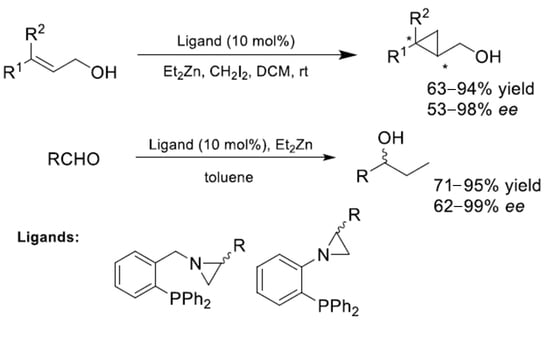
Efficient Asymmetric Simmons-Smith Cyclopropanation and Diethylzinc Addition to Aldehydes Promoted by Enantiomeric Aziridine-Phosphines
Abstract
1. Introduction
2. Results and Discussion
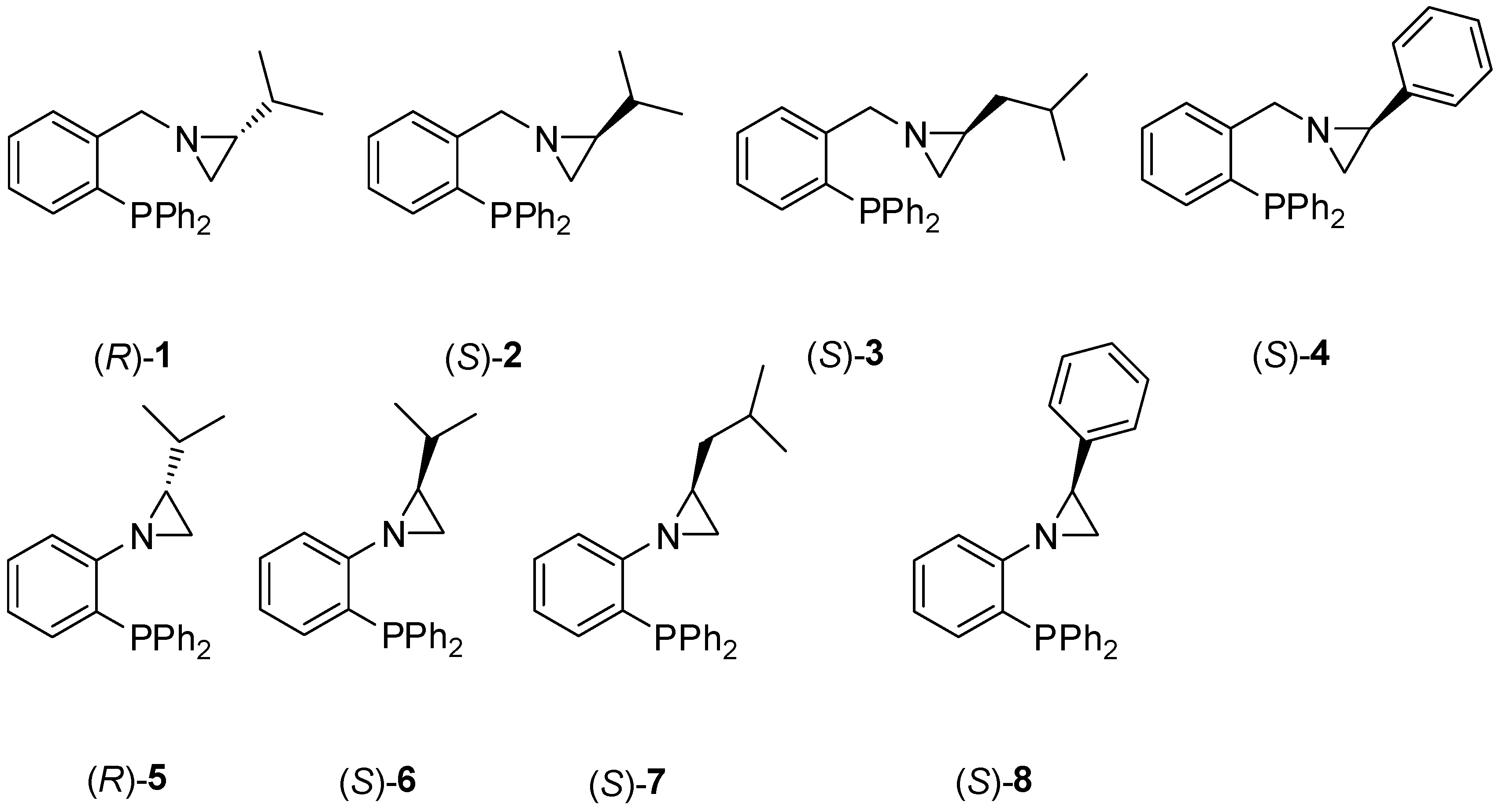
2.1. Preparation of the Aziridine-Phosphines 1–8
2.2. Synthesis of Imine-Phosphines 9–12
2.3. Asymmetric Simmons-Smith Cyclopropanation Promoted by Aziridine-Phosphines 1–8 and Imine-Phosphines 9–12
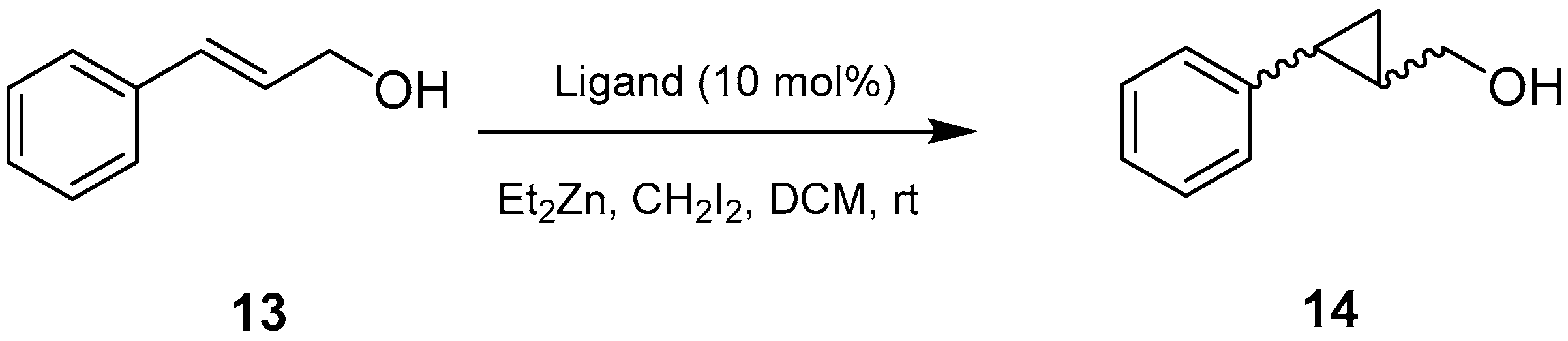
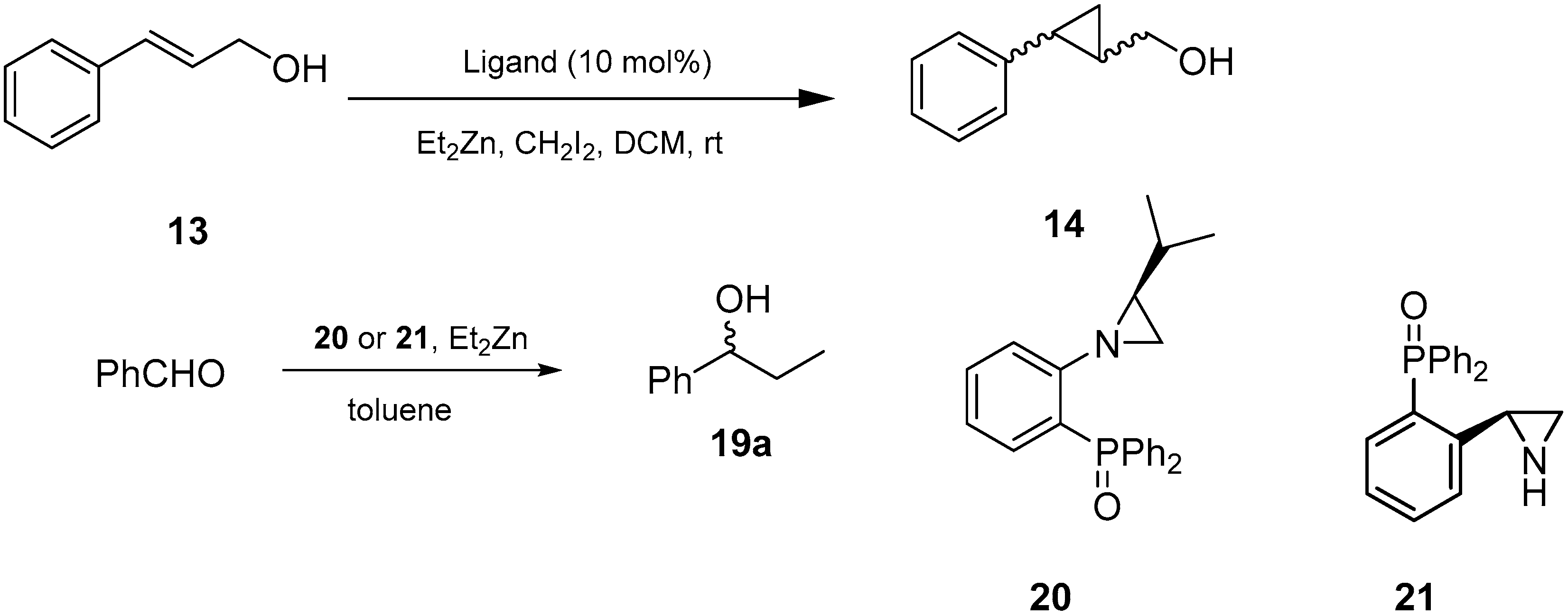
2.4. Asymmetric Simmons-Smith Cyclopropanation Promoted by Aziridine-Phosphine 6
2.5. Asymmetric Diethylzinc Addition to Benzaldehyde Catalyzed by Aziridine-Phosphines 1–8 and Imine-Phosphines 9–12
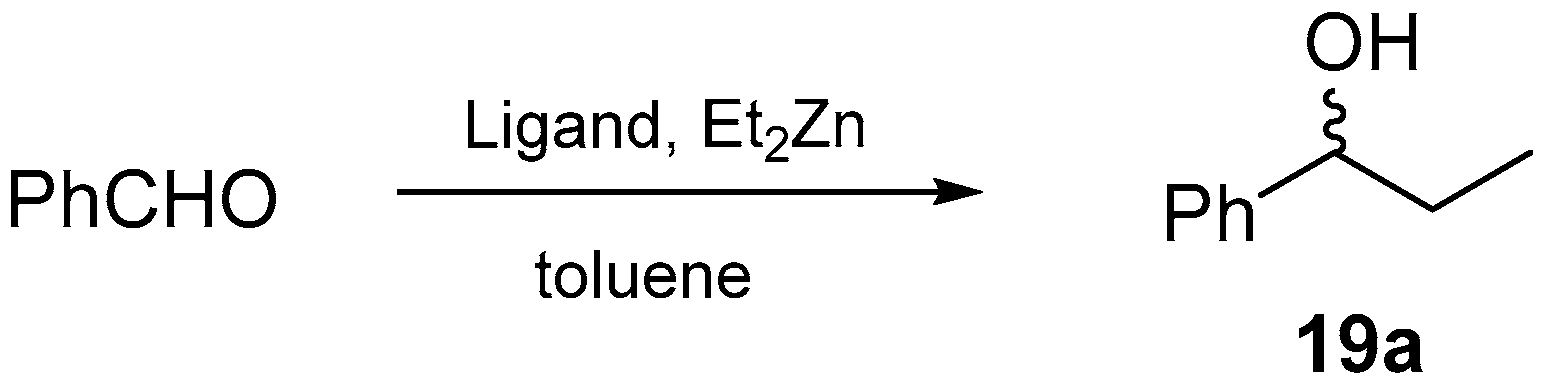
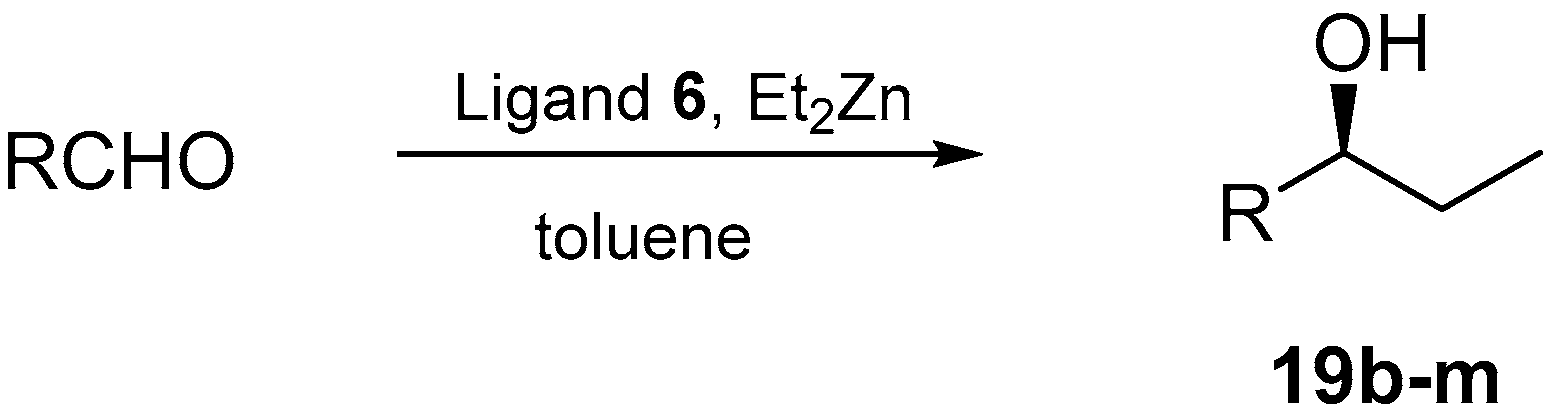
2.6. Asymmetric Addition of Et2Zn to Aldehydes Promoted by Aziridine-Phosphine 6
2.7. Asymmetric Cyclopropanation of Cinnamyl Alcohol and Diethylzinc Addition to Benzaldehyde Catalyzed by Aziridine-Phosphine Oxides
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Imine-Phosphines 9–12—General Procedure
3.2.2. Asymmetric Simmons–Smith Cyclopropanation—General Procedure
3.2.3. Asymmetric Addition of Diethylzinc to Aldehydes—General Procedure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Carroll, M.P.; Guiry, P.J. P,N ligands in asymmetric catalysis. Chem. Soc. Rev. 2014, 43, 819–833. [Google Scholar] [CrossRef]
- Li, Y.-M.; Kwong, F.-Y.; Yu, W.-Y.; Chan, A.S.C. Recent advances in developing new axially chiral phosphine ligands for asymmetric catalysis. Coord. Chem. Rev. 2007, 251, 2119–2144. [Google Scholar] [CrossRef]
- Gaunt, M.J.; Johansson, C.C.C.; McNally, A.; Vo, N.T. Enantioselective organocatalysis. Drug Discov. Today 2007, 12, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; He, X.-H.; Liu, Y.-Q.; He, G.; Peng, C.; Li, J.-L. Asymmetric organocatalysis: An enabling technology for medicinal chemistry. Chem. Soc. Rev. 2021, 50, 1522–1586. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Muñoz, G.; Miranda, I.L.; Sartori, S.K.; De Rezende, D.C.; Nogueira Diaz, M.A. Use of chiral auxiliaries in the asymmetric synthesis of biologically active compounds: A review. Chirality 2019, 31, 776–812. [Google Scholar] [CrossRef] [PubMed]
- Dixon, D.J. Bifunctional catalysis. Beilstein J. Org. Chem. 2016, 12, 1079–1080. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-H.; Han, X.; Zhu, F.-P.; Tian, J.-M.; Zhang, F.-M.; Zhang, X.-M.; Tu, Y.-Q.; Wang, S.-H.; Guo, X. Development of bifunctional organocatalysts and application to asymmetric total synthesis of naucleofficine I and II. Nat. Commun. 2019, 10, 3394. [Google Scholar] [CrossRef] [PubMed]
- Peters, R. (Ed.) Cooperative Catalysis: Designing Efficient Catalysts for Synthesis; Wiley-VCH Verlag GmbH & Co. KgaA: Weinheim, Germany, 2015; pp. 1–456. [Google Scholar]
- Werth, J.; Uyeda, C. Regioselective Simmons-Smith-type cyclopropanations of polyalkenes enabled by transition metal catalysis. Chem. Sci. 2018, 9, 1604–1609. [Google Scholar] [CrossRef]
- Časar, Z. Synthetic Approaches to Contemporary Drugs that Contain the Cyclopropyl Moiety. Synthesis 2020, 52, 1315–1345. [Google Scholar] [CrossRef]
- Ezawa, T.; Kawashima, Y.; Noguchi, T.; Jung, S.; Imai, N. Convenient synthesis of memantine analogues containing a chiral cyclopropane skeleton as a sigma-1 receptor agonist. Tetrahedron Asymmetry 2017, 28, 266–281. [Google Scholar] [CrossRef]
- Sun, M.; Li, W.-D.Z.; Qiu, F.G. Asymmetric Synthesis of the DEFG Rings of Solanoeclepin A. Org. Lett. 2019, 21, 644–647. [Google Scholar] [CrossRef]
- Talele, T.T. The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules. J. Med. Chem. 2016, 59, 8712–8756. [Google Scholar] [CrossRef] [PubMed]
- Chawner, S.J.; Cases-Thomas, M.J.; Bull, J.A. Divergent Synthesis of Cyclopropane-Containing Lead-Like Compounds, Fragments and Building Blocks through a Cobalt Catalyzed Cyclopropanation of Phenyl Vinyl Sulfide. Eur. J. Org. Chem. 2017, 5015–5024. [Google Scholar] [CrossRef] [PubMed]
- Novakov, I.A.; Babushkin, A.S.; Yablokov, A.S.; Nawrozkij, M.B.; Vostrikova, O.V.; Shejkin, D.S.; Mkrtchyan, A.S.; Balakin, K.V. Synthesis and structure—activity relationships of cyclopropane-containing analogs of pharmacologically active compounds. Russ. Chem. Bull. Int. Ed. 2018, 67, 395–418. [Google Scholar] [CrossRef]
- Wu, W.; Lin, Z.; Jiang, H. Recent advances in the synthesis of cyclopropanes. Org. Biomol. Chem. 2018, 16, 7315–7329. [Google Scholar] [CrossRef] [PubMed]
- Berkessel, A.; Ertürk, E.; Neudörfl, J.M. Asymmetric cyclopropanation of olefins catalyzed by a chiral cobalt(II) porphyrin. Org. Commun. 2017, 10, 79–89. [Google Scholar] [CrossRef]
- Meazza, M.; Ashe, M.; Shin, H.Y.; Yang, H.S.; Mazzanti, A.; Yang, J.W.; Rios, R. Enantioselective Organocatalytic Cyclopropanation of Enals Using Benzyl Chlorides. J. Org. Chem. 2016, 81, 3488–3500. [Google Scholar] [CrossRef]
- Dočekal, V.; Petrželová, S.; Císařová, I.; Veselý, J. Enantioselective Cyclopropanation of 4-Nitroisoxazole Derivatives. Adv. Synth. Catal. 2020, 362, 2597–2603. [Google Scholar] [CrossRef]
- Herchl, R.; Waser, M. Asymmetric cyclopropanation of chalcones using chiral phase-transfer catalysts. Tetrahedron Lett. 2013, 54, 2472–2475. [Google Scholar] [CrossRef]
- Rachwalski, M.; Kaczmarczyk, S.; Leśniak, S.; Kiełbasiński, P. Highly Efficient Asymmetric Simmons-Smith Cyclopropanation Promoted by Chiral Heteroorganic Aziridinyl Ligands. ChemCatChem 2014, 6, 873–875. [Google Scholar] [CrossRef]
- Pu, L.; Yu, H.-B. Catalytic Asymmetric Organozinc Additions to Carbonyl Compounds. Chem. Rev. 2001, 101, 757–824. [Google Scholar] [CrossRef]
- Rachwalski, M.; Jarzyński, S.; Leśniak, S. Aziridine ring-containing chiral ligands as highly efficient catalysts in asymmetric synthesis. Tetrahedron Asymmetry 2013, 24, 421–425. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Leśniak, S.; Jarzyński, S.; Rachwalski, M. Aziridinylethers as highly enantioselective ligands for the asymmetric addition of organozinc species to carbonyl compounds. Tetrahedron Asymmetry 2015, 26, 148–151. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Sznajder, E.; Kiełbasiński, P. New highly efficient aziridine-functionalized tridentate sulfinyl catalysts for enantioselective diethylzinc addition to carbonyl compounds. Tetrahedron Asymmetry 2009, 20, 2311–2314. [Google Scholar] [CrossRef]
- Rachwalski, M.; Jarzyński, S.; Jasiński, M.; Leśniak, S. Mandelic acid derived a-aziridinyl alcohols as highly efficient ligands for asymmetric additions of zinc organyls to aldehydes. Tetrahedron Asymmetry 2013, 24, 689–693. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Jarzyński, S.; Obijalska, E. Lactic acid derived aziridinyl alcohols as highly effective catalysts for asymmetric additions of an organozinc species to aldehydes. Tetrahedron Asymmetry 2013, 24, 1336–1340. [Google Scholar] [CrossRef]
- Rachwalski, M. Limonene oxide derived aziridinyl alcohols as highly efficient catalysts for asymmetric additions of organozinc species to aldehydes. Tetrahedron Asymmetry 2014, 25, 219–223. [Google Scholar] [CrossRef]
- Doğan, Ö.; Çağli, E. PFAM catalyzed enantioselective diethylzinc addition to imines. Turk. J. Chem. 2015, 39, 290–296. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Asymmetric Friedel-Crafts Alkylation of Indoles Catalyzed by Chiral Aziridine-Phosphines. Catalysts 2020, 10, 971. [Google Scholar] [CrossRef]
- Pieczonka, A.M.; Misztal, E.; Rachwalski, M.; Leśniak, S. Synthesis of chiral 1-(2-aminoalkyl)aziridines via a self-opening reaction of aziridine. Arkivoc 2017, 2017, 223–234. [Google Scholar] [CrossRef]
- Shitama, H.; Katsuki, T. Asymmetric Simmons-Smith Reaction of Allylic Alcohols with Al Lewis Acid/N Lewis Base Bifunctional Al(Salalen) Catalyst. Angew. Chem. Int. Ed. 2008, 47, 2450–2453. [Google Scholar] [CrossRef]
- Wujkowska, Z.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Phosphinoyl-aziridines as a new class of chiral catalysts for enantioselective Michael addition. Tetrahedron 2019, 75, 230–235. [Google Scholar] [CrossRef]
- Buchcic, A.; Zawisza, A.; Leśniak, S.; Adamczyk, J.; Pieczonka, A.M.; Rachwalski, M. Enantioselective Mannich reaction promoted by chiral phosphinoyl-aziridines. Catalysts 2019, 9, 837. [Google Scholar] [CrossRef]
- Leśniak, S.; Rachwalski, M.; Pieczonka, A.M. Optically Pure Aziridinyl Ligands as Useful Catalysts in the Stereocontrolled Synthesis. Curr. Org. Chem. 2014, 18, 3045–3065. [Google Scholar] [CrossRef]
- Zhang, C.-H.; Yan, S.-J.; Pan, S.-Q.; Huang, R.; Lin, J. Highly Enantioselective Addition of Diethylzinc to aldehydes Catalyzed by Novel Chiral tert-Amino Alcohols. Bull. Korean Chem. Soc. 2010, 31, 869–873. [Google Scholar] [CrossRef][Green Version]
- Cere, V.; Peri, F.; Pollicino, S. Regio- and Stereospecific Me3SiI-Promoted Intramolecular Displacement of Hydroxy Group by Sulfide. 2. Polyhydroxylated Thiepane Ring Contraction to Thiolane or Thiane Derivatives. Synthesis of Enantiopure Polyalcohols. J. Org. Chem. 1997, 62, 8572–8574. [Google Scholar] [CrossRef] [PubMed]
- Dilek, Ö.; Tezeren, M.A.; Tilki, T.; Ertürk, E. Chiral 2-(hydroxyaryl)alcohols (HAROLs) with a 1,4-diol scaffold as a new family of ligands and organocatalysts. Tetrahedron 2018, 74, 268–286. [Google Scholar] [CrossRef]
- Lombardo, M.; Chiarucci, M.; Trombini, C. The first enantioselective addition of diethylzinc to aldehydes in ionic liquids catalysed by a recyclable ion-tagged diphenylprolinol. Chem. Eur. J. 2008, 14, 11288–11291. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, M.; Jang, Y.-S.; Cramer, N. Chiral cyclopentadienyl iridium(III) complexes promote enantioselective cycloisomerizations giving fused cyclopropanes. Angew. Chem. Int. Ed. 2015, 54, 12149–12152. [Google Scholar] [CrossRef] [PubMed]
- Ando, K. Highly selective synthesis of (Z)-unsaturated esters by using new Horner-Emmons reagents, ethyl (diarylphosphono)acetates. J. Org. Chem. 1997, 62, 1934–1939. [Google Scholar] [CrossRef] [PubMed]
- Rachwalski, M.; Kwiatkowska, M.; Drabowicz, J.; Kłos, M.; Wieczorek, W.M.; Szyrej, M.; Sieroń, L.; Kiełbasiński, P. Enzyme-promoted desymmetrization of bis(2-hydroxymethylphenyl)sulfoxide as a route to tridentate chiral catalysts. Tetrahedron Asymmetry 2008, 19, 2096–2101. [Google Scholar] [CrossRef]


| Entry | Ligand | Product 14 | |||
|---|---|---|---|---|---|
| Yield (%) | ee (%) a | Drb | Abs. Conf. c | ||
| 1 | 1 | 70 | 62 | 5:1 | 1R,2R |
| 2 | 2 | 72 | 65 | 5:1 | 1S,2S |
| 3 | 3 | 63 | 54 | 5:1 | 1S,2S |
| 4 | 4 | 65 | 58 | 5:1 | 1S,2S |
| 5 | 5 | 90 | 92 | 12:1 | 1R,2R |
| 6 | 6 | 93 | 98 | 15:1 | 1S,2S |
| 7 | 7 | 90 | 82 | 10:1 | 1S,2S |
| 8 | 8 | 91 | 85 | 10:1 | 1S,2S |
| 9 | 9 | 85 | 72 | 10:1 | 1S,2S |
| 10 | 10 | 83 | 70 | 10:1 | 1R,2R |
| 11 | 11 | 79 | 68 | 10:1 | 1S,2S |
| 12 | 12 | 80 | 70 | 10:1 | 1S,2S |
| Entry | Substrate | Yield (%) | ee (%) a | Drb | Abs. Conf. c |
|---|---|---|---|---|---|
| 1 | 15 | 92 | 95 | 15:1 | (1S,2S) |
| 2 | 16 | 94 | 93 | 15:1 | (1S,2S) |
| 3 | 17 | 91 | 91 | 15:1 | (1S,2S) |
| 4 | 18 | 56 | 40 | 4:1 | (1R,2S) |
| Entry | Ligand | Product 19a | ||
|---|---|---|---|---|
| Yield (%) | ee (%) a | Abs. Conf. b | ||
| 1 | 1 | 75 | 66 | (R) |
| 2 | 2 | 78 | 68 | (S) |
| 3 | 3 | 71 | 62 | (S) |
| 4 | 4 | 72 | 63 | (S) |
| 5 | 5 | 92 | 96 | (R) |
| 6 | 6 | 95 | 96 | (S) |
| 7 | 7 | 91 | 86 | (S) |
| 8 | 8 | 90 | 85 | (S) |
| 9 | 9 | 85 | 80 | (S) |
| 10 | 10 | 83 | 80 | (R) |
| 11 | 11 | 81 | 73 | (S) |
| 12 | 12 | 82 | 76 | (S) |
| Entry | R | Products 19b–m | ||
|---|---|---|---|---|
| Yield (%) | ee (%) a | Abs. Conf. b | ||
| 1 | n-Pr | 90 | 89 | (S) |
| 2 | 2-MeC6H4 | 91 | 90 | (S) |
| 3 | 4-MeC6H4 | 90 | 93 | (S) |
| 4 | 2-MeOC6H4 | 93 | 93 | (S) |
| 5 | 4-MeOC6H4 | 93 | 95 | (S) |
| 6 | 2-BrC6H4 | 91 | 90 | (S) |
| 7 | 4-BrC6H4 | 93 | 92 | (S) |
| 8 | 4-CF3C6H4 | 91 | 93 | (S) |
| 9 | Vi | 87 | 85 | (S) |
| 10 | 4-O2NC6H4 | 0 | Nd c | Nd c |
| 11 | C4H3S | 70 | 68 | (S) |
| 12 | C4H3O | 0 | Nd c | Nd c |
| Entry | Ligand | Product 14 | |||
|---|---|---|---|---|---|
| Yield (%) | ee (%) a | Drb | Abs. Conf. c | ||
| 1 | 20 | 50 | 41 | 5:1 | (1S,2S) |
| 2 | 21 | 55 | 52 | 5:1 | (1S,2S) |
| Entry | Ligand | Product 19a | ||
|---|---|---|---|---|
| Yield (%) | ee (%) a | Abs. Conf. b | ||
| 1 | 20 | 60 | 56 | (S) |
| 2 | 21 | 68 | 62 | (S) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buchcic-Szychowska, A.; Adamczyk, J.; Marciniak, L.; Pieczonka, A.M.; Zawisza, A.; Leśniak, S.; Rachwalski, M. Efficient Asymmetric Simmons-Smith Cyclopropanation and Diethylzinc Addition to Aldehydes Promoted by Enantiomeric Aziridine-Phosphines. Catalysts 2021, 11, 968. https://doi.org/10.3390/catal11080968
Buchcic-Szychowska A, Adamczyk J, Marciniak L, Pieczonka AM, Zawisza A, Leśniak S, Rachwalski M. Efficient Asymmetric Simmons-Smith Cyclopropanation and Diethylzinc Addition to Aldehydes Promoted by Enantiomeric Aziridine-Phosphines. Catalysts. 2021; 11(8):968. https://doi.org/10.3390/catal11080968
Chicago/Turabian StyleBuchcic-Szychowska, Aleksandra, Justyna Adamczyk, Lena Marciniak, Adam Marek Pieczonka, Anna Zawisza, Stanisław Leśniak, and Michał Rachwalski. 2021. "Efficient Asymmetric Simmons-Smith Cyclopropanation and Diethylzinc Addition to Aldehydes Promoted by Enantiomeric Aziridine-Phosphines" Catalysts 11, no. 8: 968. https://doi.org/10.3390/catal11080968
APA StyleBuchcic-Szychowska, A., Adamczyk, J., Marciniak, L., Pieczonka, A. M., Zawisza, A., Leśniak, S., & Rachwalski, M. (2021). Efficient Asymmetric Simmons-Smith Cyclopropanation and Diethylzinc Addition to Aldehydes Promoted by Enantiomeric Aziridine-Phosphines. Catalysts, 11(8), 968. https://doi.org/10.3390/catal11080968