Heterogeneous Catalysts for the Conversion of Glucose into 5-Hydroxymethyl Furfural
Abstract
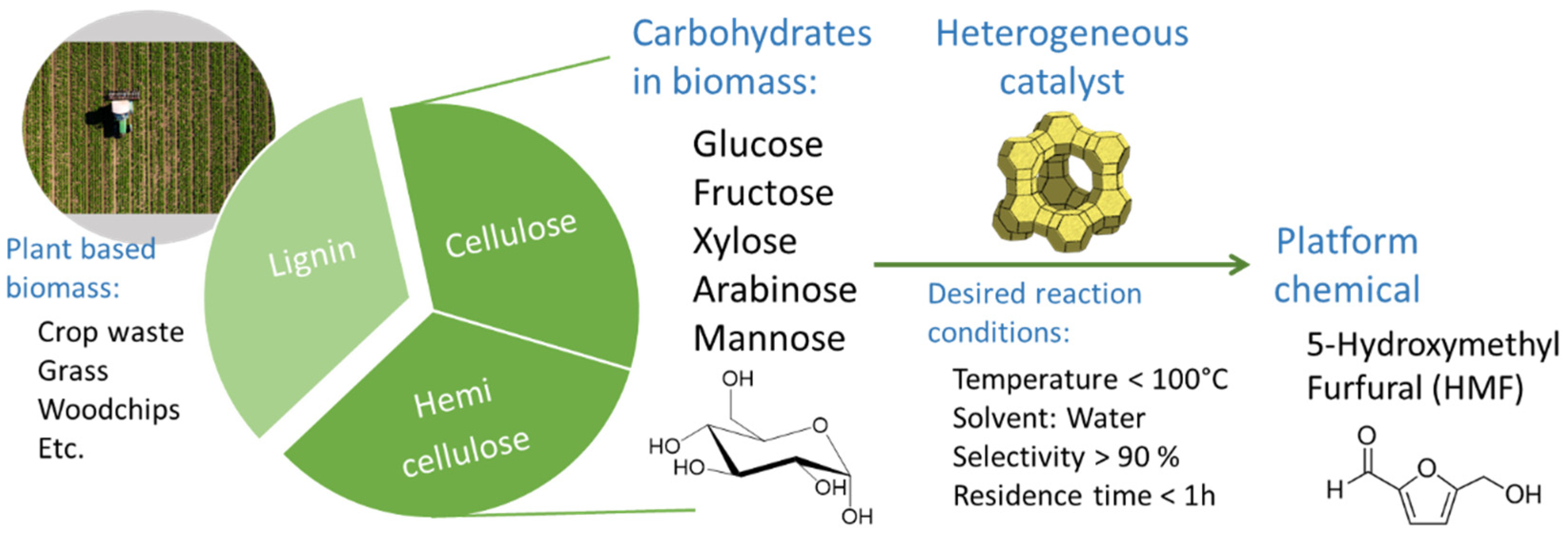
:1. Introduction
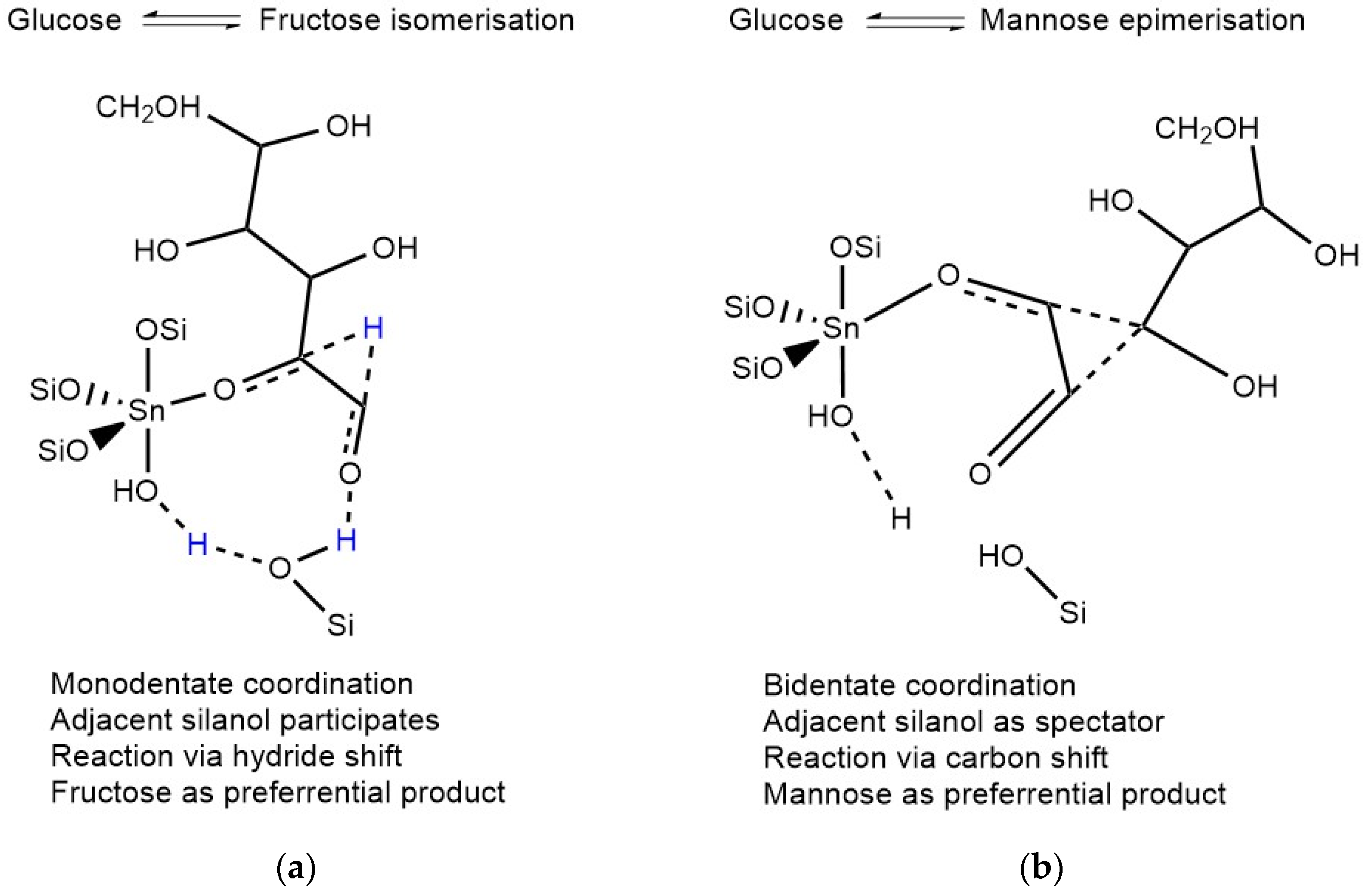
2. Mechanism of Glucose Conversion
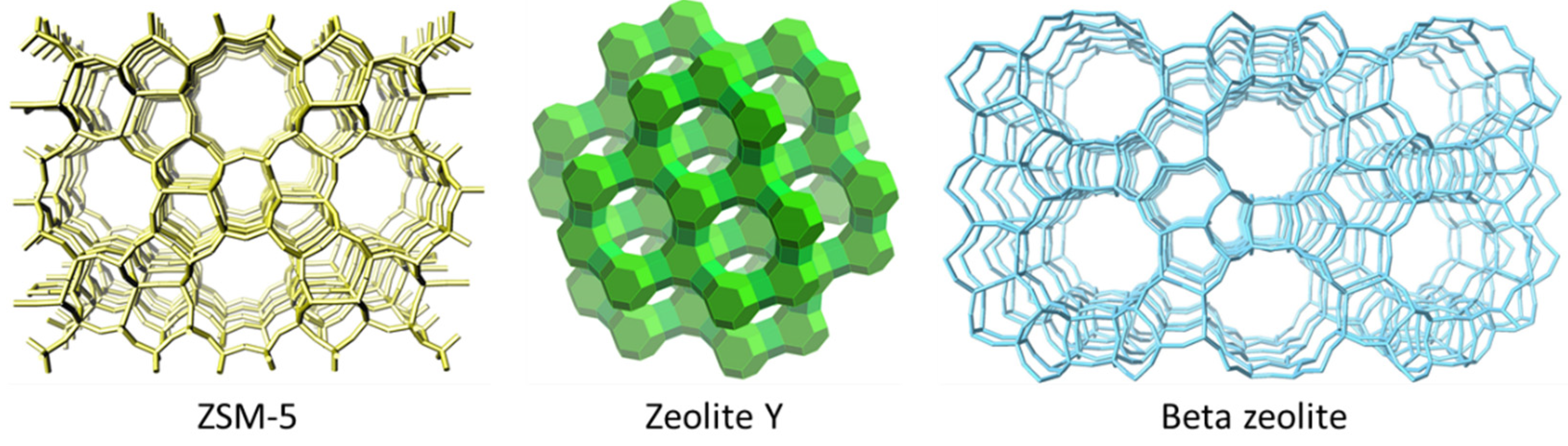
3. Zeolites
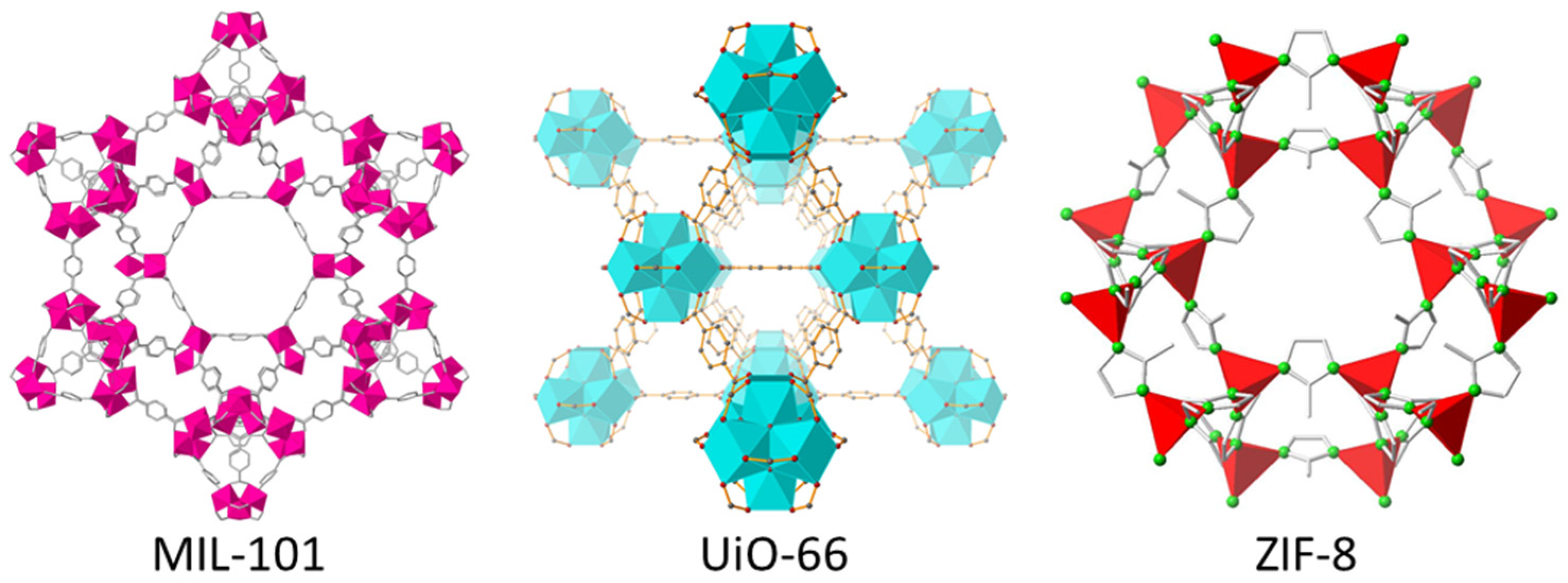
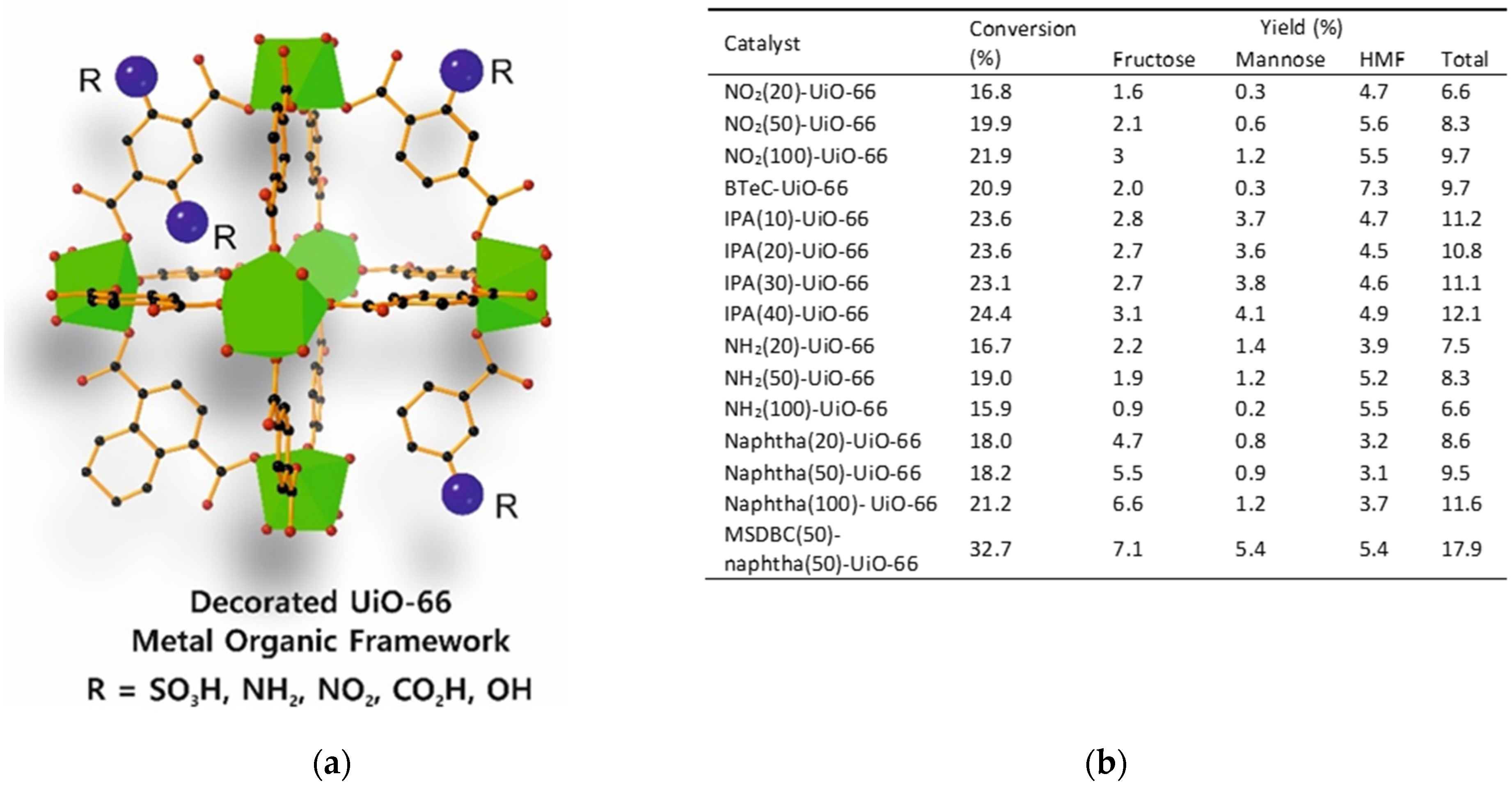
4. MOFs
5. Conventional Supported Catalysts
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Degirmenci, V.; Uner, D.; Cinlar, B.; Shanks, B.H.; Yilmaz, A.; Van Santen, R.A.; Hensen, E.J.M. Sulfated Zirconia Modified SBA-15 Catalysts for Cellobiose Hydrolysis. Catal. Lett. 2011, 141, 33–42. [Google Scholar] [CrossRef]
- Tempelman, C.; Jacobs, J.; Ramkhelawan, S.; Mok, A.; van der Zalm, W.; Degirmenci, V. Processing of agricultural apple fruit waste into sugar rich feedstocks for the catalytic production of 5-HMF over a Sn Amberlyst-15 resin catalyst. J. Ind. Eng. Chem. 2021, 99, 443–448. [Google Scholar] [CrossRef]
- Van Putten, R.-J.; Van Der Waal, J.C.; De Jong, E.; Rasrendra, C.B.; Heeres, H.J.; De Vries, J.G. Hydroxymethylfurfural, A Versatile Platform Chemical Made from Renewable Resources. Chem. Rev. 2013, 113, 1499–1597. [Google Scholar] [CrossRef]
- Gupta, K.; Rai, R.K.; Singh, S.K. Metal Catalysts for the Efficient Transformation of Biomass-derived HMF and Furfural to Value Added Chemicals. ChemCatChem 2018, 10, 2326–2349. [Google Scholar] [CrossRef]
- Yu, I.K.; Tsang, D.C. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef]
- Kong, Q.-S.; Li, X.-L.; Xu, H.-J.; Fu, Y. Conversion of 5-hydroxymethylfurfural to chemicals: A review of catalytic routes and product applications. Fuel Process. Technol. 2020, 209, 106528. [Google Scholar] [CrossRef]
- Ranoux, A.; Djanashvili, K.; Arends, I.W.C.E.; Hanefeld, U. 5-Hydroxymethylfurfural Synthesis from Hexoses Is Autocatalytic. ACS Catal. 2013, 3, 760–763. [Google Scholar] [CrossRef]
- Zhao, H.; Holladay, J.E.; Brown, H.; Zhang, Z.C. Metal Chlorides in Ionic Liquid Solvents Convert Sugars to 5-Hydroxymethylfurfural. Science 2007, 316, 1597–1600. [Google Scholar] [CrossRef] [PubMed]
- Pidko, E.; Degirmenci, V.; Van Santen, R.A.; Hensen, E.J.M. Glucose Activation by Transient Cr2+Dimers. Angew. Chem. Int. Ed. 2010, 49, 2530–2534. [Google Scholar] [CrossRef] [PubMed]
- Pidko, E.A.; Degirmenci, V.; Hensen, E.J.M. On the Mechanism of Lewis Acid Catalyzed Glucose Transformations in Ionic Liquids. ChemCatChem 2012, 4, 1263–1271. [Google Scholar] [CrossRef]
- Pidko, E.A.; Degirmenci, V.; Van Santen, R.A.; Hensen, E.J.M. Coordination Properties of Ionic Liquid-Mediated Chromium(II) and Copper(II) Chlorides and Their Complexes with Glucose. Inorg. Chem. 2010, 49, 10081–10091. [Google Scholar] [CrossRef]
- Degirmenci, V.; Hensen, E.J.M. Development of a heterogeneous catalyst for lignocellulosic biomass conversion: Glucose dehydration by metal chlorides in a silica-supported ionic liquid layer. Environ. Prog. Sustain. Energy 2013, 33, 657–662. [Google Scholar] [CrossRef]
- Degirmenci, V.; Pidko, E.; Magusin, P.C.M.M.; Hensen, E.J.M. Towards a Selective Heterogeneous Catalyst for Glucose Dehydration to 5-Hydroxymethylfurfural in Water: CrCl2 Catalysis in a Thin Immobilized Ionic Liquid Layer. ChemCatChem 2011, 3, 969–972. [Google Scholar] [CrossRef]
- Moliner, M.; Román-Leshkov, Y.; Davis, M.E. Tin-containing zeolites are highly active catalysts for the isomerization of glucose in water. Proc. Natl. Acad. Sci. USA 2010, 107, 6164–6168. [Google Scholar] [CrossRef] [Green Version]
- Davis, M.E. Heterogeneous Catalysis for the Conversion of Sugars into Polymers. Top. Catal. 2015, 58, 405–409. [Google Scholar] [CrossRef]
- Roman-Leshkov, Y.; Davis, M.E. Activation of Carbonyl-Containing Molecules with Solid Lewis Acids in Aqueous Media. ACS Catal. 2011, 1, 1566–1580. [Google Scholar] [CrossRef]
- Gounder, R.; Davis, M.E. Monosaccharide and disaccharide isomerization over Lewis acid sites in hydrophobic and hydrophilic molecular sieves. J. Catal. 2013, 308, 176–188. [Google Scholar] [CrossRef]
- Delidovich, I.; Palkovits, R. Catalytic Isomerization of Biomass-Derived Aldoses: A Review. ChemSusChem 2016, 9, 547–561. [Google Scholar] [CrossRef] [PubMed]
- De Val, R.B.; Orazov, M.; Gounder, R.; Hwang, S.-J.; Davis, M.E. Active Sites in Sn-Beta for Glucose Isomerization to Fructose and Epimerization to Mannose. ACS Catal. 2014, 4, 2288–2297. [Google Scholar] [CrossRef] [Green Version]
- Rai, N.; Caratzoulas, S.; Vlachos, D.G. Role of Silanol Group in Sn-Beta Zeolite for Glucose Isomerization and Epimerization Reactions. ACS Catal. 2013, 3, 2294–2298. [Google Scholar] [CrossRef]
- Bates, J.S.; Bukowski, B.C.; Harris, J.W.; Greeley, J.P.; Gounder, R. Distinct Catalytic Reactivity of Sn Substituted in Framework Locations and at Defect Grain Boundaries in Sn-Zeolites. ACS Catal. 2019, 9, 6146–6168. [Google Scholar] [CrossRef]
- Osmundsen, C.M.; Holm, M.S.; Dahl, S.; Taarning, E. Tin-containing silicates: Structure–activity relations. Proc. R. Soc. A Math. Phys. Eng. Sci. 2012, 468, 2000–2016. [Google Scholar] [CrossRef] [Green Version]
- Rajabbeigi, N.; Torres, A.I.; Lew, C.M.; Elyassi, B.; Ren, L.; Wang, Z.; Cho, H.J.; Fan, W.; Daoutidis, P.; Tsapatsis, M. On the kinetics of the isomerization of glucose to fructose using Sn-Beta. Chem. Eng. Sci. 2014, 116, 235–242. [Google Scholar] [CrossRef]
- Pedersen, A.T.; Ringborg, R.H.; Grotkjær, T.; Pedersen, S.; Woodley, J. Synthesis of 5-hydroxymethylfurfural (HMF) by acid catalyzed dehydration of glucose–fructose mixtures. Chem. Eng. J. 2015, 273, 455–464. [Google Scholar] [CrossRef]
- Chatterjee, S.; Degirmenci, V.; Aiouache, F.; Rebrov, E. Design of a radio frequency heated isothermal micro-trickle bed reactor. Chem. Eng. J. 2014, 243, 225–233. [Google Scholar] [CrossRef]
- Chatterjee, S.; Degirmenci, V.; Rebrov, E. Design and operation of a radio-frequency heated micro-trickle bed reactor for consecutive catalytic reactions. Chem. Eng. J. 2015, 281, 884–891. [Google Scholar] [CrossRef]
- Fernández, J.; Chatterjee, S.; Degirmenci, V.; Rebrov, E.V. Scale-up of an RF heated micro trickle bed reactor to a kg/day production scale. Green Process. Synth. 2015, 4, 343–353. [Google Scholar] [CrossRef]
- Hillen, L.; Degirmenci, V. Synthesis Methods for the Production of Hierarchically Mesoporous and Microporous Zeolites. Rev. Adv. Sci. Eng. 2015, 4, 147–162. [Google Scholar] [CrossRef]
- Li, J.; Corma, A.; Yu, J. Synthesis of new zeolite structures. Chem. Soc. Rev. 2015, 44, 7112–7127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roman-Leshkov, Y.; Moliner, M.; Labinger, M.A.; Davis, M.E. Mechanism of glucose isomerization using a solid lewis acid catalyst in water. Angew. Chem. Int. Ed. 2010, 47, 8954–8957. [Google Scholar] [CrossRef] [Green Version]
- Taarning, E.; Saravanamurugan, S.; Holm, M.S.; Xiong, J.; West, R.M.; Christensen, C.H. Zeolite-Catalyzed Isomerization of Triose Sugars. ChemSusChem 2009, 2, 625–627. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Zhang, X.; Liu, H.; Qiu, J.; Yeung, K.L. A rapid synthesis route for Sn-Beta zeolites by steam-assisted conversion and their catalytic performance in Baeyer–Villiger oxidation. Chem. Eng. J. 2013, 218, 425–432. [Google Scholar] [CrossRef]
- Kang, Z.; Zhang, X.; Liu, H.; Qiu, J.; Han, W.; Yeung, K.L. Factors affecting the formation of Sn-Beta zeolites by steam-assisted conversion method. Mater. Chem. Phys. 2013, 141, 519–529. [Google Scholar] [CrossRef]
- Dijkmans, J.; Gabriëls, D.; Dusselier, M.; De Clippel, F.; Vanelderen, P.; Houthoofd, K.; Malfliet, A.; Pontikes, Y.; Sels, B.F. Productive sugar isomerization with highly active Sn in dealuminated β zeolites. Green Chem. 2013, 15, 2777–2785. [Google Scholar] [CrossRef]
- Hammond, C.; Conrad, S.; Hermans, I. Simple and Scalable Preparation of Highly Active Lewis Acidic Sn-β. Angew. Chem. Int. Ed. 2012, 51, 11736–11739. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Liu, G.; Wu, H.; Liu, Y.; Jiang, J.-G.; Wu, P. Postsynthesis and Selective Oxidation Properties of Nanosized Sn-Beta Zeolite. J. Phys. Chem. C 2011, 115, 3663–3670. [Google Scholar] [CrossRef]
- Wolf, P.; Hammond, C.; Conrad, S.; Hermans, I. Post-synthetic preparation of Sn-, Ti- and Zr-beta: A facile route to water tolerant, highly active Lewis acidic zeolites. Dalton Trans. 2014, 43, 4514–4519. [Google Scholar] [CrossRef]
- Van Der Graaff, W.N.P.; Li, G.G.; Mezari, B.B.; Pidko, E.A.; Hensen, E.J.M. Synthesis of Sn-Beta with Exclusive and High Framework Sn Content. ChemCatChem 2015, 7, 1152–1160. [Google Scholar] [CrossRef]
- Yang, G.; Pidko, E.A.; Hensen, E.J.M. The Mechanism of Glucose Isomerization to Fructose over Sn-BEA Zeolite: A Periodic Density Functional Theory Study. ChemSusChem 2013, 6, 1688–1696. [Google Scholar] [CrossRef]
- Candu, N.; El Fergani, M.; Verziu, M.; Cojocaru, B.; Jurca, B.; Apostol, N.; Teodorescu, C.M.; Parvulescu, V.I.; Coman, S.M. Efficient glucose dehydration to HMF onto Nb-BEA catalysts. Catal. Today 2019, 325, 109–116. [Google Scholar] [CrossRef]
- Nogier, J.-P.; Millot, Y.; Man, P.P.; Shishido, T.; Che, M.; Dzwigaj, S. Probing the Incorporation of Ti(IV) into the BEA Zeolite Framework by XRD, FTIR, NMR, and DR UV−jp810722bis. J. Phys. Chem. C 2009, 113, 4885–4889. [Google Scholar] [CrossRef]
- Van Der Graaff, W.N.P.; Tempelman, C.H.L.; Pidko, E.A.; Hensen, E.J.M. Influence of pore topology on synthesis and reactivity of Sn-modified zeolite catalysts for carbohydrate conversions. Catal. Sci. Technol. 2017, 7, 3151–3162. [Google Scholar] [CrossRef] [Green Version]
- Van der Graaff, W.N.; Tempelman, C.H.; Hendriks, F.C.; Ruiz-Martinez, J.; Bals, S.; Weckhuysen, B.M.; Pidko, E.A.; Hensen, E.J.M. Deactivation of Sn-Beta during carbohydrate conversion. Appl. Catal. A Gen. 2018, 564, 113–122. [Google Scholar] [CrossRef]
- Nikolla, E.; Roman-Leshkov, Y.; Moliner, M.; Davis, M.E. “One-Pot” Synthesis of 5-(Hydroxymethyl)furfural from Carbohydrates using Tin-Beta Zeolite. ACS Catal. 2011, 1, 408–410. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Ren, L.; Alhassan, S.M.; Tsapatsis, M. Glucose isomerization in dioxane/water with Sn-β catalyst: Improved catalyst stability and use for HMF production. Chem. Commun. 2019, 55, 14942–14945. [Google Scholar] [CrossRef]
- Hu, L.; Wu, Z.; Xu, J.; Sun, Y.; Lin, L.; Liu, S. Zeolite-promoted transformation of glucose into 5-hydroxymethylfurfural in ionic liquid. Chem. Eng. J. 2014, 244, 137–144. [Google Scholar] [CrossRef]
- Dapsens, P.Y.; Mondelli, C.; Jagielski, J.; Hauert, R.; Pérez-Ramírez, J. Hierarchical Sn-MFI zeolites prepared by facile top-down methods for sugar isomerisation. Catal. Sci. Technol. 2014, 4, 2302–2311. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Guo, Q.; Yang, P.; Liu, X.; Wang, Y. Synthesis of hierarchical Sn-Beta zeolite and its catalytic performance in glucose conversion. Catal. Today 2021, 367, 117–123. [Google Scholar] [CrossRef]
- Oozeerally, R.; Pillier, J.; Kilic, E.; Thompson, P.B.; Walker, M.; Griffith, B.E.; Hanna, J.V.; Degirmenci, V. Gallium and tin exchanged Y zeolites for glucose isomerisation and 5-hydroxymethyl furfural production. Appl. Catal. A Gen. 2020, 605, 117798. [Google Scholar] [CrossRef]
- Sezgin, E.; Keçeci, M.E.; Akmaz, S.; Koc, S.N. Heterogeneous Cr-zeolites (USY and Beta) for the conversion of glucose and cellulose to 5-hydroxymethylfurfural (HMF). Cellulose 2019, 26, 9035–9043. [Google Scholar] [CrossRef]
- Mamo, W.; Chebude, Y.; Márquez-Álvarez, C.; Díaz, I.; Sastre, E. Comparison of glucose conversion to 5-HMF using different modified mordenites in ionic liquid and biphasic media. Catal. Sci. Technol. 2016, 6, 2766–2774. [Google Scholar] [CrossRef]
- Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P. Brönsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural. Chem. Eng. J. 2016, 303, 22–30. [Google Scholar] [CrossRef]
- Hoang, P.H.; Dat, N.M.; Cuong, T.D.; Tung, D.T. Production of 5-hydroxymethylfurfural (HMF) from rice-straw biomass using a HSO3–ZSM-5 zeolite catalyst under assistance of sonication. RSC Adv. 2020, 10, 13489–13495. [Google Scholar] [CrossRef] [Green Version]
- Otomo, R.; Yokoi, T.; Kondo, J.N.; Tatsumi, T. Dealuminated Beta zeolite as effective bifunctional catalyst for direct transformation of glucose to 5-hydroxymethylfurfural. Appl. Catal. A Gen. 2014, 470, 318–326. [Google Scholar] [CrossRef]
- Saenluang, K.; Thivasasith, A.; Dugkhuntod, P.; Pornsetmetakul, P.; Salakhum, S.; Namuangruk, S.; Wattanakit, C. In Situ Synthesis of Sn-Beta Zeolite Nanocrystals for Glucose to Hydroxymethylfurfural (HMF). Catalyst 2020, 10, 1249. [Google Scholar] [CrossRef]
- Wu, L.; Degirmenci, V.; Magusin, P.C.M.M.; Lousberg, N.J.H.G.M.; Hensen, E.J.M. Mesoporous SSZ-13 zeolite prepared by a dual-template method with improved performance in the methanol-to-olefins reaction. J. Catal. 2013, 298, 27–40. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Kim, W.; Degirmenci, V.; Xin, H.; Ryoo, R.; Hensen, E.J.M. Catalytic performance of sheet-like Fe/ZSM-5 zeolites for the selective oxidation of benzene with nitrous oxide. J. Catal. 2013, 299, 81–89. [Google Scholar] [CrossRef]
- Wu, L.; Magusin, P.C.M.M.; Degirmenci, V.; Li, M.; Almutairi, S.M.T.; Zhu, X.; Mezari, B.; Hensen, E.J.M. Acidic properties of nanolayered ZSM-5 zeolites. Microporous Mesoporous Mater. 2014, 189, 144–157. [Google Scholar] [CrossRef]
- Van Der Graaff, W.N.P.; Tempelman, C.H.L.; Li, G.; Mezari, B.; Kosinov, N.; Pidko, E.A.; Hensen, E.J.M. Competitive Adsorption of Substrate and Solvent in Sn-Beta Zeolite During Sugar Isomerization. ChemSusChem 2016, 9, 3145–3149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordon, M.J.; Hall, J.N.; Harris, J.W.; Bates, J.S.; Hwang, S.-J.; Gounder, R. Deactivation of Sn-Beta zeolites caused by structural transformation of hydrophobic to hydrophilic micropores during aqueous-phase glucose isomerization. Catal. Sci. Technol. 2019, 9, 1654–1668. [Google Scholar] [CrossRef] [Green Version]
- Padovan, D.; Botti, L.; Hammond, C. Active Site Hydration Governs the Stability of Sn-Beta during Continuous Glucose Conversion. ACS Catal. 2018, 8, 7131–7140. [Google Scholar] [CrossRef]
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The Chemistry and Applications of Metal-Organic Frameworks. Science 2013, 341, 1230444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal–organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef] [PubMed]
- Oozeerally, R.; Ramkhelawan, S.D.K.; Burnett, D.L.; Tempelman, C.H.L.; Degirmenci, V. ZIF-8 Metal Organic Framework for the Conversion of Glucose to Fructose and 5-Hydroxymethyl Furfural. Catalyst 2019, 9, 812. [Google Scholar] [CrossRef] [Green Version]
- Herbst, A.; Janiak, C. Selective glucose conversion to 5-hydroxymethylfurfural (5-HMF) instead of levulinic acid with MIL-101Cr MOF-derivatives. New J. Chem. 2016, 40, 7958–7967. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Chang, G.; Zhang, Z.; Xing, H.; Su, B.; Yang, Q.; Ren, Q.; Yang, Y.; Bao, Z. Catalytic dehydration of glucose to 5-hydroxymethylfurfural with a bifunctional metal-organic framework. AIChE J. 2016, 62, 4403–4417. [Google Scholar] [CrossRef]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Walton, R.I.; Degirmenci, V. Exceptionally Efficient and Recyclable Heterogeneous Metal-Organic Framework Catalyst for Glucose Isomerization in Water. ChemCatChem 2018, 10, 706–709. [Google Scholar] [CrossRef]
- Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Kashtiban, R.J.; Huband, S.; Walton, R.I.; Degirmenci, V. Systematic Modification of UiO-66 Metal-Organic Frameworks for Glucose Conversion into 5-Hydroxymethyl Furfural in Water. ChemCatChem 2021, 13, 2517–2529. [Google Scholar] [CrossRef]
- Gong, J.; Katz, M.J.; Kerton, F.M. Catalytic conversion of glucose to 5-hydroxymethylfurfural using zirconium-containing metal–organic frameworks using microwave heating. RSC Adv. 2018, 8, 31618–31627. [Google Scholar] [CrossRef] [Green Version]
- Yabushita, M.; Li, P.; Islamoglu, T.; Kobayashi, H.; Fukuoka, A.; Farha, O.K.; Katz, A. Selective Metal–Organic Framework Catalysis of Glucose to 5-Hydroxymethylfurfural Using Phosphate-Modified NU-1000. Ind. Eng. Chem. Res. 2017, 56, 7141–7148. [Google Scholar] [CrossRef]
- Riezzati, N.; Krisnandi, Y.K.; Zulys, A. Metal organic frameworks of lanthanum and iron using BDC linker as catalysts for glucose conversion into 5-hydroxymethylfurfural (5-HMF). IOP Conf. Ser. Mater. Sci. Eng. 2020, 902, 012044. [Google Scholar] [CrossRef]
- Pertiwi, R.; Oozeerally, R.; Burnett, D.L.; Chamberlain, T.W.; Cherkasov, N.; Walker, M.; Kashtiban, R.J.; Krisnandi, Y.K.; Degirmenci, V.; Walton, R.I. Replacement of Chromium by Non-Toxic Metals in Lewis-Acid MOFs: Assessment of Stability as Glucose Conversion Catalysts. Catalyst 2019, 9, 437. [Google Scholar] [CrossRef] [Green Version]
- Burnett, D.L.; Oozeerally, R.; Pertiwi, R.; Chamberlain, T.W.; Cherkasov, N.; Clarkson, G.J.; Krisnandi, Y.K.; Degirmenci, V.; Walton, R.I. A hydrothermally stable ytterbium metal-organic framework as a bifunctional solid-acid catalyst for glucose conversion. Chem. Commun. 2019, 55, 11446–11449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Degirmenci, V.; Li, C.; Hensen, E.J.M. Phosphotungstic Acid Encapsulated in Metal-Organic Framework as Catalysts for Carbohydrate Dehydration to 5-Hydroxymethylfurfural. ChemSusChem 2010, 4, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Li, K.; Chen, L.; Liu, R.; Huang, X.; Ye, D. Conversion of fructose into 5-hydroxymethylfurfural catalyzed by recyclable sulfonic acid-functionalized metal–organic frameworks. Green Chem. 2014, 16, 2490–2499. [Google Scholar] [CrossRef]
- Akiyama, G.; Matsuda, R.; Sato, H.; Kitagawa, S. Catalytic Glucose Isomerization by Porous Coordination Polymers with Open Metal Sites. Chem. Asian J. 2014, 9, 2772–2777. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, W.; Song, H.; Wei, Y.; Jin, P.; Li, B.; Yan, C.; Pan, J.; Yan, Y. Coupled acid and base UiO-66-type MOFs supported on g-C3N4 as a bi-functional catalyst for one-pot production of 5-HMF from glucose. Microporous Mesoporous Mater. 2020, 305, 110328. [Google Scholar] [CrossRef]
- Guo, Q.; Ren, L.; Kumar, P.; Cybulskis, V.J.; Mkhoyan, K.A.; Davis, M.E.; Tsapatsis, M. A Chromium Hydroxide/MIL-101(Cr) MOF Composite Catalyst and Its Use for the Selective Isomerization of Glucose to Fructose. Angew. Chem. Int. Ed. 2018, 57, 4926–4930. [Google Scholar] [CrossRef]
- Tempelman, C.; Jacobs, U.; Hut, T.; Pereira de Pina, E.P.; van Munster, M.; Cherkasov, N.; Degirmenci, V. Sn exchanged acidic ion exchange resin for the stable and continuous production of 5-HMF from glucose at low temperature. Appl. Catal. A Gen. 2019, 588, 117267. [Google Scholar] [CrossRef]
- Ravasco, J.M.J.M.; Coelho, J.A.S.; Simeonov, S.P.; Afonso, C.A.M. Bifunctional Cr3+ modified ion exchange resins as efficient reusable catalysts for the production and isolation of 5-hydroxymethylfurfural from glucose. RSC Adv. 2017, 7, 7555–7559. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Li, N.; Chen, F.; Li, G.; Wang, A.; Cong, Y.; Wang, X.; Zhang, T. Highly efficient synthesis of 5-hydroxymethylfurfural with carbohydrates over renewable cyclopentanone-based acidic resin. Green Chem. 2017, 19, 1855–1860. [Google Scholar] [CrossRef]
- Cao, X.; Teong, S.P.; Wu, D.; Yi, G.; Su, H.; Zhang, Y. An enzyme mimic ammonium polymer as a single catalyst for glucose dehydration to 5-hydroxymethylfurfural. Green Chem. 2015, 17, 2348–2352. [Google Scholar] [CrossRef]
- Zhang, T.; Li, W.; Xin, H.; Jin, L.; Liu, Q. Production of HMF from glucose using an Al3+-promoted acidic phenol-formaldehyde resin catalyst. Catal. Commun. 2019, 124, 56–61. [Google Scholar] [CrossRef]
- Marianou, A.A.; Michailof, C.M.; Pineda, A.; Iliopoulou, E.F.; Triantafyllidis, K.S.; Lappas, A.A. Effect of Lewis and Brønsted acidity on glucose conversion to 5-HMF and lactic acid in aqueous and organic media. Appl. Catal. A Gen. 2018, 555, 75–87. [Google Scholar] [CrossRef]
- Torres-Olea, B.; Mérida-Morales, S.; García-Sancho, C.; Cecilia, J.A.; Maireles-Torres, P. Catalytic Activity of Mixed Al2O3-ZrO2 Oxides for Glucose Conversion into 5-Hydroxymethylfurfural. Catalyst 2020, 10, 878. [Google Scholar] [CrossRef]
- Hou, Q.; Zhen, M.; Li, W.; Liu, L.; Liu, J.; Zhang, S.; Nie, Y.; Bai, C.; Bai, X.; Ju, M. Efficient catalytic conversion of glucose into 5-hydroxymethylfurfural by aluminum oxide in ionic liquid. Appl. Catal. B Environ. 2019, 253, 1–10. [Google Scholar] [CrossRef]
- Zhang, M.; Su, K.; Song, H.; Li, Z.; Cheng, B. The excellent performance of amorphous Cr2O3, SnO2, SrO and graphene oxide–ferric oxide in glucose conversion into 5-HMF. Catal. Commun. 2015, 69, 76–80. [Google Scholar] [CrossRef]
- Guo, J.; Zhu, S.; Cen, Y.; Qin, Z.; Wang, J.; Fan, W. Ordered mesoporous Nb–W oxides for the conversion of glucose to fructose, mannose and 5-hydroxymethylfurfural. Appl. Catal. B Environ. 2017, 200, 611–619. [Google Scholar] [CrossRef]
- Wiesfeld, J.; Sommerdijk, N.A.J.M.; Hensen, E.J.M. Early Transition Metal Doped Tungstite as an Effective Catalyst for Glucose Upgrading to 5-Hydroxymethylfurfural. Catal. Lett. 2018, 148, 3093–3101. [Google Scholar] [CrossRef] [Green Version]
- Wiesfeld, J.; Gaquere, R.; Hensen, E.J.M. Mesoporous Doped Tungsten Oxide for Glucose Dehydration to 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2019, 7, 7552–7562. [Google Scholar] [CrossRef]
- Morales, I.J.; Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Mesoporous tantalum oxide as catalyst for dehydration of glucose to 5-hydroxymethylfurfural. Appl. Catal. B Environ. 2014, 154–155, 190–196. [Google Scholar] [CrossRef]
- Guo, B.; He, L.; Tang, G.; Zhang, L.; Ye, L.; Yue, B.; Tsang, S.C.E.; He, H. Dehydration of sugars to 5-hydroxymethylfurfural and non-stoichiometric formic and levulinic acids over mesoporous Ta and Ta-W oxide solid acid catalysts. Chin. J. Catal. 2020, 41, 1248–1260. [Google Scholar] [CrossRef]
- Guo, B.; Ye, L.; Tang, G.; Zhang, L.; Yue, B.; Tsang, S.C.E.; He, H. Effect of Brønsted/Lewis Acid Ratio on Conversion of Sugars to 5-Hydroxymethylfurfural over Mesoporous Nb and Nb-W Oxides. Chin. J. Chem. 2017, 35, 1529–1539. [Google Scholar] [CrossRef]
- Njagi, E.C.; Genuino, H.C.; Kuo, C.-H.; Dharmarathna, S.; Gudz, A.; Suib, S.L. High-yield selective conversion of carbohydrates to methyl levulinate using mesoporous sulfated titania-based catalysts. Microporous Mesoporous Mater. 2015, 202, 68–72. [Google Scholar] [CrossRef]
- Saravanan, K.; Park, K.S.; Jeon, S.; Bae, J.W. Aqueous Phase Synthesis of 5-Hydroxymethylfurfural from Glucose over Large Pore Mesoporous Zirconium Phosphates: Effect of Calcination Temperature. ACS Omega 2018, 3, 808–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, A.; Shore, A.M.; Jonnalagadda, S.C.; Ramanujachary, K.V.; Mugweru, A. Conversion of fructose, glucose and sucrose to 5-hydroxymethyl-2-furfural over mesoporous zirconium phosphate catalyst. Appl. Catal. A Gen. 2015, 489, 72–76. [Google Scholar] [CrossRef]
- Dutta, A.; Gupta, D.; Patra, A.K.; Saha, B.; Bhaumik, A. Synthesis of 5-Hydroxymethylfurural from Carbohydrates using Large-Pore Mesoporous Tin Phosphate. ChemSusChem 2014, 7, 925–933. [Google Scholar] [CrossRef] [PubMed]
- Morales, I.J.; Teckchandani-Ortiz, A.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Selective dehydration of glucose to 5-hydroxymethylfurfural on acidic mesoporous tantalum phosphate. Appl. Catal. B Environ. 2014, 144, 22–28. [Google Scholar] [CrossRef]
- Peng, K.; Li, X.; Liu, X.; Wang, Y. Hydrothermally stable Nb-SBA-15 catalysts applied in carbohydrate conversion to 5-hydroxymethyl furfural. Mol. Catal. 2017, 441, 72–80. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, L.; Zhang, Y.; Cui, H.; Yi, W.; Song, F.; Zhao, P.; Sun, X.; Xie, Y.; Wang, L.; et al. AlNb/SBA-15 Catalysts with Tunable Lewis and Bronsted Acidic Sites for Glucose Conversion to HMF. ChemistrySelect 2018, 3, 3555–3560. [Google Scholar] [CrossRef]
- Morales, I.J.; Santamaría-González, J.; Jiménez-López, A.; Maireles-Torres, P. Glucose dehydration to 5-hydroxymethylfurfural on zirconium containing mesoporous MCM-41 silica catalysts. Fuel 2014, 118, 265–271. [Google Scholar] [CrossRef]
- Morales, I.J.; Moreno-Recio, M.; Santamaría-González, J.; Maireles-Torres, P.; Jiménez-López, A. Production of 5-hydroxymethylfurfural from glucose using aluminium doped MCM-41 silica as acid catalyst. Appl. Catal. B Environ. 2015, 164, 70–76. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiong, Q.; Chen, Y.; Liu, M.; Jin, P.; Yan, Y.; Pan, J. Synthesis of Ceria and Sulfated Zirconia Catalysts Supported on Mesoporous SBA-15 toward Glucose Conversion to 5-Hydroxymethylfurfural in a Green Isopropanol-Mediated System. Ind. Eng. Chem. Res. 2018, 57, 1968–1979. [Google Scholar] [CrossRef]
- Zhang, L.; Xi, G.; Chen, Z.; Qi, Z.; Wang, X. Enhanced formation of 5-HMF from glucose using a highly selective and stable SAPO-34 catalyst. Chem. Eng. J. 2017, 307, 877–883. [Google Scholar] [CrossRef]
- Rao, K.T.V.; Souzanchi, S.; Yuan, Z.; Ray, M.B.; Xu, C. (Charles) Simple and green route for preparation of tin phosphate catalysts by solid-state grinding for dehydration of glucose to 5-hydroxymethylfurfural (HMF). RSC Adv. 2017, 7, 48501–48511. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Pan, D.; Li, W.; Shen, P.; Wu, Y.; Song, X.; Zhu, Y.; Xu, N.; Gao, L.; Xiao, G. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurfural using an efficient and inexpensive manganese phosphate catalyst. Fuel Process. Technol. 2018, 181, 199–206. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Wang, J.; Wang, Y.; Wang, M.; Cui, H.; Song, F.; Sun, X.; Xie, Y.; Yi, W. Al2O3-TiO2 Modified Sulfonated Carbon with Hierarchically Ordered Pores for Glucose Conversion to 5-HMF. ChemistrySelect 2019, 4, 5724–5731. [Google Scholar] [CrossRef]
- Shaikh, M.; Singh, S.K.; Khilari, S.; Sahu, M.; Ranganath, K.V. Graphene oxide as a sustainable metal and solvent free catalyst for dehydration of fructose to 5-HMF: A new and green protocol. Catal. Commun. 2018, 106, 64–67. [Google Scholar] [CrossRef]
- Zhang, N.; Yang, M.-Q.; Liu, S.; Sun, Y.; Xu, Y.-J. Waltzing with the Versatile Platform of Graphene to Synthesize Composite Photocatalysts. Chem. Rev. 2015, 115, 10307–10377. [Google Scholar] [CrossRef]
- Hensen, E.J.M.; Poduval, D.G.; Degirmenci, V.; Ligthart, D.A.J.M.; Chen, W.; Maugé, F.; Rigutto, M.; Van Veen, J.A.R. Acidity Characterization of Amorphous Silica–Alumina. J. Phys. Chem. C 2012, 116, 21416–21429. [Google Scholar] [CrossRef]
- Koekkoek, A.J.J.; Degirmenci, V.; Hensen, E.J.M. Dry gel conversion of organosilane templated mesoporous silica: From amorphous to crystalline catalysts for benzene oxidation. J. Mater. Chem. 2011, 21, 9279–9289. [Google Scholar] [CrossRef]
- Kumar, D.; Schumacher, K.; Hohenesche, C.D.F.V.; Grün, M.; Unger, K. MCM-41, MCM-48 and related mesoporous adsorbents: Their synthesis and characterisation. Colloids Surf. A Physicochem. Eng. Asp. 2001, 187–188, 109–116. [Google Scholar] [CrossRef]
- Degirmenci, V.; Erdem, Ö.F.; Yilmaz, A.; Michel, D.; Uner, D. Sulfated zirconia in SBA-15 structures with strong Brønsted acidity as observed by 1H MAS NMR spectroscopy. Catal. Lett. 2007, 115, 79–85. [Google Scholar] [CrossRef]
- Degirmenci, V.; Erdem, Ö.F.; Ergun, O.; Yilmaz, A.; Michel, D.; Uner, D. Synthesis and NMR Characterization of Titanium and Zirconium Oxides Incorporated in SBA-15. Top. Catal. 2008, 49, 204–208. [Google Scholar] [CrossRef]



| Catalyst | T (°C) | Time (h) | Solvent | Glucose Conversion (%) | Product Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| Sn-Beta | 110 | 0.5 | Water | 55 | Fructose: 32 Mannose: 9 | [14] |
| Sn-Beta | 160 | 1.5 | pH = 1.0 HCl | 45 | Selectivity: HMF: 6 | [44] |
| Sn-Beta | 180 | 1.2 | Phase 1: pH = 1.0 HCl and NaCl Phase 2: THF | 79 | Selectivity: HMF: 72 | [44] |
| Sn-Beta | 90 | 4 | dioxane/water (95:5 w/w) | 55 | Fructose: 42 | [45] |
| H-Beta | 150 | 0.8 | [BMIM]Cl | 81 | HMF: 50 | [46] |
| Meso-Sn-MFI | 80 | 2 | Water | 37 | Fructose: 27 Mannose: 3 | [47] |
| Nb-BEA | 180 | 12 | Phase 1: water and NaCl Phase 2: MIBK * | 97 | Selectivity: HMF: 84 | [40] |
| Hierarchical Sn-Beta | 160 | 3.5 | DMSO: THF ** (3:7 v/v) | 99 | Selectivity: HMF: 42 | [48] |
| Sn-Beta | 160 | 3.6 | DMSO: THF (3:7 v/v) | 96 | Selectivity: HMF: 32 | [48] |
| Sn-Zeolite Y | 140 | 3 | Water | 36 | Fructose: 13 Mannose: 1 HMF: 4 | [49] |
| Ga-Zeolite Y | 140 | 3 | Water | 17 | Fructose: 5 HMF: 1.5 | [49] |
| Sn-Zeolite Y | 140 | 3 | DMSO | 66 | HMF: 22 | [49] |
| Ga-Zeolite Y | 140 | 3 | DMSO | 78 | HMF: 33 | [49] |
| Cr-Zeolite Y | 160 | 1.5 | DMSO | Not stated | HMF: 35 | [50] |
| Cr-Zeolite Y | 130 | 1 | *** [BMIM]Cl | Not stated | HMF: 59 | [50] |
| H-MOR | 100 | 6 | [BMIM]Br | 98 | HMF: 32 | [51] |
| H-MOR | 65 | 9 | Phase 1: acetone: water (3:2) Phase 2: Ethyl acetate (Phase 1: Phase 2 = 2:3 w/w) | 98 | HMF: 30 | [51] |
| H-ZSM-5 | 195 | 0.5 | Phase 1: water and NaCl Phase 2: MIBK (1.5:3.5 v/v) | 80 | HMF: 49 | [52] |
| ZSM-5-SO3H | 140 | 4 | DMSO:water (24:76 v/v) | 70 | HMF: 54 | [53] |
| Catalyst | T (°C) | Time (h) | Solvent | Glucose Conversion (%) | Product Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| UiO-66-SO3H | 140 | 3 | Water | 36 | HMF: 8 Fructose: 22 | [67] |
| MSBDC(50)-UiO-66 | 140 | 3 | Water | 37 | HMF: 7 Fructose: 8 Mannose: 7 | [68] |
| MSDBC(50)-naphtha(50)-UiO-66 | 140 | 3 | Water | 33 | HMF: 7 Fructose: 6 Mannose: 6 | [68] |
| ZIF-8 | 100 | 3 | Water | 24 | HMF: 16 | [64] |
| MIL-88B(Fe,Sc) | 140 | 3 | DMSO | 71 | HMF: 25 Fructose: 3.3 Mannose: 1.4 | [72] |
| Yb-BDC | 140 | 24 | Water | 28 | HMF: 18 Fructose: 1.2 | [73] |
| La-BDC | 130 | 6 | DMSO | Not stated | HMF: 19 | [71] |
| PTA-MIL-101(Cr) | 100 | 3 | [EMIM]Cl | 21 | HMF: 2 | [74] |
| MIL-101(Cr)-SO3H | 120 | 2 | DMSO | Not stated | HMF: 7 | [75] |
| MIL-101(Cr)-SO3H | 120 | 2 | [BMIM]Cl | Not stated | HMF: 8 | [75] |
| MIL-101(Cr)-SO3H | 100 | 24 | Water | 22 | Fructose: 22 | [76] |
| MIL-101(Cr)-SO3H | 150 | 2 | Phase 1: Water Phase 2: γ-valerolactone (1:9 w/w) | 90 | HMF: 40 | [66] |
| MIL-101(Cr)-SO3H | 130 | 24 | Phase 1: pH = 1.0 HCl and NaCl Phase 2: THF (1:39 v/v) | Not stated | HMF: 29 | [65] |
| NU-1000 | 140 | 5 | Water | 60 | HMF: 2.3 Fructose: 19 | [70] |
| PO4/NU-1000 | 140 | 5 | Phase 1: Water Phase 2: THF (1:9 v/v) | 97 | HMF: 25 Fructose: 5 | [70] |
| UiO-66-NH2-SO3H on g-C3N4@PDA | 120 | 6 | Isopropanol:DMSO (9:1 v/v) | 92 | HMF: 55 | [77] |
| Catalyst | T (°C) | Time (h) | Solvent | Glucose Conversion (%) | Product Yield (%) | Ref. |
|---|---|---|---|---|---|---|
| CrCl2-Im-SBA-15 | 150 | 3 | Phase 1: Water:DMSO (8:2 v/v) Phase 2: Butanol:MIBK (7:3 v/v) (Phase 1: Phase 2 = 1:1 v/v) | 51 | Selectivity: HMF: 69 | [13] |
| CrCl2-Im-SBA-15 | 150 | 3 | Phase 1: Water Phase 2: MIBK (1:1 v/v) | 67 | HMF: 28 | [12] |
| Nb-SBA-15 | 165 | 3 | Phase 1: Water and NaCl Phase 2: THF | 94 | HMF: 62 | [99] |
| AlNb/SBA-15 | 170 | 6 | Phase 1: Water Phase 2: MIBK (1:2 v/v) | 94 | HMF: 56 | [100] |
| Zr-MCM-41 | 175 | 2.5 | Phase 1: Water Phase 2: MIBK | 81 | HMF: 23 | [101] |
| Al-MCM-41 | 195 | 2.5 | Phase 1: Water and NaCl (20 wt %) Phase 2: MIBK | 98 | HMF: 63 | [102] |
| CeO2-Sulfated-ZrO2-SBA-15 | 120 | 6 | iPrOH:DMSO (9:1 v/v) | 90 | HMF: 60 | [103] |
| SAPO-34 | 170 | 0.7 | Phase 1: Water Phase 2: γ-valerolactone | 100 | HMF: 94 | [104] |
| Sn-Amberlyst-15 | 120 | 6 | Phase 1: Water Phase 2: MIBK (1:1 v/v) | 68 | Selectivity: Fructose: 6 HMF: 15 Levulinic Acid: 45 | [79] |
| Cr3+-Amberlyst-15 | 120 | 1 | Phase 1: Water Phase 2: TEAB (1:9 w/w) | Not stated | HMF: 70 | [80] |
| SCFC resin | 120 | 1.5 | DMSO | 85 | HMF: 8 | [81] |
| PBnNH3Cl | 140 | 12 | Phase 1: Water: DMSO (3:7 v/v) Phase 2: MIBK (Phase 1: Phase 2 = 1:4 v/v) | 90 | HMF: 45 | [82] |
| Al3+-SPFR resin | 180 | 1 | Phase 1: Water Phase 2: γ-valerolactone | 96 | HMF: 42 | [83] |
| Al7Zr3 | 175 | 1 | Phase 1: Water Phase 2: MIBK (1.5:3.5 v/v) | 92 | HMF: 46 | [85] |
| Al2O3 | 140 | 3 | [EMIM]Br | 96 | HMF: 50 | [86] |
| NbWO3 | 120 | 1 | Phase 1: Water Phase 2: THF (1:9 v/v) | 90 | HMF: 30 Fructose: 10 | [89] |
| Nb7W3 oxide | 140 | 2 | Phase 1: Water Phase 2: 2-Butanol (5:2 v/v) | 100 | Selectivity: HMF: 50 | [93] |
| Ta7W3 | 140 | 8 | Phase 1: Water Phase 2: 2-Butanol | 80 | Selectivity: Fructose: 2 HMF: 47 | [92] |
| SnP | 175 | 1 | Phase 1: Water and NaCl Phase 2: THF (1:3 v/v) | 98 | HMF: 61 | [105] |
| MnPO4 | 160 | 1.5 | Phase 1: Water Phase 2: THF | 90 | HMF: 59 | [106] |
| Meso-TiWO3 | 120 | 1 | Phase 1: Water Phase 2: THF (1:9 v/v) | 70 | HMF: 25 | [90] |
| Meso-Ta2O5 | 175 | 1.5 | Phase 1: Water Phase 2: MIBK | 69 | HMF: 23 | [91] |
| Meso-ZrP | 155 | 6 | Water | 84 | HMF: 47 | [95] |
| Meso-SnP | 150 | 0.3 | Phase 1: Water Phase 2: MIBK (1:2 v/v) | 50 | HMF: 50 | [97] |
| Meso-TaP | 170 | 1 | Phase 1: Water Phase 2: MIBK | 56 | HMF: 33 | [98] |
| Al−Ti Sulfonated Carbon | 130 | 5 | DMSO | 85 | HMF: 57 | [107] |
| Catalysts | Pros and Cons |
|---|---|
| Zeolites |
|
| MOFs |
|
| Conventional Supported Catalysts |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tempelman, C.H.L.; Oozeerally, R.; Degirmenci, V. Heterogeneous Catalysts for the Conversion of Glucose into 5-Hydroxymethyl Furfural. Catalysts 2021, 11, 861. https://doi.org/10.3390/catal11070861
Tempelman CHL, Oozeerally R, Degirmenci V. Heterogeneous Catalysts for the Conversion of Glucose into 5-Hydroxymethyl Furfural. Catalysts. 2021; 11(7):861. https://doi.org/10.3390/catal11070861
Chicago/Turabian StyleTempelman, Christiaan H. L., Ryan Oozeerally, and Volkan Degirmenci. 2021. "Heterogeneous Catalysts for the Conversion of Glucose into 5-Hydroxymethyl Furfural" Catalysts 11, no. 7: 861. https://doi.org/10.3390/catal11070861
APA StyleTempelman, C. H. L., Oozeerally, R., & Degirmenci, V. (2021). Heterogeneous Catalysts for the Conversion of Glucose into 5-Hydroxymethyl Furfural. Catalysts, 11(7), 861. https://doi.org/10.3390/catal11070861








