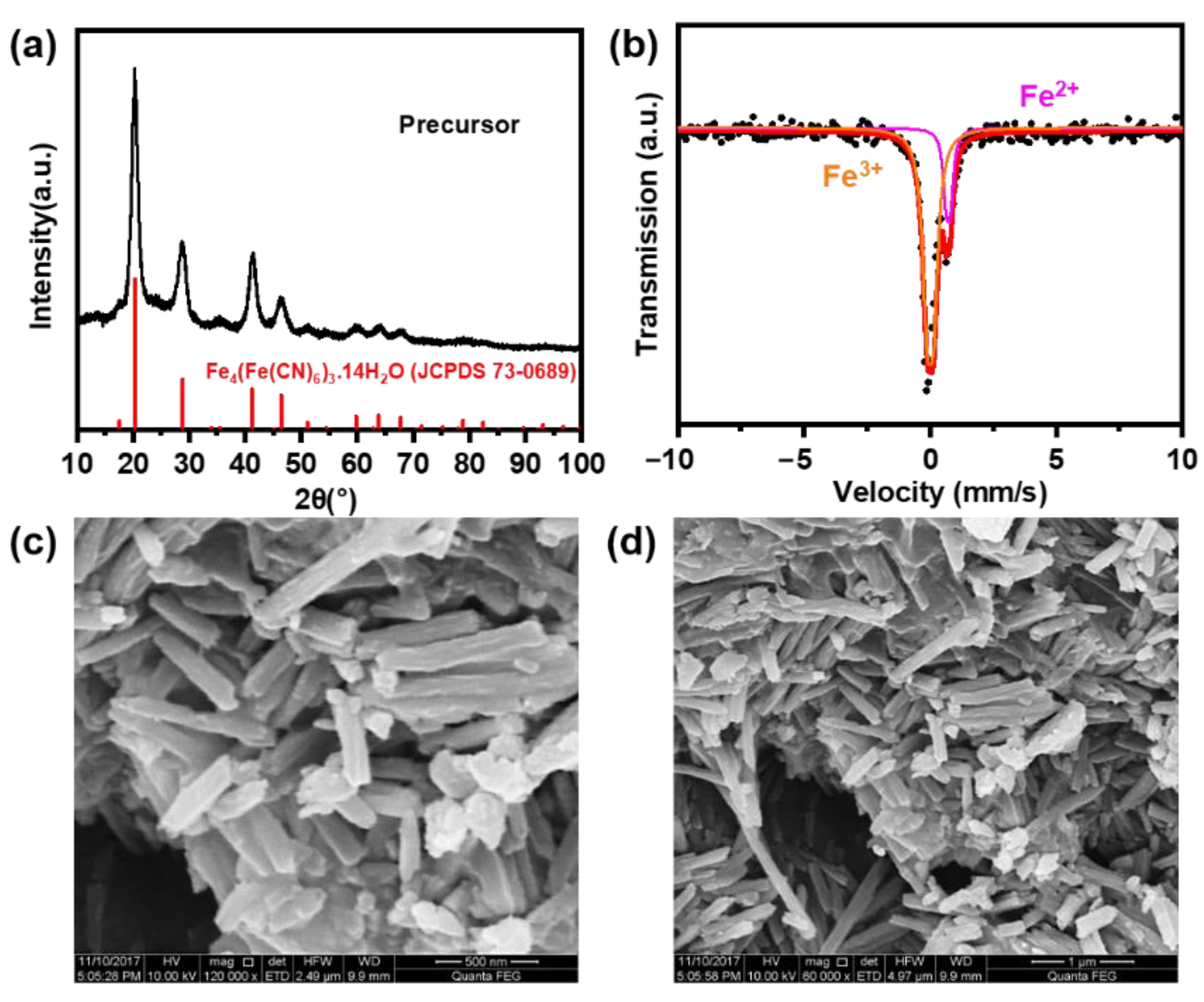

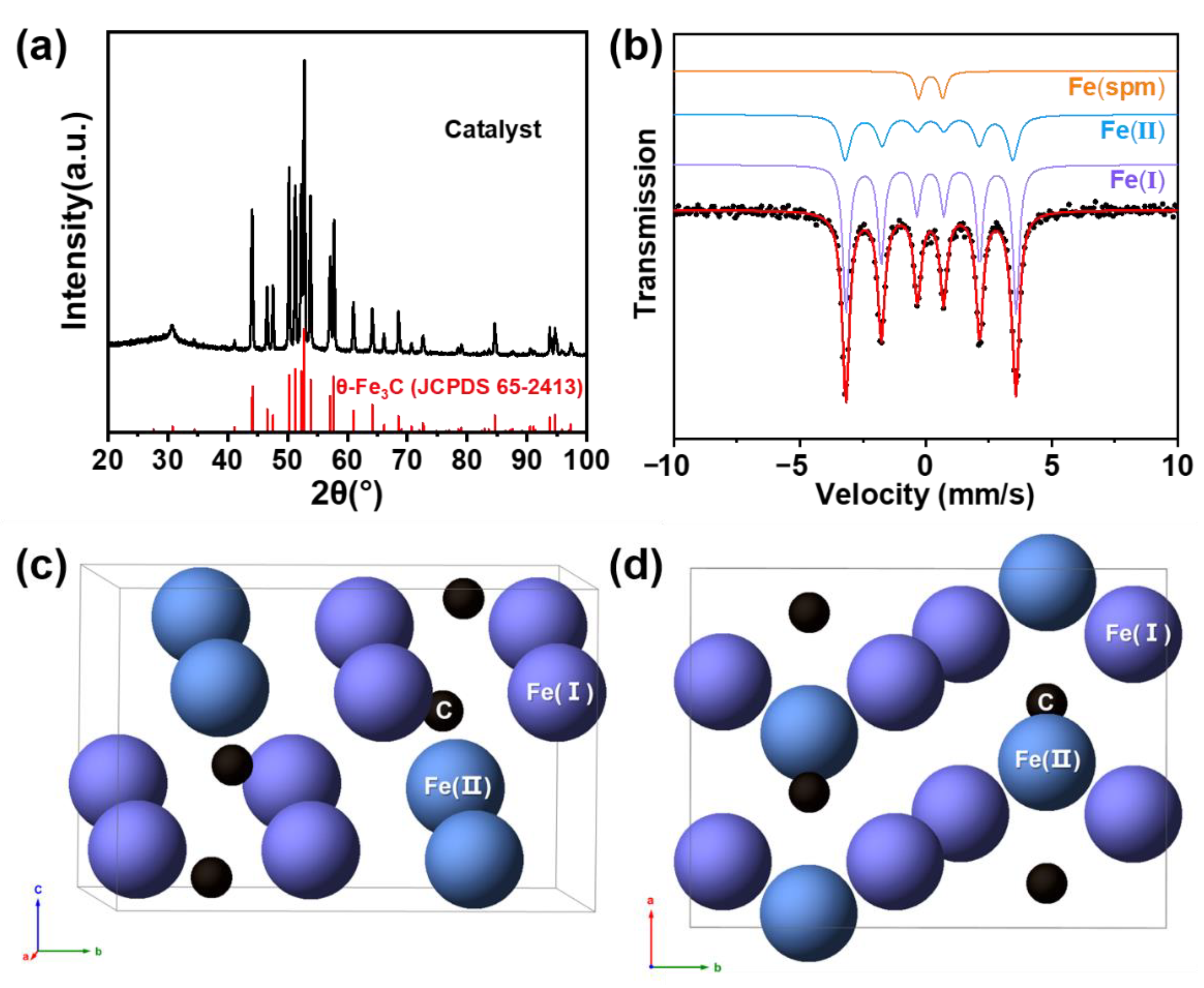
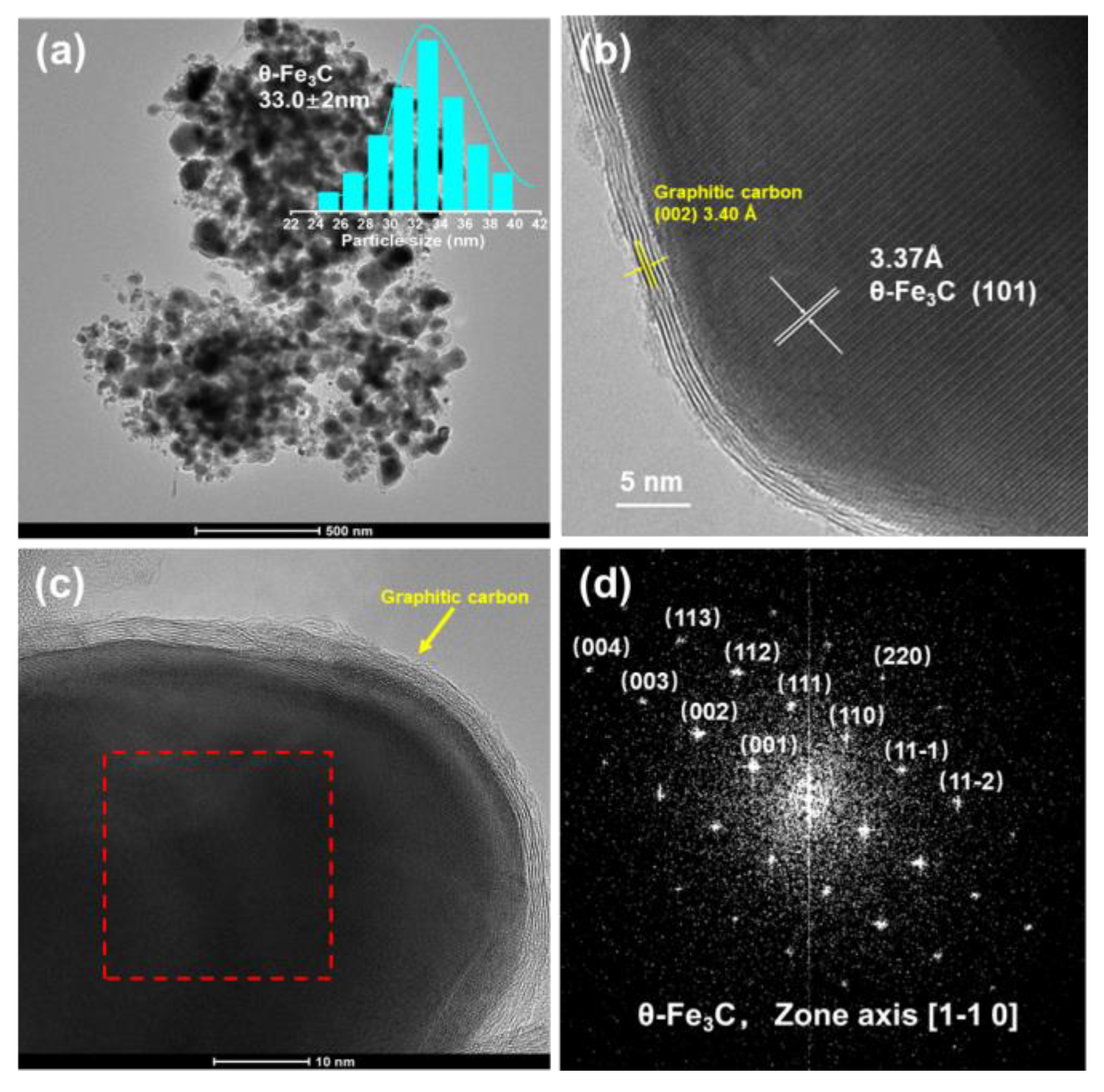

| Phases | Area (%) | IS (mm/s) | QS (mm/s) |
|---|---|---|---|
| Fe2+ | 35.7 | 0.72 | 0.16 |
| Fe3+ | 64.3 | 0.00 | −0.26 |
| Samples | IS (mm/s) | QS (mm/s) | Hhf (kOe) | Area (%) | Phases | Composition (%) |
|---|---|---|---|---|---|---|
| Sample-450 °C | 0.32 | 0.14 | 169 | 26.7 | ε-Fe2.2C (I) | 69.5 |
| 0.07 | 0.21 | 235 | 10.7 | ε-Fe2.2C (II) | ||
| 0.32 | 0.18 | 138 | 32.1 | ε-Fe2.2C (III) | ||
| 0.07 | 0.08 | 170 | 3.6 | h-Fe7C3 (I) | 20.2 | |
| 0.37 | 0.03 | 211 | 8.3 | h-Fe7C3 (II) | ||
| −0.14 | 1.46 | 246 | 8.3 | h-Fe7C3 (III) | ||
| 0.29 | 1.05 | - | 10.3 | Fe (spm) | 10.3 | |
| Sample-500 °C | 0.22 | 0.1 | 209 | 36.7 | θ-Fe3C (I) | 55.0 |
| 0.14 | −0.09 | 207 | 18.3 | θ-Fe3C (II) | ||
| 0.37 | −0.23 | 225 | 16.3 | χ-Fe5C2 (I) | 40.8 | |
| 0.24 | 0.02 | 180 | 16.3 | χ-Fe5C2 (II) | ||
| 0.4 | −0.3 | 105 | 8.2 | χ-Fe5C2 (III) | ||
| 0.26 | 0.82 | - | 4.2 | Fe (spm) | 4.2 | |
| Sample-550 °C | 0.19 | 0.07 | 210 | 63.6 | θ-Fe3C (I) | 95.4 |
| 0.17 | −0.12 | 207 | 31.8 | θ-Fe3C (II) | ||
| 0.18 | −0.92 | - | 4.6 | Fe (spm) | 4.6 | |
| Sample-600 °C | 0.19 | 0.07 | 210 | 63.3 | θ-Fe3C (I) | 95.0 |
| 0.17 | −0.11 | 206 | 31.7 | θ-Fe3C (II) | ||
| 0.18 | −1.01 | - | 5.0 | Fe (spm) | 5.0 | |
| Sample-650 °C | 0.19 | 0.03 | 210 | 62.6 | θ-Fe3C (I) | 93.9 |
| 0.15 | −0.07 | 207 | 31.3 | θ-Fe3C (II) | ||
| 0.19 | −0.96 | - | 6.1 | Fe (spm) | 6.1 |
| Phase | Area (%) | IS (mm/s) | QS (mm/s) | Hhf (kOe) |
|---|---|---|---|---|
| θ-Fe3C (I) | 63.5 | 0.19 | 0.07 | 210 |
| θ-Fe3C (II) | 31.9 | 0.17 | −0.12 | 207 |
| Fe (spm) | 4.6 | 0.19 | −0.96 | - |
| CO Conv. (%) | CO2 Selec. (C mol%) | HC Distribution (wt%) 2 | C=2-4/C02-4 | |||
|---|---|---|---|---|---|---|
| CH4 | C2-C4 | C5+ | ||||
| θ-Fe3C 1 | 30 | 9.9 | 9.4 | 39.4 | 51.2 | 3.5 |
| 6 wt% Mn/Fe3C 1 | 21.5 | 14.8 | 8.8 | 34.6 | 56.6 | 4.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Ma, C.; Liu, X.; Yang, Y.; Li, Y.; Wen, X. Single-Phase θ-Fe3C Derived from Prussian Blue and Its Catalytic Application in Fischer-Tropsch Synthesis. Catalysts 2022, 12, 1140. https://doi.org/10.3390/catal12101140
Zhang W, Ma C, Liu X, Yang Y, Li Y, Wen X. Single-Phase θ-Fe3C Derived from Prussian Blue and Its Catalytic Application in Fischer-Tropsch Synthesis. Catalysts. 2022; 12(10):1140. https://doi.org/10.3390/catal12101140
Chicago/Turabian StyleZhang, Wei, Caiping Ma, Xingwu Liu, Yong Yang, Yongwang Li, and Xiaodong Wen. 2022. "Single-Phase θ-Fe3C Derived from Prussian Blue and Its Catalytic Application in Fischer-Tropsch Synthesis" Catalysts 12, no. 10: 1140. https://doi.org/10.3390/catal12101140
APA StyleZhang, W., Ma, C., Liu, X., Yang, Y., Li, Y., & Wen, X. (2022). Single-Phase θ-Fe3C Derived from Prussian Blue and Its Catalytic Application in Fischer-Tropsch Synthesis. Catalysts, 12(10), 1140. https://doi.org/10.3390/catal12101140







