Transition Metal Ions as Ozonation Catalysts: An Alternative Process of Heterogeneous Catalytic Ozonation
Abstract
1. Introduction
2. Results and Discussion
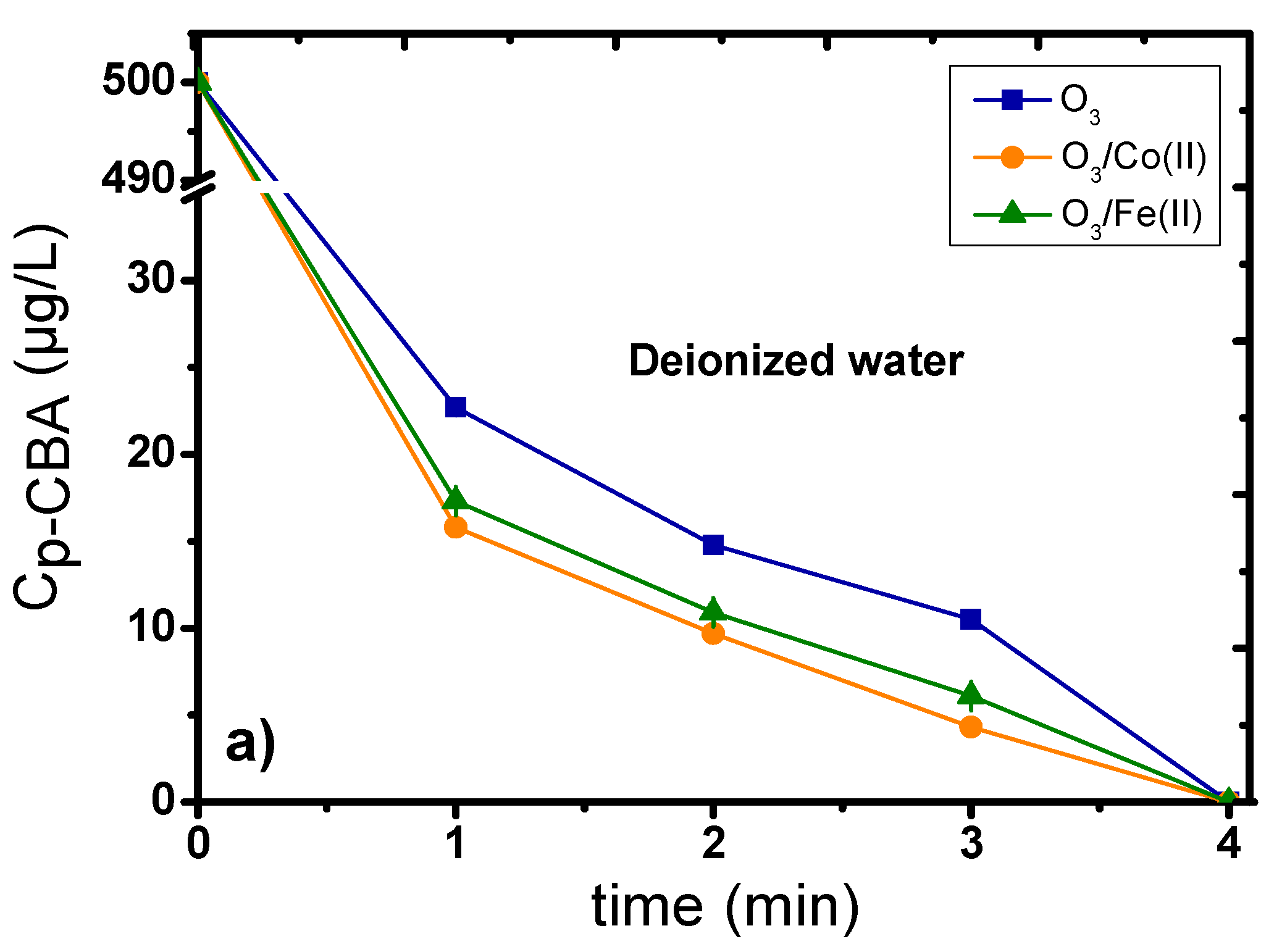
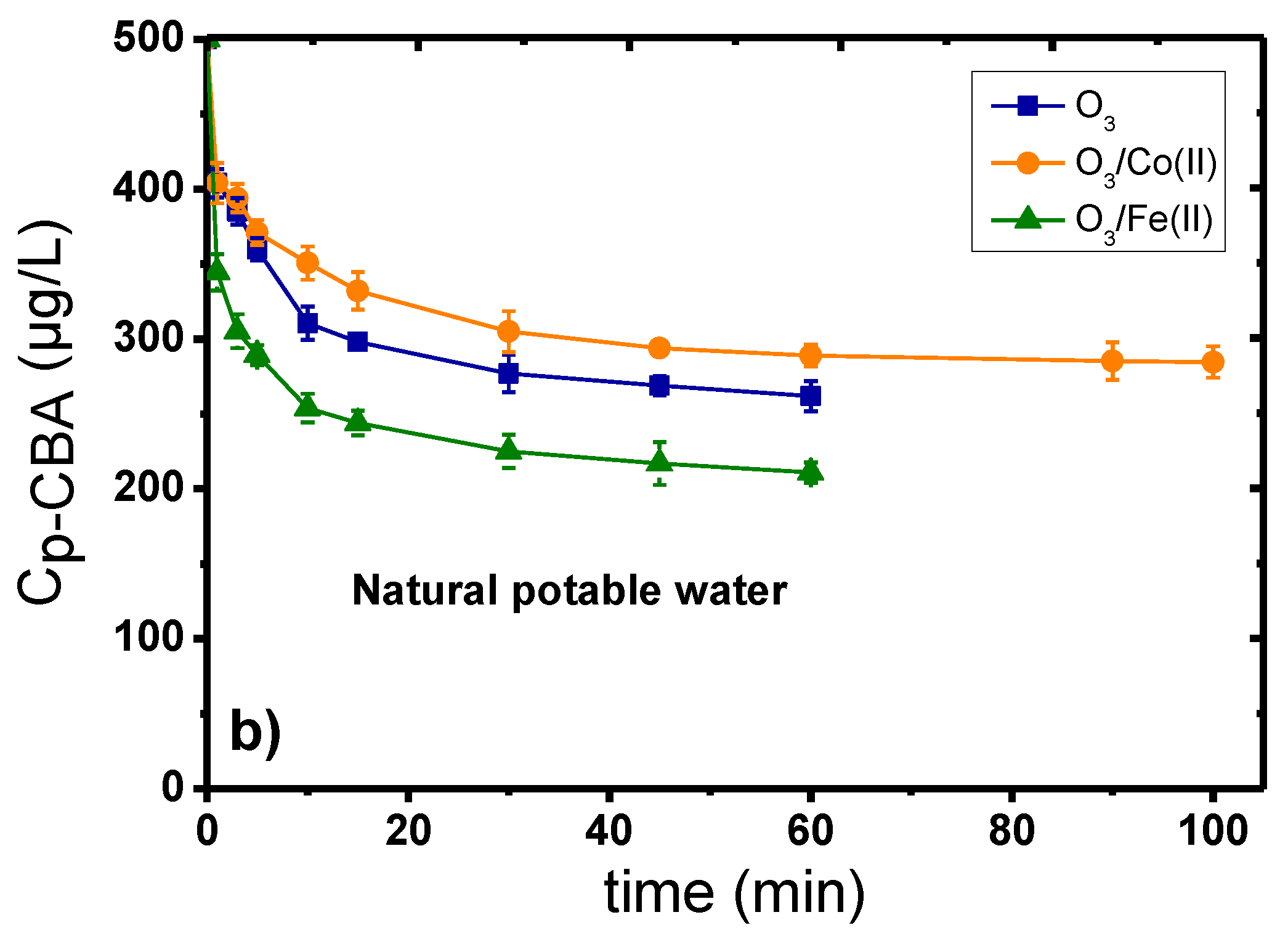
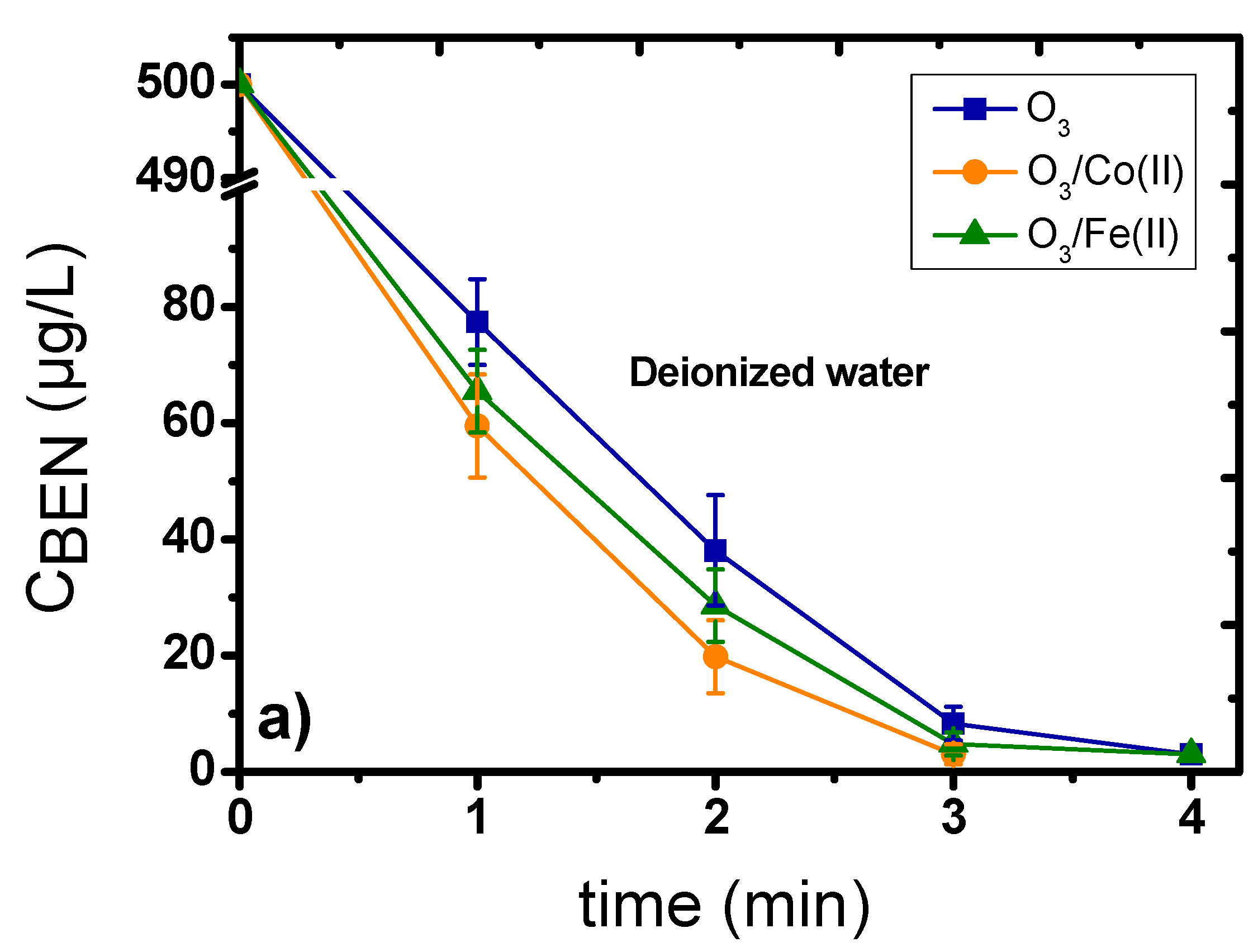
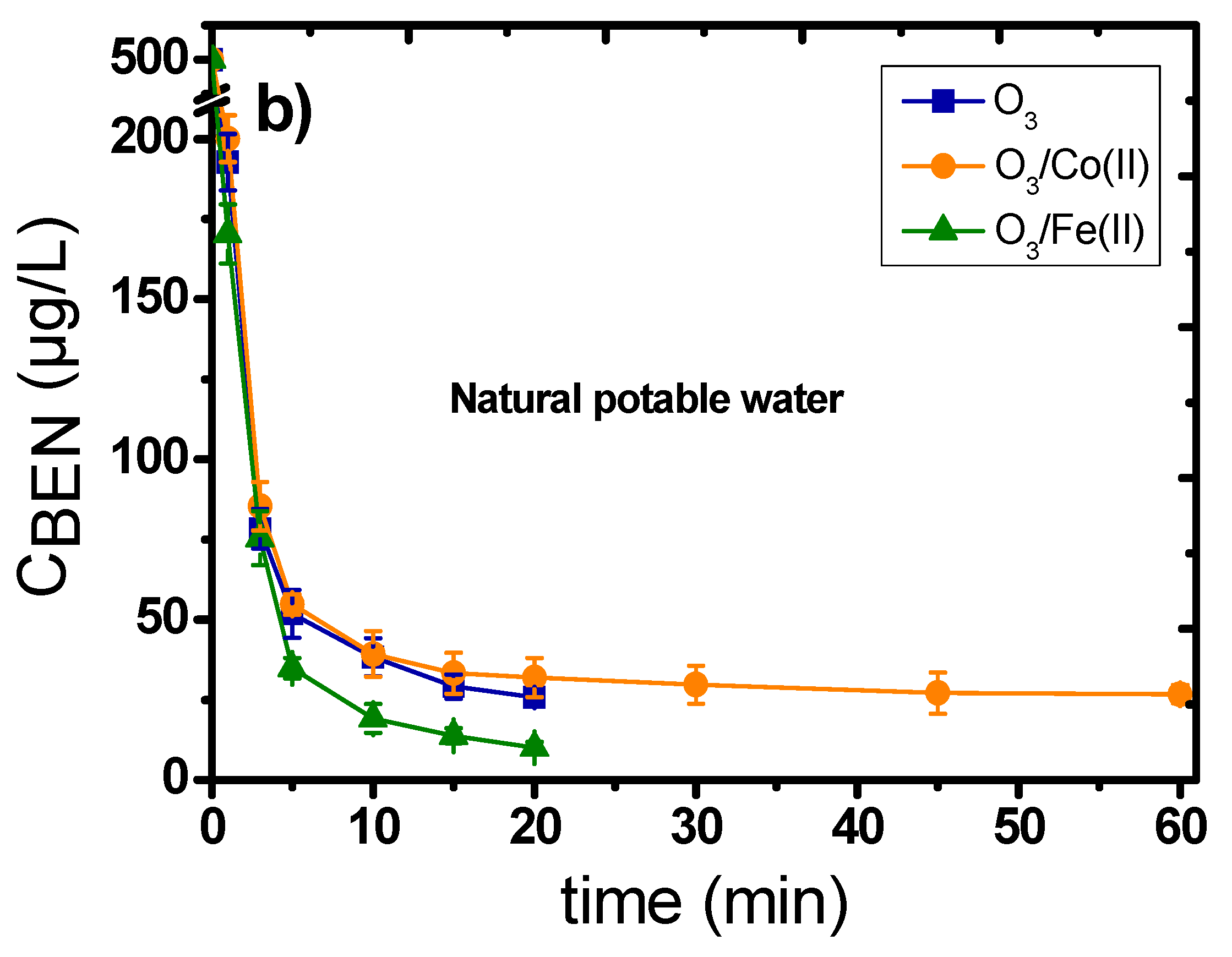
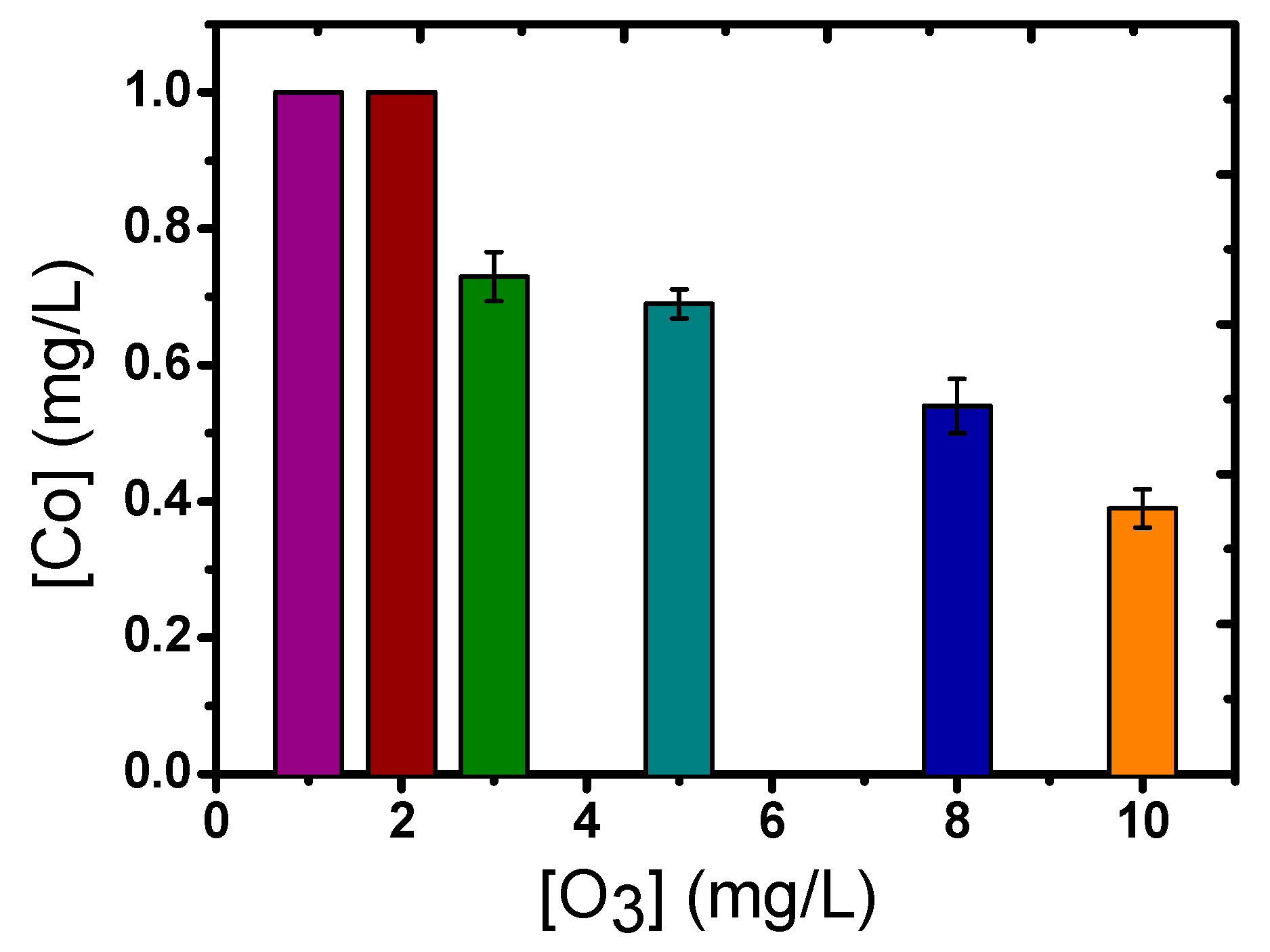
2.1. Catalytic Ozonation with Fe(II) and Co(II) as Catalysts—The Role of Aqueous Matrix
- The presence of dissolved metal ions can decompose ozone, enhancing the production of •OH, which can subsequently more effectively oxidize the organic compounds/pollutants.
- The added metal ions can create intermediate complexes with the micropollutants; hence, subsequently favoring their more efficient oxidation by the ozone molecules.
- The oxidation of dissolved metals under the presence of highly oxidative conditions can lead to the formation of oxides/oxy-hydroxides at the nano-scale range (1–5 nm), which can further improve the decomposition of ozone and the subsequent production of the more oxidative •OH agents.
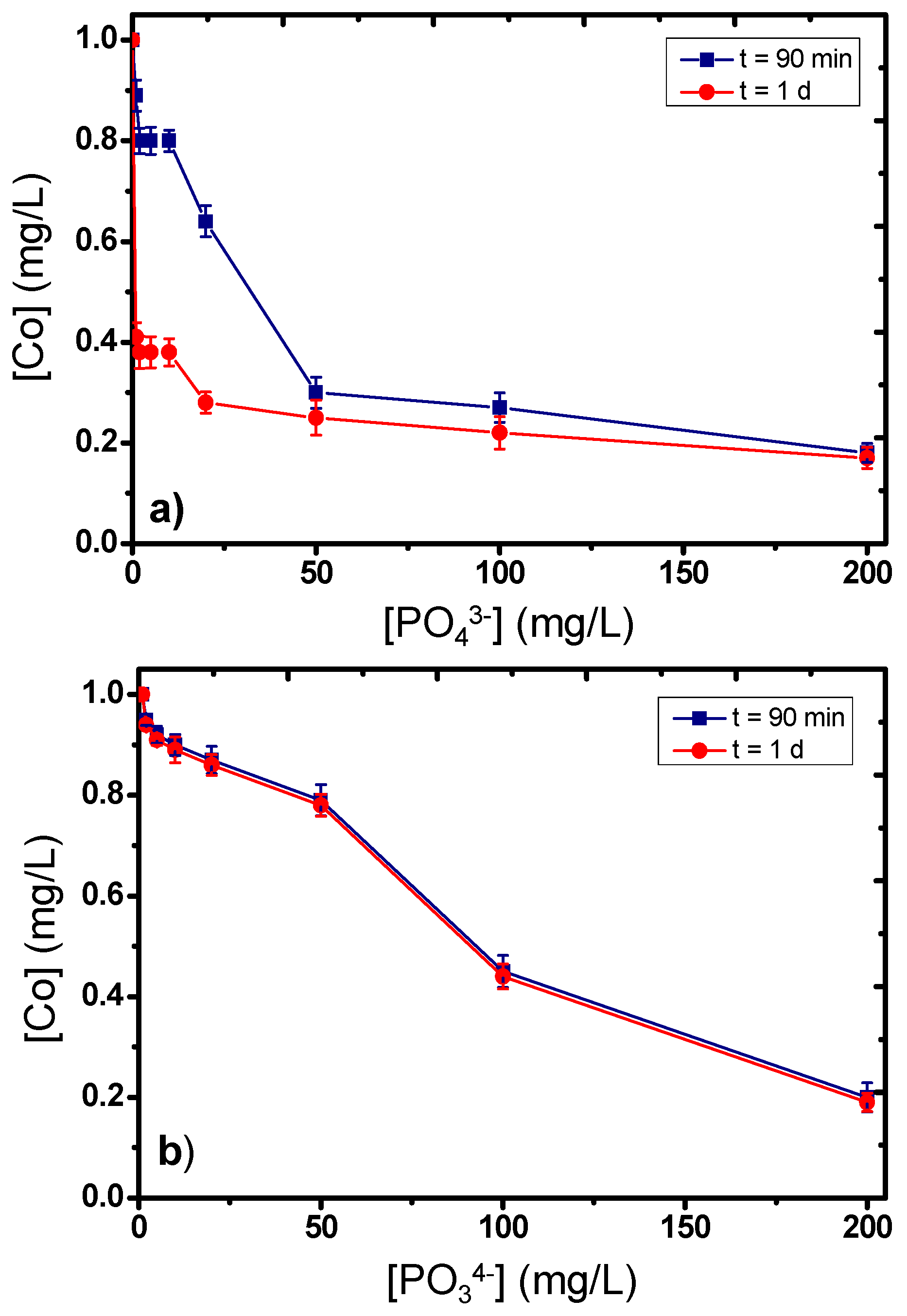
2.2. The Influence of Phosphate Ions Regarding the Catalytic Activity of Co(II)
3. Materials and Methods
3.1. Materials
3.2. Ozonation Procedure
3.3. Analytical Determinations
4. Conclusions
Supplementary Materials
Author Contributions
Funding

Conflicts of Interest
References
- Hammad Khan, M.; Jung, J.Y. Ozonation catalyzed by homogeneous and heterogeneous catalysts for degradation of DEHP in aqueous phase. Chemosphere 2008, 72, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.H.; Chen, J.N.; Yang, P.Y. Catalytic ozonation of 2-dichlorophenol by metallic ions. Water Sci. Technol. 2003, 47, 77–82. [Google Scholar] [CrossRef] [PubMed]
- Pines, D.S.; Reckhow, D.A. Effect of Dissolved cobalt(II) on the ozonation of oxalic acid. Environ. Sci. Technol. 2002, 36, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Liu, R.; Zhao, X.; Qu, J. Effect of manganese ion on the mineralization of 2,4-dichlorophenol by ozone. Chemosphere 2008, 72, 1006–1012. [Google Scholar] [CrossRef] [PubMed]
- von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- El-Raady, A.A.A.; Nakajima, T. Decomposition of carboxylic acids in water by O3, O3/H2O2, and O3/catalyst. Ozone Sci. Eng. 2005, 27, 11–18. [Google Scholar] [CrossRef]
- Rivas, J.; Rodríguez, E.; Beltrán, F.J.; García-Araya, J.F.; Alvarez, P. Homogeneouscatalyzed ozonation of simazine. Effect of Mn(II) and Fe(II). J. Environ. Sci. Health B 2001, 36, 317–330. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Chang, C.L. Homogeneous catalytic ozonation of C.I. Reactive Red 2 by metallic ions in a bubble column reactor. J. Hazard Mat. 2008, 154, 748–755. [Google Scholar] [CrossRef]
- Grochowska, J. Assessment of water buffer capacity of two morphometrically different, degraded, urban lakes. Water 2020, 12, 1512. [Google Scholar] [CrossRef]
- Yıldırım, A.Ö.; Gül, Ş.; Eren, O.; Kuşvuran, E. A comparative study of ozonation, homogeneous catalytic ozonation, and photocatalytic ozonation for C.I. Reactive Red 194 Azo dye degradation. Clean Soil Air Water 2011, 39, 795–805. [Google Scholar] [CrossRef]
- Psaltou, S.; Karapatis, A.; Mitrakas, M.; Zouboulis, A. The role of metal ions on p-CBA degradation by catalytic ozonation. J. Environ. Chem. Eng. 2019, 7, 103324. [Google Scholar] [CrossRef]
- Psaltou, S.; Kaprara, E.; Mitrakas, M.; Zouboulis, A. Calcite mineral catalyst capable of enhancing micropollutant degradation during the ozonation process at pH 7. Environ. Sci. Proc. 2020, 2, 26. [Google Scholar] [CrossRef]
- Al jibouri, A.K.H.; Wu, J.; Upreti, S.R. Heterogeneous catalytic ozonation of naphthenic acids in water. Can. J. Chem. Eng. 2019, 97, 67–73. [Google Scholar] [CrossRef]
- Mandal, S. Reaction rate constants of hydroxyl radicals with micropollutants and their significance in advanced oxidation Processes. J. Adv. Oxid. Technol. 2018, 21, 178–195. [Google Scholar] [CrossRef]
- Pi, Y.; Schumacher, J.; Jekel, M. The use of para-chlorobenzoic acid (pCBA) as an ozone/hydroxyl radical probe compound. Ozone Sci. Eng. 2005, 27, 431–436. [Google Scholar] [CrossRef]
- Maier, A.C.; Iglebaek, E.H.; Jonsson, M. Confirming the formation of hydroxyl radicals in the catalytic decomposition of H2O2 on metal oxides using coumarin as a probe. Chem. Cat. Chem. 2019, 11, 5435–5438. [Google Scholar] [CrossRef]
- Fandrey, J.; Frede, S.; Ehleben, W.; Porwol, T.; Acker, H.; Jelkmann, W. Cobalt chloride and desferrioxamine antagonize the inhibition of erythropoietin production by reactive oxygen species. Kidney Int. 1997, 51, 492–496. [Google Scholar] [CrossRef]
- Martins, R.C.; Quinta-Ferreira, R.M. A review on the applications of ozonation for the treatment of real agro-industrial wastewaters. Ozone Sci. Eng. 2014, 36, 3–35. [Google Scholar] [CrossRef]
- Sugimori, H.; Kanzaki, Y.; Yokota, K.; Murakami, T. Nonlinear dependence of the oxidation rate of Fe(II) on dissolved oxygen under low-O2 conditions in aqueous solutions. J. Miner. Petroll. Sci. 2011, 106, 142–152. [Google Scholar] [CrossRef][Green Version]
- Tian, Q.; Guo, X.; Yi, Y.; Li, Z. Kinetics of oxidation-precipitation of cobalt(II) from solution by ozone. Trans. Nonferr. Met. Soc. 2010, 20, 42–45. [Google Scholar] [CrossRef]
- Tian, Q.H.; Xin, Y.T.; Jiao, C.Y.; Guo, X.Y. Recovery of cobalt from cobalt chloride solution under the action of ozone. AMR 2012, 460, 317–320. [Google Scholar] [CrossRef]
- Rekab, K.; Lepeytre, C.; Goettmann, F.; Dunand, M.; Guillard, C.; Herrmann, J.M. Degradation of a cobalt(II)–EDTA complex by photocatalysis and H2O2/UV-C. Application to nuclear wastes containing 60Co. J. Radioanal. Nucl. Chem. 2015, 303, 131–137. [Google Scholar] [CrossRef]
- Agbaba, J.; Molnar, J.; Tubić, A.; Watson, M.; Maletić, S.; Dalmacija, B. Effects of water matrix and ozonation on natural organic matter fractionation and corresponding disinfection by-products formation. Water Supply 2015, 15, 75–83. [Google Scholar] [CrossRef]
- Manasfi, T. Ozonation in drinking water treatment: An overview of general and practical aspects, mechanisms, kinetics, and byproduct formation. Compr. Anal. Chem. 2021, 92, 85–116. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhao, D.; Li, J.; Wu, F.; Brigante, M.; Mailhot, G. Rapid oxidation of paracetamol by Cobalt(II) catalyzed sulfite at alkaline pH. Catal. Today 2018, 313, 155–160. [Google Scholar] [CrossRef]
- Najafpour, M.M.; Feizi, H. Water oxidation catalyzed by two cobalt complexes: New challenges and questions. Catal. Sci. Technol. 2018, 8, 1840–1848. [Google Scholar] [CrossRef]
- Morozov, P.A.; Ershov, B.G. The influence of phosphates on the decomposition of ozone in water: Chain process inhibition. Russ. J. Phys. Chem. 2010, 84, 1136–1140. [Google Scholar] [CrossRef]
- Hoigné, J.; Bader, H.; Haag, W.R.; Staehelin, J. Rate constants of reactions of ozone with organic and inorganic compounds in water—III. Inorganic compounds and radicals. Water Res. 1985, 19, 993–1004. [Google Scholar] [CrossRef]
- Eberhardt, M.K.; Santos, C.; Ann Soto, M. Formation of hydroxyl radicals and Co3+ in the reaction of Co2+-EDTA with hydrogen peroxide catalytic effect of Fe3+. Biochim. Biophys. Acta (BBA) Gen. Subj. 1993, 1157, 102–106. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Trussell, R.R.; Greenberg, A. Standard Methods: For the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989. [Google Scholar]


| Constituent | pH | DOC (mg/L) | Conductivity (μS/cm) | NO3− (mg/L) | PO43− (mg/L) |
|---|---|---|---|---|---|
| Value | 7.8 | 0.5 | 790 | 0.18 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Psaltou, S.; Sioumpoura, K.; Kaprara, E.; Mitrakas, M.; Zouboulis, A. Transition Metal Ions as Ozonation Catalysts: An Alternative Process of Heterogeneous Catalytic Ozonation. Catalysts 2021, 11, 1091. https://doi.org/10.3390/catal11091091
Psaltou S, Sioumpoura K, Kaprara E, Mitrakas M, Zouboulis A. Transition Metal Ions as Ozonation Catalysts: An Alternative Process of Heterogeneous Catalytic Ozonation. Catalysts. 2021; 11(9):1091. https://doi.org/10.3390/catal11091091
Chicago/Turabian StylePsaltou, Savvina, Konstantina Sioumpoura, Efthimia Kaprara, Manassis Mitrakas, and Anastasios Zouboulis. 2021. "Transition Metal Ions as Ozonation Catalysts: An Alternative Process of Heterogeneous Catalytic Ozonation" Catalysts 11, no. 9: 1091. https://doi.org/10.3390/catal11091091
APA StylePsaltou, S., Sioumpoura, K., Kaprara, E., Mitrakas, M., & Zouboulis, A. (2021). Transition Metal Ions as Ozonation Catalysts: An Alternative Process of Heterogeneous Catalytic Ozonation. Catalysts, 11(9), 1091. https://doi.org/10.3390/catal11091091









