Naturally Occurring Oxazole Structural Units as Ligands of Vanadium Catalysts for Ethylene-Norbornene (Co)polymerization
Abstract
:1. Introduction
2. Results and Discussion
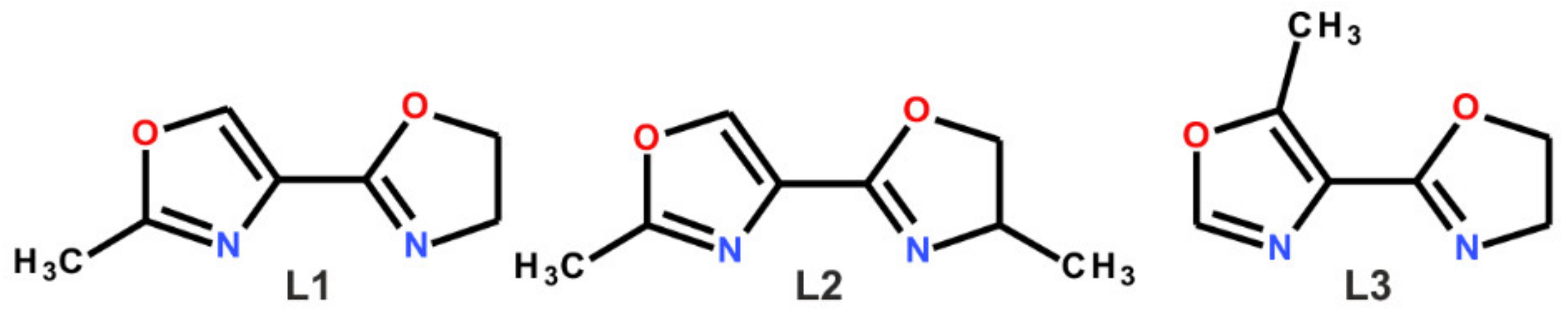
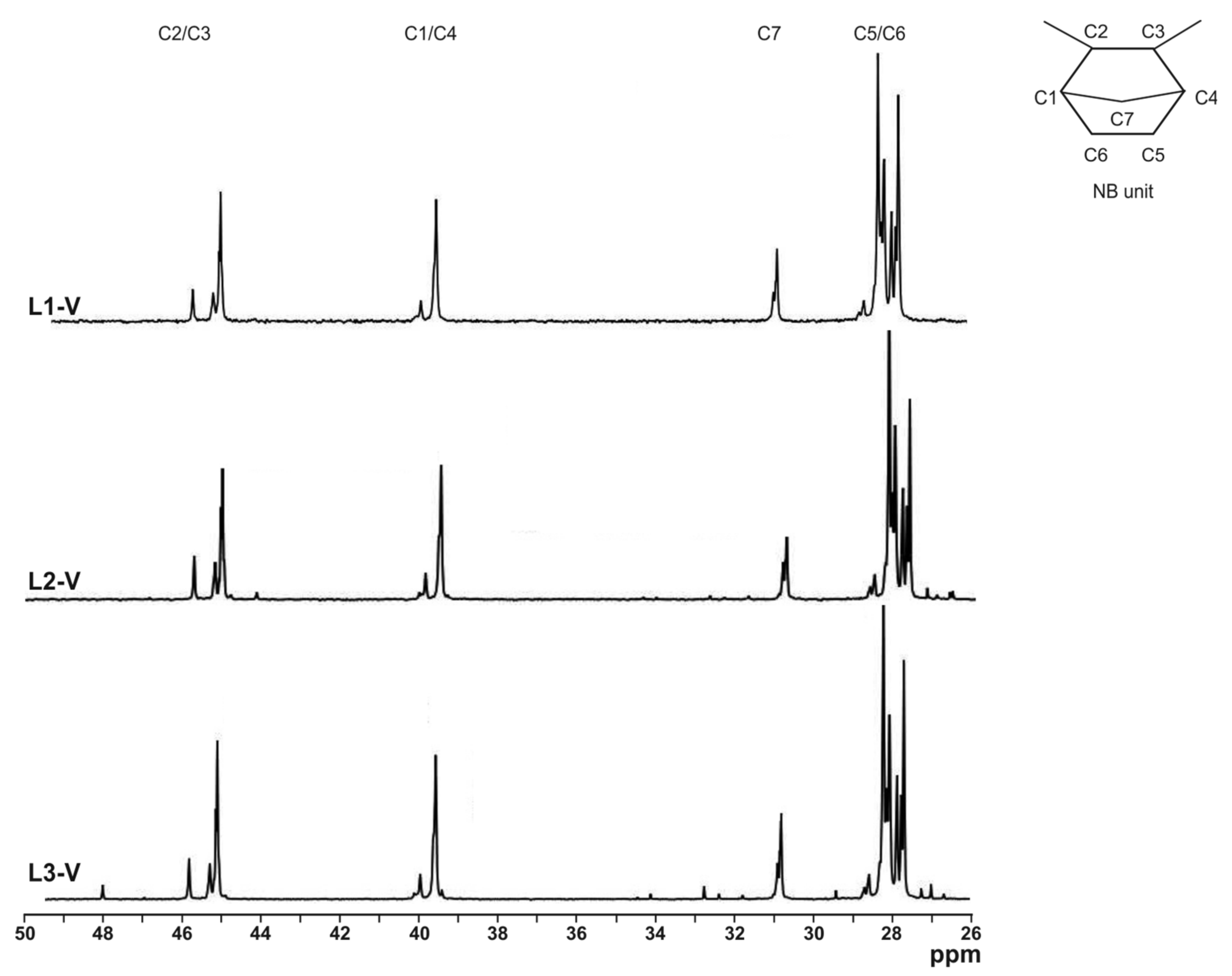
2.1. Synthesis and Analysis of the Catalysts
2.2. Polymerization and Copolymerization
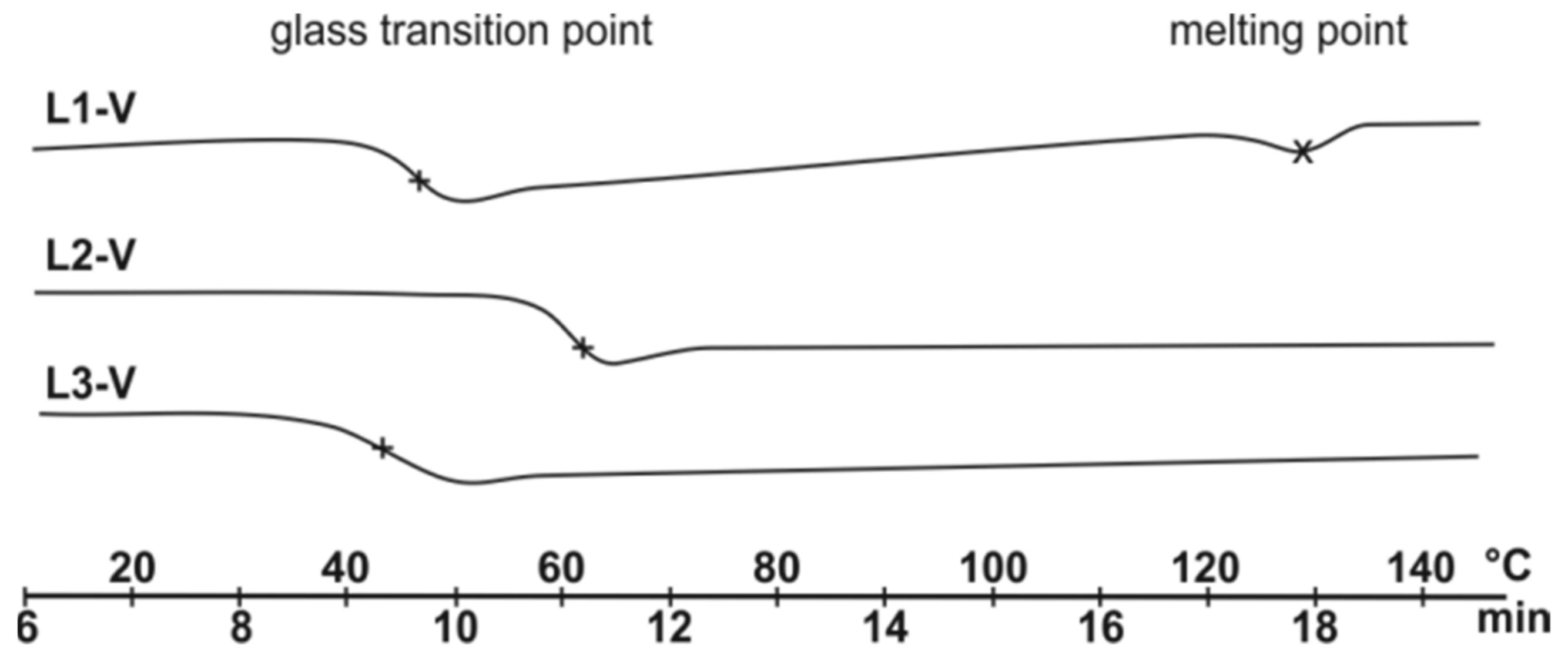
2.3. Polymer Properties
3. Materials and Methods
3.1. Materials and Ligand Synthesis
3.2. Catalysts Preparation
3.3. Polymerization Procedure
3.4. Instruments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dawooda, K.M.; Nomura, K. Recent Developments in z-selective olefin metathesis reactions by molybdenum, tungsten, ruthenium, and vanadium catalysts. Adv. Synth. Catal. 2021, 363, 1970–1997. [Google Scholar] [CrossRef]
- Yuan, S.-F.; Yan, Y.; Solan, G.A.; Ma, Y.; Sun, W.-H. Recent advancements in N-ligated group 4 molecular catalysts for the (co)polymerization of ethylene. Coord. Chem. Rev. 2020, 411, 213254. [Google Scholar] [CrossRef]
- Phillips, A.M.F.; Suo, H.; da Silva, M.F.C.G.; Pombeiro, A.J.L.; Sun, W.-H. Recent developments in vanadium-catalyzed olefin coordination polymerization. Coord. Chem. Rev. 2020, 416, 213332. [Google Scholar] [CrossRef]
- Ishikura, H.; Neven, R.; Lange, T.; Galetová, A.; Blom, B.; Romano, D. Developments in vanadium-catalysed polymerisation reactions: A review. Inorg. Chim. Acta 2021, 515, 120047. [Google Scholar] [CrossRef]
- Spronck, M.; Klein, A.; Blom, B.; Romano, D. Synthesis of disentangled ultra-high molecular weight polyethylene using vanadium(V)-based catalysts. Z. Anorg. Allg. Chem. 2018, 644, 993–998. [Google Scholar] [CrossRef]
- Bihun-Kisiel, A.; Ochędzan-Siodłak, W. Vanadium catalysts for ethylene-norbornene copolymerization. Polimery 2020, 65, 11–12. [Google Scholar] [CrossRef]
- Shakeri, S.E.; Mortazavi, S.M.M.; Ahmadjo, S.; Zohuri, G.H. Comparison of mono and dinuclear α–diimine Ni based catalysts for synthesis of polynorbornene and its microstructure study. J. Macromol. Sci. Part A 2020, 57, 837–843. [Google Scholar] [CrossRef]
- Ochędzan-Siodłak, W.; Bihun-Kisiel, A.; Siodłak, D.; Poliwoda, A.; Dziuk, B. Titanium and vanadium catalysts with oxazoline ligands for ethylenenorbornene (co)polymerization. Eur. Polym. J. 2018, 106, 148–155. [Google Scholar] [CrossRef]
- Guo, J.; Liu, H.; Bi, J.; Zhang, C.; Zhang, H.; Bai, C.; Hua, Y.; Zhang, X. Pyridine–oxazoline and quinoline–oxazoline ligated cobalt complexes: Synthesis, characterization, and 1,3-butadiene polymerization behaviors. Inorg. Chim. Acta 2015, 435, 305–312. [Google Scholar] [CrossRef]
- Yun, B.-S.; Hidaka, T.; Furihata, K.; Seto, H. Microbial metabolites with tipA promoter inducing activity-II: Geninthiocin, a novel thiopeptide produced by Streptomyces sp. DD84. J. Antibiot. 1994, 47, 969–975. [Google Scholar] [CrossRef] [Green Version]
- Kodani, S.; Ninomiya, A. Isolation of new thiopeptide berninamycin E from Streptomyces atroolivaceus. Asian J. Chem. 2013, 25, 490–492. [Google Scholar] [CrossRef]
- Vijaya Kumar, E.K.S.; Kenia, J.; Mukhopadhyay, T.; Nadkarni, S.R. Methylsulfomycin I, a new cyclic peptide antibiotic from a streptomyces sp. HIL Y-9420704. J. Nat. Prod. 1999, 62, 1562–1564. [Google Scholar] [CrossRef] [PubMed]
- Castro Rodríguez, J.; Holgado, G.G.; Santamaría Sánchez, R.I.; Cañedo, L.M. Radamycin, a novel thiopeptide produced by Streptomyces sp. RSP9. II. Physico-chemical properties and structure determination. J. Antibiot. 2002, 55, 391–395. [Google Scholar] [CrossRef] [Green Version]
- Engelhardt, K.; Degnes, K.F.; Kemmler, M.; Bredholt, H.; Fjærvik, E.; Klinkenberg, G.; Sletta, H.; Ellingsen, T.E.; Zotchev, S.B. Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl. Environ. Microbiol. 2010, 76, 4969–4976. [Google Scholar] [CrossRef] [Green Version]
- Ochędzan-Siodłak, W.; Siodłak, D.; Piontek, A.; Doležal, K. Titanium and vanadium catalysts with 2-hydroxyphenyloxazoline and oxazine ligands for ethylene-norbornene (co)polymerization. Catalysts 2019, 9, 1041. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Mu, R.; Liu, Z.-J.; Lu, S.-C.; Liu, G. Scalable total synthesis of a mycobactin T analogue utilizing a novel synthetic and protection strategy. Org. Chem. Front. 2019, 6, 2467–2470. [Google Scholar] [CrossRef]
- Yokokawa, F.; Izumi, K.; Omata, J.; Shioiri, T. Total synthesis of amamistatin A, an antiproliferative linear peptide from an actinomycete. Tetrahedron 2000, 56, 3027–3034. [Google Scholar] [CrossRef]
- Miller, M.J.; Zhu, H.; Xu, Y.; Wu, C.; Walz, A.J.; Vergne, A.; Roosenberg, J.M.; Moraski, G.; Minnick, A.A.; McKee-Dolence, J.; et al. Utilization of microbial iron assimilation processes for the development of new antibiotics and inspiration for the design of new anticancer agents. Biometals 2009, 22, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mhlongo, J.T.; Brasil, E.; de la Torre, B.G.; Albericio, F. Naturally occurring oxazole-containing peptides. Mar. Drugs 2020, 18, 203. [Google Scholar] [CrossRef]
- Nagatsu, A.; Kajitani, H.; Sakakibara, J. Muscoride A: A new oxazole peptide alkaloid from freshwater cyanobacterium Nostoc muscorum. Tetrahedron Lett. 1995, 36, 4097–4100. [Google Scholar] [CrossRef]
- Smith, T.E.; Kuo, W.; Balskus, E.P.; Bock, V.D.; Roizen, J.L.; Theberge, A.B.; Carroll, K.A.; Kurihara, T.; Wessler, J.D. Total synthesis of (-)-hennoxazole A. J. Org. Chem. 2008, 73, 142–150. [Google Scholar] [CrossRef]
- Fernández, R.; Martín, M.J.; Rodríguez-Acebes, R.; Reyes, F.; Francesch, A.; Cuevas, C. Diazonamides C–E, new cytotoxic metabolites from the ascidian Diazona sp. Tetrahedron Lett. 2008, 49, 2283–2285. [Google Scholar] [CrossRef]
- Scholz, R.; Molohon, K.J.; Nachtigall, J.; Vater, J.; Markley, A.L.; Süssmuth, R.D.; Mitchell, D.A.; Borriss, R. Plantazolicin, a novel microcin B17/streptolysin S-like natural product from Bacillus amyloliquefaciens FZB42. J. Bacteriol. 2011, 193, 215–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pattenden, G.; Ashweek, N.J.; Baker-Glenn, C.A.G.; Kempson, J.; Walker, G.M.; Yee, J.G.K. Total synthesis of (-)-ulapualide A, a novel tris-oxazole macrolide from marine nudibranchs, based on some biosynthesis speculation. Org. Biomol. Chem. 2008, 6, 1478–1497. [Google Scholar] [CrossRef]
- Liu, N.; Song, L.; Liu, M.; Shang, F.; Anderson, Z.; Fox, D.J.; Challis, G.L.; Huang, Y. Unique post-translational oxime formation in the biosynthesis of the azolemycin complex of novel ribosomal peptides from Streptomyces sp. FXJ1.264. Chem. Sci. 2016, 7, 482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin-ya, K.; Wierzba, K.; Matsuo, K.-i.; Ohtani, T.; Yamada, Y.; Furihata, K.; Hayakawa, Y.; Seto, H. Telomestatin, a novel telomerase inhibitor from Streptomyces anulatus. J. Am. Chem. Soc. 2001, 123, 1262–1263. [Google Scholar] [CrossRef] [PubMed]
- Sarada, G.; Sim, B.; Cho, W.; Yoon, J.; Gal, Y.S.; Kim, J.J.; Jin, S.H. New sky-blue and bluishegreen emitting Ir(III) complexes containing an azoline ancillary ligand for highly efficient PhOLEDs. Dye. Pigment. 2016, 131, 60–68. [Google Scholar] [CrossRef]
- Comito, R.J.; Wu, Z.; Zhang, G.; Lawrence, J.A., III; Korzyński, M.D.; Kehl, J.A.; Miller, J.T.; Dincă, M. Stabilized vanadium catalyst for olefin polymerization by site isolation in a metal–organic framework. Angew. Chem. Int. Ed. 2018, 57, 8135–8139. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Tang, Y.; Gao, H. Homo- and copolymerization of ethylene and norbornene with anilido–imine chromium catalysts. Polymers 2016, 8, 69. [Google Scholar] [CrossRef] [Green Version]
- Boggioni, L.; Losio, S.; Tritto, I. Microstructure of Copolymers of Norbornene Based on Assignments of 13C NMR Spectra: Evolution of a Methodology. Polymers 2018, 10, 647. [Google Scholar] [CrossRef] [Green Version]
- Leone, G.; Mauri, M.; Losio, S.; Bertini, F.; Ricci, G.; Porri, L. Copolymerization of ethylene with a-olefins and cyclic olefins catalyzed by a Ti(IV) diisopropoxy complex bearing a tridentate [O,S,O]-type bis(phenolato) ligand. Polym. Chem. 2014, 5, 3412–3423. [Google Scholar] [CrossRef]
| Catalyst | Item | NB | Yield | Activity | NB | Tg | Tm | Mw·10−3 | Mw/Mn |
|---|---|---|---|---|---|---|---|---|---|
| (mol/dm3) | (g) | (kg/molV/h) | (mol%) | (°C) | (°C) | (g/mol) | |||
| L1-V | 1 | - | 29.80 | 4198 | - | - | 136.2 | 630 | 2.0 |
| 2 | 0.5 | 12.88 | 1814 | 17.3 | 11.7 | 128.7 | 360 | 2.7 | |
| 3 | 1.0 | 15.05 | 2120 | 21.4 | 28.3 | 128.5 | 240 | 2.4 | |
| 4 | 1.5 | 13.45 | 1895 | 26.3 | 46.4 | 128.6 | 210 | 2.5 | |
| L2-V | 5 | - | 22.36 | 3150 | - | - | 138.3 | 890 | 2.1 |
| 6 | 0.5 | 8.80 | 1240 | 25.0 | 35.5 | - | 330 | 2.2 | |
| 7 | 1.0 | 9.83 | 1385 | 26.6 | 42.7 | - | 230 | 2.4 | |
| 8 | 1.5 | 13.23 | 1864 | 32.4 | 61.7 | - | 180 | 2.8 | |
| L3-V | 9 | - | 34.10 | 4804 | - | - | 135.3 | 720 | 2.1 |
| 10 | 0.5 | 12.11 | 1706 | 11.4 | 9.8 | - | 410 | 2.5 | |
| 11 | 1.0 | 17.38 | 2448 | 21.7 | 30.8 | - | 280 | 2.6 | |
| 12 | 1.5 | 19.35 | 2726 | 24.1 | 42.0 | - | 220 | 2.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ochędzan-Siodłak, W.; Siodłak, D.; Banaś, K.; Halikowska, K.; Wierzba, S.; Doležal, K. Naturally Occurring Oxazole Structural Units as Ligands of Vanadium Catalysts for Ethylene-Norbornene (Co)polymerization. Catalysts 2021, 11, 923. https://doi.org/10.3390/catal11080923
Ochędzan-Siodłak W, Siodłak D, Banaś K, Halikowska K, Wierzba S, Doležal K. Naturally Occurring Oxazole Structural Units as Ligands of Vanadium Catalysts for Ethylene-Norbornene (Co)polymerization. Catalysts. 2021; 11(8):923. https://doi.org/10.3390/catal11080923
Chicago/Turabian StyleOchędzan-Siodłak, Wioletta, Dawid Siodłak, Karolina Banaś, Katarzyna Halikowska, Sławomir Wierzba, and Karel Doležal. 2021. "Naturally Occurring Oxazole Structural Units as Ligands of Vanadium Catalysts for Ethylene-Norbornene (Co)polymerization" Catalysts 11, no. 8: 923. https://doi.org/10.3390/catal11080923
APA StyleOchędzan-Siodłak, W., Siodłak, D., Banaś, K., Halikowska, K., Wierzba, S., & Doležal, K. (2021). Naturally Occurring Oxazole Structural Units as Ligands of Vanadium Catalysts for Ethylene-Norbornene (Co)polymerization. Catalysts, 11(8), 923. https://doi.org/10.3390/catal11080923








