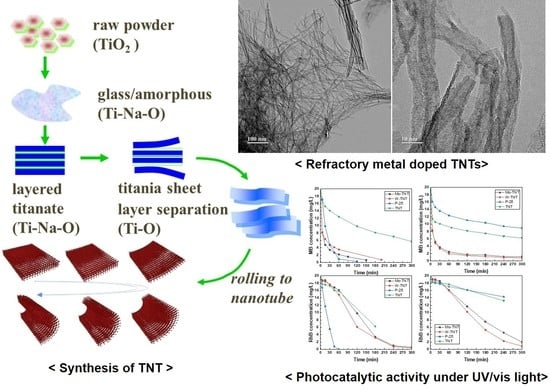
Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range
Abstract
:1. Introduction
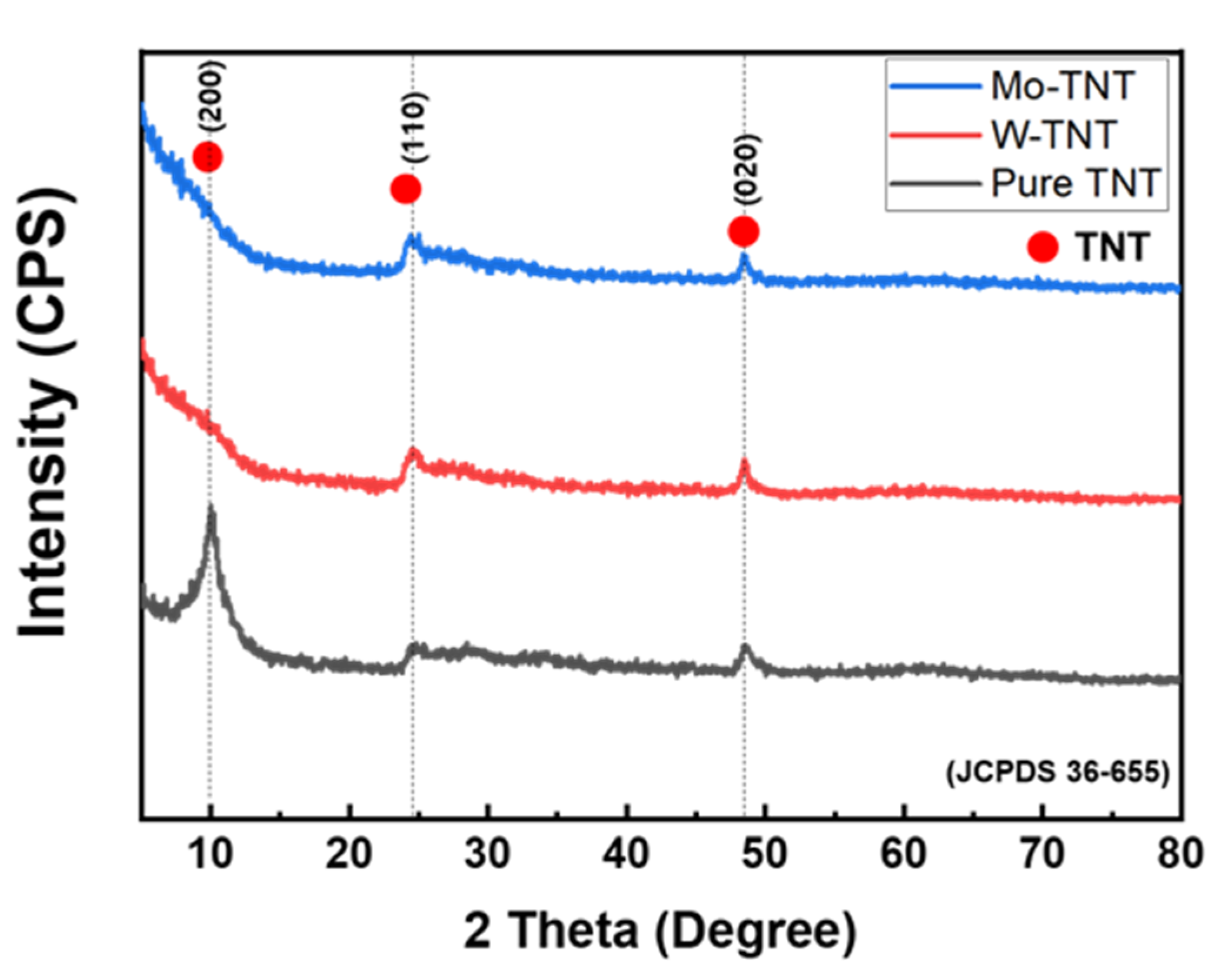
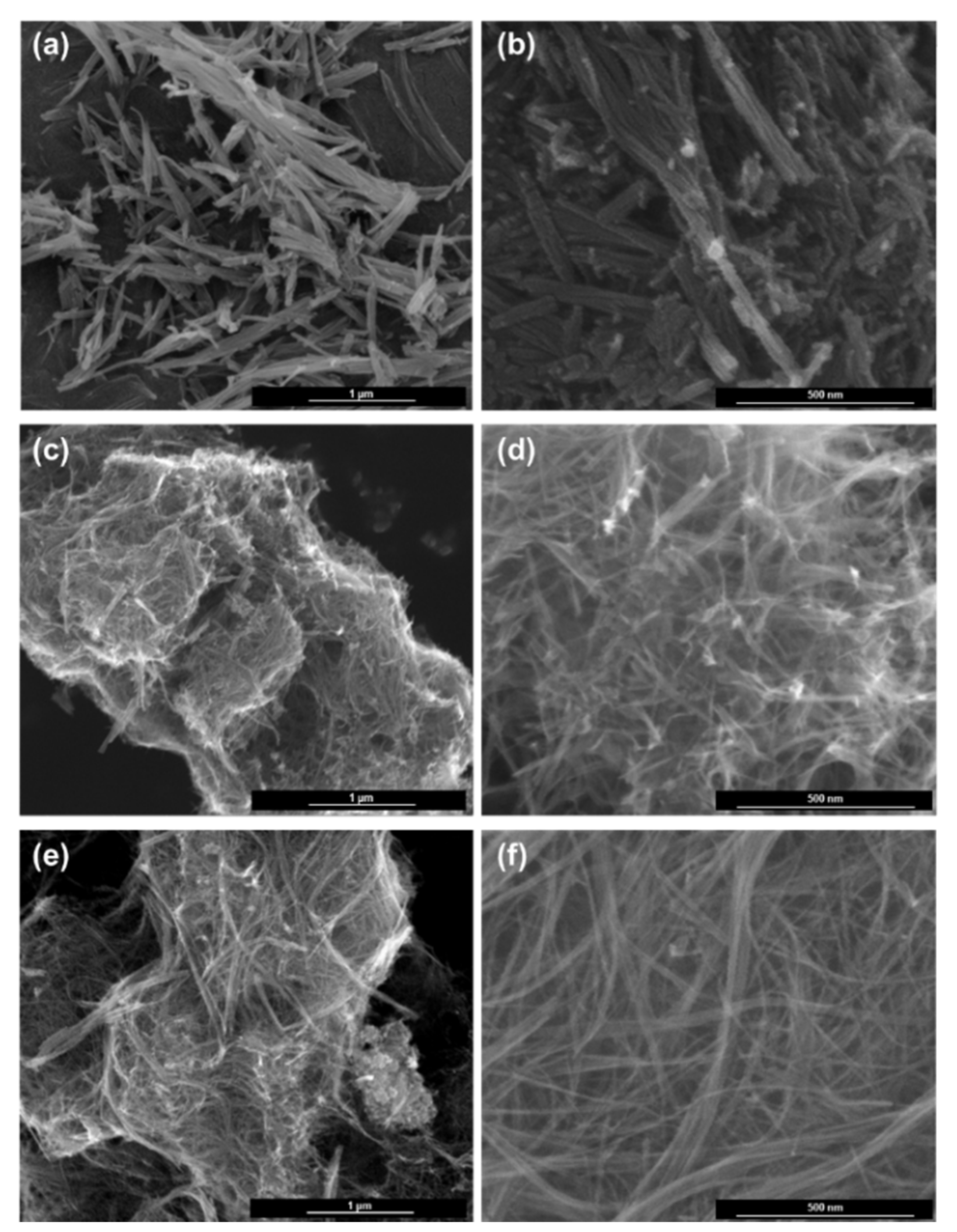
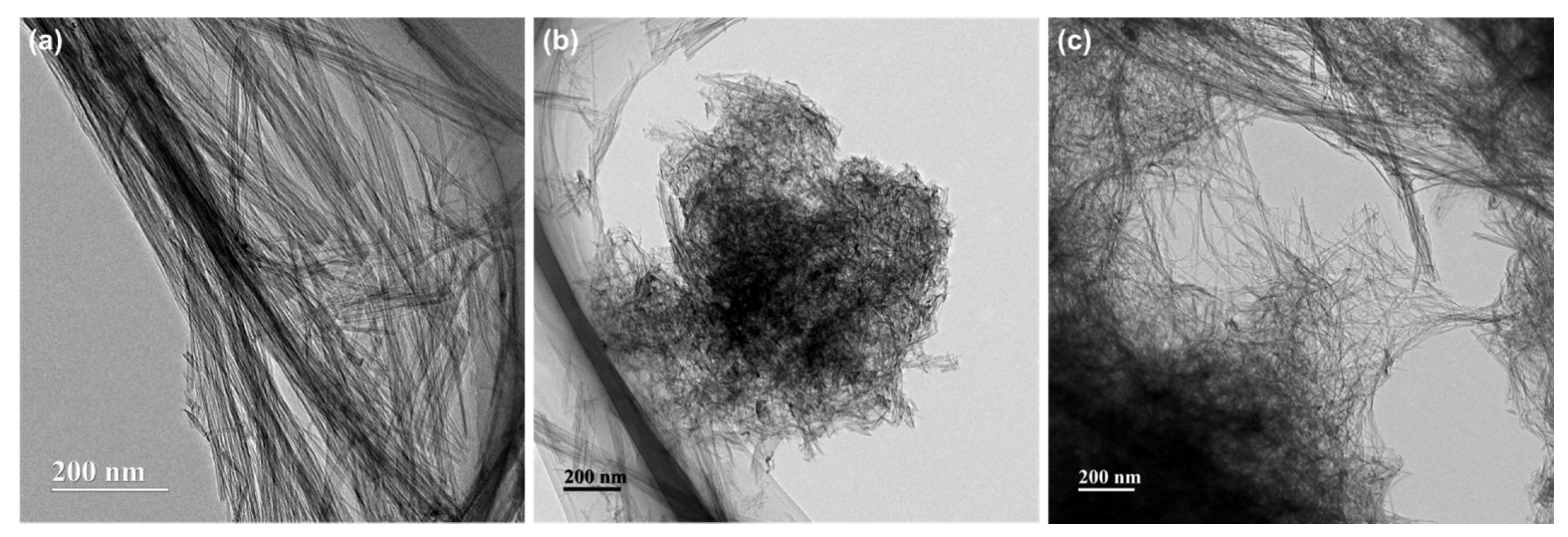
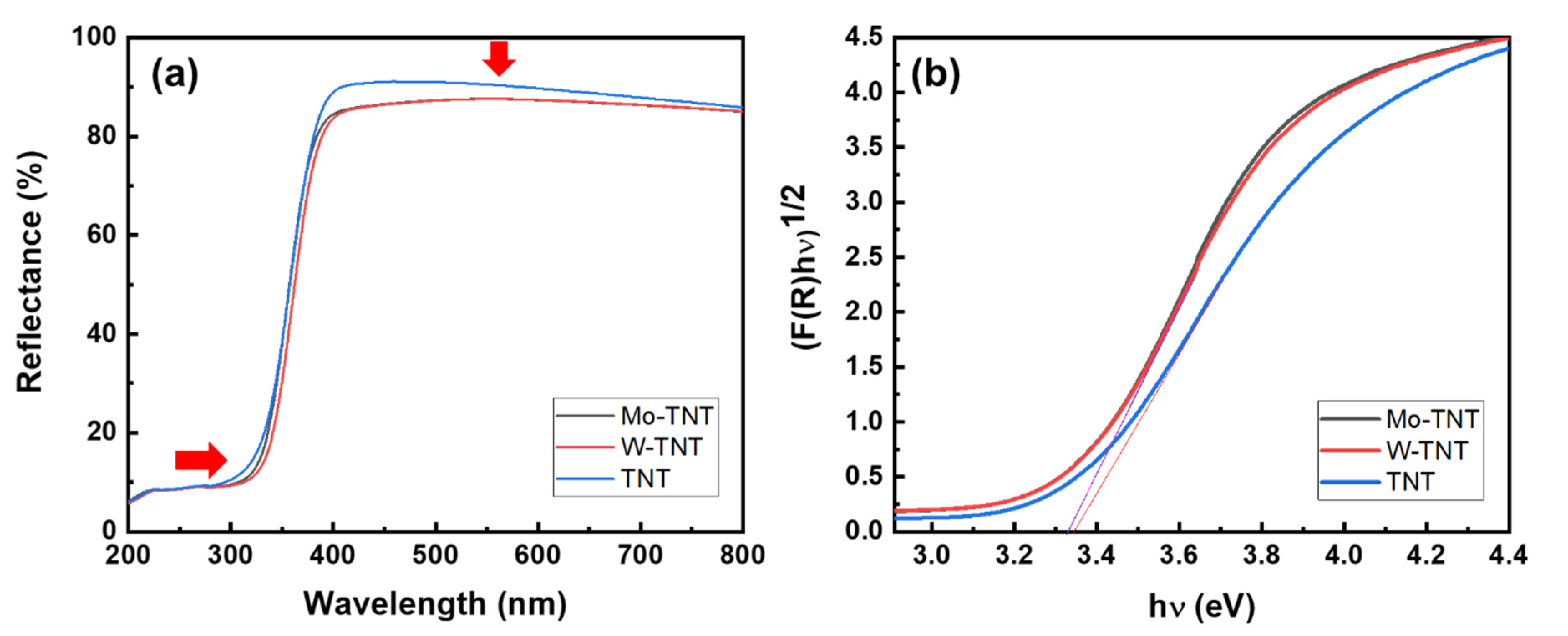
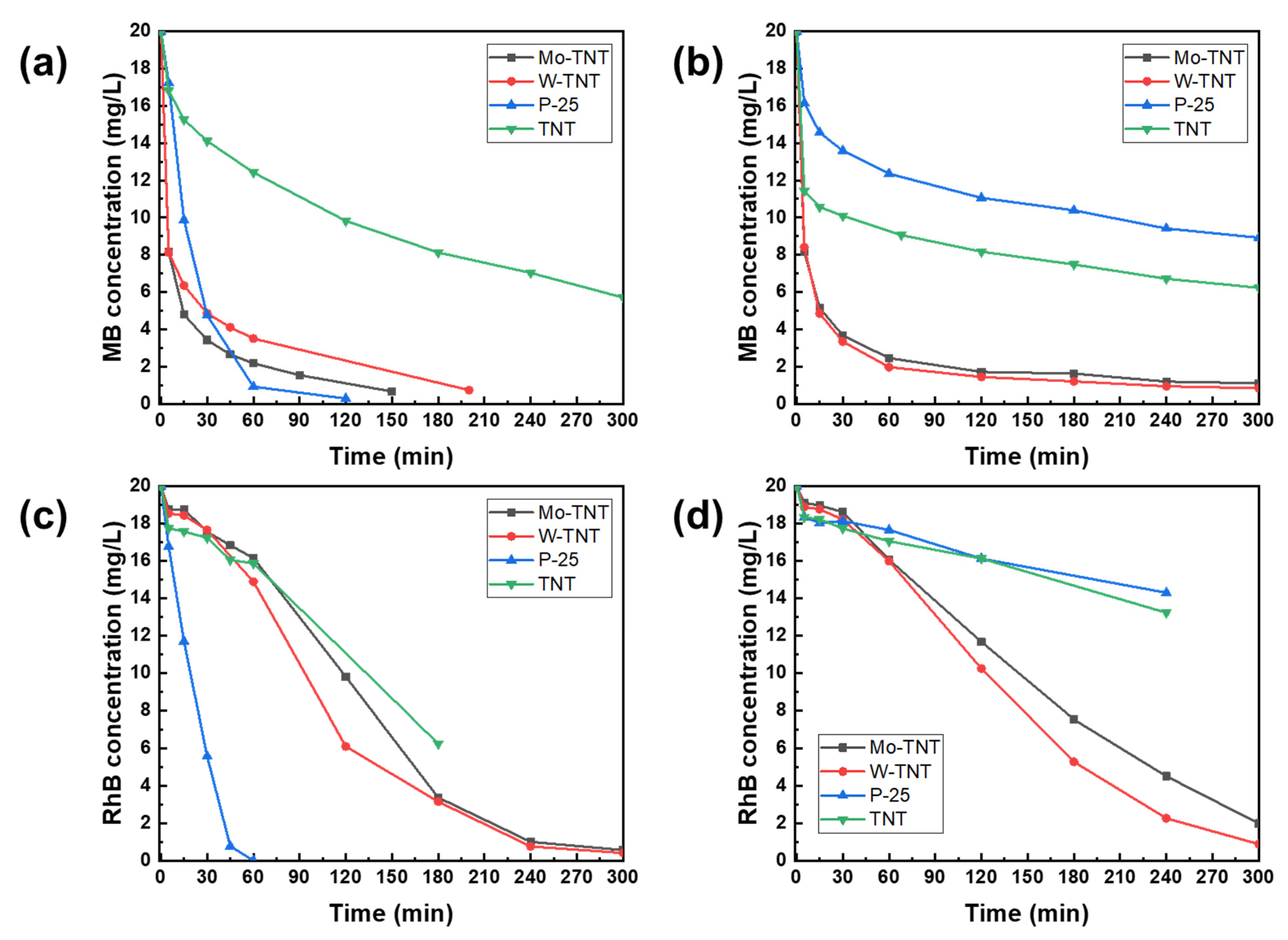
2. Results and Discussion
3. Materials and Methods
3.1. Sample Preparation
3.2. Analysis of Phase and Microstructures
3.3. Characterization of Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Byun, J.M.; Park, C.W.; Kim, Y.I.; Kim, Y.D. Photocatalytic activity of rutile TiO2 powder coupled with anatase TiO2 nanoparticles using surfactant. J Korean Powder Metall. Inst. 2018, 25, 257–262. [Google Scholar] [CrossRef]
- Fu, C.; Li, M.; Li, H.; Li, C.; Wu, X.; Yang, B. Fabrication of Au nanoparticle/TiO2 hybrid films for photoelectrocatalytic degradation of methyl orange. J. Alloys Compd. 2017, 692, 727–733. [Google Scholar] [CrossRef]
- Mor, G.K.; Shankar, K.; Paulose, M.; Varghese, O.K.; Grimes, C.A. Use of highly-ordered TiO2 nanotube arrays in dye-sensitized solar cells. Nano Lett. 2006, 6, 215–218. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Li, W.; Liang, W.; Yang, F. Au nanocrystals decorated TiO2 nanotube arrays as anode material for lithium ion batteries. Appl. Surf. Sci. 2019, 476, 948–958. [Google Scholar] [CrossRef]
- Xu, H.; Vanamu, G.; Nie, Z.; Konishi, H.; Teredla, R.; Phillips, J.; Wang, Y. Photocatalytic oxidation of a volatile organic component of acetaldehyde using titanium oxide nanotubes. J. Nanomater. 2006, 2006, 78902. [Google Scholar] [CrossRef] [Green Version]
- Hoyer, P. Formation of a titanium dioxide nanotube array. Langmuir 1996, 12, 1411–1413. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Formation of titanium oxide nanotube. Langmuir 1998, 14, 3160–3163. [Google Scholar] [CrossRef]
- Chao, W.; Huiqing, F.; Xiaohu, R.; Yun, W.; Weijia, W. Highly dispersed PtO nanodots as efficient co-catalyst for photocatalytic hydrogen evolution. Appl. Surf. Sci. 2018, 462, 423–431. [Google Scholar]
- Imai, H.; Takei, Y.; Shimizu, K.; Matsuda, M.; Hirashima, H. Direct preparation of anatase TiO2 nanotubes in porous alumina membranes. J. Mater. Chem. 1999, 9, 2971–2972. [Google Scholar] [CrossRef]
- Sekino, T.; Okamoto, T.; Kasuga, T.; Kusunose, T.; Nakayama, T.; Niihara, K. Synthesis and properties of titania nanotube doped with small amount of cations. Key Eng. Mater. 2006, 317–318, 251–254. [Google Scholar] [CrossRef]
- Michailowski, A.; AlMawlawi, D.; Cheng, G.; Moskovits, M. Highly regular anatase nanotubule arrays fabricated in porous anodic templates. Chem. Phys. Lett. 2001, 349, 1–5. [Google Scholar] [CrossRef]
- Liu, S.M.; Gan, L.M.; Liu, L.H.; Zhang, W.D.; Zeng, H.C. Synthesis of single-crystalline TiO2 nanotubes. Chem. Mater. 2002, 14, 1391–1397. [Google Scholar] [CrossRef]
- Eder, D.; Windle, A.H. Carbon–Inorganic Hybrid Materials: The Carbon-Nanotube/TiO2 Interface. Adv. Mater. 2008, 20, 1787–1793. [Google Scholar] [CrossRef]
- Kasuga, T.; Hiramatsu, M.; Hoson, A.; Sekino, T.; Niihara, K. Titania nanotubes prepared by chemical processing. Adv. Mater. 1999, 11, 1307–1311. [Google Scholar] [CrossRef]
- Tacchini, I.; Anson-Casaos, A.; Yu, Y.; Martinez, M.T.; Lira-Cantu, M. Hydrothermal synthesis of 1D TiO2 nanostructures for dye sensitized solar cells. Mater. Sci. Eng. B 2012, 177, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Lu, C.; Ni, Y.; Su, M.; Xu, Z. Hydrothermal synthesis and enhanced photocatalytic activity of flower-like TiO2 on carbon nanotubes. Mater. Lett. 2012, 79, 11–13. [Google Scholar] [CrossRef]
- Duong, T.T.; Nguyen, Q.D.; Hong, S.K.; Kim, D.; Yoon, S.G.; Pham, T.H. Enhanced photoelectrochemical activity of the TiO2/ITO nanocomposites grown onto single-walled carbon nanotubes at a low temperature by nanocluster deposition. Adv. Mater. 2011, 23, 5557–5562. [Google Scholar] [CrossRef] [PubMed]
- Antony, R.P.; Mathews, T.; Dasgupta, A.; Dash, S.; Tyagi, A.K.; Raj, B. Rapid breakdown anodization technique for the synthesis of high aspect ratio and high surface area anatase TiO2 nanotube powders. J. Solid State Chem. 2011, 184, 624–632. [Google Scholar] [CrossRef]
- Wang, L.; Wu, X.; Zhang, S. Electrochemical properties of highly ordered TiO2 nanotube arrays as an anode materials for lithium-ion batteries. Appl. Mech. Mater. 2012, 130–134, 1281–1285. [Google Scholar] [CrossRef]
- Gong, D.; Grimes, C.A.; Varghese, O.K.; Hu, W.; Singh, R.S.; Chen, Z.; Dickey, E.C. Titanium oxide nanotube arrays prepared by anodic oxidation. J. Mater. Res. 2001, 16, 3331–3334. [Google Scholar] [CrossRef] [Green Version]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. Photobiol. C Photochem. Rev. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Mohapatra, S.K.; Misra, M.; Mahajan, V.K.; Raja, K.S. Design of a highly efficient photoelectrolytic cell for hydrogen generation by water splitting: Application of TiO2−xCx nanotubes as a photoanode and Pt/TiO2 nanotubes as a cathode. J. Phys. Chem. C 2007, 111, 8677–8685. [Google Scholar] [CrossRef]
- Wang, J.; Han, X.; Zhang, W. Controlled growth of monocrystalline rutile nanoshuttles in anatase TiO2 particles under mild conditions. Cryst. Eng. Comm. 2009, 11, 564–566. [Google Scholar] [CrossRef]
- Tian, B.; Li, C.; Zhang, J. One-step preparation, characterization and visible-light photocatalytic activity of Cr-doped TiO2 with anatase and rutile bicrystalline phases. Chem. Eng. J. 2012, 191, 402–409. [Google Scholar] [CrossRef]
- Kim, H.C.; Han, J.K. Synthesis and photo catalytic activity of 10 wt%: 20 wt% Li-TiO2 composite powders. J. Korean Powder Metall. Inst. 2016, 23, 33–37. [Google Scholar] [CrossRef]
- Chen, X.; Kuo, D.H.; Lu, D. N-doped mesoporous TiO2 nanoparticles synthesized by using biological renewable nanocrystalline cellulose as template for the degradation of pollutants under visible and sun light. Chem. Eng. J. 2016, 295, 192–200. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, X.; Li, J.; Burda, C. Photocatalytic degradation of azo dyes by nitrogen-doped TiO2 nanocatalysts. Chemosphere 2005, 61, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. A 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Corbel, G.; Laligant, Y.; Goutenoire, F.; Suard, E.; Lacorre, P. Effects of partial substitution of Mo6+ by Cr6+ and W6+ on the crystal structure of the fast oxide-ion conductor structural effects of W6+. Chem. Mater. 2005, 17, 4678–4684. [Google Scholar] [CrossRef]
- Kletzin, A.; Adams, M.W.W. Tungsten in biological systems. FEMS Microbiol. Rev. 1996, 18, 5–63. [Google Scholar] [CrossRef] [PubMed]
- Byun, J.M.; Choi, H.R.; Kim, Y.D.; Sekino, T.; Kim, S.H. Photocatalytic activity under UV/Visible light range of Nb-doped titanate nanostructures synthesized with Nb oxide. Appl. Surf. Sci. 2017, 415, 126–131. [Google Scholar] [CrossRef]
- Yang, D.; Liu, H.; Liu, L.; Sarina, S.; Zheng, Z.; Zhu, H. Silver oxide nanocrystals anchored on titanate nanotubes and nanofibers: Promising candidates for entrapment of radioactive iodine anions. Nanoscale 2013, 5, 11011–11018. [Google Scholar] [CrossRef] [PubMed]
- Jose, M.; Sienkiewicz, P.; Szymanska, K.; Darowna, D.; Moszynski, D.; Lendzion-Bielun, Z.; Szymanski, K.; Mozia, S. Influence of Preparation Procedure on Physicochemical and Antibacterial Properties of Titanate Nanotubes Modified with Silver. Nanomaterials 2019, 9, 795. [Google Scholar] [CrossRef] [Green Version]
- Rahimnejad, S.; He, J.H.; Pan, F.; Lee, X.; Chen, W.; Wu, K.; Xu, G.Q. Enhancement of the photocatalytic efficiency of WO3 nanoparticles via hydrogen plasma treatment. Mat. Res. Express 2014, 1, 045044. [Google Scholar] [CrossRef]
- Yun-Ju, L.; Barrera, D.; Luo, K.; Hsu, J.W.P. In situ chemical oxidation of ultrasmall MoOx nanoparticles in suspensions. J. Nanotechnol. 2012, 2012, 195761. [Google Scholar]
- Kiatkittipong, K.; Scott, J.; Amal, R. Hydrothermally synthesized titanate nanostructures: Impact of heat treatment on particle characteristics and photocatalytic properties. ACS Appl. Mater. Interfaces 2011, 3, 3988–3996. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Galvan, M.; Celaya, C.A.; Jaramillo-Quintero, O.; Muniz, J.; Diaz, G.; Lara-Garcia, H.A. Tuning the band gap of M-doped titanate nanotubes (M=Fe, Co, Ni, and Cu):an experimental and theoretical study. Nanoscale Adv. 2021, 3, 1382–1391. [Google Scholar] [CrossRef]
- Fernandez-Werner, L.; Pignanelli, F.; Montenegro, B.; Romero, M.; Pardo, H.; Faccio, R.; Mombru, A.W. Characterization of titanate nanotubes for energy applications. J. Energy Storage 2017, 12, 66–77. [Google Scholar] [CrossRef]
- Li, L.; Liu, X.; Zhang, Y. Visible-light photochemical activity of heterostructured core–shell materials composed of selected Ternary titanates and ferrites coated by TiO2. Appl. Mater. Interfaces 2013, 5, 5064–5071. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.-S.; Choi, H.-J.; Sekino, T.; Kim, Y.-D.; Kim, S.-H. Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range. Catalysts 2021, 11, 987. https://doi.org/10.3390/catal11080987
Kim M-S, Choi H-J, Sekino T, Kim Y-D, Kim S-H. Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range. Catalysts. 2021; 11(8):987. https://doi.org/10.3390/catal11080987
Chicago/Turabian StyleKim, Min-Sang, Hyun-Joo Choi, Tohru Sekino, Young-Do Kim, and Se-Hoon Kim. 2021. "Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range" Catalysts 11, no. 8: 987. https://doi.org/10.3390/catal11080987
APA StyleKim, M.-S., Choi, H.-J., Sekino, T., Kim, Y.-D., & Kim, S.-H. (2021). Refractory Metal Oxide–Doped Titanate Nanotubes: Synthesis and Photocatalytic Activity under UV/Visible Light Range. Catalysts, 11(8), 987. https://doi.org/10.3390/catal11080987