Low Temperature Ozonation of Acetone by Transition Metals Derived Catalysts: Activity and Sulfur/Water Resistance
Abstract
:1. Introduction
2. Results and Discussion
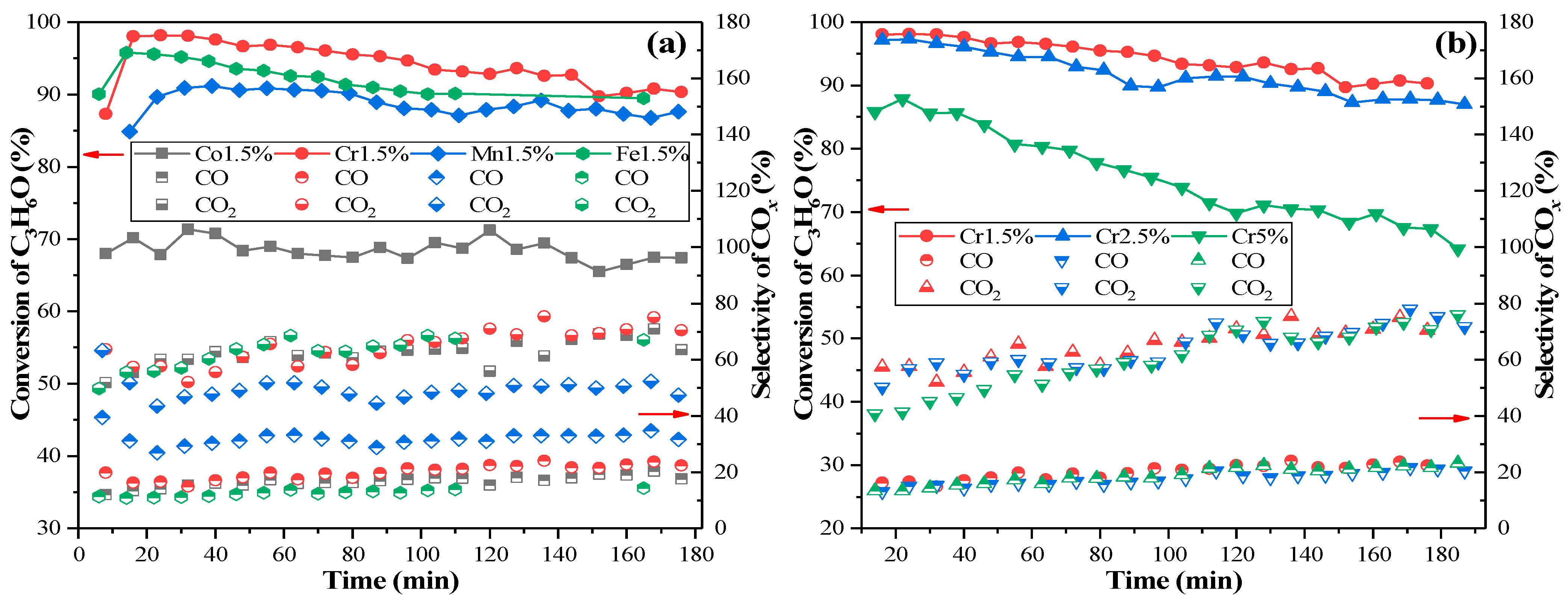
2.1. Activity Evaluation of Different Metal Oxides Catalysts
2.2. Catalyst Characterization Analysis
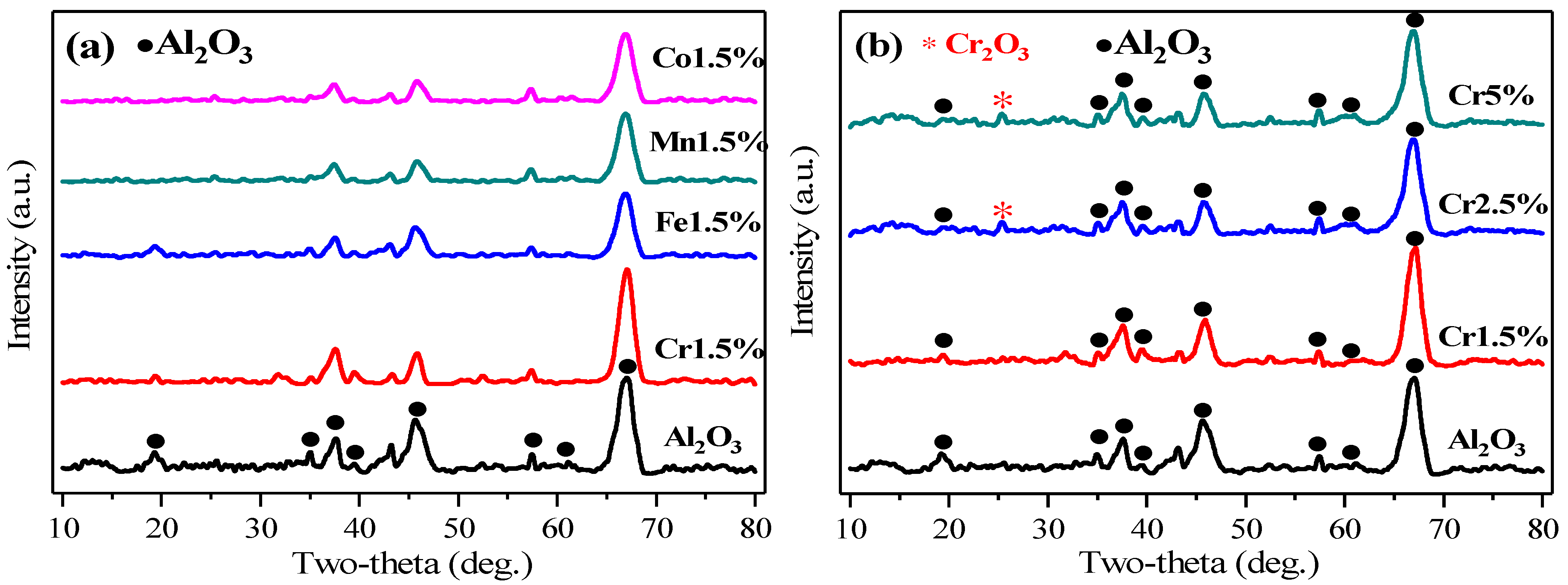
2.2.1. Crystalline Structures
2.2.2. Textural Properties
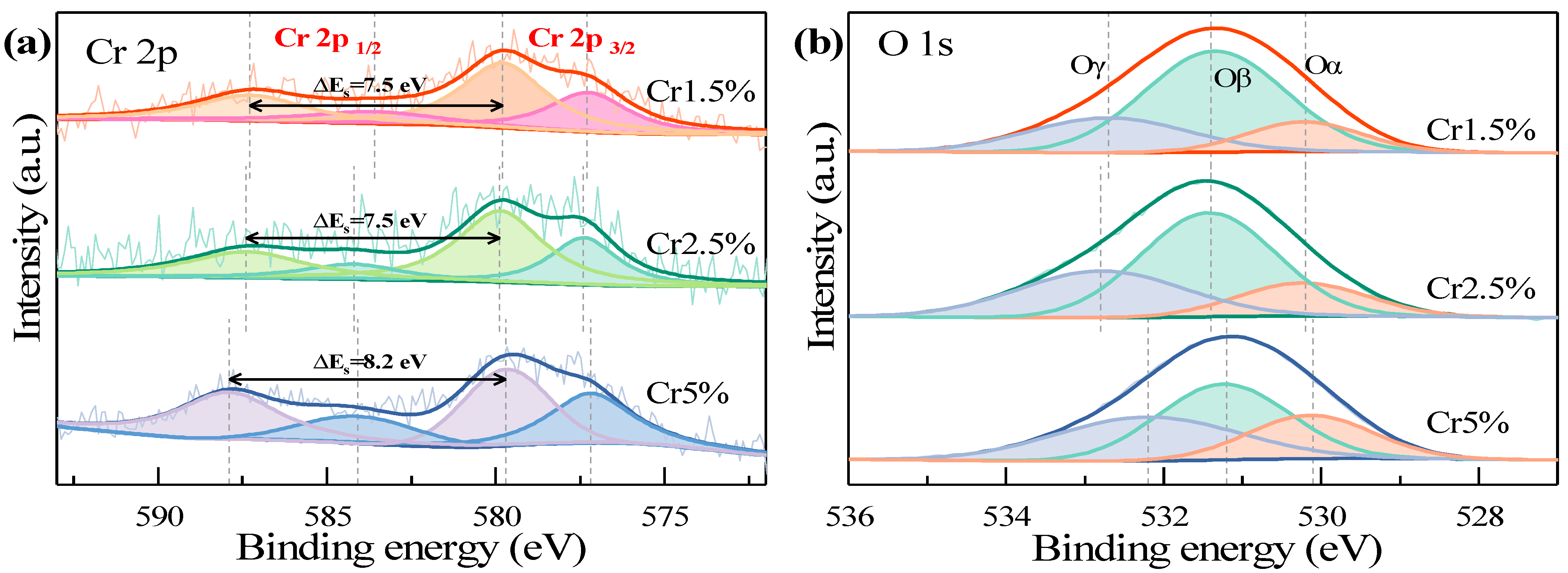
2.2.3. Surface Properties
2.2.4. Temperature Programmed Studies
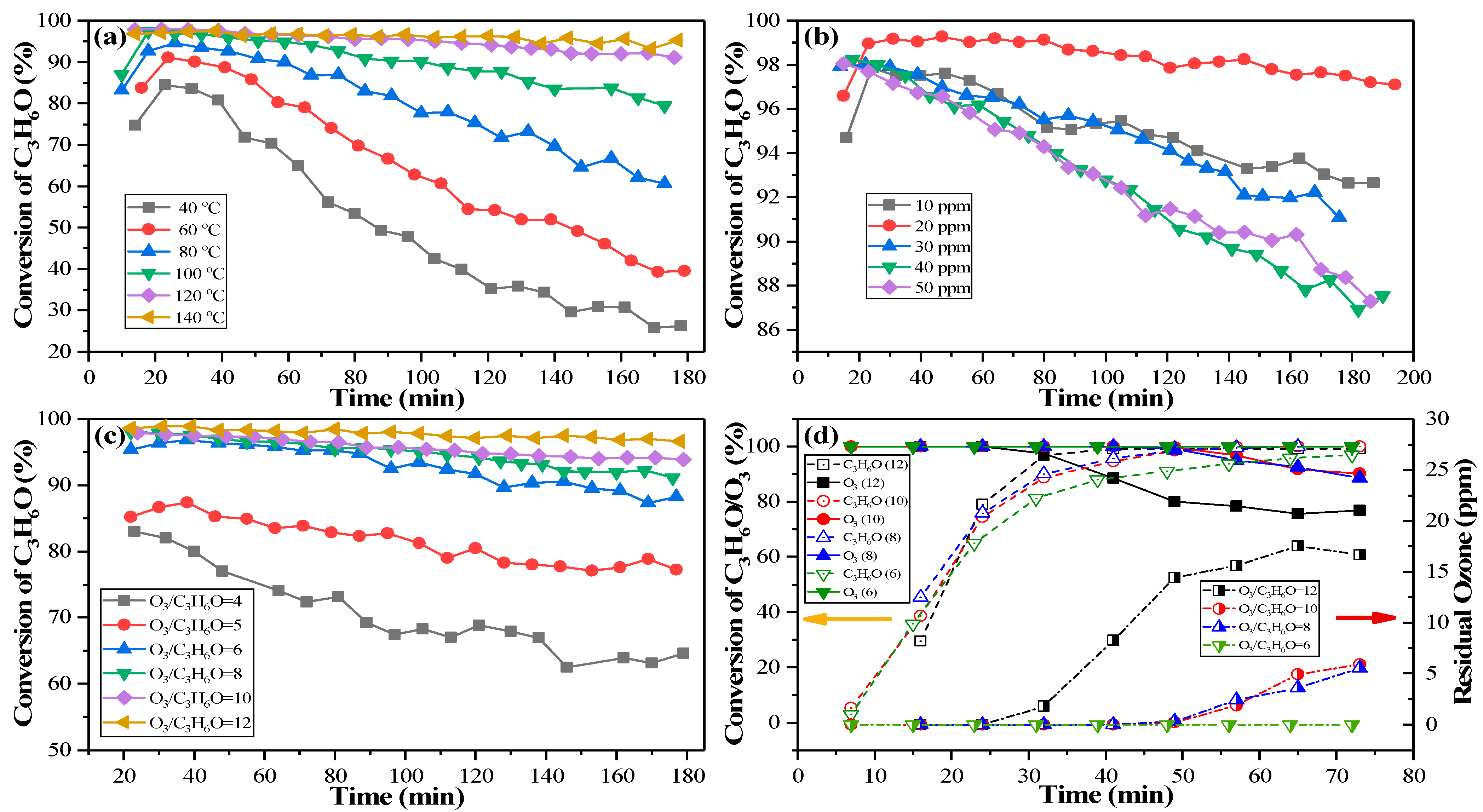
2.3. Effects of Temperature, C3H6O Concentration and O3/C3H6O Molar Ratio
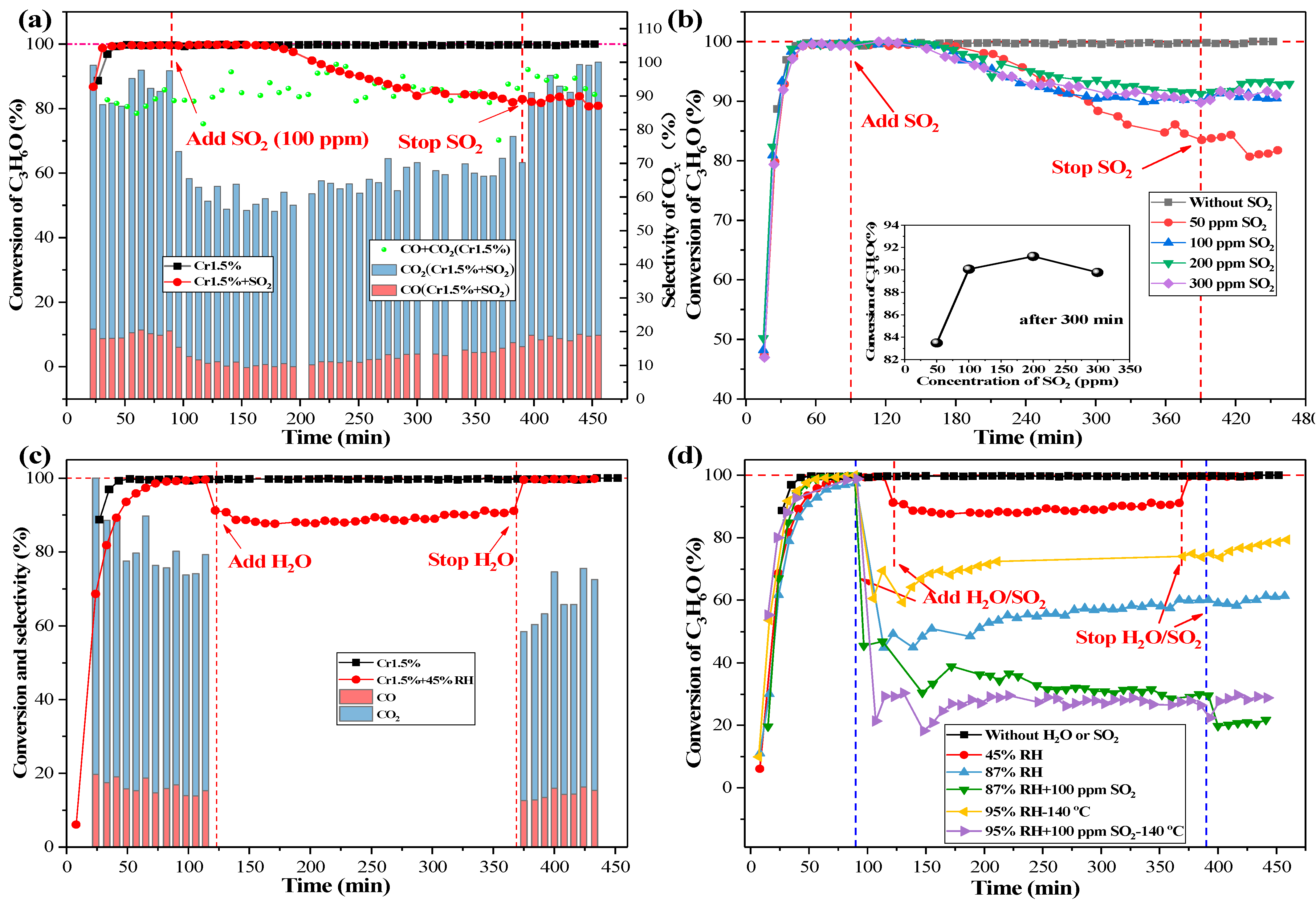
2.4. Catalyst Stability and Effects of SO2/H2O
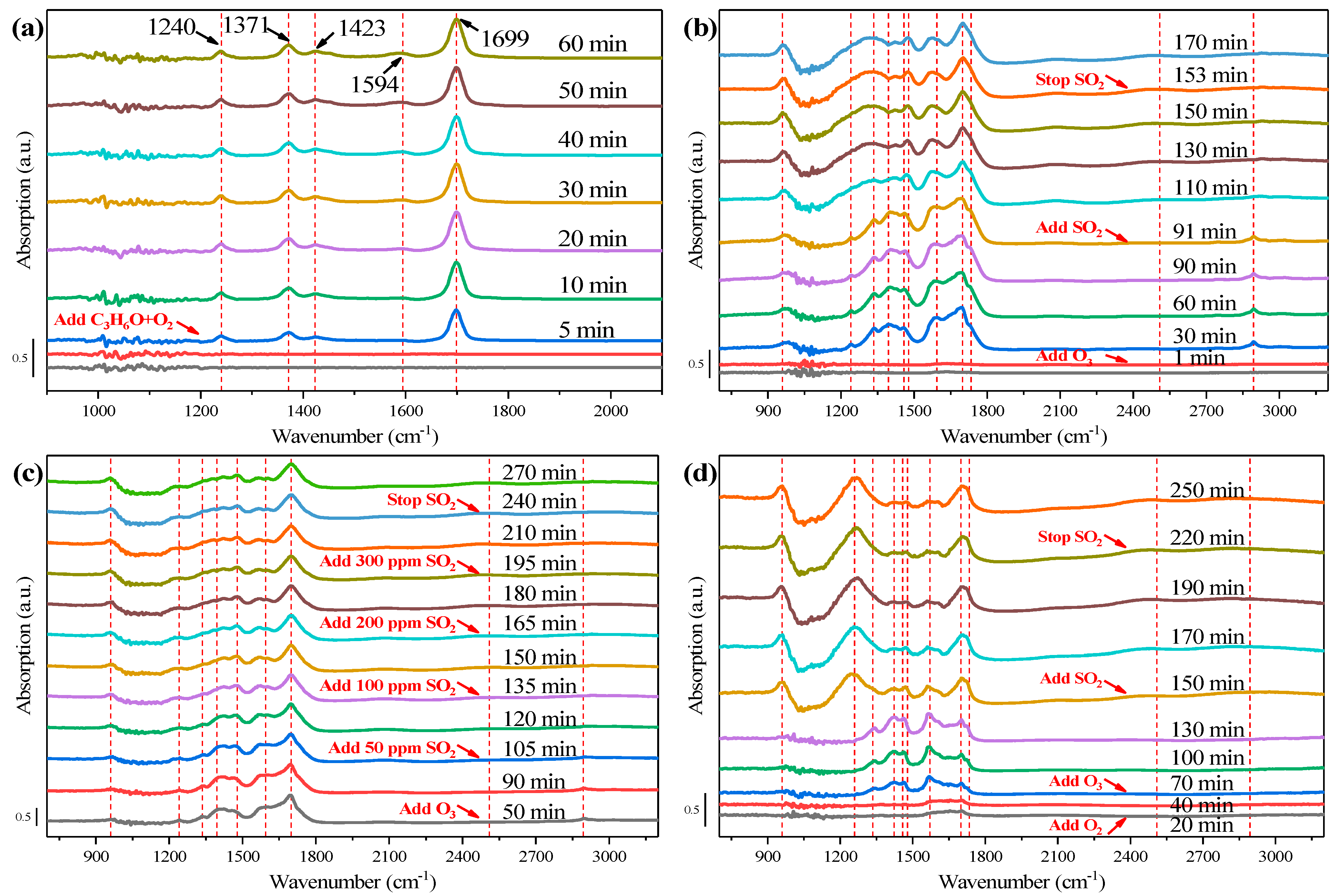
2.5. In-Situ DRIFTS Measurement
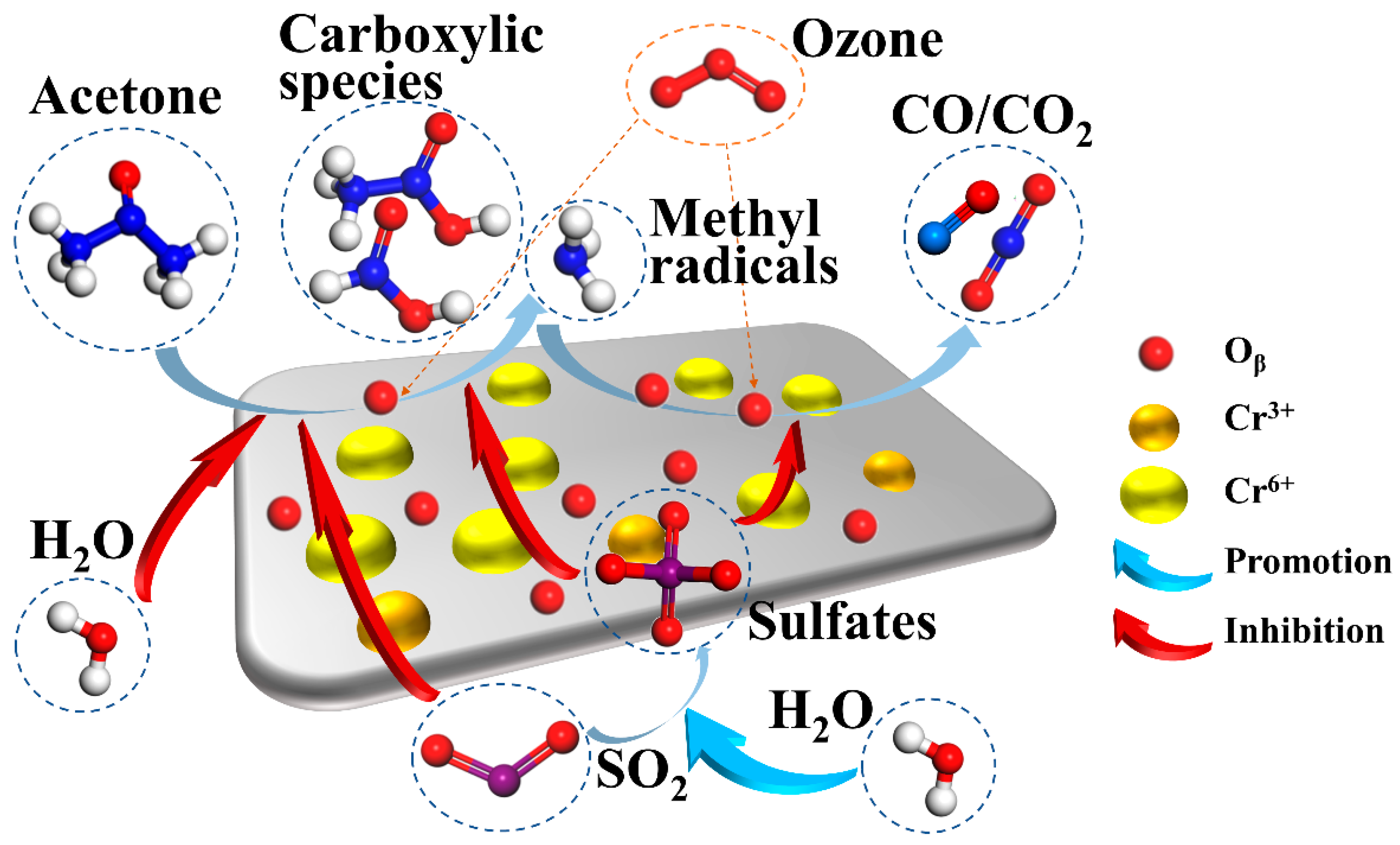
2.6. Mechanisms of Acetone Catalytic Ozonation
3. Experiments and Methods
3.1. Catalyst Preparation
3.2. Catalyst Characterization
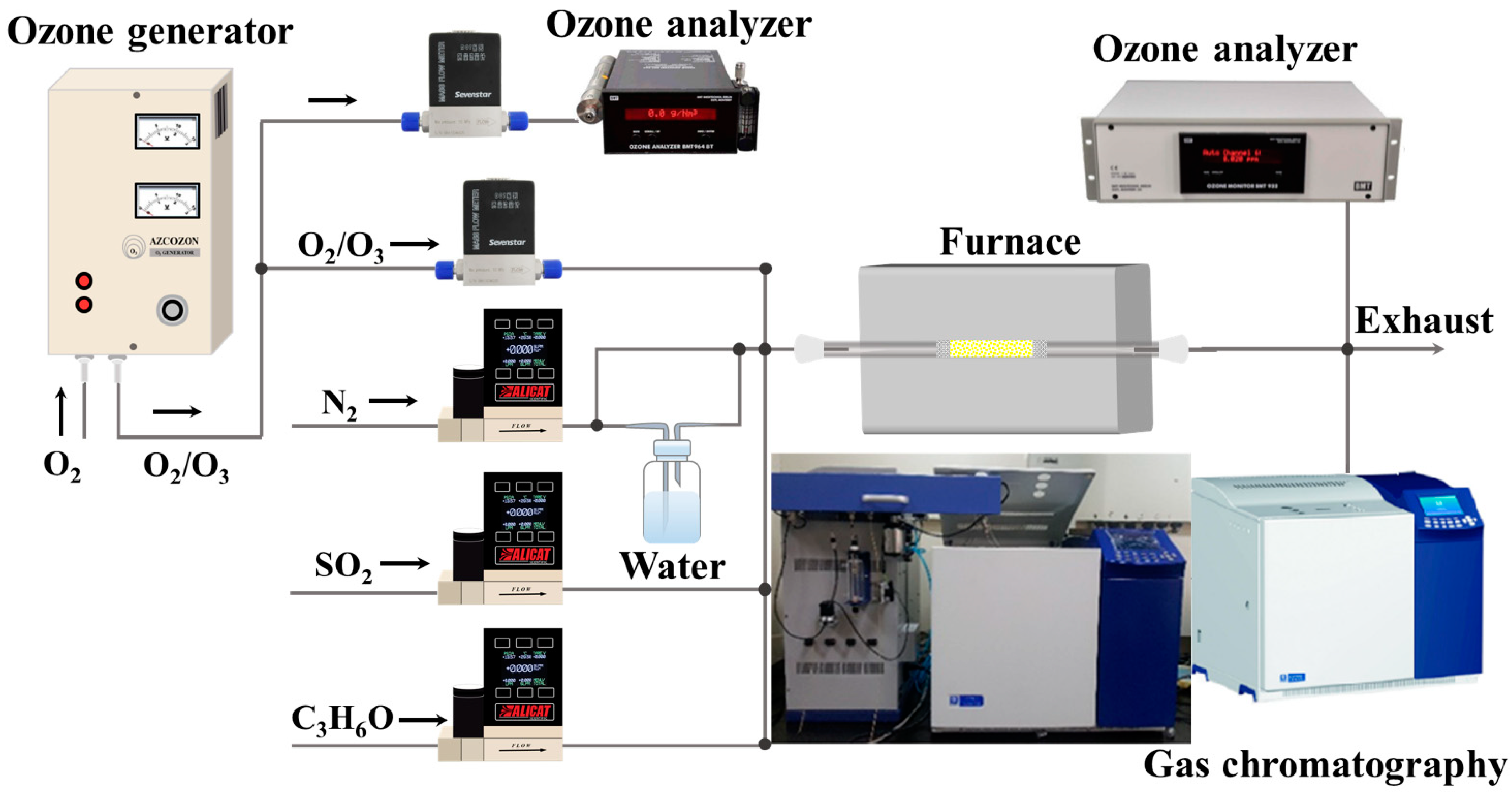
3.3. Experimental Setup and Activity Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ling, Z.H.; Guo, H.; Cheng, H.R.; Yu, Y.F. Sources of ambient volatile organic compounds and their contributions to photochemical ozone formation at a site in the Pearl River Delta, southern China. Environ. Pollut. 2011, 159, 2310–2319. [Google Scholar] [CrossRef]
- Atkinson, R. Atmospheric chemistry of VOCs and NOx. Atmos. Environ. 2000, 34, 2063–2101. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, L.; Liu, S.; Qian, L.; Yu, Z.; Wang, H.; Lei, J.; Gao, Z.; Long, H.; Xu, C.C. Volatile organic compounds (VOC) emissions control in iron ore sintering process: Recent progress and future development. Chem. Eng. J. 2022, 448, 137601. [Google Scholar] [CrossRef]
- Pathak, A.K.; Viphavakit, C. A review on all-optical fiber-based VOC sensors: Heading towards the development of promising technology. Sens. Actuators A Phys. 2022, 338, 113455. [Google Scholar] [CrossRef]
- Wang, C.; Bai, H. Catalytic incineration of acetone on mesoporous silica supported metal oxides prepared by one-step aerosol method. Ind. Eng. Chem. Res. 2011, 50, 3842–3848. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, Q.; Han, J.; Lu, S.; Su, Y.; Song, C.; Ji, N.; Lu, X.; Ma, D.; Cheung, O. The effect of cerium incorporation on the catalytic performance of cobalt and manganese containing layer double oxides for acetone oxidation. J. Chem. Technol. Biotechnol. 2018, 94, 3753–3762. [Google Scholar] [CrossRef]
- Abdelhamid, H.N.; Mathew, A.P. Cellulose–metal organic frameworks (CelloMOFs) hybrid materials and their multifaceted applications: A review. Coord. Chem. Rev. 2022, 451, 214263. [Google Scholar] [CrossRef]
- Ge, Y.; Fu, K.; Zhao, Q.; Ji, N.; Song, C.; Ma, D.; Liu, Q. Performance study of modified Pt catalysts for the complete oxidation of acetone. Chem. Eng. Sci. 2019, 206, 499–506. [Google Scholar] [CrossRef]
- Corey, R. Structure and oxidation state of silica-supported manganese oxide catalysts and reactivity for acetone oxidation with ozone. J. Phys. Chem. B 2006, 110, 4207–4216. [Google Scholar]
- Xi, Y.; Reed, C.; Lee, Y.-K.; Oyama, S.T. Acetone oxidation using ozone on manganese oxide catalysts. J. Phys. Chem. B 2005, 109, 17587–17596. [Google Scholar] [CrossRef]
- Pradier, M.C.; Rodrigues, F.; Marcus, P.; Landau, M.V.; Kaliya, M.L.; Gutman, A.; Herskowitz, M. Supported chromia catalysts for oxidation of organic compounds: The state of chromia phase and catalytic performance. Appl. Catal. B Environ. 2000, 27, 73–85. [Google Scholar] [CrossRef]
- Su, J. Experimental Study on Catalytic Oxidation of DCM over HZSM-5 Supported Transition Metal Oxides; Zhejiang University: Hangzhou, China, 2016. [Google Scholar]
- Wang, Z.; Li, S.; Xie, S.; Liu, Y.; Dai, H.; Guo, G.; Deng, J. Supported ultralow loading Pt catalysts with high H2O-, CO2-, and SO2-resistance for acetone removal. Appl. Catal. A Gen. 2019, 579, 106–115. [Google Scholar] [CrossRef]
- Li, X.; Guo, T.; Peng, Z.; Xu, L.; Dong, J.; Cheng, P.; Zhou, Z. Real-time monitoring and quantification of organic by-products and mechanism study of acetone decomposition in a dielectric barrier discharge reactor. Environ. Sci. Pollut. Res. 2019, 26, 6773–6781. [Google Scholar] [CrossRef]
- Aghbolaghy, M.; Ghavami, M.; Soltan, J.; Chen, N. Effect of active metal loading on catalyst structure and performance in room temperature oxidation of acetone by ozone. J. Ind. Eng. Chem. 2019, 77, 118–127. [Google Scholar] [CrossRef]
- Aghbolaghy, M.; Soltan, J.; Sutarto, R. The role of surface carboxylates in catalytic ozonation of acetone on alumina-supported manganese oxide. Chem. Eng. Res. Des. 2017, 128, 73–84. [Google Scholar] [CrossRef]
- Ghavami, M.; Aghbolaghy, M.; Soltan, J.; Chen, N. Room temperature oxidation of acetone by ozone over alumina-supported manganese and cobalt mixed oxides. Front. Chem. Sci. Eng. 2020, 14, 937–947. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, Y.; Song, X. Review on the catalytic oxidation of volatile organic compounds. Sichuan Chem. Ind. 2019, 22, 55–58. [Google Scholar]
- Shao, J.; Lin, F.; Wang, Z.; Liu, P.; Tang, H.; He, Y.; Cen, K. Low temperature catalytic ozonation of toluene in flue gas over Mn-based catalysts: Effect of support property and SO2/water vapor addition. Appl. Catal. B Environ. 2020, 266, 118662. [Google Scholar] [CrossRef]
- Rezaei, E.; Soltan, J.; Chen, N. Catalytic oxidation of toluene by ozone over alumina supported manganese oxides: Effect of catalyst loading. Appl. Catal. B Environ. 2013, 136–137, 239–247. [Google Scholar] [CrossRef]
- Reed, C.; Xi, Y.; Oyama, S. Distinguishing between reaction intermediates and spectators: A kinetic study of acetone oxidation using ozone on a silica-supported manganese oxide catalyst. J. Catal. 2005, 235, 378–392. [Google Scholar] [CrossRef]
- Konova, P.; Stoyanova, M.; Naydenov, A.; Christoskova, S.; Mehandjiev, D. Catalytic oxidation of VOCs and CO by ozone over alumina supported cobalt oxide. Appl. Catal. A Gen. 2006, 298, 109–114. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Zeng, Y.; Zhang, M.; Zou, H.; Zhong, Q.; Li, Y. Promotional effect of surface fluorine on TiO2: Catalytic conversion of O3 and H2O2 into ·OH and ·O2− radicals for high-efficiency NO oxidation. Chem. Eng. J. 2021, 424, 130358. [Google Scholar] [CrossRef]
- Rochard, G.; Olivet, L.; Tannous, M.; Poupin, C.; Siffert, S.; Cousin, R. Recent advances in the catalytic treatment of volatile organic compounds: A review based on the mixture effect. Catalysts 2021, 11, 1218. [Google Scholar] [CrossRef]
- Salari, D.; Niaei, A.; Seyed, A.H.; Leshzadeh, R.; Afshary, H. Remediation of various naturally oxygenated volatile organic compounds (O-VOCs) by Mn- and Cr-supported g-Al2O3 nanocatalysts. Turk. J. Chem. 2011, 35, 793–802. [Google Scholar]
- Yang, H.H.; Du, J.; Wu, M.; Zhou, H.; Yi, X.; Zhan, J.; Liu, Y. Tin-modified α-MnO2 catalyst with high performance for benzene oxidation, ozone decomposition and particulate matter filtration. Chem. Eng. J. 2022, 427, 132075. [Google Scholar] [CrossRef]
- Shao, J.; Lin, F.; Li, Y.; Tang, H.; Wang, Z.; Liu, P.; Chen, G. Co-precipitation synthesized MnOx-CeO2 mixed oxides for NO oxidation and enhanced resistance to low concentration of SO2 by metal addition. Catalysts 2019, 9, 519. [Google Scholar] [CrossRef]
- KSing, S.W.; Everett, D.H.; Haul, R.A.W.; Moscou, L.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar]
- Lin, F.; Jiang, X.; Boreriboon, N.; Wang, Z.; Song, C.; Cen, K. Effects of supports on bimetallic Pd-Cu catalysts for CO2 hydrogenation to methanol. Appl. Catal. A Gen. 2019, 585, 117210. [Google Scholar] [CrossRef]
- Tan, J.; He, Y.; Yuan, Y.; Wang, Z.; Liu, J.; Cen, K. Structure and combustion characteristics of semi-cokes from a pilot-scale entrained flow gasifier using oxygen-enriched air. J. Energy Inst. 2021, 97, 80–91. [Google Scholar] [CrossRef]
- Li, X.; Cao, J.; Zhang, W. Stoichiometry of Cr(VI) immobilization using nanoscale zerovalent iron (NZVI): A study with high-resolution X-ray photoelectron spectroscopy (HR-XPS). Ind. Eng. Chem. Res. 2008, 47, 2131–2139. [Google Scholar] [CrossRef]
- Yang, P.; Shi, Z.; Tao, F.; Yang, S.; Zhou, R. Synergistic performance between oxidizability and acidity/texture properties for 1,2-dichloroethane oxidation over (Ce,Cr)xO2/zeolite catalysts. Chem. Eng. Sci. 2015, 134, 340–347. [Google Scholar] [CrossRef]
- Lee, D.K.; Yoon, W.L. Ru-promoted CrOx/Al2O3 catalyst for the low-temperature oxidative decomposition of trichloroethylene in air. Catal. Lett. 2002, 81, 3–4. [Google Scholar] [CrossRef]
- Ma, R.; Hu, P.; Jin, L.; Wang, Y.; Lu, J.; Luo, M. Characterization of CrOx/Al2O3 catalysts for dichloromethane oxidation. Catal. Today 2011, 175, 598–602. [Google Scholar] [CrossRef]
- Hardcastle, F.D.; Wachs, I.E. Raman spectroscopy of chromium oxide supported on Al2O3, TiO2 and SiO2: A comparative study. J. Mol. Catal. 1988, 46, 173–186. [Google Scholar] [CrossRef]
- Asami, K.; Hashimoto, K. An XPS study of the surfaces on Fe-Cr, Fe-Co and Fe-Ni alloys after mechanical polishing. Corros. Sci. 1984, 24, 83–97. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, Y.; Yang, T.; Deng, J.; Xie, S.; Dai, H. Catalytic performance of cobalt oxide-supported gold-palladium nanocatalysts for the removal of toluene and o-xylene. Chin. J. Catal. 2017, 38, 207–216. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Zhang, T.; Luo, Y.; Lan, Z.; Zhang, K.; Zuo, J.; Jiang, L.; Wang, R. Geometrical-site-dependent catalytic activity of ordered mesoporous Co-based spinel for benzene oxidation: In situ DRIFTS study coupled with Raman and XAFS spectroscopy. ACS Catal. 2017, 7, 1626–1636. [Google Scholar] [CrossRef]
- Sun, M.; Li, W.; Zhang, B.; Cheng, G.; Lan, B.; Ye, F.; Zheng, Y.; Cheng, X.; Yu, L. Enhanced catalytic performance by oxygen vacancy and active interface originated from facile reduction of OMS-2. Chem. Eng. J. 2018, 331, 626–635. [Google Scholar] [CrossRef]
- Zhu, G.; Zhu, J.; Jiang, W.; Zhang, Z.; Wang, J.; Zhu, Y.; Zhang, Q. Surface oxygen vacancy induced α-MnO2 nanofiber for highly efficient ozone elimination. Appl. Catal. B Environ. 2017, 209, 729–737. [Google Scholar] [CrossRef]
- Lin, F.; Shao, J.; Tang, H.; Li, Y.; Wang, Z.; Chen, G.; Yuan, D.; Cen, K. Enhancement of NO oxidation activity and SO2 resistance over LaMnO3+δ perovskites catalysts with metal substitution and acid treatment. Appl. Surf. Sci. 2019, 479, 234–246. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, X.; Li, N.; Xing, X.; Yang, H.; Zhang, F.; Cheng, J.; Zhang, Z.; Hao, Z. Surface properties enhanced MnxAlO oxide catalysts derived from MnxAl layered double hydroxides for acetone catalytic oxidation at low temperature. Appl. Catal. B Environ. 2019, 251, 295–304. [Google Scholar] [CrossRef]
- Long, L.; Zhao, J.; Yang, L.; Fu, M.; Wu, J.; Huang, B.; Ye, D. Room temperature catalytic ozonation of toluene over MnO2/Al2O3. Chin. J. Catal. 2011, 32, 904–916. [Google Scholar] [CrossRef]
- Aghbolaghy, M.; Soltan, J.; Chen, N. Low temperature catalytic oxidation of binary mixture of toluene and acetone in the presence of ozone. Catal. Lett. 2018, 148, 3431–3444. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiang, L.; Lin, F.; Wang, Z.; Yan, B.; Chen, G. Catalytic deep degradation of Cl-VOCs with the assistance of ozone at low temperature over MnO2 catalysts. Chem. Eng. J. 2021, 426, 130814. [Google Scholar] [CrossRef]

| Catalysts | Measurement Conditions | Acetone Conversion | CO2 Selectivity |
|---|---|---|---|
| Cr1.5%/γ-Al2O3 a | 30 ppm acetone, 240 ppm O3, 5% O2, 240,000 mL/(g·h), 120 °C | 90% | 60% |
| Fe1.5%/γ-Al2O3 a | 30 ppm acetone, 240 ppm O3, 5% O2, 240,000 mL/(g·h), 120 °C | 90% | 55% |
| 10 wt.% MnOx/SiO2 [10] | 1000 ppm acetone, 5000 ppm O3, 15,000 h−1, 177 °C | 60% | / |
| 10 wt.% MnOx/Al2O3 [10] | 1000 ppm acetone, 5000 ppm O3, 15,000 h−1, 177 °C | 60% | / |
| Co2.5% [15] | 150 ppm acetone, 1200 ppm O3, 230,000 mL/(g·h), 25 °C | 85% | 90% (COx yield) |
| Co5% [15] | 150 ppm acetone, 1200 ppm O3, 230,000 mL/(g·h), 25 °C | 66% | 84% (COx yield) |
| Co10% [15] | 150 ppm acetone, 1200 ppm O3, 230,000 mL/(g·h), 25 °C | 53% | 85% (COx yield) |
| MnOx/γ-Al2O3 [16] | 144 ppm acetone, 1150 ppm O3, 250,000 mL/(g·h), 25 °C | 54% | 34% |
| MnOx/γ-Al2O3 [16] | 144 ppm acetone, 1150 ppm O3, 250,000 mL/(g·h), 90 °C | 87% | 56% |
| Mn10%-Co2.5% [17] | 150 ppm acetone, 1200 ppm O3, 230,769 mL/(g·h), 25 °C | 55% | / |
| MnAl-LDO [6] | 1000 ppm acetone, 20% O2, 33,000 mL/(g·h) | T50% = 170 °C; T90% = 200 °C | / |
| Catalysts | Metal Phase | 2θ (440) (°) | FWHM | Size (nm) | Lattice Parameters (nm) |
|---|---|---|---|---|---|
| Al2O3 | Al2O3 | 66.96 | 1.644 | 5.8 | 0.7902 |
| Fe1.5% | Al2O3 | 66.99 | 1.849 | 5.2 | 0.7895 |
| Mn1.5% | Al2O3 | 67.03 | 1.822 | 5.2 | 0.7891 |
| Co1.5% | Al2O3 | 66.96 | 1.783 | 5.4 | 0.7901 |
| Cr1.5% | Al2O3 | 67.07 | 1.759 | 5.4 | 0.7888 |
| Cr2.5% | Al2O3 | 67.02 | 1.974 | 4.8 | 0.7892 |
| Cr5% | Al2O3 | 67.01 | 1.760 | 5.4 | 0.7896 |
| Catalysts | Metal Phase | 2θ (012) (°) | FWHM | Size (nm) |
|---|---|---|---|---|
| Cr1.5% | Cr2O3 | 25.47 | 1.514 | 5.4 |
| Cr2.5% | Cr2O3 | 25.27 | 1.199 | 6.8 |
| Cr5% | Cr2O3 | 25.25 | 0.692 | 11.9 |
| Catalysts | Surface Area a (m2/g) | Pore Volume b (cm3/g) | Average Pore Diameter c (nm) |
|---|---|---|---|
| Al2O3 | 230 | 0.473 | 7.5 |
| Fe1.5% | 232 | 0.480 | 7.7 |
| Mn1.5% | 222 | 0.474 | 8.1 |
| Co1.5% | 200 | 0.427 | 8.0 |
| Cr1.5% | 234 | 0.473 | 7.5 |
| Cr2.5% | 210 | 0.406 | 8.1 |
| Cr5% | 199 | 0.377 | 8.1 |
| Catalysts | Cr Distribution | O Distribution | Oβ/Oα | |||
|---|---|---|---|---|---|---|
| Cr6+/Cr | Cr3+/Cr | Oα | Oβ | Oγ | ||
| Cr1.5% | 0.64 | 0.36 | 0.15 | 0.61 | 0.24 | 4.07 |
| Cr2.5% | 0.66 | 0.34 | 0.18 | 0.53 | 0.29 | 2.94 |
| Cr5% | 0.60 | 0.40 | 0.42 | 0.35 | 0.23 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, P.; Chen, L.; Tang, H.; Shao, J.; Lin, F.; He, Y.; Zhu, Y.; Wang, Z. Low Temperature Ozonation of Acetone by Transition Metals Derived Catalysts: Activity and Sulfur/Water Resistance. Catalysts 2022, 12, 1090. https://doi.org/10.3390/catal12101090
Liu P, Chen L, Tang H, Shao J, Lin F, He Y, Zhu Y, Wang Z. Low Temperature Ozonation of Acetone by Transition Metals Derived Catalysts: Activity and Sulfur/Water Resistance. Catalysts. 2022; 12(10):1090. https://doi.org/10.3390/catal12101090
Chicago/Turabian StyleLiu, Peixi, Lichun Chen, Hairong Tang, Jiaming Shao, Fawei Lin, Yong He, Yanqun Zhu, and Zhihua Wang. 2022. "Low Temperature Ozonation of Acetone by Transition Metals Derived Catalysts: Activity and Sulfur/Water Resistance" Catalysts 12, no. 10: 1090. https://doi.org/10.3390/catal12101090
APA StyleLiu, P., Chen, L., Tang, H., Shao, J., Lin, F., He, Y., Zhu, Y., & Wang, Z. (2022). Low Temperature Ozonation of Acetone by Transition Metals Derived Catalysts: Activity and Sulfur/Water Resistance. Catalysts, 12(10), 1090. https://doi.org/10.3390/catal12101090