Iron-Zinc Co-Doped Titania Nanocomposite: Photocatalytic and Photobiocidal Potential in Combination with Molecular Docking Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis Parameters Optimization
2.1.1. Effect NC Synthesis Methods
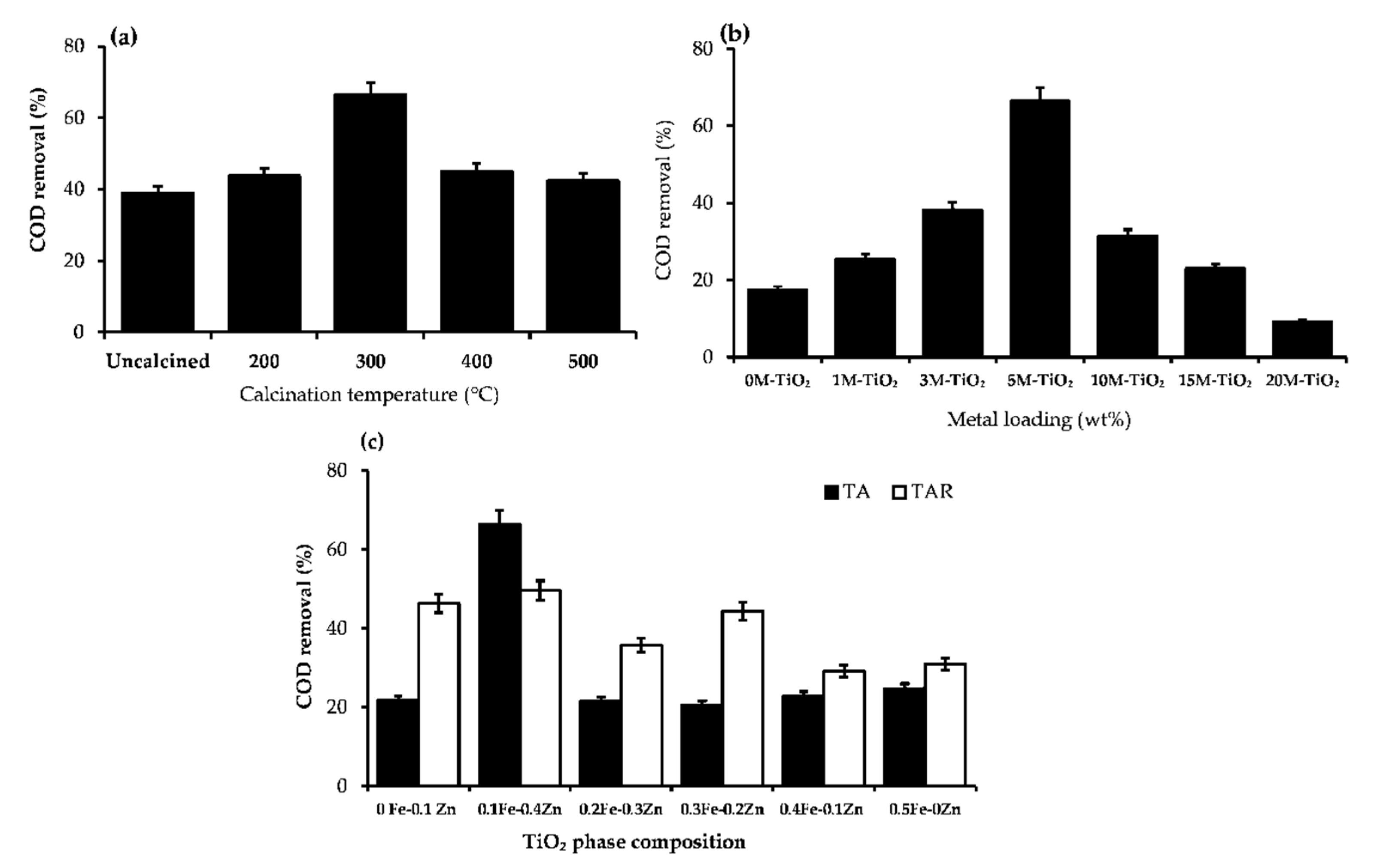
2.1.2. Effect of Calcination Temperature
2.1.3. Effect of Metal Loading (wt%)
2.1.4. Effect of Titania Phase
2.2. Reaction Parameters Optimization
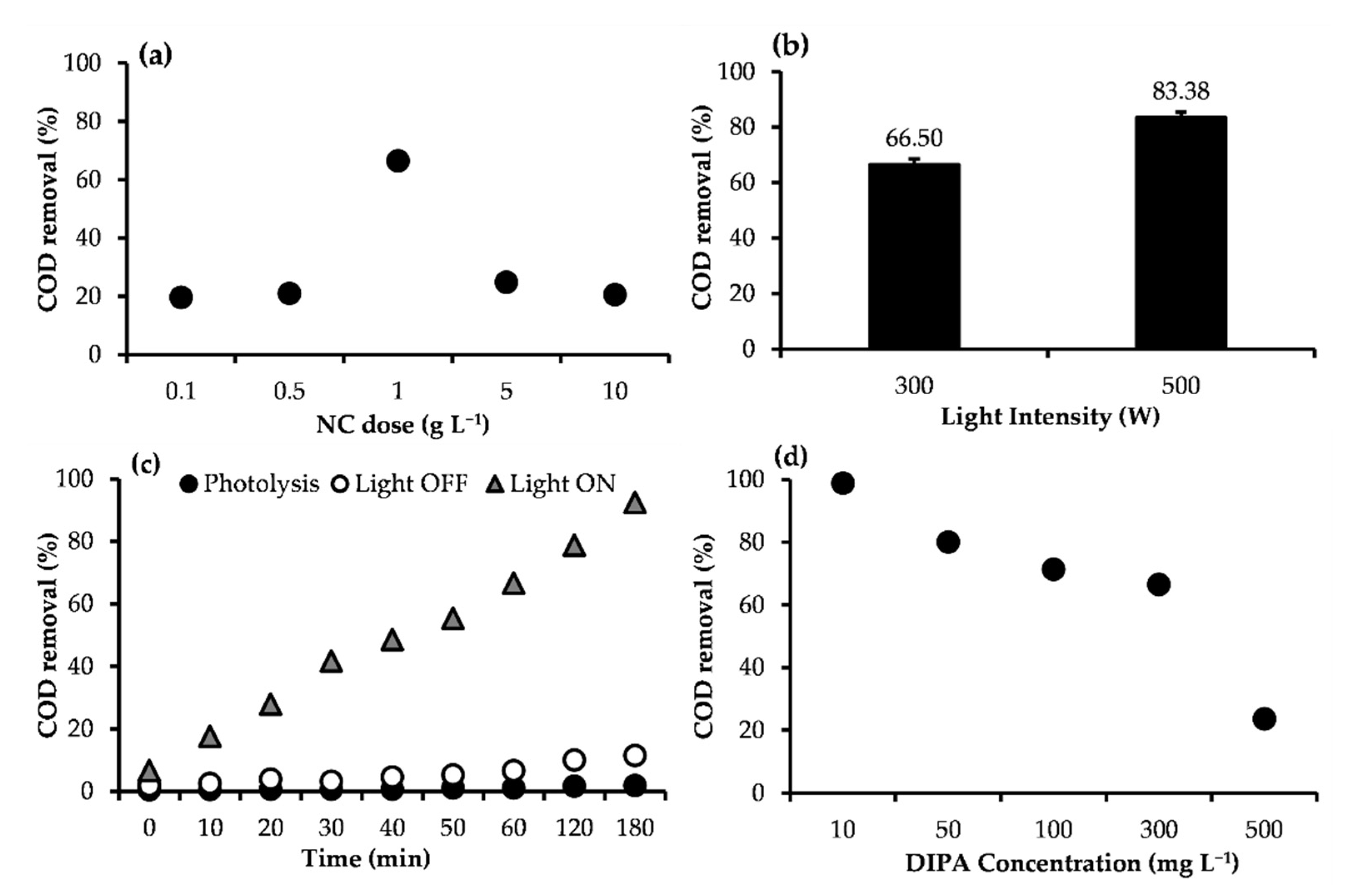
2.2.1. Effect of NC Dose
2.2.2. Effect of Light Intensity
2.2.3. Effect of Irradiation Time
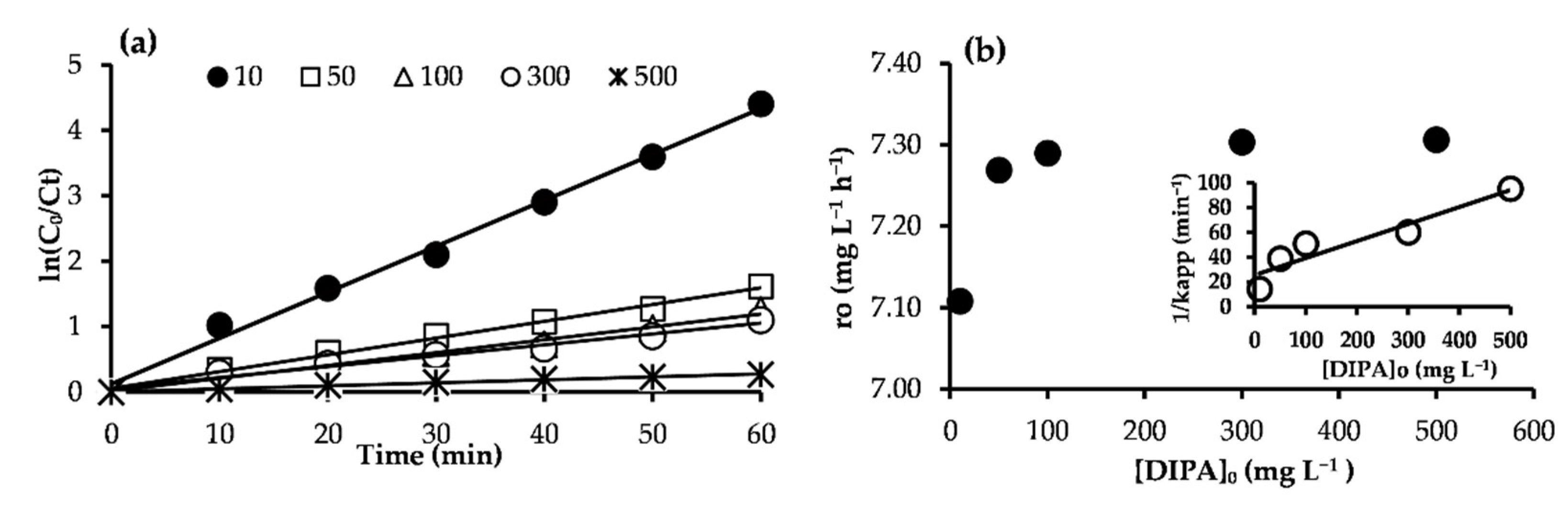
2.3. Photodegradation Kinetics
2.4. Photocatalytic Bactericidal Performance Evaluation
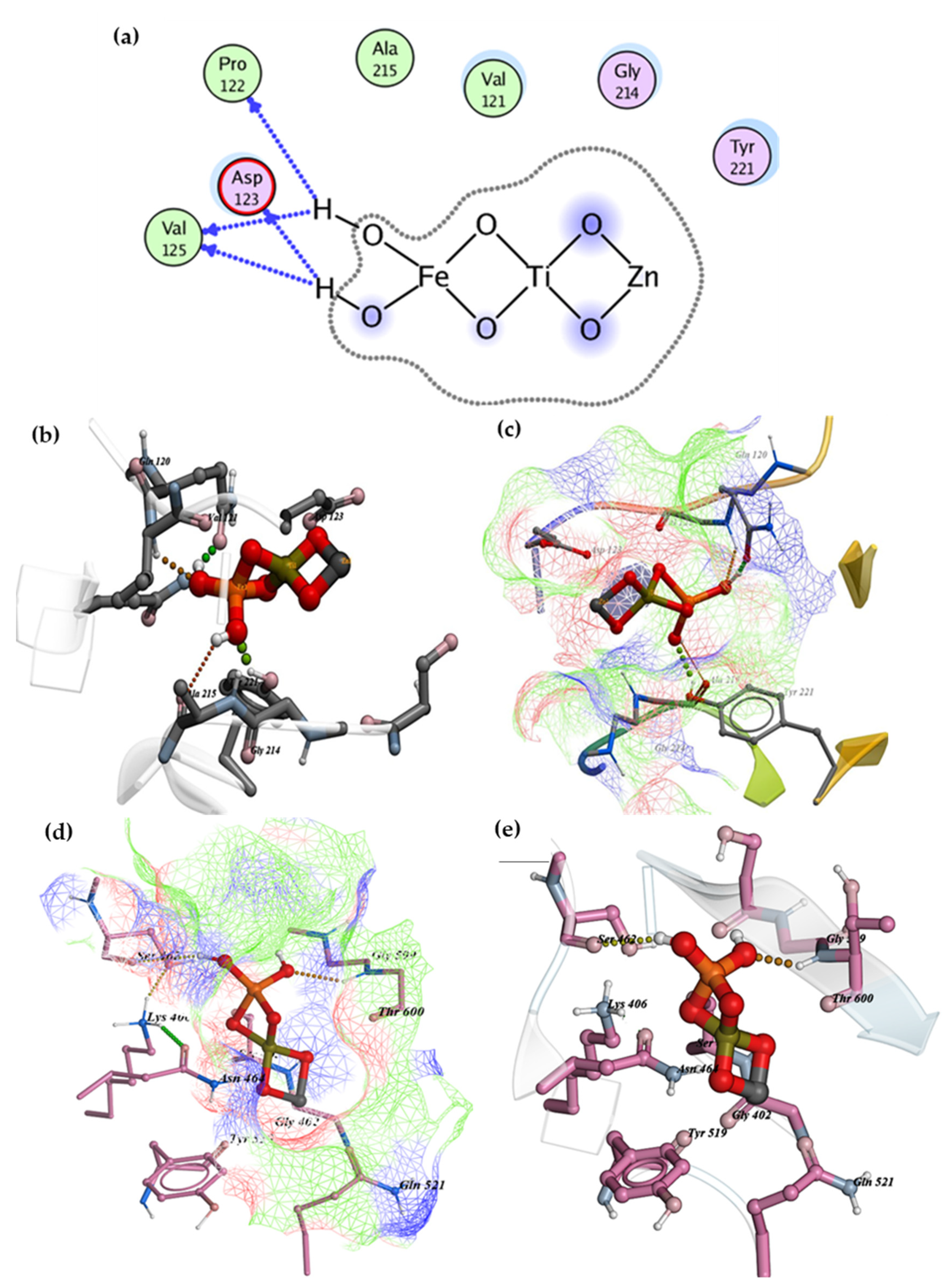
2.5. Molecular Docking Studies
2.6. Energy Efficiency Analysis
2.7. Physico-Chemical Characterization
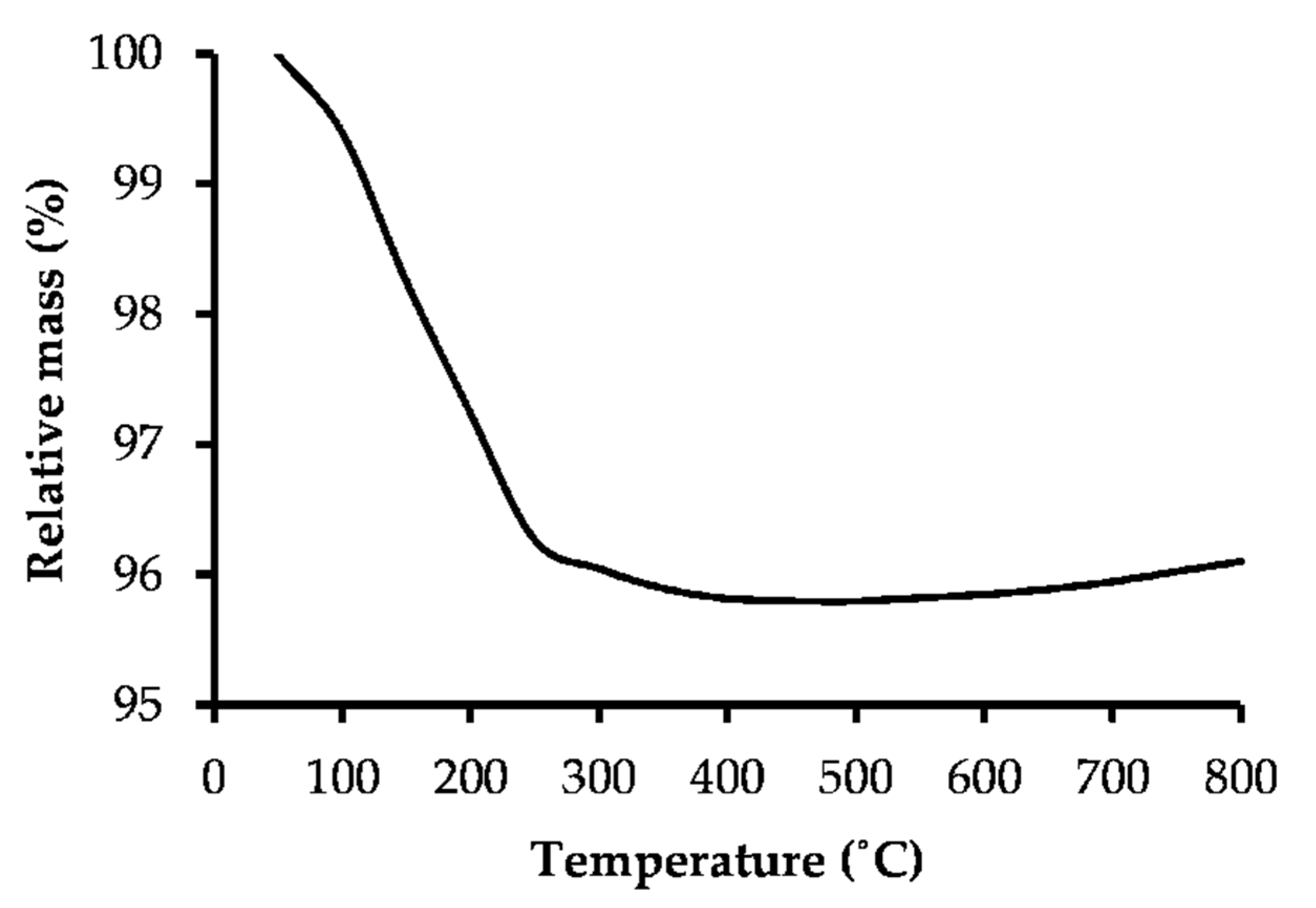
2.7.1. Thermal Stability
2.7.2. Surface Functional Groups (FTIR)
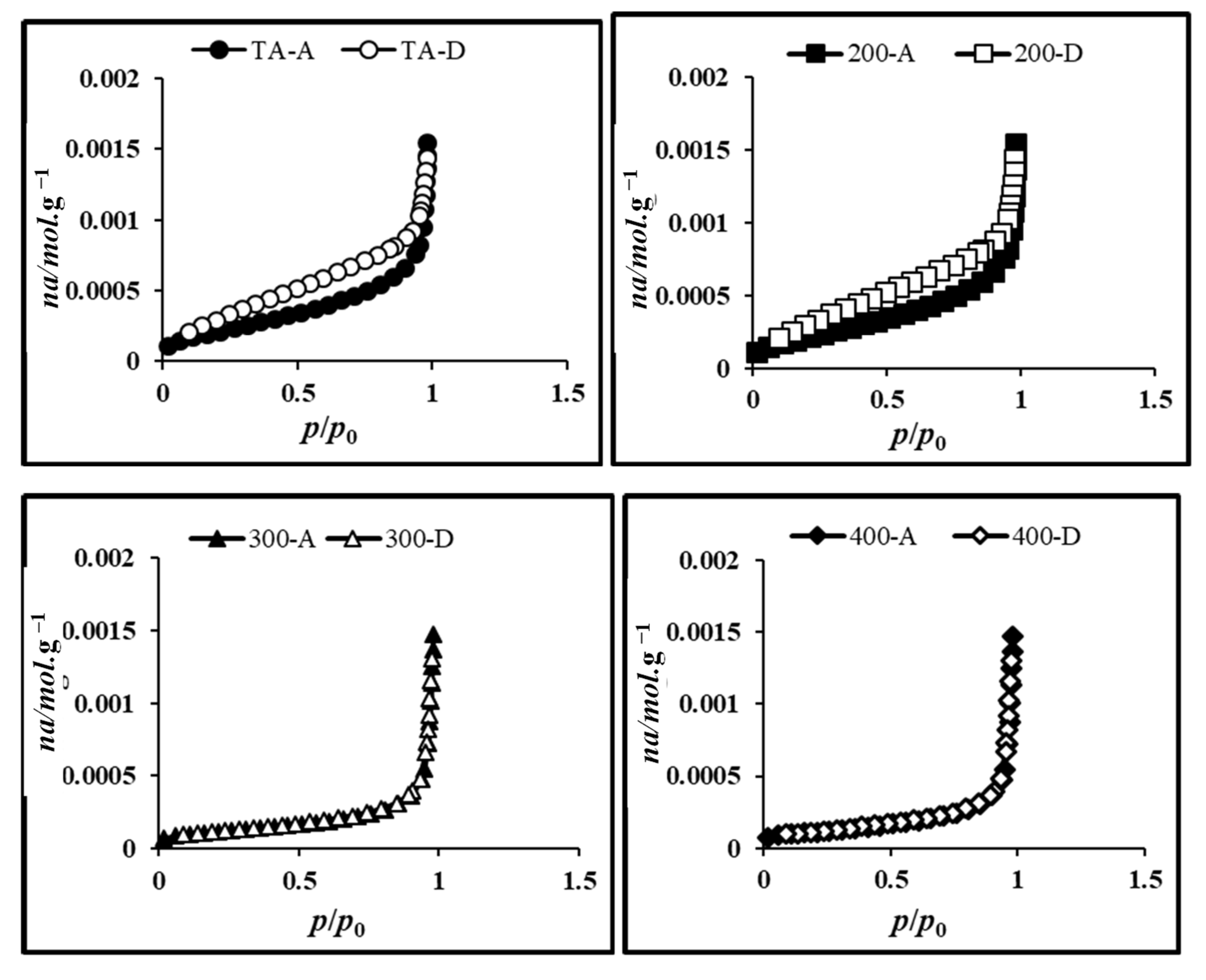
2.7.3. BET Surface Area
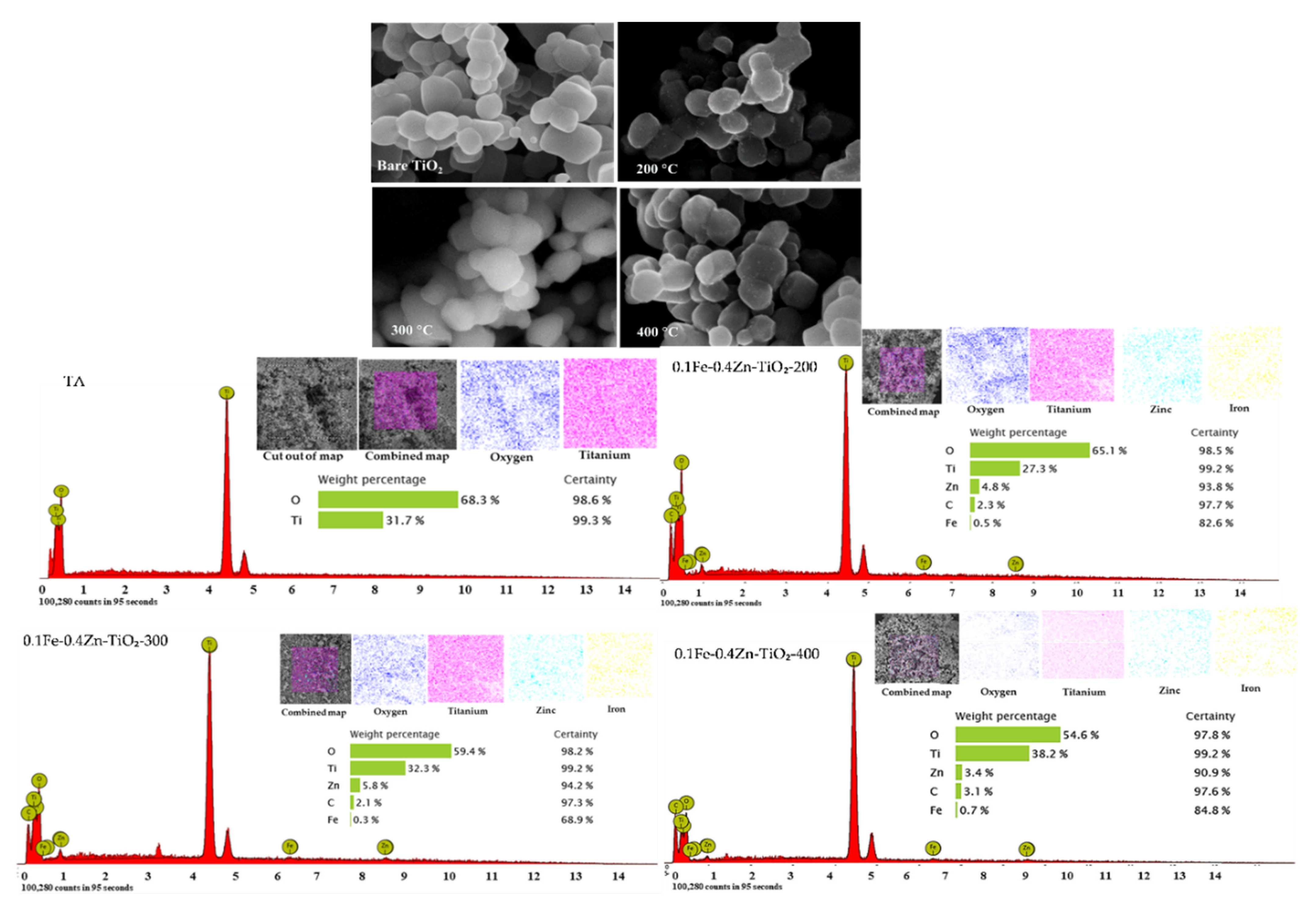
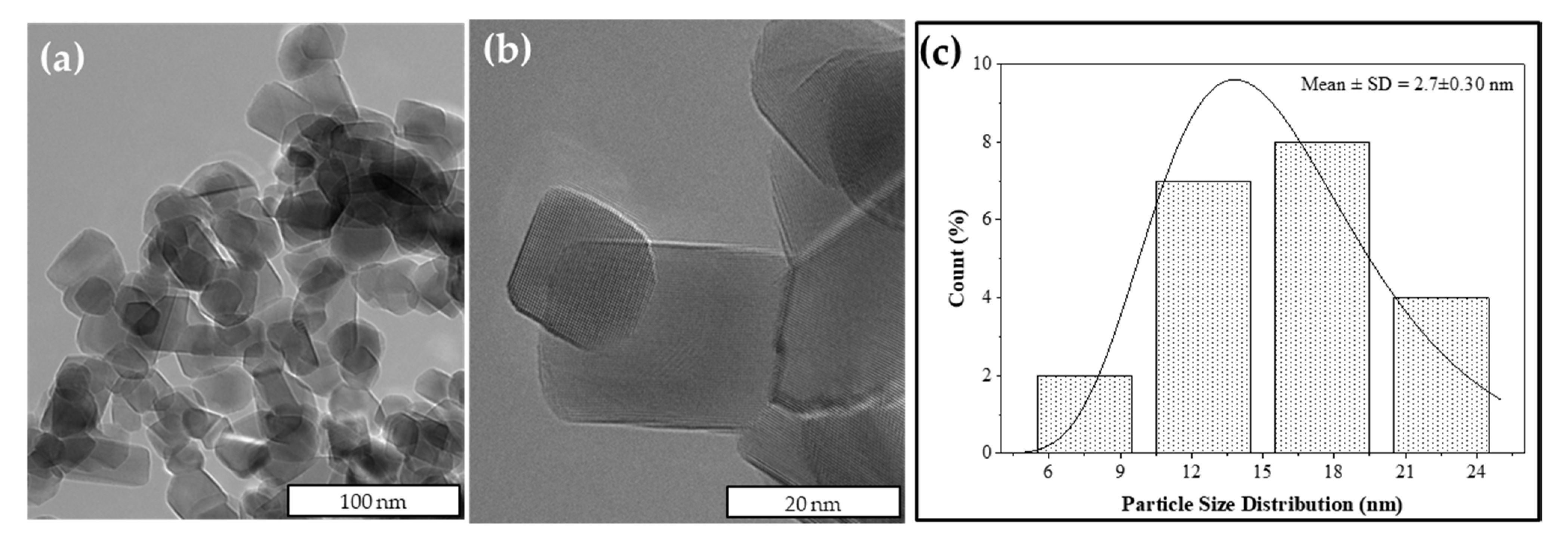
2.7.4. Morphology
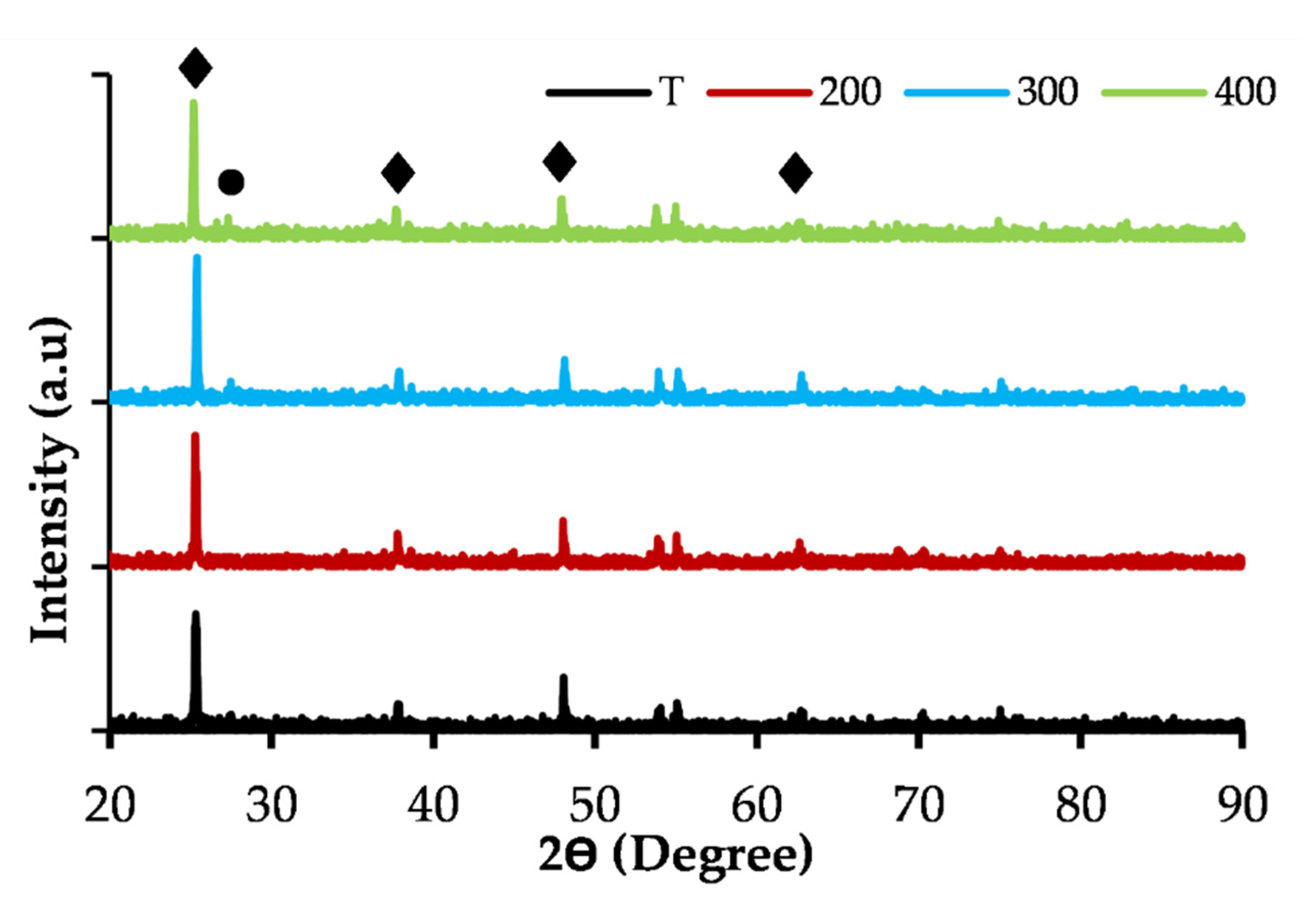
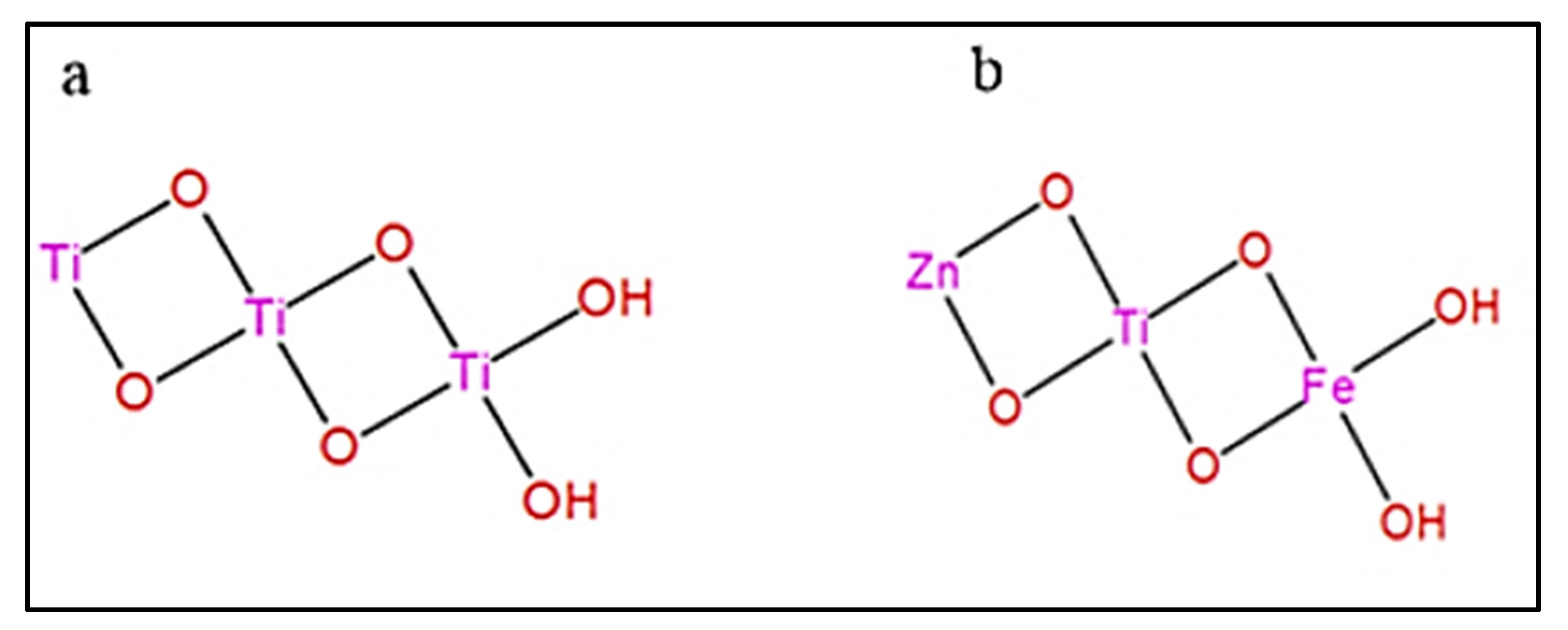
2.7.5. Phase and Crystal Structure
2.7.6. Diffuse Reflectance UV-Visible Spectrophotometry
3. Materials and Methods
3.1. Materials
3.2. Nanocomposite Synthesis
3.3. Performance Evaluation of Fe-Zn-TiO2 Nanocomposite
3.3.1. Screening and Optimization Studies
3.3.2. Kinetic Studies
3.3.3. Photocatalytic Bactericidal Studies
3.4. Energy Efficiency Analysis of Synthesized NC
3.5. Molecular Docking Simulation Studies
3.6. Physico-Chemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aaron, D.; Tsouris, C. Separation of CO2 from Flue Gas: A Review. Sep. Sci. Technol. 2005, 40, 321–348. [Google Scholar] [CrossRef]
- Bradley, A.; Duan, H.; Elion, W.; van Soest-Vercammen, E.; Nagelvoort, R.K. Innovation in the LNG industry: Shell’s approach. In Proceedings of the 24th World Gas Conference, Buenos Aires, Argentina, 5–9 October 2009. [Google Scholar]
- Poste, A.E.; Grung, M.; Wright, R.F. Amines and amine-related compounds in surface waters: A review of sources, concentrations and aquatic toxicity. Sci. Total Environ. 2014, 481, 274–279. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Eide-Haugmo, I.; Brakstad, O.G.; Hoff, K.A.; Sørheim, K.R.; da Silva, E.F.; Svendsen, H.F. Environmental impact of amines. Energy Procedia 2009, 1, 1297–1304. [Google Scholar] [CrossRef]
- Haroon, H.; Shah, J.A.; Khan, M.S.; Alam, T.; Khan, R.; Asad, S.A.; Ali, M.A.; Farooq, G.; Iqbal, M.; Bilal, M. Activated carbon from a specific plant precursor biomass for hazardous Cr(VI) adsorption and recovery studies in batch and column reactors: Isotherm and kinetic modeling. J. Water Process Eng. 2020, 38, 101577. [Google Scholar] [CrossRef]
- World Health Organization. The Top 10 Causes of Death. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 3 September 2021).
- World Health Organization. Diarrhoeal Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/diarrhoeal-disease (accessed on 15 July 2021).
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner Jr, R.C.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909–948. [Google Scholar] [CrossRef]
- Khan, M.S.; Shah, J.A.; Arshad, M.; Halim, S.A.; Khan, A.; Shaikh, A.J.; Riaz, N.; Khan, A.J.; Arfan, M.; Shahid, M. Photocatalytic Decolorization and Biocidal Applications of Nonmetal Doped TiO2: Isotherm, Kinetic Modeling and In Silico Molecular Docking Studies. Molecules 2020, 25, 4468. [Google Scholar] [CrossRef]
- Khan, M.S.; Shah, J.A.; Riaz, N.; Butt, T.A.; Khan, A.J.; Khalifa, W.; Gasmi, H.H.; Latifee, E.R.; Arshad, M.; Al-Naghi, A.A.A.; et al. Synthesis and Characterization of Fe-TiO2 Nanomaterial: Performance Evaluation for RB5 Decolorization and In Vitro Antibacterial Studies. Nanomaterials 2021, 11, 436. [Google Scholar] [CrossRef]
- Riaz, N.; Hassan, M.; Siddique, M.; Mahmood, Q.; Farooq, U.; Sarwar, R.; Khan, M.S. Photocatalytic degradation and kinetic modeling of azo dye using bimetallic photocatalysts: Effect of synthesis and operational parameters. Environ. Sci. Pollut. Res. 2020, 27, 2992–3006. [Google Scholar] [CrossRef]
- Riaz, N.; Kait, C.F.; Man, Z.; Dutta, B.K.; Ramli, R.M.; Khan, M.S. Visible Light Photodegradation of Azo Dye by Cu/TiO2. Adv. Mater. Res. 2014, 917, 151–159. [Google Scholar] [CrossRef]
- Yoon, K.-Y.; Hoon Byeon, J.; Park, J.-H.; Hwang, J. Susceptibility constants of Escherichia coli and Bacillus subtilis to silver and copper nanoparticles. Sci. Total Environ. 2007, 373, 572–575. [Google Scholar] [CrossRef]
- Erdem, A.; Metzler, D.; Cha, D.; Huang, C.P. Inhibition of bacteria by photocatalytic nano-TiO2 particles in the absence of light. Int. J. Environ. Sci. Technol. 2015, 12, 2987–2996. [Google Scholar] [CrossRef][Green Version]
- Long, M.; Wang, J.; Zhuang, H.; Zhang, Y.; Wu, H.; Zhang, J. Performance and mechanism of standard nano-TiO2 (P-25) in photocatalytic disinfection of foodborne microorganisms—Salmonella typhimurium and Listeria monocytogenes. Food Control 2014, 39, 68–74. [Google Scholar] [CrossRef]
- Foster, H.A.; Ditta, I.B.; Varghese, S.; Steele, A. Photocatalytic disinfection using titanium dioxide: Spectrum and mechanism of antimicrobial activity. Appli. Microbiol. Biotechnol. 2011, 90, 1847–1868. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.C.; Christensen, P.A.; Egerton, T.A.; Curtis, T.P.; Gunlazuardi, J. Effect of catalyst type on the kinetics of the photoelectrochemical disinfection of water inoculated with E. coli. J. Appl. Electrochem. 2001, 31, 623–628. [Google Scholar] [CrossRef]
- Drawz, S.M.; Bonomo, R.A. Three Decades of β-Lactamase Inhibitors. Clin. Microbiol. Rev. 2010, 23, 160–201. [Google Scholar] [CrossRef] [PubMed]
- Hanaor, D.A.; Assadi, M.H.; Li, S.; Yu, A.; Sorrell, C.C. Ab initio study of phase stability in doped TiO2. Comput. Mech. 2012, 50, 185–194. [Google Scholar] [CrossRef]
- Assadi, M.H.N.; Hanaor, D.A.H. The effects of copper doping on photocatalytic activity at (101) planes of anatase TiO2: A theoretical study. Appl. Surf. Sci. 2016, 387, 682–689. [Google Scholar] [CrossRef]
- Doustkhah, E.; Assadi, M.H.N.; Komaguchi, K.; Tsunoji, N.; Esmat, M.; Fukata, N.; Tomita, O.; Abe, R.; Ohtani, B.; Ide, Y. In situ Blue titania via band shape engineering for exceptional solar H2 production in rutile TiO2. Appl. Catal. B Environ. 2021, 297, 120380. [Google Scholar] [CrossRef]
- Yodsomnuk, P.; Junjeam, K.; Termtanun, M. Photoactivity of Fe and Zn-doped TiO2 in phenol degradation under visible light. MATEC Web Conf. 2018, 192, 03047. [Google Scholar] [CrossRef]
- Liu, W.-J.; Qian, T.-T.; Jiang, H. Bimetallic Fe nanoparticles: Recent advances in synthesis and application in catalytic elimination of environmental pollutants. Chem. Eng. J. 2014, 236, 448–463. [Google Scholar] [CrossRef]
- Saleh, R.; Djaja, N.F. UV light photocatalytic degradation of organic dyes with Fe-doped ZnO nanoparticles. Superlattices Microstruct. 2014, 74, 217–233. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–117. [Google Scholar] [CrossRef]
- Coleman, H.M.; Chiang, K.; Amal, R. Effects of Ag and Pt on photocatalytic degradation of endocrine disrupting chemicals in water. Chem. Eng. J. 2005, 113, 65–72. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Shokri, M.; Rad, B. Enhancement of photocatalytic activity of TiO2 nanoparticles by silver doping: Photodeposition versus liquid impregnation methods. Glob. NEST J. 2008, 10, 1–7. [Google Scholar]
- Nurlaela, E. Development of Cu-Ni/TiO2 Bimetallic Catalyst For Photohydrogen Production under Visible Light Illumination. Master’s Thesis, Universiti Teknologi PETRONAS, Tronoh, Bandar Seri Iskandar, Malaysia, 2011. [Google Scholar]
- Yoong, L.S.; Chong, F.K.; Dutta, B.K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661. [Google Scholar] [CrossRef]
- Ramli, R.M.; Chong, F.K.; Omar, A.A.; Murugesan, T. Performance of Surfactant Assisted Synthesis of Fe/TiO2 on the Photodegradation of Diisopropanolamine. CLEAN—Soil Air Water 2015, 43, 690–697. [Google Scholar] [CrossRef]
- Blake, D.M.; Webb, J.; Turchi, C.; Magrini, K. Kinetic and mechanistic overview of TiO2-photocatalyzed oxidation reactions in aqueous solution. Solar Energy Mater. 1991, 24, 584–593. [Google Scholar] [CrossRef]
- Li, Y.; Sun, S.; Ma, M.; Ouyang, Y.; Yan, W. Kinetic study and model of the photocatalytic degradation of rhodamine B (RhB) by a TiO2-coated activated carbon catalyst: Effects of initial RhB content, light intensity and TiO2 content in the catalyst. Chem. Eng. J. 2008, 142, 147–155. [Google Scholar] [CrossRef]
- Dominguez, S.; Ribao, P.; Rivero, M.J.; Ortiz, I. Influence of radiation and TiO2 concentration on the hydroxyl radicals generation in a photocatalytic LED reactor. Application to dodecylbenzenesulfonate degradation. Appl. Catal. B Environ. 2015, 178, 165–169. [Google Scholar] [CrossRef]
- Giraldo-Aguirre, A.L.; Erazo-Erazo, E.D.; Flórez-Acosta, O.A.; Serna-Galvis, E.A.; Torres-Palma, R.A. TiO2 photocatalysis applied to the degradation and antimicrobial activity removal of oxacillin: Evaluation of matrix components, experimental parameters, degradation pathways and identification of organics by-products. J. Photochem. Photobiol. A Chem. 2015, 311, 95–103. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Weng, C.-H.; Chen, F.-Y. Key operating parameters affecting photocatalytic activity of visible-light-induced C-doped TiO2 catalyst for ethylene oxidation. Chem. Eng. J. 2014, 248, 175–183. [Google Scholar] [CrossRef]
- Sun, Z.; Zheng, L.; Zheng, S.; Frost, R.L. Preparation and characterization of TiO2/acid leached serpentinite tailings composites and their photocatalytic reduction of Chromium (VI). J. Colloid Interface Sci. 2013, 404, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Albrbar, A.J.; Bjelajac, A.; Đokić, V.; Miladinović, J.; Janaćković, Đ.; Petrović, R. Photocatalytic efficiency of titania photocatalysts in saline waters. J. Serb. Chem. Soc. 2014, 79, 1127–1140. [Google Scholar] [CrossRef]
- Hernández, R.; Olvera-Rodríguez, I.; Guzmán, C.; Medel, A.; Escobar-Alarcón, L.; Brillas, E.; Sirés, I.; Esquivel, K. Microwave-assisted sol-gel synthesis of an Au-TiO2 photoanode for the advanced oxidation of paracetamol as model pharmaceutical pollutant. Electrochem. Commun. 2018, 96, 42–46. [Google Scholar] [CrossRef]
- Kumar, K.V.; Porkodi, K.; Rocha, F. Langmuir–Hinshelwood kinetics—A theoretical study. Catal. Commun. 2008, 9, 82–84. [Google Scholar] [CrossRef]
- Tanaka, H.; Sadamoto, T. The simultaneous determination of the kinetics and thermodynamics of Cu(OH)2 decomposition by means of TG and DSC. Thermochim. Acta 1982, 54, 273–280. [Google Scholar] [CrossRef]
- Sun, J.; Jing, Y.; Jia, Y.; Tillard, M.; Belin, C. Mechanism of preparing ultrafine copper powder by polyol process. Mater. Lett. 2005, 59, 3933–3936. [Google Scholar] [CrossRef]
- Li, D.; Haneda, H.; Hishita, S.; Ohashi, N. Visible-light-driven nitrogen-doped TiO2 photocatalysts: Effect of nitrogen precursors on their photocatalysis for decomposition of gas-phase organic pollutants. Mater. Sci. Eng. B 2005, 117, 67–75. [Google Scholar] [CrossRef]
- Yan, X.; He, J.; Evans, D.G.; Zhu, Y.; Duan, X. Preparation, Characterization and Photocatalytic Activity of TiO2 Formed from a Mesoporous Precursor. J. Porous Mater. 2004, 11, 131–139. [Google Scholar] [CrossRef]
- Linacero, R.; Aguado-Serrano, J.; Rojas-Cervantes, M. Preparation of mesoporous TiO2 by the sol-gel method assisted by surfactants. J. Mater. Sci. 2006, 41, 2457–2464. [Google Scholar] [CrossRef]
- Porkodi, K.; Arokiamary, S.D. Synthesis and spectroscopic characterization of nanostructured anatase titania: A photocatalyst. Mater. Charact. 2007, 58, 495–503. [Google Scholar] [CrossRef]
- Mohan, J. Organic Spectroscopy Principles and Applications, 2nd ed.; Alpha Science International Ltd.: Harrow, UK, 2007. [Google Scholar]
- Li, J.L.; Inui, T. Characterization of precursors of methanol synthesis catalysts, copper/zinc/aluminum oxides, precipitated at different pHs and temperatures. Appl. Catal. A Gen. 1996, 137, 105–117. [Google Scholar] [CrossRef]
- Caudillo-Flores, U.; Muñoz-Batista, M.J.; Kubacka, A.; Zárate-Medina, J.; Cortés, J.A.; Fernández-García, M. Measuring and interpreting quantum efficiency of acid blue 9 photodegradation using TiO2-based catalysts. Appl. Catal. A Gen. 2018, 550, 38–47. [Google Scholar] [CrossRef]
- Brunauer, S.; Deming, L.S.; Deming, W.E.; Teller, E. On a theory of the van der Waals adsorption of gases. J. Am. Chem. Soc. 1940, 62, 1723–1732. [Google Scholar] [CrossRef]
- Chen, Y.-F.; Lee, C.-Y.; Yeng, M.-Y.; Chiu, H.-T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Jiang, F.; Zheng, S.; An, L.; Chen, H. Effect of calcination temperature on the adsorption and photocatalytic activity of hydrothermally synthesized TiO2 nanotubes. Appl. Surface Sci. 2012, 258, 7188–7194. [Google Scholar] [CrossRef]
- Bauer, A.; Lee, K.; Song, C.; Xie, Y.; Zhang, J.; Hui, R. Pt nanoparticles deposited on TiO2 based nanofibers: Electrochemical stability and oxygen reduction activity. J. Power Sour. 2010, 195, 3105–3110. [Google Scholar] [CrossRef]
- Xie, M.; Jing, L.; Zhou, J.; Lin, J.; Fu, H. Synthesis of nanocrystalline anatase TiO2 by one-pot two-phase separated hydrolysis-solvothermal processes and its high activity for photocatalytic degradation of rhodamine B. J. Hazard. Mater. 2010, 176, 139–145. [Google Scholar] [CrossRef]
- Sreethawong, T.; Suzuki, Y.; Yoshikawa, S. Photocatalytic evolution of hydrogen over mesoporous TiO2 supported NiO photocatalyst prepared by single-step sol-gel process with surfactant template. Int. J. Hydrogen Energy 2005, 30, 1053–1062. [Google Scholar] [CrossRef]
- Fernández-García, M.; Wang, X.; Belver, C.; Hanson, J.C.; Rodriguez, J.A. Anatase-TiO2 nanomaterials: Morphological/size dependence of the crystallization and phase behavior phenomena. J. Phys. Chem. C 2007, 111, 674–682. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, Y.; Zhao, X.; Wan, H.; Yang, L.; Hong, J.; Yu, Q.; Dong, L.; Chen, Y.; Jian, C.; et al. Influence of impregnation times on the dispersion of CuO on anatase. J. Mol. Catal. A Chem. 2006, 243, 24–30. [Google Scholar] [CrossRef]
- Pang, S.; Huang, J.G.; Su, Y.; Geng, B.; Lei, S.Y.; Huang, Y.T.; Lyu, C.; Liu, X.J. Synthesis and Modification of Zn-doped TiO2 Nanoparticles for the Photocatalytic Degradation of Tetracycline. Photochem. Photobiol. 2016, 92, 651–657. [Google Scholar] [CrossRef]
- Li, P.; Liu, C.; Wu, G.; Heng, Y.; Lin, S.; Ren, A.; Lv, K.; Xiao, L.; Shi, W. Solvothermal synthesis and visible light-driven photocatalytic degradation for tetracycline of Fe-doped SrTiO3. RSC Adv. 2014, 4, 47615–47624. [Google Scholar] [CrossRef]
- Riaz, N.; Bustam, M.A.; Chong, F.K.; Man, Z.B.; Khan, M.S.; Shariff, A.M. Photocatalytic Degradation of DIPA Using Bimetallic Cu-Ni/TiO2 Photocatalyst under Visible Light Irradiation. Sci. World J. 2014, 2014, 8. [Google Scholar] [CrossRef]
- Riaz, N.; Chong, F.K.; Man, Z.B.; Khan, M.S.; Dutta, B.K. Photodegradation of Orange II under Visible Light Using Cu–Ni/TiO2: Influence of Cu:Ni Mass Composition, Preparation, and Calcination Temperature. Ind. Eng. Chem. Res. 2013, 52, 4491–4503. [Google Scholar] [CrossRef]
- Kobayakawa, K.; Sato, C.; Sato, Y.; Fujishima, A. Continuous-flow photoreactor packed with titanium dioxide immobilized on large silica gel beads to decompose oxalic acid in excess water. J. Photochem. Photobiol. A Chem. 1998, 118, 65–69. [Google Scholar] [CrossRef]
- Mushtaq, S.; Khan, J.A.; Rabbani, F.; Latif, U.; Arfan, M.; Yameen, M.A. Biocompatible biodegradable polymeric antibacterial nanoparticles for enhancing the effects of a third-generation cephalosporin against resistant bacteria. J. Med. Microbiol. 2017, 66, 318–327. [Google Scholar] [CrossRef]
- Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.; Bartlett, J.; Nolan, M.; Pillai, S. Cu-doped TiO2: Visible light assisted photocatalytic antimicrobial activity. Appl. Sci. 2018, 8, 2067. [Google Scholar] [CrossRef]
- Iftikhar, A.; Khan, M.S.; Rashid, U.; Mahmood, Q.; Zafar, H.; Bilal, M.; Riaz, N. Influence of metallic species for efficient photocatalytic water disinfection: Bactericidal mechanism of in vitro results using docking simulation. Environ. Sci. Pollut. Res. 2020, 27, 39819–39831. [Google Scholar] [CrossRef]
- Thakur, I.; Örmeci, B.; verma, A. Inactivation of E. coli in water employing Fe-TiO2 composite incorporating in-situ dual process of photocatalysis and photo-Fenton in fixed-mode. J. Water Process Eng. 2020, 33, 101085. [Google Scholar] [CrossRef]
- Azbar, N.; Yonar, T.; Kestioglu, K. Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 2004, 55, 35–43. [Google Scholar] [CrossRef]
- Gaber, A.; Refat, M.S.; Belal, A.A.M.; El-Deen, I.M.; Hassan, N.; Zakaria, R.; Alhomrani, M.; Alamri, A.S.; Alsanie, W.F.; Saied, M.E. New Mononuclear and Binuclear Cu(II), Co(II), Ni(II), and Zn(II) Thiosemicarbazone complexes with potential biological activity: Antimicrobial and molecular docking study. Molecules 2021, 26, 2288. [Google Scholar] [CrossRef] [PubMed]
- Barelier, S.; Eidam, O.; Fish, I.; Hollander, J.; Figaroa, F.; Nachane, R.; Irwin, J.J.; Shoichet, B.K.; Siegal, G. Increasing chemical space coverage by combining empirical and computational fragment screens. ACS Chem. Biol. 2014, 9, 1528–1535. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Strynadka, N.C. Structural basis for the β lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Biol. 2002, 9, 870–876. [Google Scholar] [CrossRef] [PubMed]
- Chemical Computing Group, M. Molecular Operating Environment; Chemical Computing Group: Montreal, QC, Canada, 2008. [Google Scholar]
- Scherrer, P. Determination of the size and internal structure of colloid particles by X-rays. Nachr. Ges. Wiss. Gött. 1918, 2, 98–100. [Google Scholar]




| Metal Composition | Calcination (°C) | % COD Removal | ||
|---|---|---|---|---|
| NC Preparation Methods | ||||
| WI | Co–P | SG | ||
| 0Fe-0.1Zn | 300 | 21.70 | 20.60 | 18.96 |
| 0.1Fe-0.4Zn | 300 | 66.50 | 30.49 | 21.84 |
| 0.2Fe-0.3Zn | 300 | 21.57 | 18.54 | 18.96 |
| 0.3Fe-0.2Zn | 300 | 20.60 | 21.70 | 21.70 |
| 0.4Fe-0.1Zn | 300 | 22.80 | 22.66 | 22.12 |
| 0.5Fe-0Zn | 300 | 24.73 | 28.43 | 27.20 |
| NC | Calcination (°C) | Energy Efficiency of NC with Respect to Synthesis Method (KW h m−3) | ||
|---|---|---|---|---|
| WI | Co-P | SG | ||
| 0.0Fe-0.1Zn-TiO2 | 300 | 2043.61 | 2167.06 | 2378.93 |
| 0.1Fe-0.4Zn-TiO2 | 300 | 457.40 | 1374.52 | 2029.05 |
| 0.2Fe-0.3Zn-TiO2 | 300 | 2058.36 | 2437.76 | 2378.93 |
| 0.3Fe-0.2Zn-TiO2 | 300 | 2167.06 | 2043.61 | 2043.61 |
| 0.4Fe-0.1Zn-TiO2 | 300 | 1932.00 | 1945.36 | 2000.47 |
| 0.5Fe-0.0Zn-TiO2 | 300 | 1760.40 | 1494.54 | 1575.18 |
| Parameters | Calcination Temperature | |||
|---|---|---|---|---|
| TA | 200 | 300 | 400 | |
| SABET (m2·g−1) | 17.43 | 7.42 | 55.99 | 8.37 |
| Total pore volume (cm3·g−1) | 0.054 | 0.054 | 0.100 | 0.051 |
| Average pore diameter (nm) | 12.06 | 29.19 | 7.160 | 24.346 |
| Photocatalysts | Crystallite Size (nm) | Composition of Phases (%) | ||
|---|---|---|---|---|
| Anatase | Rutile | Anatase (ωA) | Rutile (ωR) | |
| TiO2 (A) | 38.39 | 0.00 | 100.00 | 0.00 |
| TiO2 (A&R) | 14.78 | 15.47 | 80.07 | 19.93 |
| SG-0 | 18.75 | 0.00 | 100.00 | 0.00 |
| 0.1Fe-0.4Zn-TiO2-200 | 41.53 | 41.21 | 75.66 | 24.34 |
| 0.1Fe-0.4Zn-TiO2-300 | 41.11 | 35.49 | 74.01 | 25.99 |
| 0.1Fe-0.4Zn-TiO2-400 | 41.10 | 37.62 | 73.59 | 26.41 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, N.; Fen, D.A.C.S.; Khan, M.S.; Naz, S.; Sarwar, R.; Farooq, U.; Bustam, M.A.; Batiha, G.E.-S.; El Azab, I.H.; Uddin, J.; et al. Iron-Zinc Co-Doped Titania Nanocomposite: Photocatalytic and Photobiocidal Potential in Combination with Molecular Docking Studies. Catalysts 2021, 11, 1112. https://doi.org/10.3390/catal11091112
Riaz N, Fen DACS, Khan MS, Naz S, Sarwar R, Farooq U, Bustam MA, Batiha GE-S, El Azab IH, Uddin J, et al. Iron-Zinc Co-Doped Titania Nanocomposite: Photocatalytic and Photobiocidal Potential in Combination with Molecular Docking Studies. Catalysts. 2021; 11(9):1112. https://doi.org/10.3390/catal11091112
Chicago/Turabian StyleRiaz, Nadia, Debra Adelina Chia Siew Fen, Muhammad Saqib Khan, Sadia Naz, Rizwana Sarwar, Umar Farooq, Mohamad Azmi Bustam, Gaber El-Saber Batiha, Islam H. El Azab, Jalal Uddin, and et al. 2021. "Iron-Zinc Co-Doped Titania Nanocomposite: Photocatalytic and Photobiocidal Potential in Combination with Molecular Docking Studies" Catalysts 11, no. 9: 1112. https://doi.org/10.3390/catal11091112
APA StyleRiaz, N., Fen, D. A. C. S., Khan, M. S., Naz, S., Sarwar, R., Farooq, U., Bustam, M. A., Batiha, G. E.-S., El Azab, I. H., Uddin, J., & Khan, A. (2021). Iron-Zinc Co-Doped Titania Nanocomposite: Photocatalytic and Photobiocidal Potential in Combination with Molecular Docking Studies. Catalysts, 11(9), 1112. https://doi.org/10.3390/catal11091112










