Catalytic Activity Enhancement of Cu-Zn-Based Catalyst for Methanol Steam Reforming with Magnetic Inducement
Abstract
:1. Introduction
2. Results
2.1. Effect of CeO2-Al2O3 Supports Prepared under Magnetic Inducement on H2 Production
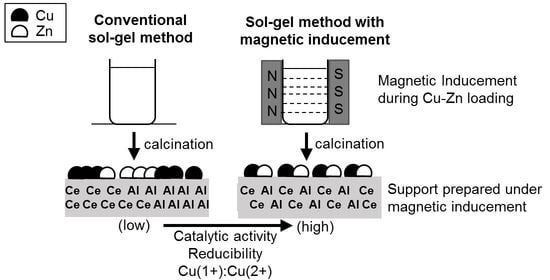
2.2. Effects of Magnetic Inducement during Cu-Zn Active Metal Loading
2.2.1. Hydrogen Production of Cu-Zn Catalysts Prepared with Magnetic Inducement during Metal Loading
2.2.2. Temperature-Programmed Reduction (TPR) Profiles of Catalysts Prepared under Magnetic Inducement
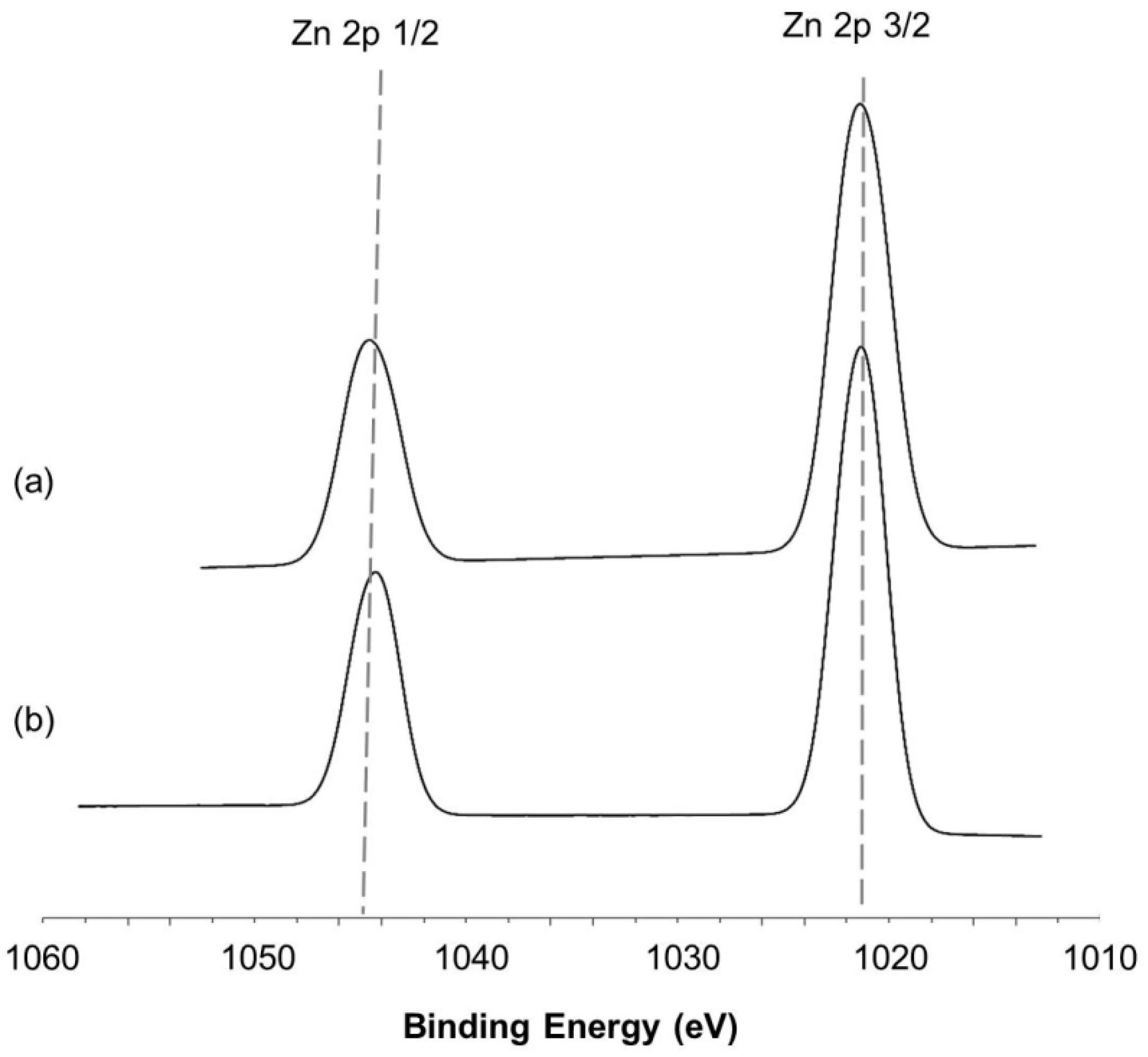
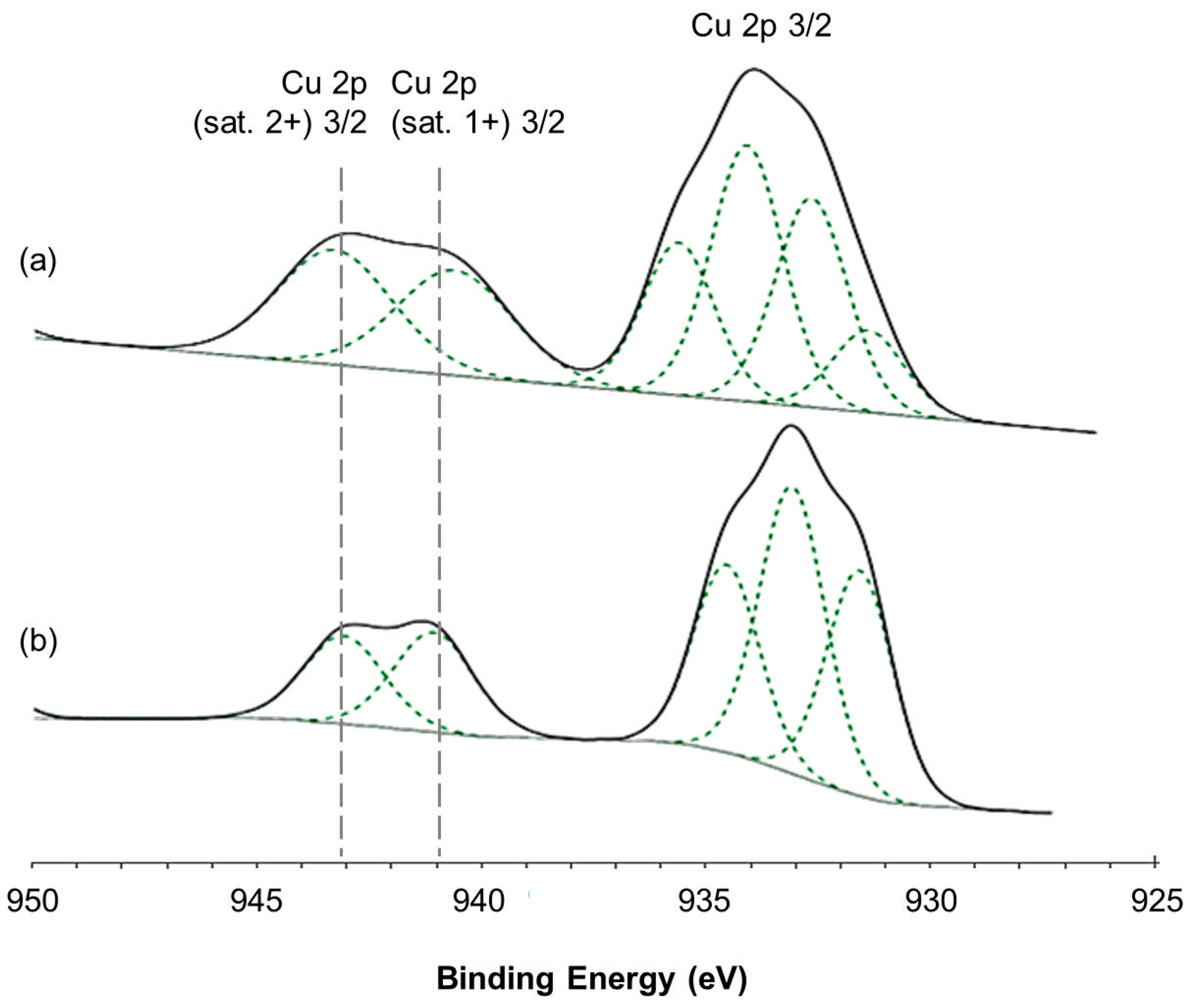
2.2.3. X-ray Photoelectron Spectroscopy (XPS) Spectra of Catalysts Loaded under Magnetic Inducement
3. Discussion
4. Materials and Methods
4.1. Preparation of γ-Al2O3 Support
4.2. Preparation of CeO2-Al2O3 Supports under Magnetic Inducement
4.3. Cu-Zn Metal Loading under Magnetic Inducement on γ-Al2O3 and CeO2-Al2O3 Supports
4.4. Catalytic Activity Testing for H2 Production from MSR
4.5. Support and Catalyst Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sá, S.; Silva, H.; Brandão, L.; Sousa, J.M.; Mendes, A. Catalysts for methanol steam reforming—A review. Appl. Catal. B Environ. 2010, 99, 43–57. [Google Scholar] [CrossRef]
- Pérez-Hernández, R.; Mendoza-Anaya, D.; Gutiérrez Martínez, A.; Gómez-Cortés, A. Catalytic Steam Reforming of Methanol to Produce Hydrogen on Supported Metal Catalysts. In Hydrogen Energy—Challenges and Perspectives; Minić, D., Ed.; IntechOpen: London, UK, 2012; pp. 149–174. ISBN 978-953-51-4271-3. [Google Scholar]
- Madej-Lachowska, M.; Kulawska, M.; Słoczyński, J. Methanol as a high purity hydrogen source for fuel cells: A brief review of catalysts and rate expressions. Chem. Process Eng. 2017, 38, 147–162. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol Steam Reforming for Hydrogen Production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef]
- Papavasiliou, J.; Paxinou, A.; Słowik, G.; Neophytides, S.; Avgouropoulos, G. Steam Reforming of Methanol over Nanostructured Pt/TiO2 and Pt/CeO2 Catalysts for Fuel Cell Applications. Catalysts 2018, 8, 544. [Google Scholar] [CrossRef] [Green Version]
- Tahay, P.; Khani, Y.; Jabari, M.; Bahadoran, F.; Safari, N. Highly porous monolith/TiO2 supported Cu, Cu-Ni, Ru, and Pt catalysts in methanol steam reforming process for H2 generation. Appl. Catal. A Gen. 2018, 554, 44–53. [Google Scholar] [CrossRef]
- Rameshan, C.; Stadlmayr, W.; Penner, S.; Lorenz, H.; Memmel, N.; Hävecker, M.; Blume, R.; Teschner, D.; Rocha, T.; Zemlyanov, D.; et al. Hydrogen Production by Methanol Steam Reforming on Copper Boosted by Zinc-Assisted Water Activation. Angew. Chem. Int. Ed. Engl. 2012, 51, 3002–3006. [Google Scholar] [CrossRef] [Green Version]
- Mrad, M.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E. Cu/Zn-based catalysts for H2 production via steam reforming of methanol. Catal. Today 2011, 176, 88–92. [Google Scholar] [CrossRef]
- Lindström, B.; Pettersson, L.J.; Menon, P.G. Activity and characterization of Cu/Zn, Cu/Cr and Cu/Zr on γ-alumina for methanol reforming for fuel cell vehicles. Appl. Catal. A Gen. 2002, 234, 111–125. [Google Scholar] [CrossRef]
- Turco, M.; Bagnasco, G.; Costantino, U.; Marmottini, F.; Montanari, T.; Ramis, G.; Busca, G. Production of hydrogen from oxidative steam reforming of methanol I. Preparation and characterization of Cu/ZnO/Al2O3 catalysts from a hydrotalcite-like LDH precursor. J. Catal. 2004, 228, 43–55. [Google Scholar] [CrossRef]
- Shishido, T.; Yamamoto, Y.; Morioka, H.; Takaki, K.; Takehira, K. Active Cu/ZnO and Cu/ZnO/Al2O3 catalysts prepared by homogeneous precipitation method in steam reforming of methanol. Appl. Catal. A Gen. 2004, 263, 249–253. [Google Scholar] [CrossRef]
- Mrad, M.; Hammoud, D.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E. A comparative study on the effect of Zn addition to Cu/Ce and Cu/Ce-Al catalysts in the steam reforming of methanol. Appl. Catal. A Gen. 2014, 471, 84–90. [Google Scholar] [CrossRef]
- Chiu, K.L.; Kwong, F.L.; Ng, D.H.L. Oxidation states of Cu in the CuO/CeO2/Al2O3 catalyst in the methanol steam reforming process. Curr. Appl. Phys. 2012, 12, 1195–1198. [Google Scholar] [CrossRef]
- Oguchi, H.; Kanai, H.; Utani, K.; Matsumura, Y.; Imamura, S. Cu2O as active species in the steam reforming of methanol by CuO/ZrO2 catalysts. Appl. Catal. A Gen. 2005, 293, 64–70. [Google Scholar] [CrossRef]
- Kurr, P.; Kasatkin, I.; Girgsdies, F.; Trunschke, A.; Schlögl, R.; Ressler, T. Microstructural characterization of Cu/ZnO/Al2O3 catalysts for methanol steam reforming—A comparative study. Appl. Catal. A Gen. 2008, 348, 153–164. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Huang, C.; Zong, L.; Lu, K.; Wang, X.; Cai, J. Hydrogen Production from Methanol Steam Reforming over TiO2 and CeO2 Pillared Clay Supported Au Catalysts. Appl. Sci. 2018, 8, 176. [Google Scholar] [CrossRef] [Green Version]
- Yao, C.Z.; Wang, L.C.; Liu, Y.M.; Wu, G.S.; Cao, Y.; Dai, W.L.; He, H.Y.; Fan, K.N. Effect of preparation method on the hydrogen production from methanol steam reforming over binary Cu/ZrO2 catalysts. Appl. Catal. A Gen. 2006, 297, 151–158. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Co-precipitation, impregnation and so-gel preparation of Ni catalysts for pyrolysis-catalytic steam reforming of waste plastics. Appl. Catal. B Environ. 2018, 239, 565–577. [Google Scholar] [CrossRef]
- Sharifi, M.; Haghighi, M.; Rahmani, F.; Karimipour, S. Syngas production via dry reforming of CH4 over Co- and Cu-promoted Ni/Al2O3-ZrO2 nanocatalysts synthesized via sequential impregnation and sol-gel methods. J. Nat. Gas Sci. Eng. 2014, 21, 993–1004. [Google Scholar] [CrossRef]
- Fornari, A.C.; Menechini Neto, R.; Lenzi, G.G.; dos Santos, O.A.A.; de Matos Jorge, L.M. Utilization of sol-gel CuO-ZnO-Al2O3 catalysts in the methanol steam reforming for hydrogen production. Can. J. Chem. Eng. 2017, 95, 2258–2271. [Google Scholar] [CrossRef]
- Li, H.; Liao, J.; Du, Y.; You, T.; Liao, W.; Wena, L. Magnetic-field-induced deposition to fabricate multifunctional nanostructured Co, Ni, and CoNi alloy films as catalysts, ferromagnetic and superhydrophobic materials. Chem. Commun. 2013, 49, 1768–1770. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wei, J.; Bonville, P.; Pileni, M.P. Engineering the Magnetic Dipolar Interactions in 3D Binary Supracrystals Via Mesoscale Alloying. Adv. Funct. Mater. 2015, 25, 4908–4915. [Google Scholar] [CrossRef]
- Lalatonne, Y.; Motte, L.; Russier, V.; Ngo, A.T.; Bonville, P.; Pileni, M.P. Mesoscopic Structures of Nanocrystals: Collective Magnetic Properties Due to the Alignment of Nanocrystals. J. Phys. Chem. B 2004, 108, 1848–1854. [Google Scholar] [CrossRef]
- Yang, Z.; Wei, J.; Bonville, P.; Pileni, M.P. Beyond Entropy: Magnetic Forces Induce Formation of Quasicrystalline Structure in Binary Nanocrystal Superlattices. J. Am. Chem. Soc. 2015, 137, 4487–4493. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Chie, K.; Sawai, J.; Shimizu, D.; Tanimoto, Y. On the Movement of Paramagnetic Ions in an Inhomogeneous Magnetic Field. J. Phys. Chem. B 2004, 108, 3531–3534. [Google Scholar] [CrossRef]
- Fujiwara, M.; Mitsuda, K.; Tanimoto, Y. Movement and Diffusion of Paramagnetic Ions in a Magnetic Field. J. Phys. Chem. B 2006, 110, 13965–13969. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.-P.; Hejiazan, M.; Huang, X.; Nguyen, N.-T. Magnetophoresis of diamagnetic microparticles in a weak magnetic field. Lab Chip 2014, 14, 4609–4615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vacharapong, P.; Arayawate, S.; Henpraserttae, S.; Katanyutanon, S.; Charojrochkul, S.; Lawtrakul, L.; Toochinda, P. Effect of Magnetic Inducement in Preparation of Ni/Ce-doped Al2O3 for Ammonia Decomposition. ChemistrySelect 2019, 4, 11913–11919. [Google Scholar] [CrossRef]
- Vacharapong, P.; Arayawate, S.; Katanyutanon, S.; Toochinda, P.; Lawtrakul, L.; Charojrochkul, S. Enhancement of Ni Catalyst Using CeO2–Al2O3 Support Prepared with Magnetic Inducement for ESR. Catalysts 2020, 10, 1357. [Google Scholar] [CrossRef]
- Mrad, M.; Gennequin, C.; Aboukaïs, A.; Abi-Aad, E. The performance of the Cu-Zn/CeO2 catalysts in the steam reforming of methanol reaction. Adv. Mater. Res. 2011, 324, 157–161. [Google Scholar] [CrossRef]
- Liu, Y.; Hayakawa, T.; Tsunoda, T.; Suzuki, K.; Hamakawa, S.; Murata, K.; Shiozaki, R.; Ishii, T.; Kumagai, M. Steam reforming of methanol over Cu/CeO2 catalysts studied in comparison with Cu/ZnO and Cu/Zn(Al)O catalysts. Top. Catal. 2003, 22, 205–213. [Google Scholar] [CrossRef]
- Avgouropoulos, G.; Ioannides, T. Selective CO oxidation over CuO-CeO2 catalysts prepared via the urea-nitrate combustion method. Appl. Catal. A Gen. 2003, 244, 155–167. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, B.; Li, Y.; Xu, Y.; Xin, Q.; Shen, W. Carbon monoxide oxidation over CuO/CeO2 catalysts. Catal. Today 2004, 93–95, 191–198. [Google Scholar] [CrossRef]
- Zheng, X.; Zhang, X.; Wang, X.; Wang, S.; Wu, S. Preparation and characterization of CuO/CeO2 catalysts and their applications in low-temperature CO oxidation. Appl. Catal. A Gen. 2005, 295, 142–149. [Google Scholar] [CrossRef]
- Li, L.; Zhan, Y.; Zheng, Q.; Zheng, Y.; Lin, X.; Li, D.; Zhu, J. Water-Gas Shift Reaction Over Aluminum Promoted Cu/CeO2 Nanocatalysts Characterized by XRD, BET, TPR and Cyclic Voltammetry (CV). Catal. Lett. 2007, 118, 91–97. [Google Scholar] [CrossRef]
- Tonelli, F.; Gorriz, O.; Arrúa, L.; Abello, M.C. Methanol steam reforming over Cu/CeO2 catalysts. Influence of zinc addition. Quim. Nova 2011, 34, 1334–1338. [Google Scholar] [CrossRef] [Green Version]
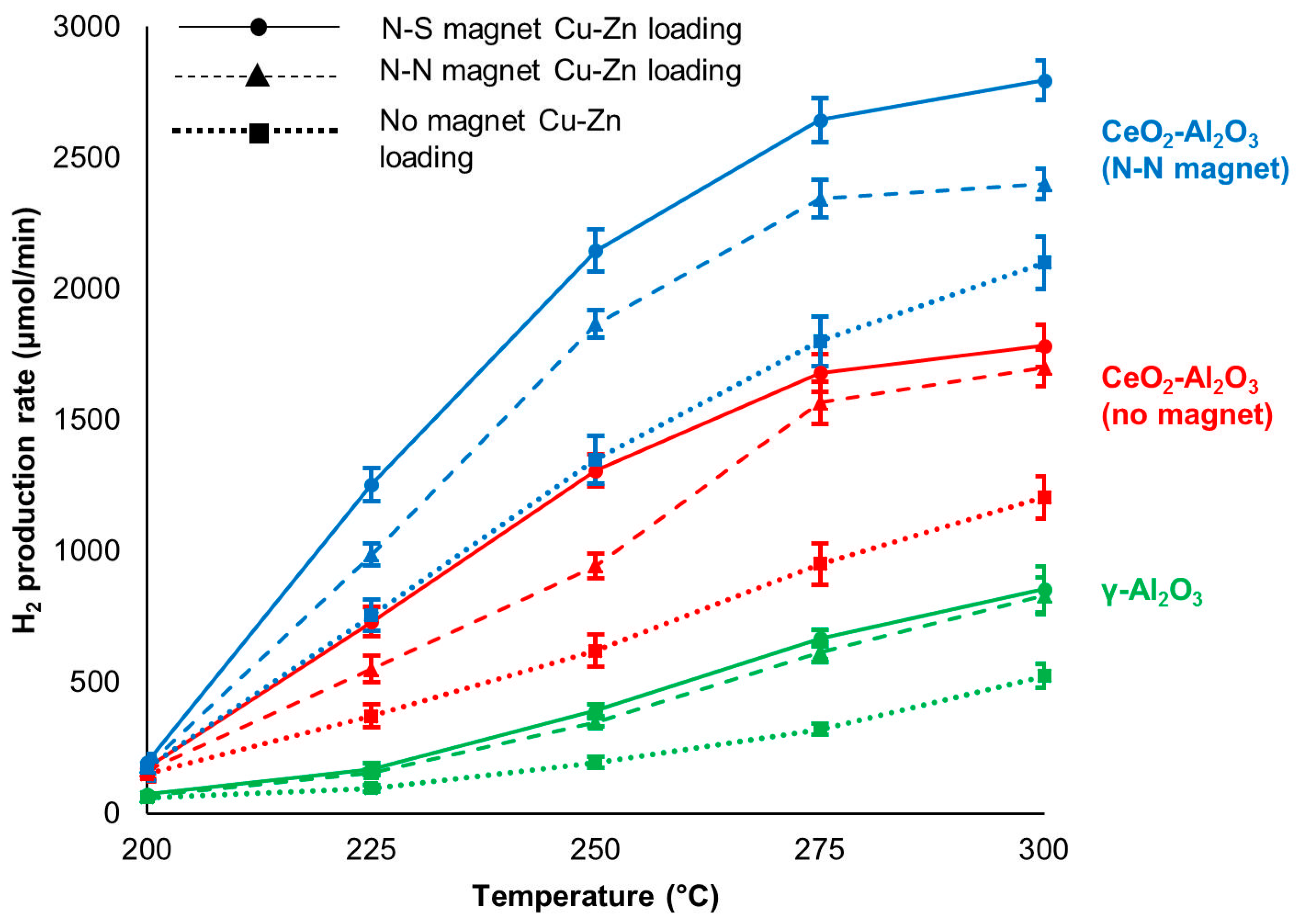

| Magnetic Inducement | H2 Production Rate (µmol/min) | ||||
|---|---|---|---|---|---|
| 200 °C | 225 °C | 250 °C | 275 °C | 300 °C | |
| No magnet | 150 ± 30 | 371 ± 43 | 619 ± 61 | 850 ± 80 | 1095 ± 81 |
| N-N | 175 ± 50 | 754 ± 60 | 1349 ± 99 | 1800 ± 97 | 2099 ± 98 |
| N-S | 167 ± 42 | 530 ± 40 | 916 ± 42 | 1388 ± 72 | 2002 ± 76 |
| Support | Magnetic Inducement | Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter |
|---|---|---|---|---|
| γ-Al2O3 | No magnet | 180.5 | 0.31 | 69.2 |
| CeO2 | No magnet | 46.6 | 0.11 | 80.0 |
| CeO2-Al2O3 | No magnet | 142.1 | 0.32 | 86.3 |
| N-N | 134.2 | 0.31 | 84.6 | |
| N-S | 139.5 | 0.33 | 85.4 |
| Catalysts | Peak Area (%) | Area Ratio Cu(1+):Cu(2+) | |
|---|---|---|---|
| Cu (sat. 1+) | Cu (sat. 2+) | ||
| Cu-Zn (no magnet) /CeO2-Al2O3 (no magnet) | 10.68 | 11.67 | 0.92 |
| Cu-Zn (N-S) /CeO2-Al2O3 (N-N) | 8.69 | 7.67 | 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katanyutanon, S.; Samarasinghe, D.; Lawtrakul, L.; Toochinda, P. Catalytic Activity Enhancement of Cu-Zn-Based Catalyst for Methanol Steam Reforming with Magnetic Inducement. Catalysts 2021, 11, 1110. https://doi.org/10.3390/catal11091110
Katanyutanon S, Samarasinghe D, Lawtrakul L, Toochinda P. Catalytic Activity Enhancement of Cu-Zn-Based Catalyst for Methanol Steam Reforming with Magnetic Inducement. Catalysts. 2021; 11(9):1110. https://doi.org/10.3390/catal11091110
Chicago/Turabian StyleKatanyutanon, Sasimas, Dilpium Samarasinghe, Luckhana Lawtrakul, and Pisanu Toochinda. 2021. "Catalytic Activity Enhancement of Cu-Zn-Based Catalyst for Methanol Steam Reforming with Magnetic Inducement" Catalysts 11, no. 9: 1110. https://doi.org/10.3390/catal11091110
APA StyleKatanyutanon, S., Samarasinghe, D., Lawtrakul, L., & Toochinda, P. (2021). Catalytic Activity Enhancement of Cu-Zn-Based Catalyst for Methanol Steam Reforming with Magnetic Inducement. Catalysts, 11(9), 1110. https://doi.org/10.3390/catal11091110