Well-Dispersed MgAl2O4 Supported Ni Catalyst with Enhanced Catalytic Performance and the Reason of Its Deactivation for Long-Term Dry Methanation Reaction
Abstract
:1. Introduction
2. Results
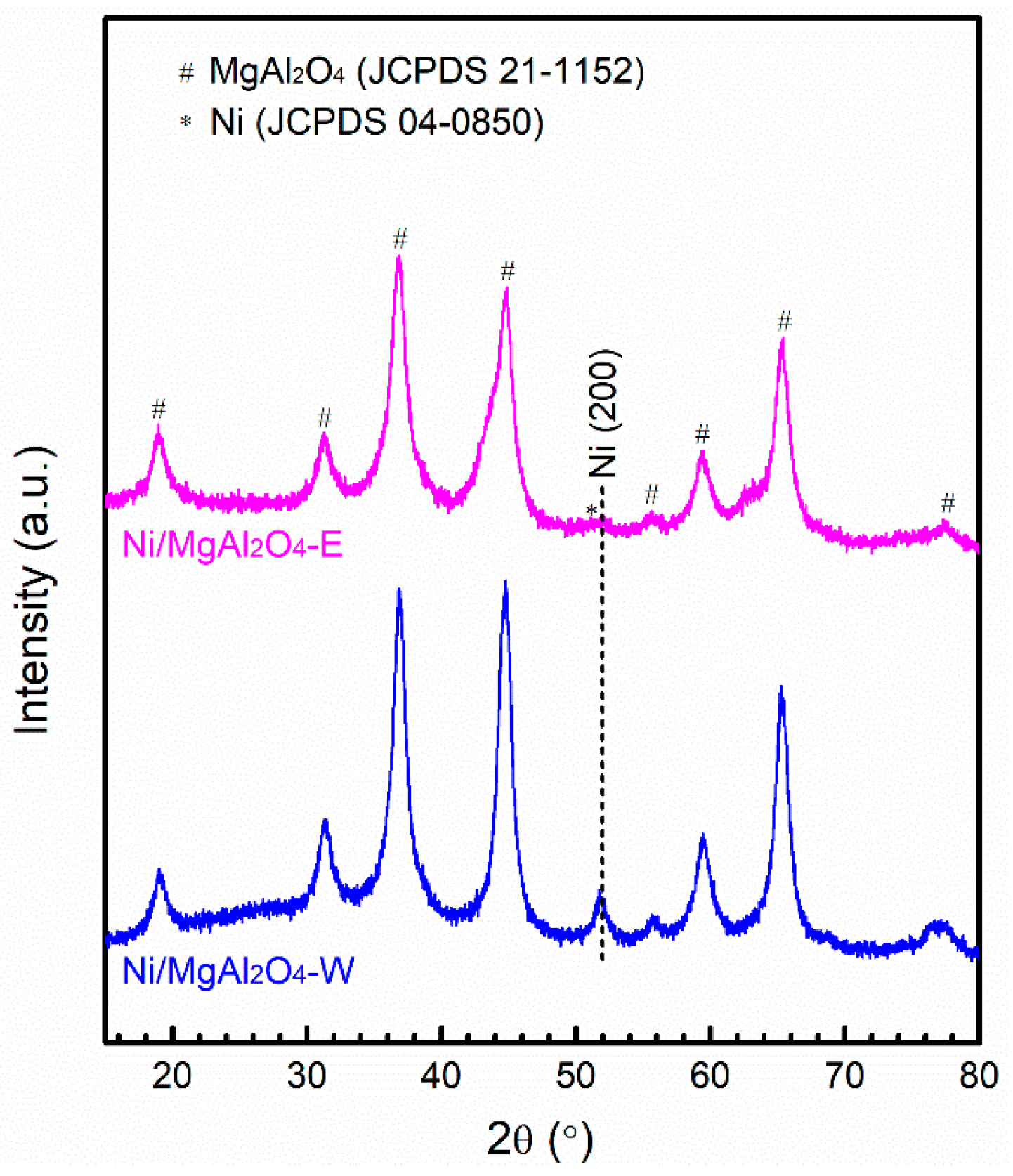
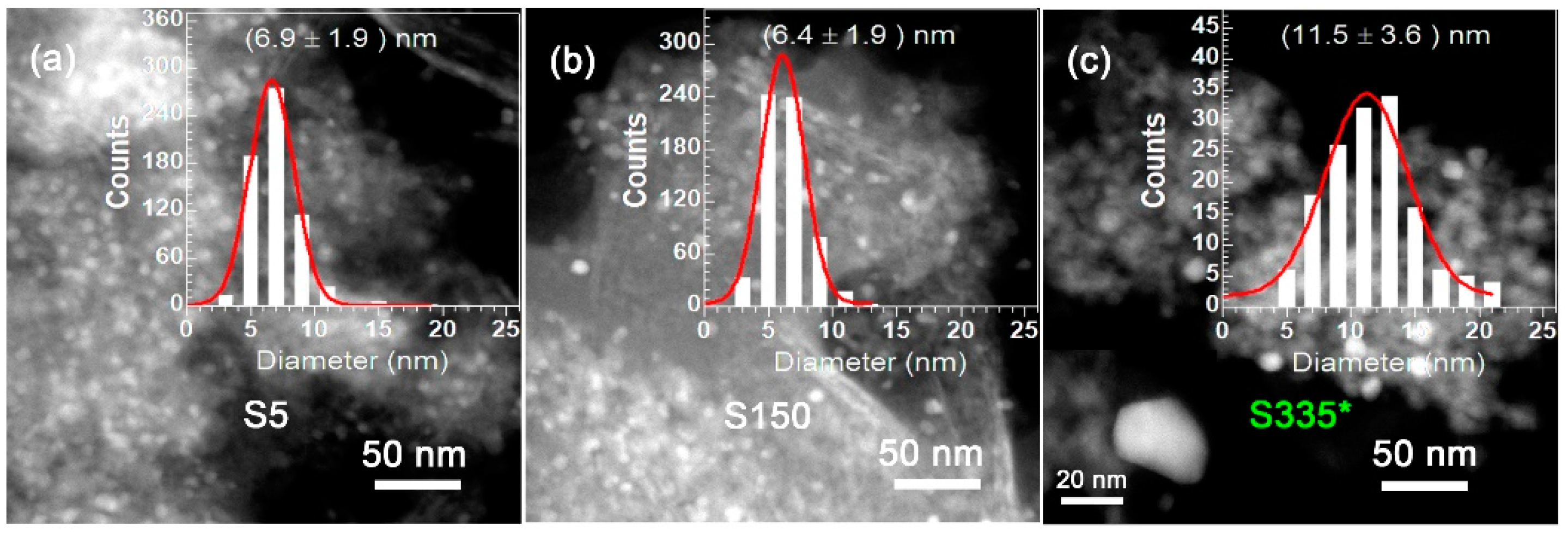
2.1. Catalyst Characterization
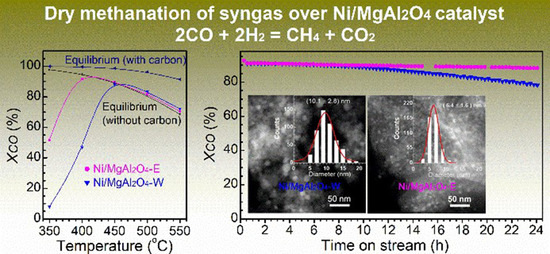
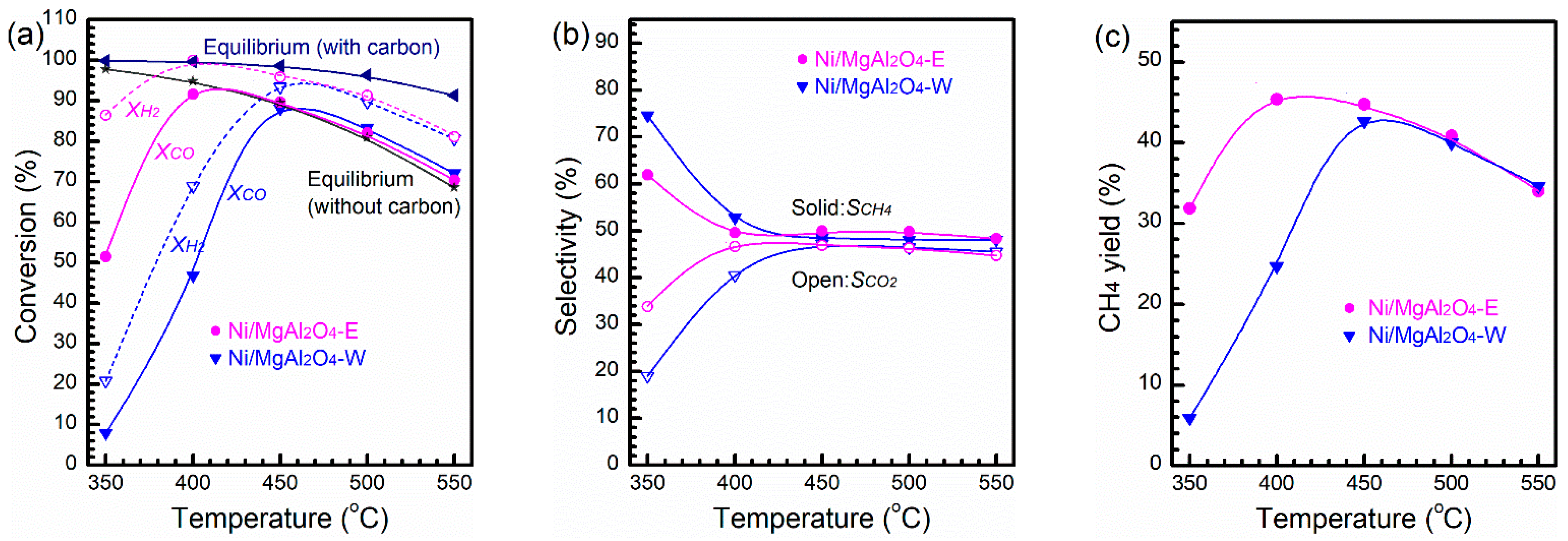
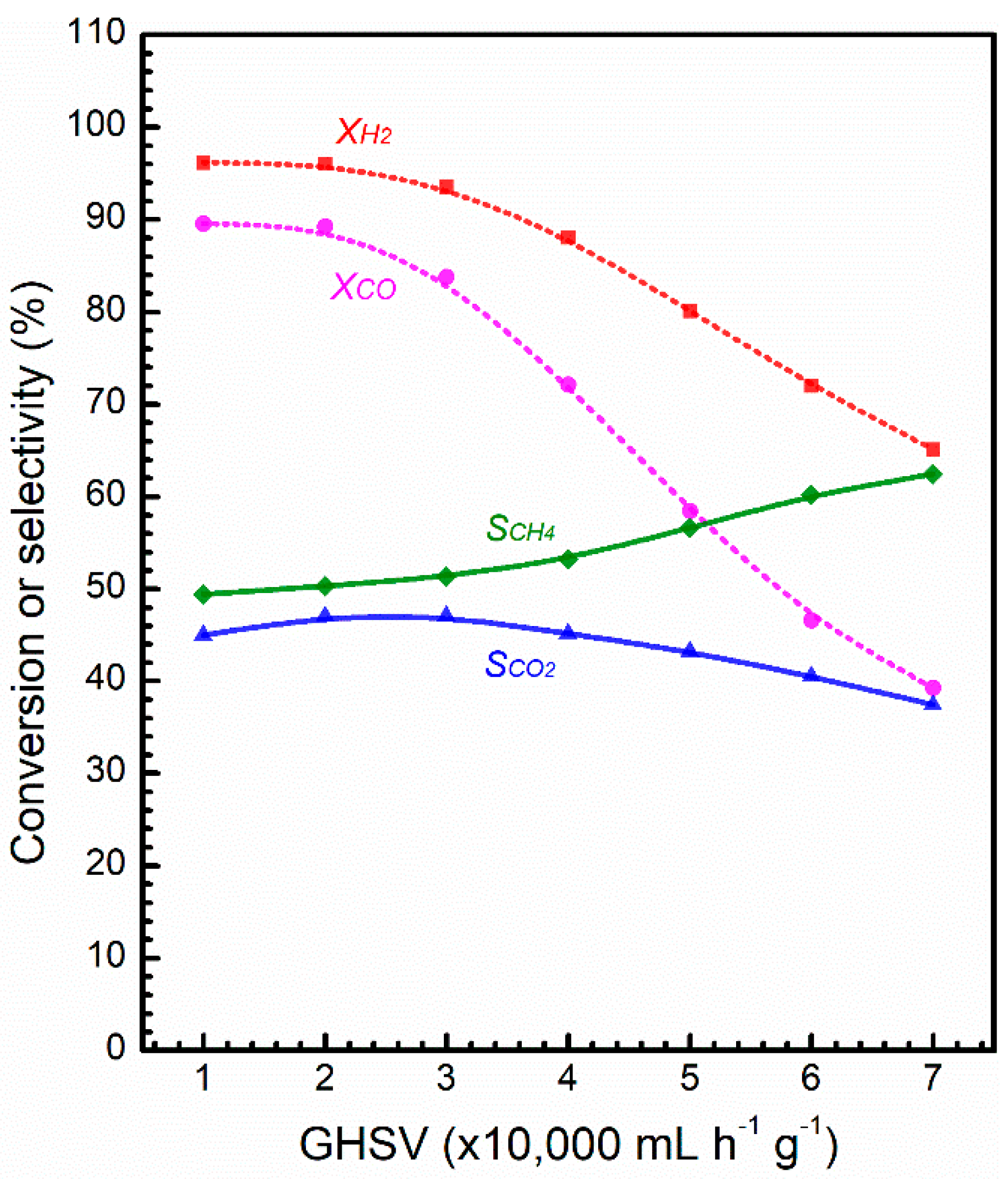
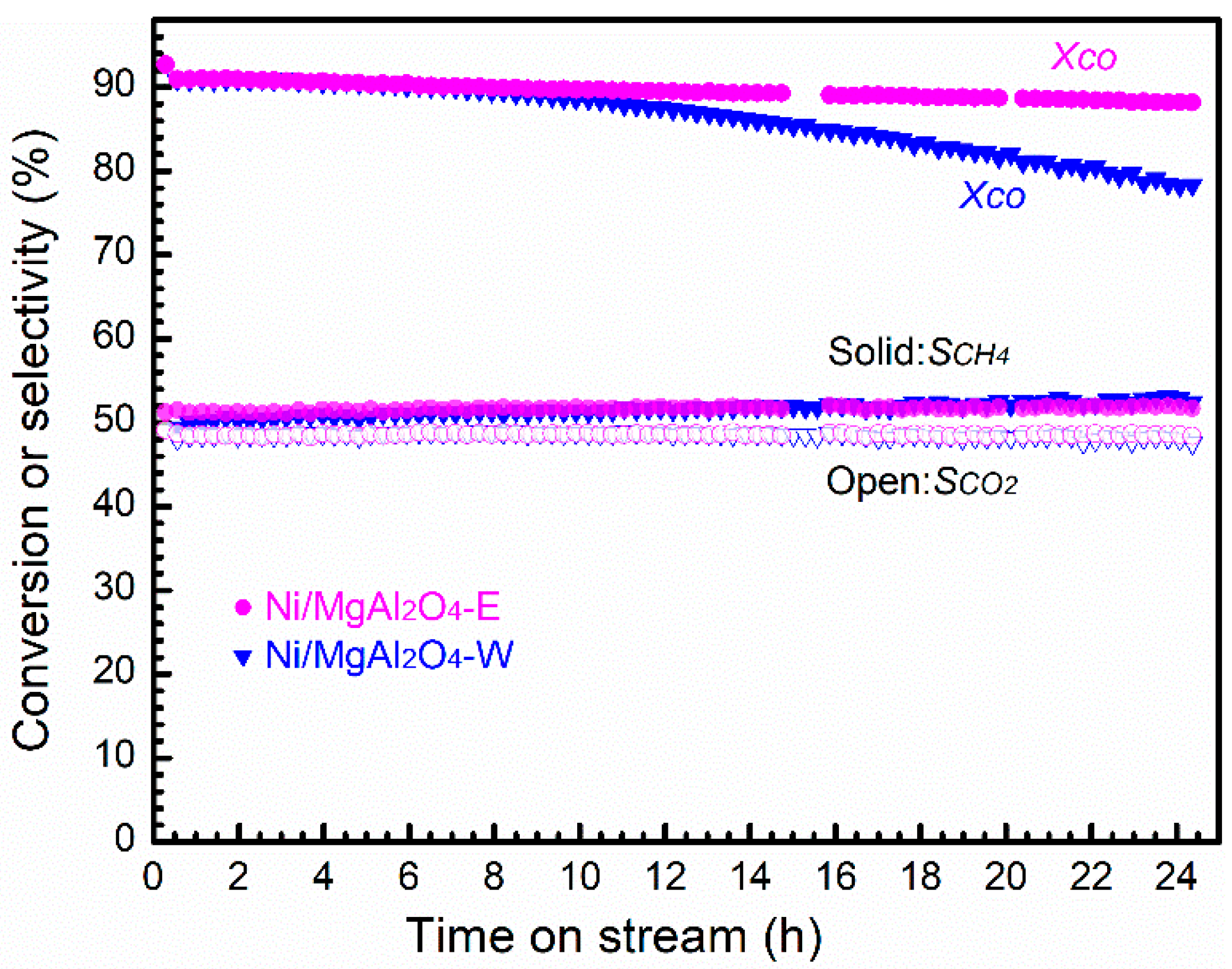
2.2. Catalytic Activity and Stability
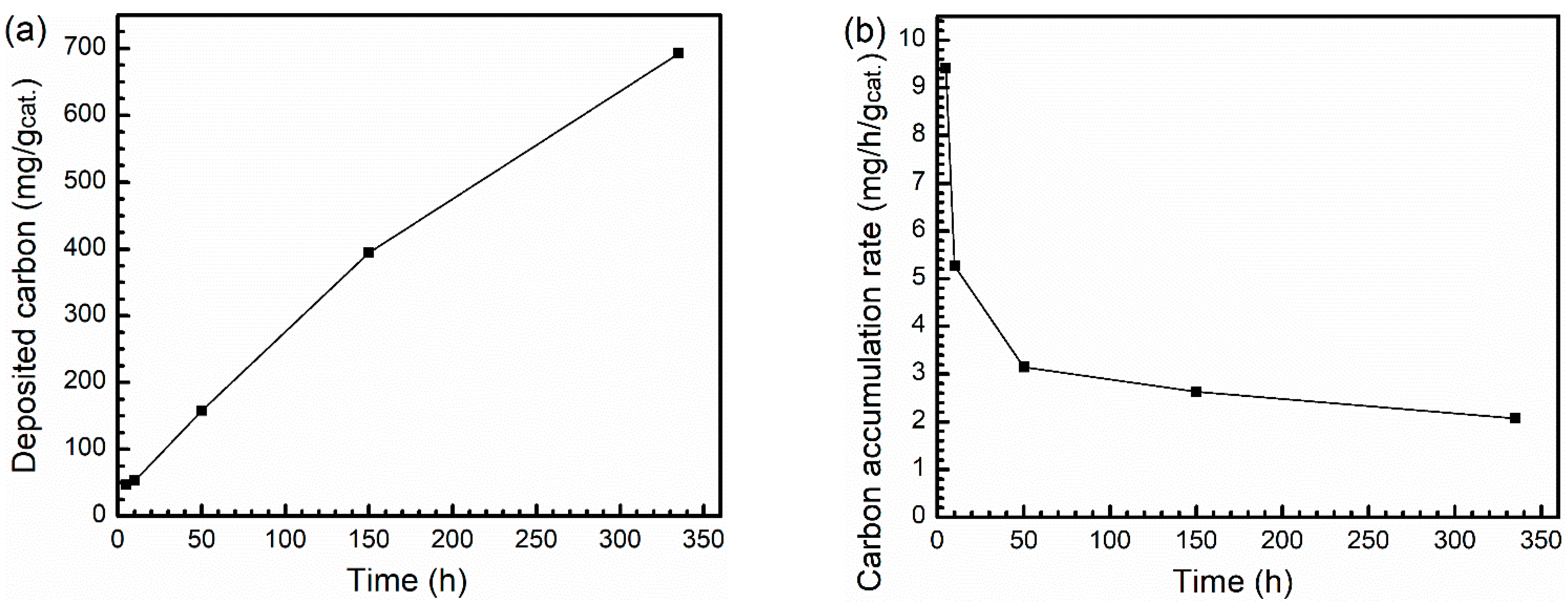
3. Discussion
4. Materials and Methods
4.1. Catalyst Preparation
4.2. Catalyst Characterization
4.3. Catalytic Performance Tests
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rahmaninejad, F.; Gavaskar, V.S.; Abbasian, J. Dry regenerable CuO/γ-Al2O3 catalyst for simultaneous removal of SOx and NOx from flue gas. Appl. Catal. B Environ. 2012, 119–120, 297–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Schauer, J.J.; Zhang, Y.; Zeng, L.; Wei, Y.; Liu, Y.; Shao, M. Characteristics of particulate carbon emissions from real-world Chinese coal combustion. Environ. Sci. Technol. 2008, 42, 5068–5073. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Z.; Wang, G.; Li, H. Study on the impacts of natural gas supply cost on gas flow and infrastructure deployment in China. Appl. Energy 2016, 162, 1385–1398. [Google Scholar] [CrossRef]
- Ding, Y.; Han, W.; Chai, Q.; Yang, S.; Shen, W. Coal-based synthetic natural gas (SNG): A solution to China’s energy security and CO2 reduction? Energy Policy 2013, 55, 445–453. [Google Scholar] [CrossRef]
- Gao, L.; Fu, Q.; Wei, M.; Zhu, Y.; Liu, Q.; Crumlin, E.; Liu, Z.; Bao, X. Enhanced Nickel-catalyzed methanation confined under hexagonal boron nitride shells. ACS Catal. 2016, 6, 6814–6822. [Google Scholar] [CrossRef]
- Shinde, V.M.; Madras, G. CO methanation toward the production of synthetic natural gas over highly active Ni/TiO2 catalyst. AIChE J. 2014, 60, 1027–1035. [Google Scholar] [CrossRef]
- Ren, J.; Li, H.; Jin, Y.; Zhu, J.; Liu, S.; Lin, J.; Li, Z. Silica/titania composite-supported Ni catalysts for CO methanation: Effects of Ti species on the activity, anti-sintering, and anti-coking properties. Appl. Catal. B Environ. 2017, 201, 561–572. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Chen, Z.; Lin, J.; Li, W.; Wang, Y.; Chen, B. Water-saving dry methanation for direct conversion of syngas to synthetic natural gas over robust Ni0.1Mg0.9Al2O4 catalyst. J. Catal. 2019, 375, 466–477. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.; Li, W.; Chen, B. Coke-resistant Au–Ni/MgAl2O4 catalyst for direct methanation of syngas. J. Energy Chem. 2019, 39, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Lebarbier, V.M.; Dagle, R.A.; Kovarik, L.; Albrecht, K.O.; Li, X.; Li, L.; Taylor, C.E.; Bao, X.; Wang, Y. Sorption-enhanced synthetic natural gas (SNG) production from syngas: A novel process combining CO methanation, water-gas shift, and CO2 capture. Appl. Catal. B Environ. 2014, 144, 223–232. [Google Scholar] [CrossRef]
- Gao, J.; Jia, C.; Zhang, M.; Gu, F.; Xu, G.; Su, F. Effect of nickel nanoparticle size in Ni/α-Al2O3 on CO methanation reaction for the production of synthetic natural gas. Catal. Sci. Technol. 2013, 3, 2009–2015. [Google Scholar] [CrossRef]
- Li, J.; Li, P.; Li, J.; Tian, Z.; Yu, F. Highly-dispersed Ni-NiO nanoparticles anchored on an SiO2 support for an enhanced CO methanation performance. Catalysts 2019, 9, 506. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.; Jo, S.; Lee, Y.; Kang, S.; Kim, J.; Lee, S.; Kim, J. Influence of Ni on Fe and Co-Fe based catalysts for high-calorific synthetic natural gas. Catalysts 2021, 11, 697. [Google Scholar] [CrossRef]
- Du, G.; Lim, S.; Yang, Y.; Wang, C.; Pfefferle, L.; Haller, G.L. Methanation of carbon dioxide on Ni-incorporated MCM-41 catalysts: The influence of catalyst pretreatment and study of steady-state reaction. J. Catal. 2007, 249, 370–379. [Google Scholar] [CrossRef]
- Czekaj, I.; Loviat, F.; Raimondi, F.; Wambach, J.; Biollaz, S.; Wokaun, A. Characterization of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas: X-ray photoelectron spectroscopy (XPS). Appl. Catal. A Gen. 2007, 329, 68–78. [Google Scholar] [CrossRef]
- Kim, J.-H.; Suh, D.J.; Park, T.-J.; Kim, K.-L. Effect of metal particle size on coking during CO2 reforming of CH4 over Ni–alumina aerogel catalysts. Appl. Catal. A Gen. 2000, 197, 191–200. [Google Scholar] [CrossRef]
- Zhan, Y.; Song, K.; Shi, Z.; Wan, C.; Pan, J.; Li, D.; Au, C.; Jiang, L. Influence of reduction temperature on Ni particle size and catalytic performance of Ni/Mg(Al)O catalyst for CO2 reforming of CH4. Int. J. Hydrog. Energy 2020, 45, 2794–2807. [Google Scholar] [CrossRef]
- Jung, Y.; Yoon, W.; Seo, Y.; Rhee, Y. The effect of precipitants on Ni-Al2O3 catalysts prepared by a co-precipitation method for internal reforming in molten carbonate fuel cells. Catal. Commun. 2012, 26, 103–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Chen, T.; Chang, D.; Chen, D.; Kong, D.; Zou, X.; Frost, R.L. Effect of preparation method of palygorskite-supported Fe and Ni catalysts on catalytic cracking of biomass tar. Chem. Eng. J. 2012, 188, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Li, C.; Wang, F.; He, S.; Chen, H.; Zhao, Y.; Wei, M.; Evans, D.G.; Duan, X. Enhanced low-temperature activity of CO2 methanation over highly-dispersed Ni/TiO2 catalyst. Catal. Sci. Technol. 2013, 3, 2627–2633. [Google Scholar] [CrossRef]
- Bitter, J.H.; van der Lee, M.K.; Slotboom, A.G.T.; van Dillen, A.J.; de Jong, K.P. Synthesis of highly loaded highly dispersed nickel on carbon nanofibers by homogeneous deposition–precipitation. Catal. Lett. 2003, 89, 139–142. [Google Scholar] [CrossRef]
- Wu, C.; Williams, P.T. A novel nano-Ni/SiO2 catalyst for hydrogen production from steam reforming of ethanol. Environ. Sci. Technol. 2010, 44, 5993–5998. [Google Scholar] [CrossRef]
- Zhang, W.D.; Liu, B.S.; Zhan, Y.P.; Tian, Y.L. Syngas production via CO2 reforming of methane over Sm2O3−La2O3-supported Ni catalyst. Ind. Eng. Chem. Res. 2009, 48, 7498–7504. [Google Scholar] [CrossRef]
- Jiang, C.; Shang, Z.; Liang, X. Chemoselective transfer hydrogenation of nitroarenes catalyzed by highly dispersed, supported nickel nanoparticles. ACS Catal. 2015, 5, 4814–4818. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Yan, W.; Duan, F.; Zhang, B.; Gao, Z.; Qin, Y. Ni nanoparticles supported on CNTs with excellent activity produced by atomic layer deposition for hydrogen generation from the hydrolysis of ammonia borane. Catal. Sci. Technol. 2016, 6, 2112–2119. [Google Scholar] [CrossRef]
- He, L.; Liang, B.; Li, L.; Yang, X.; Huang, Y.; Wang, A.; Wang, X.; Zhang, T. Cerium-oxide-modified nickel as a non-noble metal catalyst for selective decomposition of hydrous hydrazine to hydrogen. ACS Catal. 2015, 5, 1623–1628. [Google Scholar] [CrossRef]
- Le, T.A.; Kim, J.; Kang, J.K.; Park, E.D. CO and CO2 methanation over Ni/Al@Al2O3 core–shell catalyst. Catal. Today 2020, 356, 622–630. [Google Scholar] [CrossRef]
- Lakshmanan, P.; Kim, M.S.; Park, E.D. A highly loaded Ni@SiO2 core–shell catalyst for CO methanation. Appl. Catal. A Gen. 2016, 513, 98–105. [Google Scholar] [CrossRef]
- Gould, T.D.; Lubers, A.M.; Neltner, B.T.; Carrier, J.V.; Weimer, A.W.; Falconer, J.L.; Will Medlin, J. Synthesis of supported Ni catalysts by atomic layer deposition. J. Catal. 2013, 303, 9–15. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The sol-gel process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Xiao, Z.; Ma, Z.; Wang, X.; Williams, C.T.; Liang, C. Effects of synthetic parameters on the structure and catalytic performance of Cu−Cr catalysts prepared by a non-alkoxide sol−gel route. Ind. Eng. Chem. Res. 2011, 50, 2031–2039. [Google Scholar] [CrossRef]
- Hakeem, A.A.; Zhao, Z.; Kapteijn, F.; Makkee, M. Revisiting the synthesis of Au/TiO2 P25 catalyst and application in the low temperature water–gas shift under realistic conditions. Catal. Today 2015, 244, 19–28. [Google Scholar] [CrossRef]
- Wolf, A.; Schüth, F. A systematic study of the synthesis conditions for the preparation of highly active gold catalysts. Appl. Catal. A Gen. 2002, 226, 1–13. [Google Scholar] [CrossRef]
- Leskelä, M.; Ritala, M. Atomic layer deposition chemistry: Recent developments and future challenges. Angew. Chem. Int. Edit. 2003, 42, 5548–5554. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lang, R.; Qiao, B.; Wang, A.; Zhang, T. Highlights of the major progress in single-atom catalysis in 2015 and 2016. Chin. J. Catal. 2017, 38, 1498–1507. [Google Scholar] [CrossRef]
- Liu, J. Catalysis by supported single metal atoms. ACS Catal. 2017, 7, 34–59. [Google Scholar] [CrossRef]
- Ganesh, I. A review on magnesium aluminate (MgAl2O4) spinel: Synthesis, processing and applications. Int. Mater. Rev. 2013, 58, 63–112. [Google Scholar] [CrossRef]
- Seeman, V.; Feldbach, E.; Kärner, T.; Maaroos, A.; Mironova-Ulmane, N.; Popov, A.I.; Shablonin, E.; Vasil′chenko, E.; Lushchik, A. Fast-neutron-induced and as-grown structural defects in magnesium aluminate spinel crystals with different stoichiometry. Opt. Mater. 2019, 91, 42–49. [Google Scholar] [CrossRef]
- Luisetto, I.; Tuti, S.; Battocchio, C.; Mastro, S.L.; Sodo, A. Ni/CeO2–Al2O3 catalysts for the dry reforming of methane: The effect of CeAlO3 content and nickel crystallite size on catalytic activity and coke resistance. Appl. Catal. A Gen. 2015, 500, 12–22. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Lin, J.; Chen, Z.; Wang, Y. Crucial support effect on the durability of Pt/MgAl2O4 for partial oxidation of methane to syngas. Appl. Catal. B Environ. 2018, 231, 292–298. [Google Scholar] [CrossRef]



| Sample | Ni Content (wt.%) | dNi (200) (nm) | SBET (m2/g) | Pore Size * (nm) | Pore Volume (cm3/g) |
|---|---|---|---|---|---|
| MgAl2O4 | - | - | 327.6 | 8.6 | 0.89 |
| Ni/MgAl2O4-E | 4.9 | 5.6 (±1.4) | 220.3 | 8.6 | 0.57 |
| Ni/MgAl2O4-W | 5.2 | 9.6 (±2.0) | 242.7 | 3.5 | 0.40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Yang, X.; Zhang, J. Well-Dispersed MgAl2O4 Supported Ni Catalyst with Enhanced Catalytic Performance and the Reason of Its Deactivation for Long-Term Dry Methanation Reaction. Catalysts 2021, 11, 1117. https://doi.org/10.3390/catal11091117
Wang F, Yang X, Zhang J. Well-Dispersed MgAl2O4 Supported Ni Catalyst with Enhanced Catalytic Performance and the Reason of Its Deactivation for Long-Term Dry Methanation Reaction. Catalysts. 2021; 11(9):1117. https://doi.org/10.3390/catal11091117
Chicago/Turabian StyleWang, Fen, Xiumiao Yang, and Jingcai Zhang. 2021. "Well-Dispersed MgAl2O4 Supported Ni Catalyst with Enhanced Catalytic Performance and the Reason of Its Deactivation for Long-Term Dry Methanation Reaction" Catalysts 11, no. 9: 1117. https://doi.org/10.3390/catal11091117
APA StyleWang, F., Yang, X., & Zhang, J. (2021). Well-Dispersed MgAl2O4 Supported Ni Catalyst with Enhanced Catalytic Performance and the Reason of Its Deactivation for Long-Term Dry Methanation Reaction. Catalysts, 11(9), 1117. https://doi.org/10.3390/catal11091117