Encapsulation of Combi-CLEAs of Glycosidases in Alginate Beads and Polyvinyl Alcohol for Wine Aroma Enhancement
Abstract
:1. Introduction
2. Results and Discussion
2.1. Entrapment of Combi-CLEAs in Alginate Beads
2.2. Entrapment of Combi-CLEAs in Polyvinyl Alcohol Lens-Shaped Particles
2.3. Operational Stability of the Biocatalysts in White Wine
3. Materials and Methods
3.1. Specific Activity Analysis
3.2. Synthesis of Combi-CLEAs of Glycosidases
3.3. Encapsulation of Combi-CLEAs in Alginate Beads
3.4. Encapsulation of Combi-CLEAs in Polyvinyl Alcohol Lens-Shaped Particles
3.5. Operational Stability of the Biocatalysts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabaroglu, T.; Selli, S.; Canbas, A.; Lepoutre, J.-P.; Günata, Z. Wine flavor enhancement through the use of exogenous fungal glycosidases. Enzym. Microb. Technol. 2003, 33, 581–587. [Google Scholar] [CrossRef]
- Maicas, S.; Mateo, J.J. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: A review. Appl. Microbiol. Biotechnol. 2005, 67, 322–335. [Google Scholar] [CrossRef] [PubMed]
- Schwab, W.; Wüst, M. Understanding the Constitutive and Induced Biosynthesis of Mono- and Sesquiterpenes in Grapes (Vitis vinifera): A Key to Unlocking the Biochemical Secrets of Unique Grape Aroma Profiles. J. Agric. Food Chem. 2015, 63, 10591–10603. [Google Scholar] [CrossRef]
- Tavernini, L.; Ottone, C.; Illanes, A.; Wilson, L. Entrapment of enzyme aggregates in chitosan beads for aroma release in white wines. Int. J. Biol. Macromol. 2020, 154, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, R.; Spagna, G. β-Glucosidase in cellular and acellular form for winemaking application. Enzym. Microb. Technol. 2007, 40, 382–389. [Google Scholar] [CrossRef]
- Ottone, C.; Romero, O.; Aburto, C.; Illanes, A.; Wilson, L. Biocatalysis in the winemaking industry: Challenges and opportunities for immobilized enzymes. Compr. Rev. Food Sci. Food Saf. 2020, 19, 595–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madhavan, A.; Sindhu, R.; Binod, P.; Sukumaran, R.K.; Pandey, A. Strategies for design of improved biocatalysts for industrial applications. Bioresour. Technol. 2017, 245, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; Van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahumada, K.; Urrutia, P.; Illanes, A.; Wilson, L. Production of combi-CLEAs of glycosidases utilized for aroma enhancement in wine. Food Bioprod. Process. 2015, 94, 555–560. [Google Scholar] [CrossRef]
- Ahumada, K.; Martínez-Gil, A.; Moreno-Simunovic, Y.; Illanes, A.; Wilson, L. Aroma Release in Wine Using Co-Immobilized Enzyme Aggregates. Molecules 2016, 21, 1485. [Google Scholar] [CrossRef] [Green Version]
- Sheldon, R.A. Cross-Linked Enzyme Aggregates as Industrial Biocatalysts. Org. Process. Res. Dev. 2011, 15, 213–223. [Google Scholar] [CrossRef]
- Sheldon, R.A. CLEAs, Combi-CLEAs and ‘Smart’ Magnetic CLEAs: Biocatalysis in a Bio-Based Economy. Catalysts 2019, 9, 261. [Google Scholar] [CrossRef] [Green Version]
- Illanes, A.; Cauerhff, A.; Wilson, L.; Castro, G.R. Recent trends in biocatalysis engineering. Bioresour. Technol. 2012, 115, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Betancor, L.; Luckarift, H.R. Co-immobilized coupled enzyme systems in biotechnology. Biotechnol. Genet. Eng. Rev. 2010, 27, 95–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, L.; Illanes, A.; Pessela, B.C.C.; Abian, O.; Fernández-Lafuente, R.; Guisán, J.M. Encapsulation of crosslinked penicillin G acylase aggregates in lentikats: Evaluation of a novel biocatalyst in organic media. Biotechnol. Bioeng. 2004, 86, 558–562. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Merlis, C.; Schoneker, D. Review of the oral toxicity of polyvinyl alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319–326. [Google Scholar] [CrossRef]
- Aslam, M.; Kalyar, M.A.; Raza, Z.A. Polyvinyl alcohol: A review of research status and use of polyvinyl alcohol based nanocomposites. Polym. Eng. Sci. 2018, 58, 2119–2132. [Google Scholar] [CrossRef]
- Klein, J.; Stock, J.; Vorlop, K.-D. Pore size and properties of spherical Ca-alginate biocatalysts. Appl. Microbiol. Biotechnol. 1983, 18, 86–91. [Google Scholar] [CrossRef]
- Bidmanova, S.; Hrdlickova, E.; Jaros, J.; Ilkovics, L.; Hampl, A.; Damborsky, J.; Prokop, Z. Microscopic monitoring provides information on structure and properties during biocatalyst immobilization. Biotechnol. J. 2014, 9, 852–860. [Google Scholar] [CrossRef]
- Krasňan, V.; Stloukal, R.; Rosenberg, M.; Rebroš, M. Immobilization of cells and enzymes to LentiKats®. Appl. Microbiol. Biotechnol. 2016, 100, 2535–2553. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, S.; Shimasaki, T.; Kusakabe, I. Purification and Some Properties of Intracellular α-l-Arabinofuranosidase from Aspergillus Niger 5–16. Biosci. Biotechnol. Biochem. 1993, 57, 1161–1165. [Google Scholar] [CrossRef] [PubMed]
- Narasimha, G.; Sridevi, A.; Ramanjaneyulu, G.; Reddy, B.R. Purification and Characterization of β-Glucosidase from Aspergillus Niger. Int. J. Food Prop. 2015, 19, 652–661. [Google Scholar] [CrossRef]
- Rashid, M.H.; Siddiqui, K.S. Purification and characterization of a β-Glucosidase from Aspergillus Niger. Folia Microbiol. 1997, 42, 544–550. [Google Scholar] [CrossRef] [PubMed]
- Afandizadeh, S.; Foumeny, E. Design of packed bed reactors: Guides to catalyst shape, size, and loading selection. Appl. Therm. Eng. 2001, 21, 669–682. [Google Scholar] [CrossRef]
- Jovanovic-Malinovska, R.; Fernandes, P.; Winkelhausen, E.; Fonseca, L.P. Galacto-oligosaccharides Synthesis from Lactose and Whey by β-Galactosidase Immobilized in PVA. Appl. Biochem. Biotechnol. 2012, 168, 1197–1211. [Google Scholar] [CrossRef]
 ) 2.8 mm average diameter and (
) 2.8 mm average diameter and ( ) 4.2 mm average diameter. Results are presented as mean ± margin of error, n = 2.
) 4.2 mm average diameter. Results are presented as mean ± margin of error, n = 2.
 ) 2.8 mm average diameter and (
) 2.8 mm average diameter and ( ) 4.2 mm average diameter. Results are presented as mean ± margin of error, n = 2.
) 4.2 mm average diameter. Results are presented as mean ± margin of error, n = 2.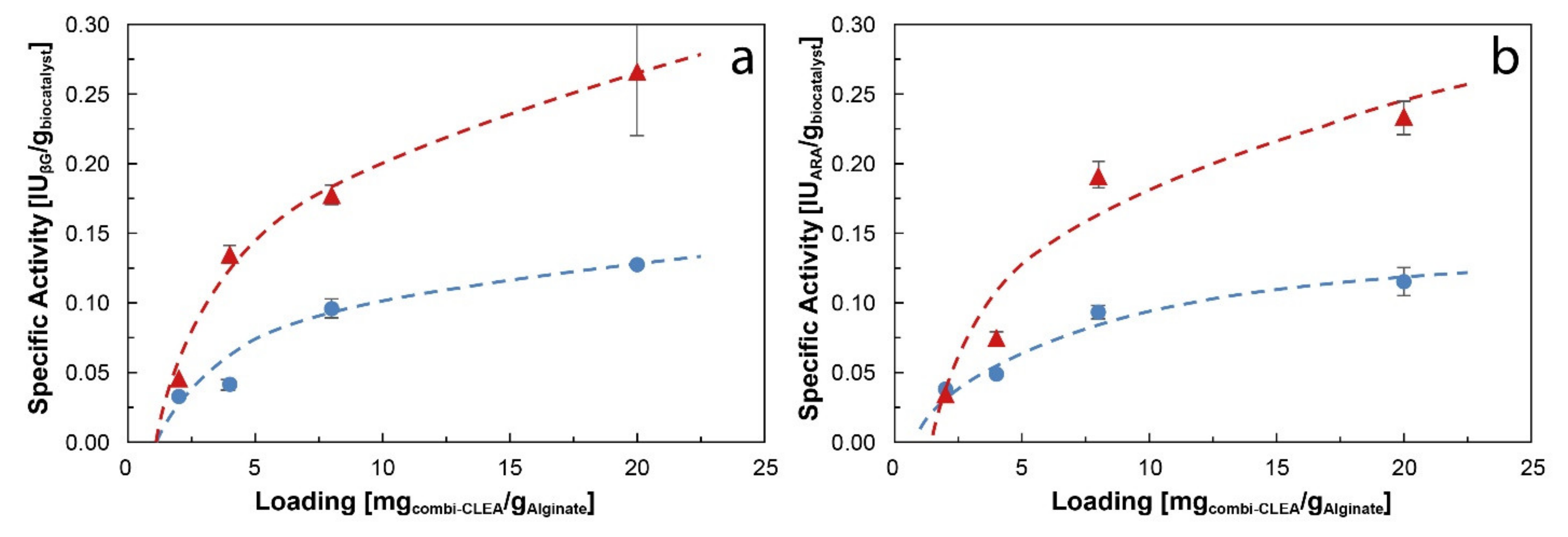
 ) 2.6 mm average diameter and (
) 2.6 mm average diameter and ( ) 6.0 mm average diameter. Results are presented as mean ± margin of error, n = 2.
) 6.0 mm average diameter. Results are presented as mean ± margin of error, n = 2.
 ) 2.6 mm average diameter and (
) 2.6 mm average diameter and ( ) 6.0 mm average diameter. Results are presented as mean ± margin of error, n = 2.
) 6.0 mm average diameter. Results are presented as mean ± margin of error, n = 2.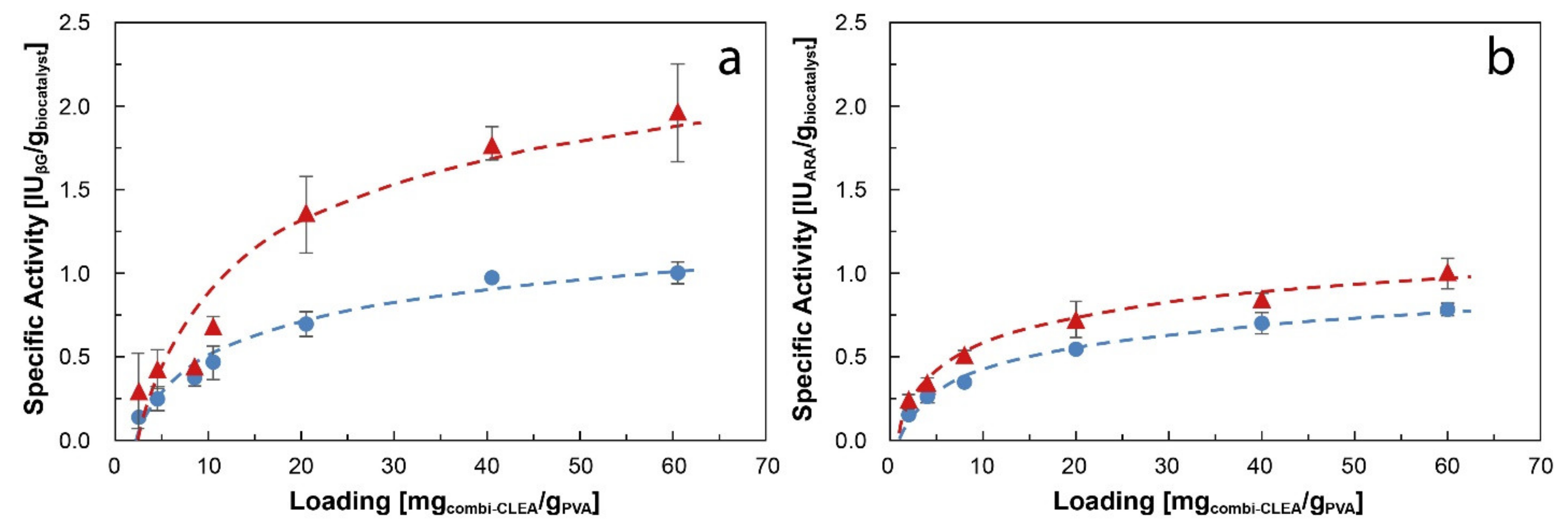
 ) soluble enzymes, (
) soluble enzymes, ( ) combi-CLEAs of glycosidases, (
) combi-CLEAs of glycosidases, ( ) combi-CLEAs encapsulated in alginate beads, and (
) combi-CLEAs encapsulated in alginate beads, and ( ) combi-CLEAs encapsulated in polyvinyl alcohol, carried out in white wine at 16 °C. Results are expressed as residual activity (A/A0) of (a) βG and (b) ARA.
) combi-CLEAs encapsulated in polyvinyl alcohol, carried out in white wine at 16 °C. Results are expressed as residual activity (A/A0) of (a) βG and (b) ARA.
 ) soluble enzymes, (
) soluble enzymes, ( ) combi-CLEAs of glycosidases, (
) combi-CLEAs of glycosidases, ( ) combi-CLEAs encapsulated in alginate beads, and (
) combi-CLEAs encapsulated in alginate beads, and ( ) combi-CLEAs encapsulated in polyvinyl alcohol, carried out in white wine at 16 °C. Results are expressed as residual activity (A/A0) of (a) βG and (b) ARA.
) combi-CLEAs encapsulated in polyvinyl alcohol, carried out in white wine at 16 °C. Results are expressed as residual activity (A/A0) of (a) βG and (b) ARA.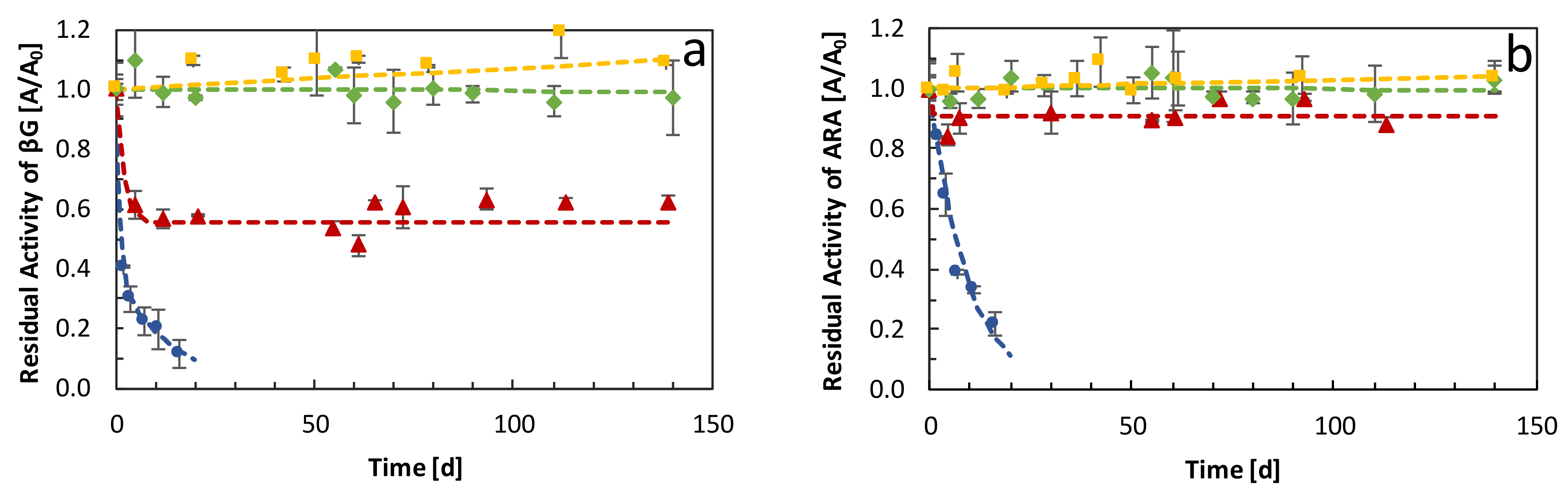
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavernini, L.; Aburto, C.; Romero, O.; Illanes, A.; Wilson, L. Encapsulation of Combi-CLEAs of Glycosidases in Alginate Beads and Polyvinyl Alcohol for Wine Aroma Enhancement. Catalysts 2021, 11, 866. https://doi.org/10.3390/catal11070866
Tavernini L, Aburto C, Romero O, Illanes A, Wilson L. Encapsulation of Combi-CLEAs of Glycosidases in Alginate Beads and Polyvinyl Alcohol for Wine Aroma Enhancement. Catalysts. 2021; 11(7):866. https://doi.org/10.3390/catal11070866
Chicago/Turabian StyleTavernini, Luigi, Carla Aburto, Oscar Romero, Andrés Illanes, and Lorena Wilson. 2021. "Encapsulation of Combi-CLEAs of Glycosidases in Alginate Beads and Polyvinyl Alcohol for Wine Aroma Enhancement" Catalysts 11, no. 7: 866. https://doi.org/10.3390/catal11070866
APA StyleTavernini, L., Aburto, C., Romero, O., Illanes, A., & Wilson, L. (2021). Encapsulation of Combi-CLEAs of Glycosidases in Alginate Beads and Polyvinyl Alcohol for Wine Aroma Enhancement. Catalysts, 11(7), 866. https://doi.org/10.3390/catal11070866







