Controlled Synthesis of Chromium-Oxide-Based Protective Layers on Pt: Influence of Layer Thickness on Selectivity
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef] [PubMed]
- Pinaud, B.A.; Benck, J.D.; Seitz, L.C.; Forman, A.J.; Chen, Z.; Deutsch, T.G.; James, B.D.; Baum, K.N.; Baum, G.N.; Ardo, S.; et al. Technical and economic feasibility of centralized facilities for solar hydrogen production via photocatalysis and photoelectrochemistry. Energy Environ. Sci. 2013, 6, 1983. [Google Scholar] [CrossRef]
- Zini, G.; Tartarini, P. Solar-Hydrogen Energy Systems; Elsevier: Amsterdam, The Netherlands, 1979; pp. 25–33. [Google Scholar]
- Rajeshwar, K. Fundamentals of Semiconductor Electrochemistry and Photoelectrochemistry; American Cancer Society: Atlanta, GA, USA, 2007. [Google Scholar]
- Bard, A.J.; Stratmann, M. (Eds.) Encyclopedia of Electrochemistry: Online, 1st ed.; Wiley: Hoboken, NJ, USA, 2007. [Google Scholar]
- Kudo, A. Photocatalysis and solar hydrogen production. Pure Appl. Chem. 2007, 79, 1917–1927. [Google Scholar] [CrossRef]
- Mei, B.; Mul, G.; Seger, B. Beyond water splitting: Efficiencies of photo-electrochemical devices producing hydrogen and valuable oxidation products. Adv. Sustain. Syst. 2017, 1, 1600035. [Google Scholar] [CrossRef]
- Yang, J.; Wang, D.; Han, H.; Li, C. Roles of cocatalysts in photocatalysis and photoelectrocatalysis. Acc. Chem. Res. 2013, 46, 1900–1909. [Google Scholar] [CrossRef]
- Li, X.; Hao, X.; Abudula, A.; Guan, G. Nanostructured catalysts for electrochemical water splitting: Current state and prospects. J. Mater. Chem. A 2016, 4, 11973–12000. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniec, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787–7812. [Google Scholar] [CrossRef]
- Takanabe, K.; Domen, K. Toward Visible Light Response: Overall Water Splitting Using Heterogeneous Photocatalysts. Green 2011, 1, 313–322. [Google Scholar] [CrossRef]
- Juodkazyte, J.; Seniutinas, G.; Sebeka, B.; Savickaja, I.; Malinauskas, T.; Badokas, K.; Juodkazis, K.; Juodkazis, S. Solar water splitting: Efficiency discussion. Int. J. Hydrogen Energy 2016, 41, 11941–11948. [Google Scholar] [CrossRef]
- Qureshi, M.; Garcia-Esparza, A.T.; Jeantelot, G.; Ould-Chikh, S.; Aguilar-Tapia, A.; Hazemann, J.-L.; Basset, J.-M.; Loffreda, D.; le Bahers, T.; Takanabe, K. Catalytic consequences of ultrafine Pt clusters supported on SrTiO3 for photocatalytic overall water splitting. J. Catal. 2019, 376, 180–190. [Google Scholar] [CrossRef]
- Han, K.; Kreuger, T.; Mei, B.; Mul, G. Transient Behavior of Ni@NiOx Functionalized SrTiO3 in Overall Water Splitting. ACS Catal. 2017, 7, 1610–1614. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Q.; Aoki, T.; Crozier, P.A. Structural evolution during photocorrosion of Ni/NiO core/shell cocatalyst on TiO2. J. Phys. Chem. C 2015, 119, 7207–7214. [Google Scholar] [CrossRef]
- Garcia-Esparza, A.T.; Shinagawa, T.; Ould-Chikh, S.; Qureshi, M.; Peng, X.; Wei, N.; Anjum, D.H.; Clo, A.; Weng, T.-C.; Nordlund, D.; et al. An Oxygen-Insensitive Hydrogen Evolution Catalyst Coated by a Molybdenum-Based Layer for Overall Water Splitting. Angew. Chem. Int. Ed. 2017, 56, 5780–5784. [Google Scholar] [CrossRef]
- Gustavsson, J.; Li, G.; Hummelgard, C.; Backstrom, J.; Cornell, A. On the suppression of cathodic hypochlorite reduction by electrolyte additions of molybdate and chromate ions. J. Electrochem. Sci. Eng. 2012, 2, 185–198. [Google Scholar] [CrossRef]
- Busser, G.W.; Mei, B.; Weide, P.; Vesborg, P.C.K.; Stührenberg, K.; Bauer, M.; Huang, X.; Willinger, M.-G.; Chorkendorff, I.; Schlögl, R.; et al. Cocatalyst designing: A regenerable molybdenum-containing ternary cocatalyst system for efficient photocatalytic water splitting. ACS Catal. 2015, 5, 5530–5539. [Google Scholar] [CrossRef]
- Endrődi, B.; Diaz-Morales, O.; Mattinen, U.; Cuartero, M.; Padinjarethil, A.K.; Simic, N.; Wildlock, M.; Crespo, G.A.; Cornell, A. Selective electrochemical hydrogen evolution on cerium oxide protected catalyst surfaces. Electrochim. Acta 2020, 341, 136022. [Google Scholar] [CrossRef]
- Bhardwaj, A.A.; Vos, J.G.; Beatty, M.E.S.; Baxter, A.F.; Koper, M.T.M.; Yip, N.Y.; Esposito, D.V. Ultrathin silicon oxide overlayers enable selective oxygen evolution from acidic and unbuffered pH-neutral seawater. ACS Catal. 2021, 11, 1316–1330. [Google Scholar] [CrossRef]
- Takata, T.; Pan, C.; Nakabayashi, M.; Shibata, N.; Domen, K. Fabrication of a core–shell-type photocatalyst via photodeposition of group IV and V transition metal oxyhydroxides: An effective surface modification method for overall water splitting. J. Am. Chem. Soc. 2015, 137, 9627–9634. [Google Scholar] [CrossRef]
- Yoshida, M.; Maeda, K.; Lu, D.; Kubota, J.; Domen, K. Lanthanoid Oxide Layers on Rhodium-Loaded (Ga1−xZnx)(N1−xOx) Photocatalyst as a Modifier for Overall Water Splitting under Visible-Light Irradiation. J. Phys. Chem. C 2013, 117, 14000–14006. [Google Scholar] [CrossRef]
- Gomes, A.S.O.; Busch, M.; Wildlock, M.; Simic, N.; Ahlberg, E. Understanding Selectivity in the Chlorate Process: A Step towards Efficient Hydrogen Production. ChemistrySelect 2018, 3, 6683–6690. [Google Scholar] [CrossRef]
- Gustavsson, J.; Nylén, L.; Cornell, A. Rare earth metal salts as potential alternatives to Cr (VI) in the chlorate process. J. Appl. Electrochem. 2010, 40, 1529–1536. [Google Scholar] [CrossRef]
- Hedenstedt, K.; Gomes, A.S.O.; Busch, M.; Ahlberg, E. Study of hypochlorite reduction related to the sodium chlorate process. Electrocatalysis 2016, 7, 326–335. [Google Scholar] [CrossRef]
- Tidblad, A.A.; Lindbergh, G. Surface analysis with ESCA and GD-OES of the film formed by cathodic reduction of chromate. Electrochim. Acta 1991, 36, 1605–1610. [Google Scholar] [CrossRef]
- Cornell, A.; Lindbergh, G.; Simonsson, D. The effect of addition of chromate on the hydrogen evolution reaction and on iron oxidation in hydroxide and chlorate solutions. Electrochim. Acta 1992, 37, 1873–1881. [Google Scholar] [CrossRef]
- Maeda, K.; Teramura, K.; Lu, D.; Saito, N.; Inoue, Y.; Domen, K. Noble-metal/Cr(2)O(3) core/shell nanoparticles as a cocatalyst for photocatalytic overall water splitting. Angew. Chem. Int. Ed. 2006, 45, 7806–7809. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Teramura, K.; Lu, D.; Saito, N.; Inoue, Y.; Domen, K. Roles of Rh/Cr2O3 (Core/Shell) Nanoparticles Photodeposited on Visible-Light-Responsive (Ga1−xZnx)(N1−xOx) Solid Solutions in Photocatalytic Overall Water Splitting. J. Phys. Chem. C 2007, 111, 7554–7560. [Google Scholar] [CrossRef]
- Dionigi, F.; Vesborg, P.C.; Pedersen, T.; Hansen, O.; Dahl, S.; Xiong, A.; Maeda, K.; Domen, K.; Chorkendorff, I. Suppression of the water splitting back reaction on GaN: ZnO photocatalysts loaded with core/shell cocatalysts, investigated using a μ-reactor. J. Catal. 2012, 292, 26–31. [Google Scholar] [CrossRef]
- Busser, G.W.; Mei, B.; Pougin, A.; Strunk, J.; Gutkowski, R.; Schuhmann, W.; Willinger, M.-G.; Schlögl, R.; Muhler, M. Photodeposition of Copper and Chromia on Gallium Oxide: The Role of Co-Catalysts in Photocatalytic Water Splitting. ChemSusChem 2014, 7, 1030–1034. [Google Scholar] [CrossRef]
- Chiang, T.H.; Lyu, H.; Hisatomi, T.; Goto, Y.; Takata, T.; Katayama, M.; Minegishi, T.; Domen, K. Efficient photocatalytic water splitting using Al-doped SrTiO3 coloaded with molybdenum oxide and rhodium–chromium oxide. ACS Catal. 2018, 8, 2782–2788. [Google Scholar] [CrossRef]
- Godin, R.; Hisatomi, T.; Domen, K.; Durrant, J.R. Understanding the visible-light photocatalytic activity of GaN:ZnO solid solution: The role of Rh2−yCryO3 cocatalyst and charge carrier lifetimes over tens of seconds. Chem. Sci. 2018, 9, 7546–7555. [Google Scholar] [CrossRef]
- Kanazawa, T.; Maeda, K. Light-induced synthesis of heterojunctioned nanoparticles on a semiconductor as durable cocatalysts for hydrogen evolution. ACS Appl. Mater. Interfaces 2016, 8, 7165–7172. [Google Scholar] [CrossRef]
- European Chemicals Agency (ECHA). Authorization List (Annex XIV of REACH). Available online: https://echa.europa.eu/authorisation-list (accessed on 4 January 2022).
- Yoshida, M.; Takanabe, K.; Maeda, K.; Ishikawa, A.; Kubota, J.; Sakata, Y.; Ikezawa, Y.; Domen, K. Role and function of noble-metal/Cr-layer core/shell structure cocatalysts for photocatalytic overall water splitting studied by model electrodes. J. Phys. Chem. C 2009, 113, 10151–10157. [Google Scholar] [CrossRef]
- Lindbergh, G.; Simonsson, D. Inhibition of cathode reactions in sodium hydroxide solution containing chromate. Electrochim. Acta 1991, 36, 1985–1994. [Google Scholar] [CrossRef]
- Qureshi, M.; Shinagawa, T.; Tsiapis, N.; Takanabe, K. Exclusive Hydrogen Generation by Electrocatalysts Coated with an Amorphous Chromium-Based Layer Achieving Efficient Overall Water Splitting. ACS Sustain. Chem. Eng. 2017, 5, 8079–8088. [Google Scholar] [CrossRef]
- Robinson, J.E.; Labrador, N.Y.; Chen, H.; Sartor, B.E.; Esposito, D.V. Silicon oxide-encapsulated platinum thin films as highly active electrocatalysts for carbon monoxide and methanol oxidation. ACS Catal. 2018, 8, 11423–11434. [Google Scholar] [CrossRef]
- Smulders, V.; Simic, N.; Gomes, A.S.; Mei, B.; Mul, G. Electrochemical formation of Cr (III)-based films on Au electrodes. Electrochim. Acta 2019, 296, 1115–1121. [Google Scholar] [CrossRef]
- Aguilar, M.; Barrera, E.; Palomar-Pardavé, M.; Huerta, L.; Muhl, S. Characterization of black and white chromium electrodeposition films: Surface and optical properties. J. Non Cryst. Solids 2003, 329, 31–38. [Google Scholar] [CrossRef]
- Aguilar-Sánchez, M.; Palomar-Pardavé, M.; Romero-Romo, M.; Ramírez-Silva, M.; Barrera, E.; Scharifker, B. Electrochemical nucleation and growth of black and white chromium deposits onto stainless steel surfaces. J. Electroanal. Chem. 2010, 647, 128–132. [Google Scholar] [CrossRef]
- Endrodi, B.; Simic, N.; Wildlock, M.; Cornell, A. A review of chromium (VI) use in chlorate electrolysis: Functions, challenges and suggested alternatives. Electrochim. Acta 2017, 234, 108–122. [Google Scholar] [CrossRef]
- Tidblad, A.A.; Mirtensson, J. In situ ellipsometric characterization of films formed by cathodic reduction of chromate. Electrochim. Acta 1997, 42, 389–398. [Google Scholar] [CrossRef]
- Kita, H. Periodic variation of exchange current density of hydrogen electrode reaction with atomic number and reaction mechanism. J. Electrochem. Soc. 1966, 113, 1095. [Google Scholar] [CrossRef]
- Moffat, T.P.; Yang, H.; Fan, F.-R.F.; Bard, A.J. Electron-Transfer Reactions on Passive Chromium. J. Electrochem. Soc. 1992, 139, 3158–3167. [Google Scholar] [CrossRef]
- Jo, W.J.; Katsoukis, G.; Frei, H. Ultrathin Amorphous Silica Membrane Enhances Proton Transfer across Solid-to-Solid Interfaces of Stacked Metal Oxide Nanolayers while Blocking Oxygen. Adv. Funct. Mater. 2020, 30, 1909262. [Google Scholar] [CrossRef]
- Gough, D.A.; Leypoldt, J.K. Membrane-covered, rotated disk electrode. Anal. Chem. 1979, 51, 439–444. [Google Scholar] [CrossRef]
- Ogumi, Z.; Takehara, Z.; Yoshizawa, S. Gas permeation in SPE method: I. Oxygen permeation through Nafion and NEOSEPTA. J. Electrochem. Soc. 1984, 131, 769–773. [Google Scholar] [CrossRef]
- Watanabe, M.; Igarashi, H.; Yosioka, K. An experimental prediction of the preparation condition of Nafion-coated catalyst layers for PEFCs. Electrochim. Acta 1995, 40, 329–334. [Google Scholar] [CrossRef]
- Mello, R.M.; Ticianelli, E.A. Kinetic study of the hydrogen oxidation reaction on platinum and Nafion® covered platinum electrodes. Electrochim. Acta 1997, 42, 1031–1039. [Google Scholar] [CrossRef]
- Seo, T.; Kurokawa, R.; Sato, B. A convenient method for determining the concentration of hydrogen in water: Use of methylene blue with colloidal platinum. Med. Gas Res. 2012, 2, 1. [Google Scholar] [CrossRef]
- Cuesta, A.; Couto, A.; Rincón, A.; Pérez, M.; López-Cudero, A.; Gutiérrez, C. Potential dependence of the saturation CO coverage of Pt electrodes: The origin of the pre-peak in CO-stripping voltammograms. Part 3: Pt(poly). J. Electroanal. Chem. 2006, 586, 184–195. [Google Scholar] [CrossRef]
- Łukaszewski, M.; Soszko, M.; Czerwinski, A. Electrochemical methods of real surface area determination of noble metal electrodes—An overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Cuesta, A.; Gutiérrez, C. Catalysis in Electrochemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 339–373. [Google Scholar]
- Hanawa, H.; Kunimatsu, K.; Uchida, H.; Watanabe, M. In situ ATR-FTIR study of bulk CO oxidation on a polycrystalline Pt electrode. Electrochim. Acta 2009, 54, 6276–6285. [Google Scholar] [CrossRef]
- McPherson, I.J.; Ash, P.A.; Jones, L.; Varambhia, A.; Jacobs, R.M.J.; Vincent, K.A. Electrochemical CO Oxidation at Platinum on Carbon Studied through Analysis of Anomalous in Situ IR Spectra. J. Phys. Chem. C 2017, 121, 17176–17187. [Google Scholar] [CrossRef] [PubMed]
- Spiccia, L.; Marty, W. The fate of “active” chromium hydroxide, Cr(OH)3·3H2O, in aqueous suspension. Study of the chemical changes involved in its aging. Inorg. Chem. 1986, 25, 266–271. [Google Scholar] [CrossRef]
- Spiccia, L.; Stoeckli-Evans, H.; Marty, W.; Giovanoli, R. A new “active” chromium(III) hydroxide: Cr2(.mu.-OH)2(OH)4(OH2)4·2H2O. Characterization and use in the preparation of salts of the (H2O)4Cr(.mu.-OH)2Cr(OH2)44+ ion. Crystal structure of [(H2O)4Cr(.mu.-OH)2Cr(OH2)4][(H3C)3C6H2SO3]4·4H2O. Inorg. Chem. 1987, 26, 474–482. [Google Scholar] [CrossRef]
- Spiccia, L.; Marty, W.; Giovanoli, R. Hydrolytic trimer of chromium (III). Synthesis through chromite cleavage and use in the preparation of the “active” trimer hydroxide. Inorg. Chem. 1988, 27, 2660–2666. [Google Scholar] [CrossRef]
- Torapava, N.; Radkevich, A.; Davydov, D.; Titov, A.; Persson, I. Composition and structure of polynuclear chromium (III) hydroxo complexes. Inorg. Chem. 2009, 48, 10383–10388. [Google Scholar] [CrossRef]
- Stuenzi, H.; Spiccia, L.; Rotzinger, F.P.; Marty, W. Early stages of the hydrolysis of chromium(III) in aqueous solution. 4. The stability constants of the hydrolytic dimer, trimer, and tetramer at 25.degree.C and I = 1.0 M. Inorg. Chem. 1989, 28, 66–71. [Google Scholar] [CrossRef]
- Kankare, J. Sauerbrey equation of quartz crystal microbalance in liquid medium. Langmuir 2002, 18, 7092–7094. [Google Scholar] [CrossRef]
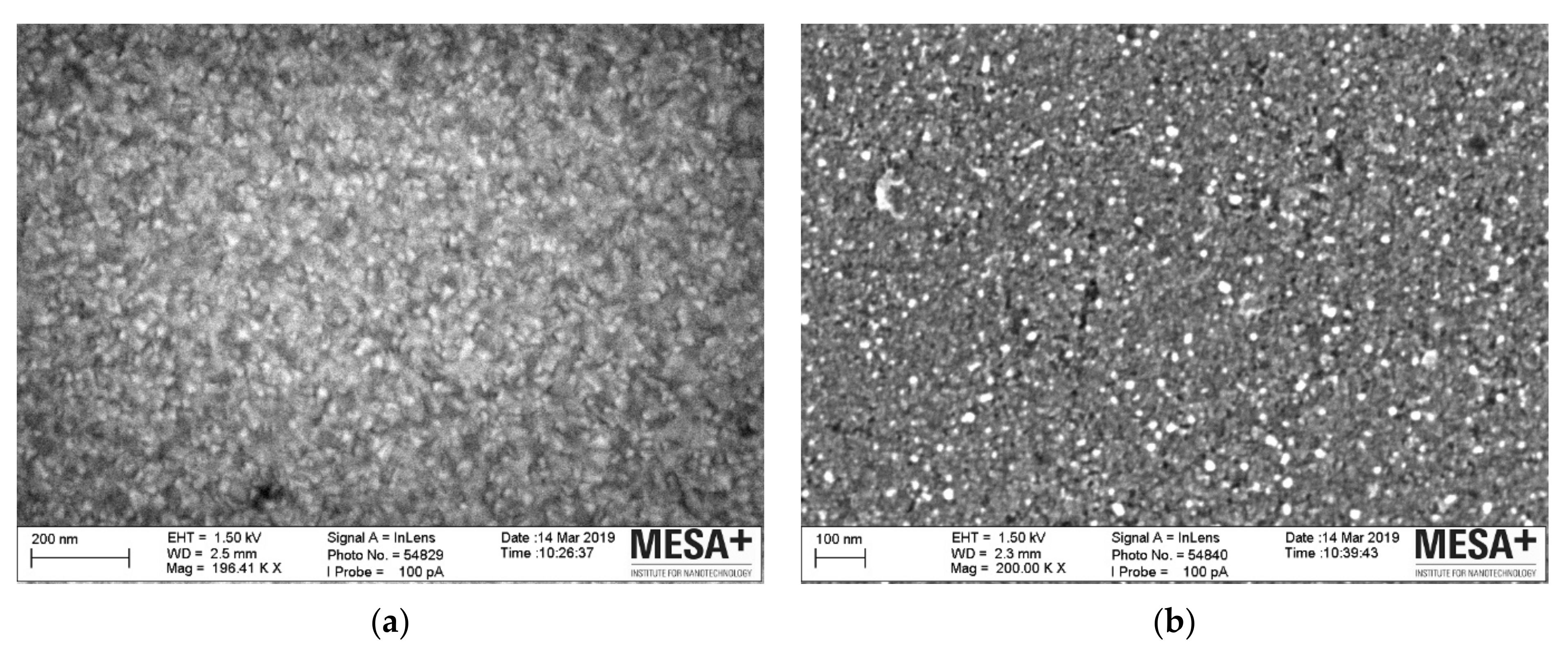




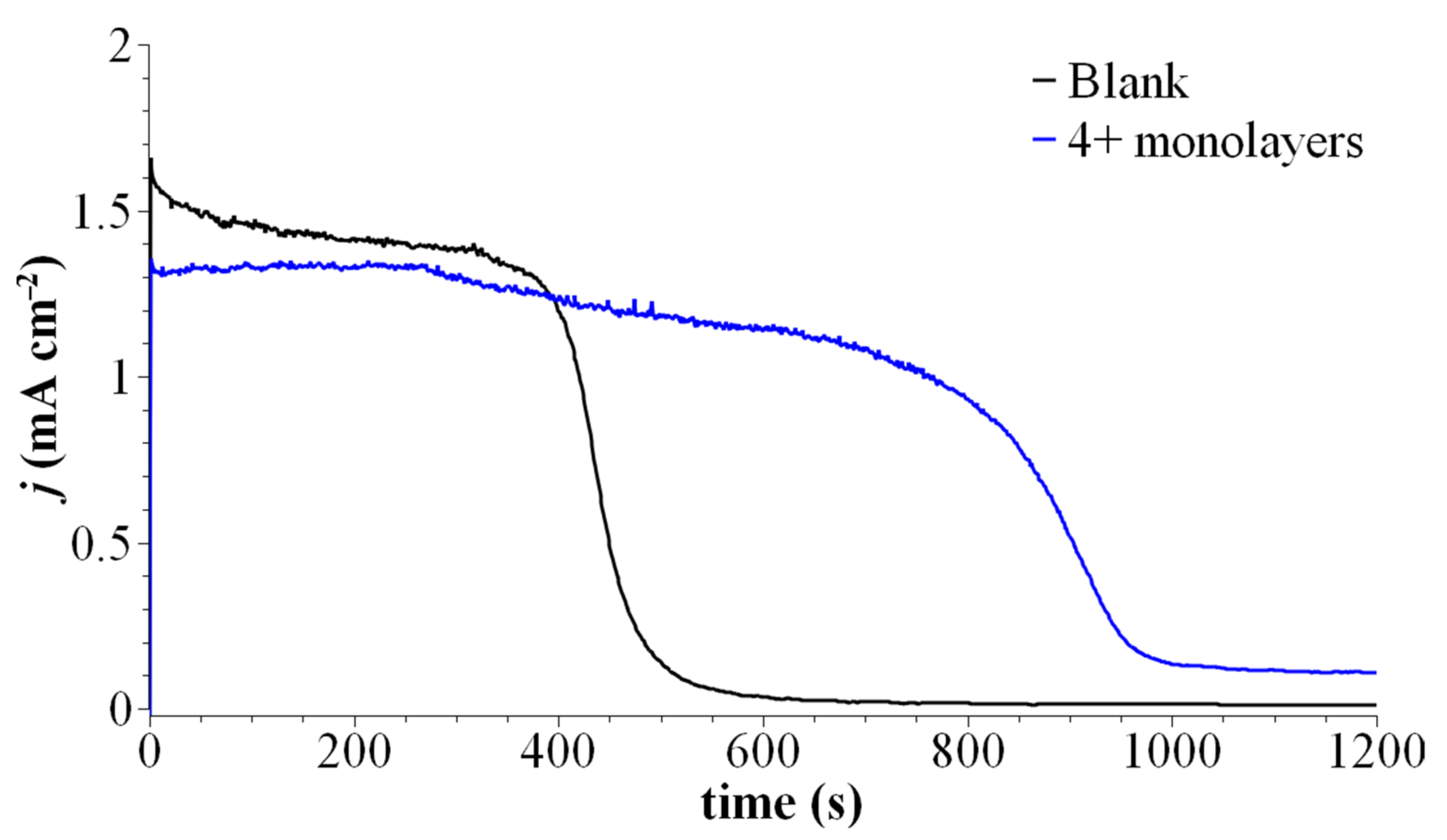
| Conc. (µM) | τmin (nm) | τmax (nm) | No. of Monolayers |
|---|---|---|---|
| 1 | 0.10 ± 0.02 | 0.17 ± 0.03 | <1 monolayer |
| 10 | 0.46 ± 0.09 | 0.77 ± 0.14 | 1 monolayer, approx. |
| 100 | 1.26 ± 0.07 | 2.11 ± 0.12 | 2–4 monolayers |
| 1000 | 1.76 ± 0.04 | 2.95 ± 0.07 | 4+ monolayers |
| τav (nm) | j0 (A cm−2) | b (mV dec−1) |
|---|---|---|
| 0 (Pt only) | −3.33 | 64 |
| 0.62 (1 monolayer, approx.) | −3.45 | 60 |
| 1.69 (2–4 monolayers) | −3.85 | 57 |
| 2.36 (4+ monolayers) | −4.00 | 55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Worsley, M.; Smulders, V.; Mei, B. Controlled Synthesis of Chromium-Oxide-Based Protective Layers on Pt: Influence of Layer Thickness on Selectivity. Catalysts 2022, 12, 1077. https://doi.org/10.3390/catal12101077
Worsley M, Smulders V, Mei B. Controlled Synthesis of Chromium-Oxide-Based Protective Layers on Pt: Influence of Layer Thickness on Selectivity. Catalysts. 2022; 12(10):1077. https://doi.org/10.3390/catal12101077
Chicago/Turabian StyleWorsley, Myles, Vera Smulders, and Bastian Mei. 2022. "Controlled Synthesis of Chromium-Oxide-Based Protective Layers on Pt: Influence of Layer Thickness on Selectivity" Catalysts 12, no. 10: 1077. https://doi.org/10.3390/catal12101077
APA StyleWorsley, M., Smulders, V., & Mei, B. (2022). Controlled Synthesis of Chromium-Oxide-Based Protective Layers on Pt: Influence of Layer Thickness on Selectivity. Catalysts, 12(10), 1077. https://doi.org/10.3390/catal12101077







