Catalytic Effect of 1,4-Dioxane on the Kinetics of the Oxidation of Iodide by Dicyanobis(bipyridine)iron(III) in Water
Abstract
:1. Introduction
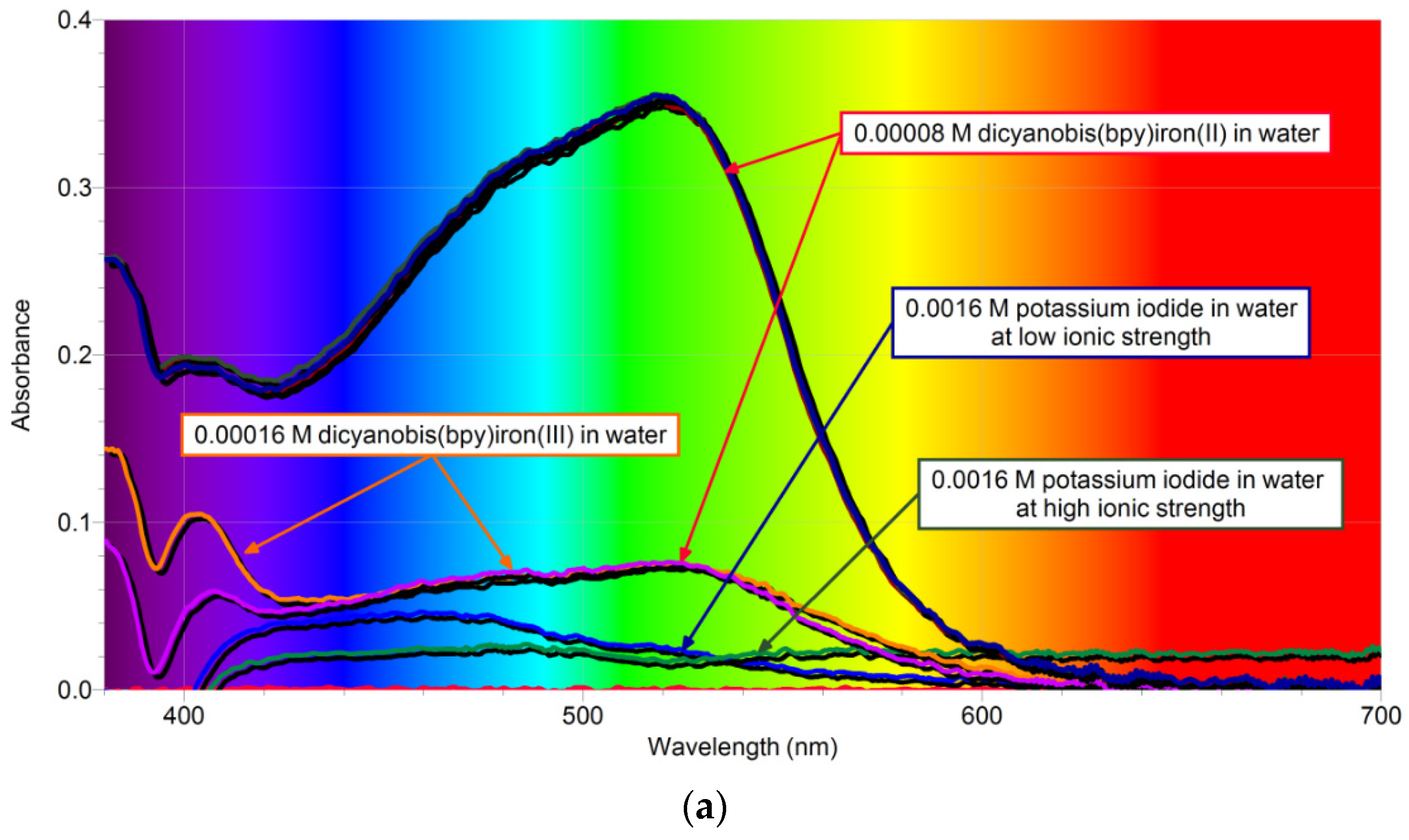
2. Results and Discussion
2.1. Kinetic Parameters of Uncatalyzed Reaction in Water
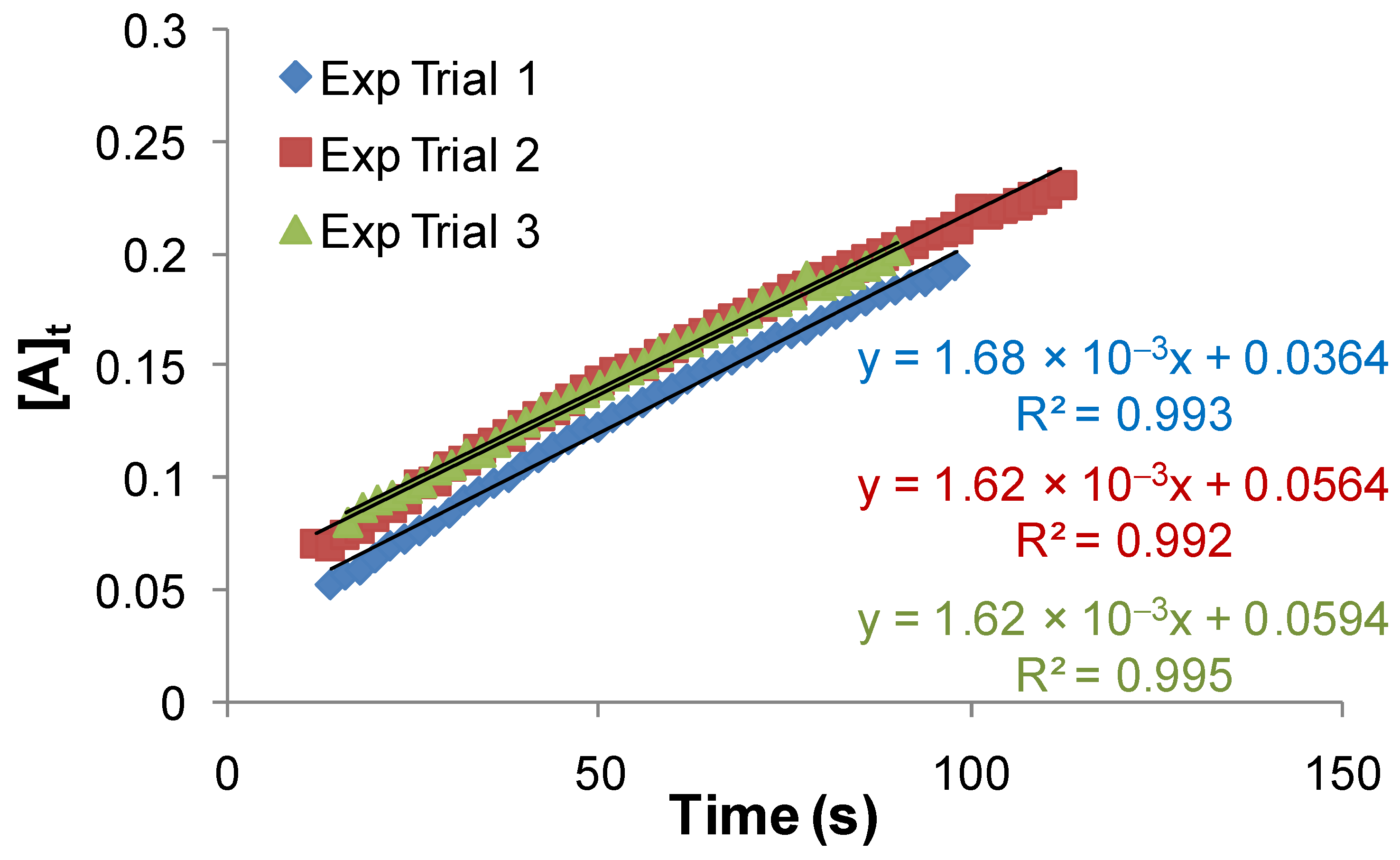
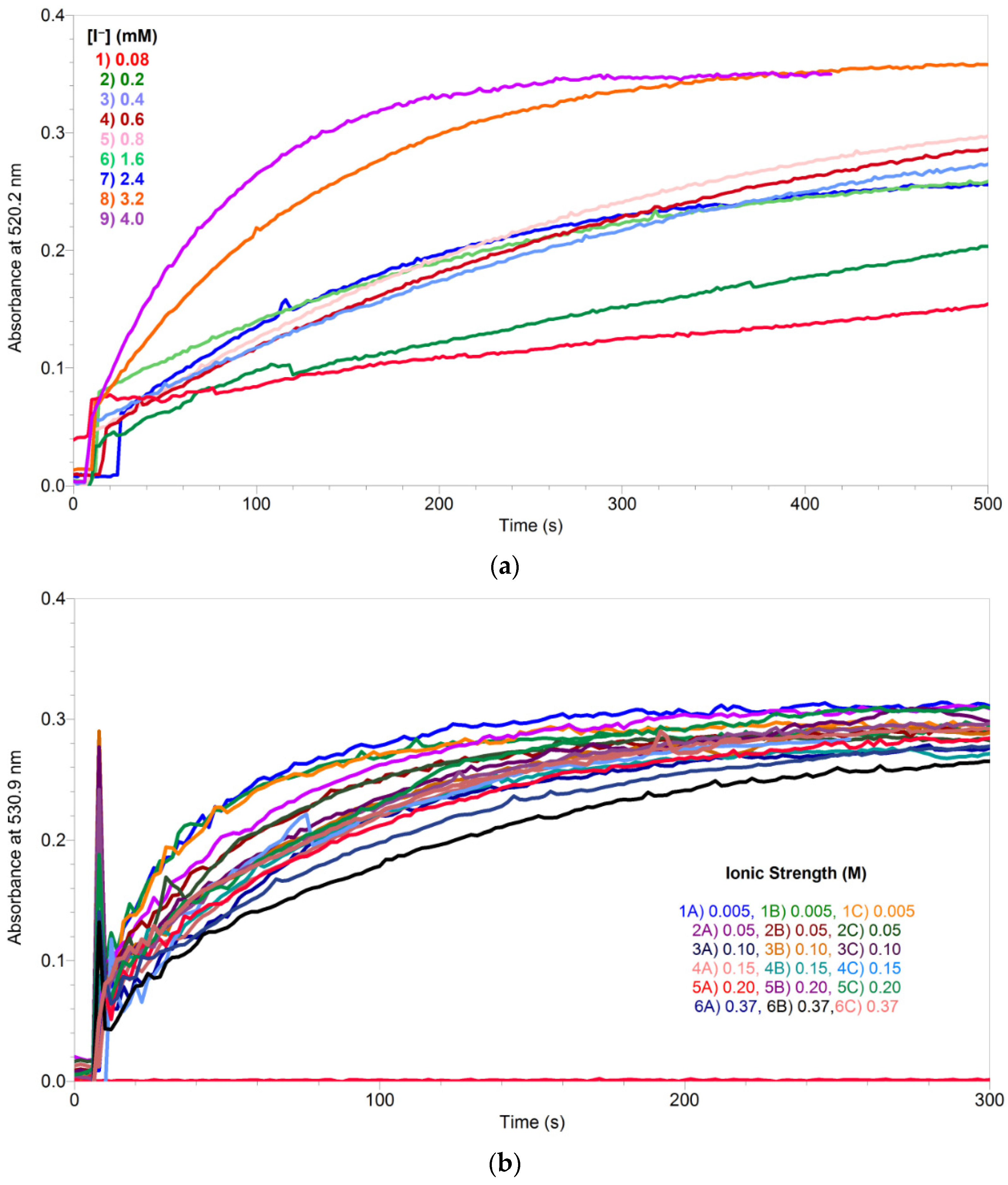
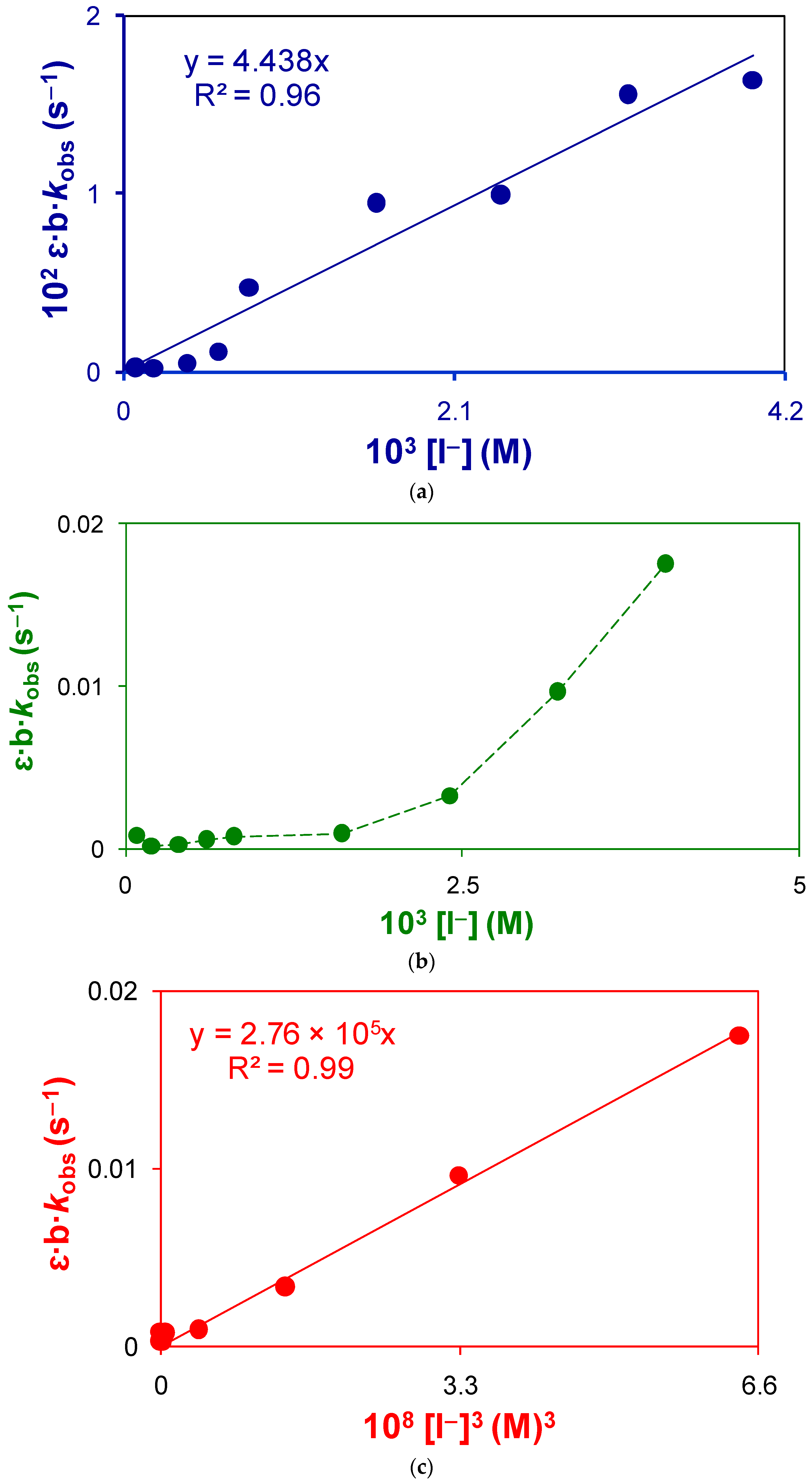
2.1.1. Overall Order of Reaction and the Overall Rate Constant of Uncatalyzed Reaction
- At moderate concentration of iodide:
- At fairly high concentration of iodide:
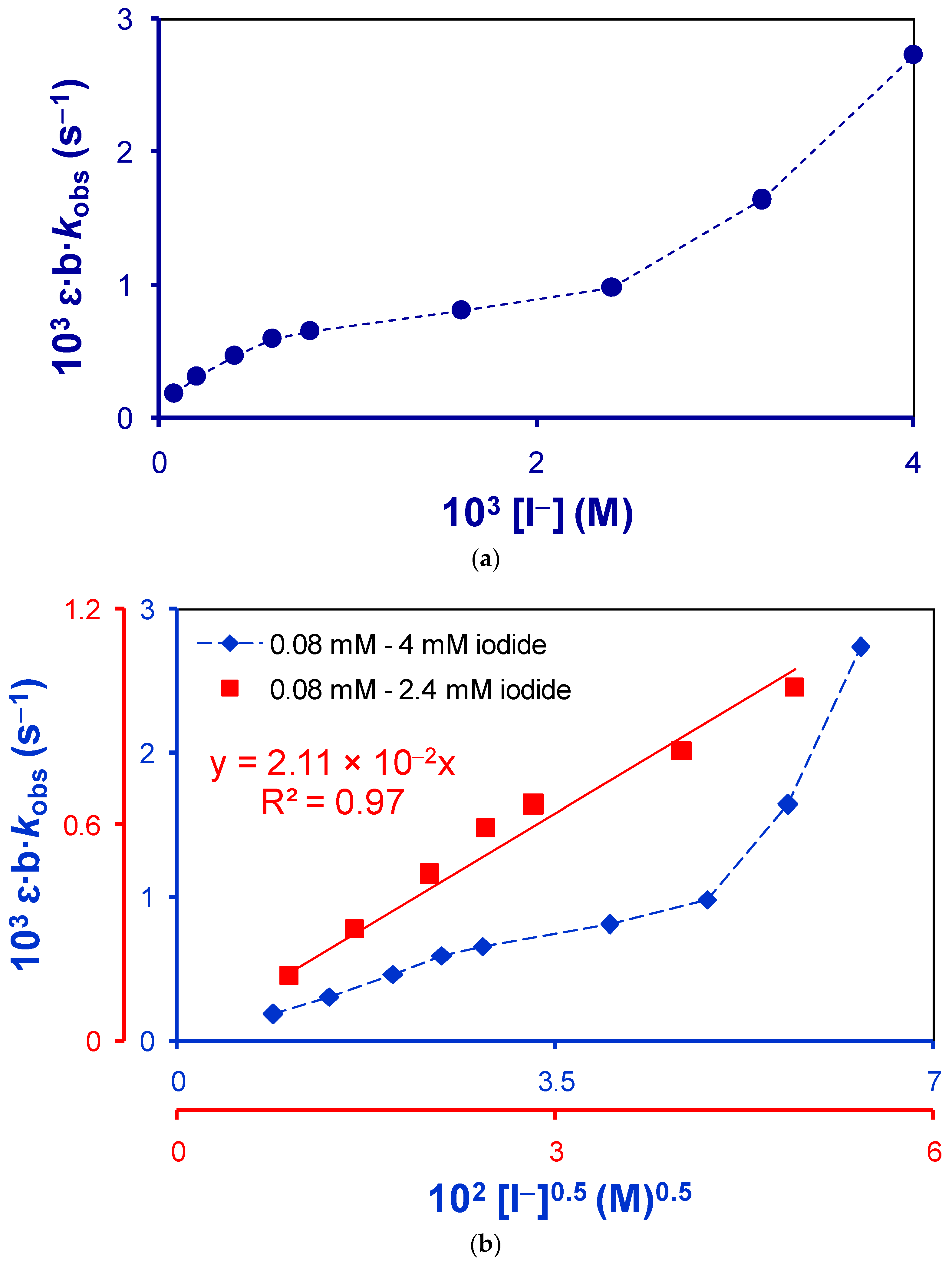
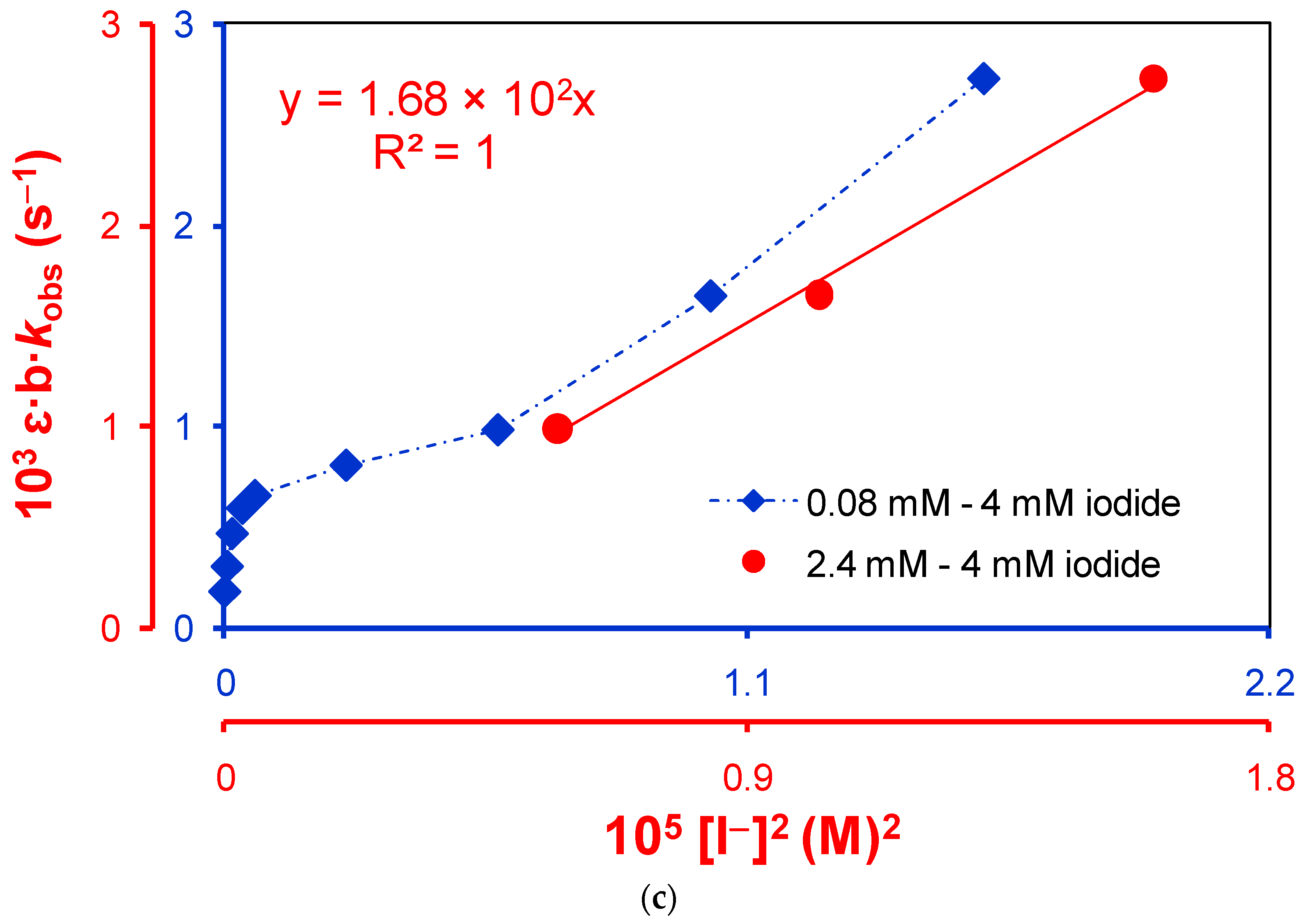
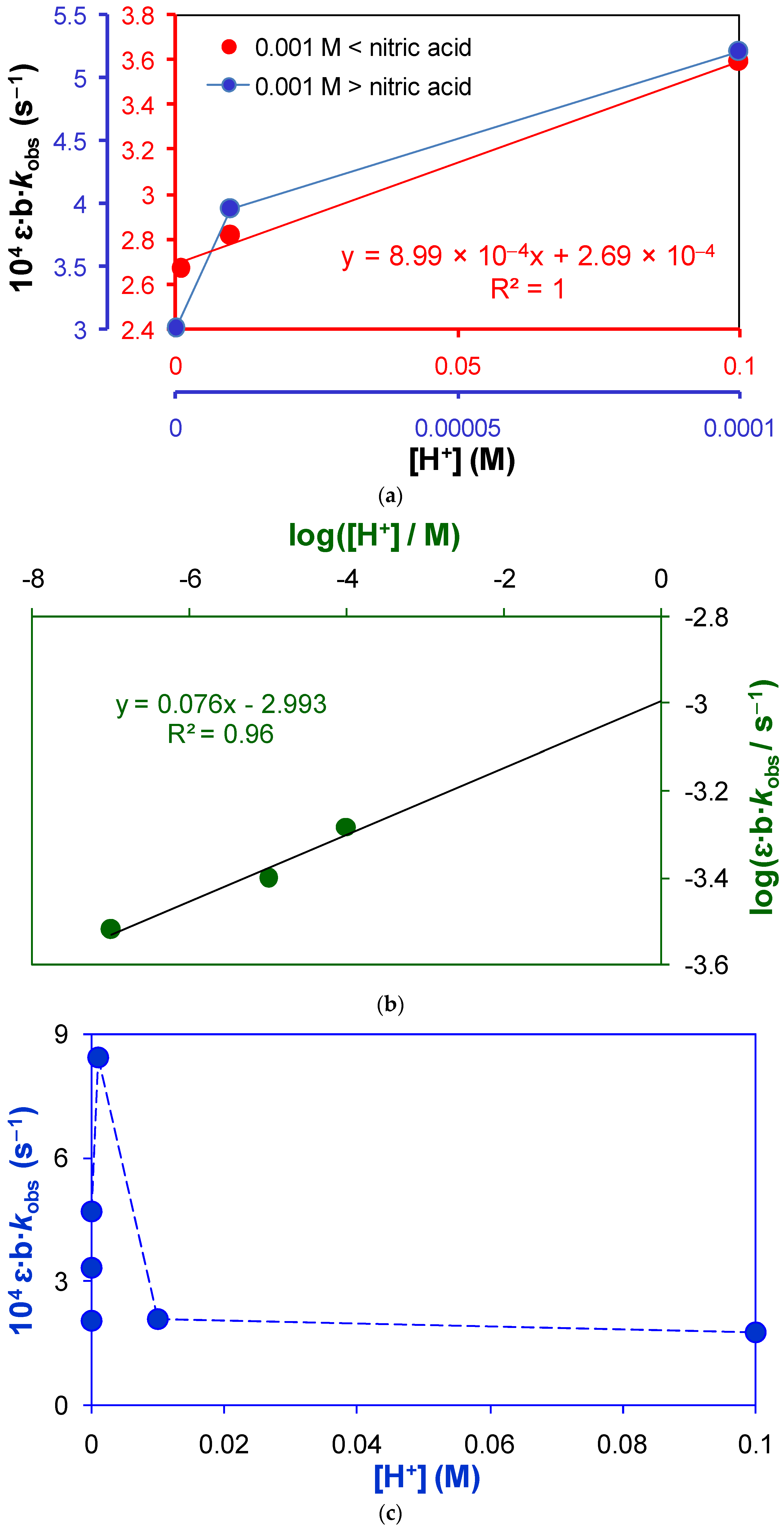
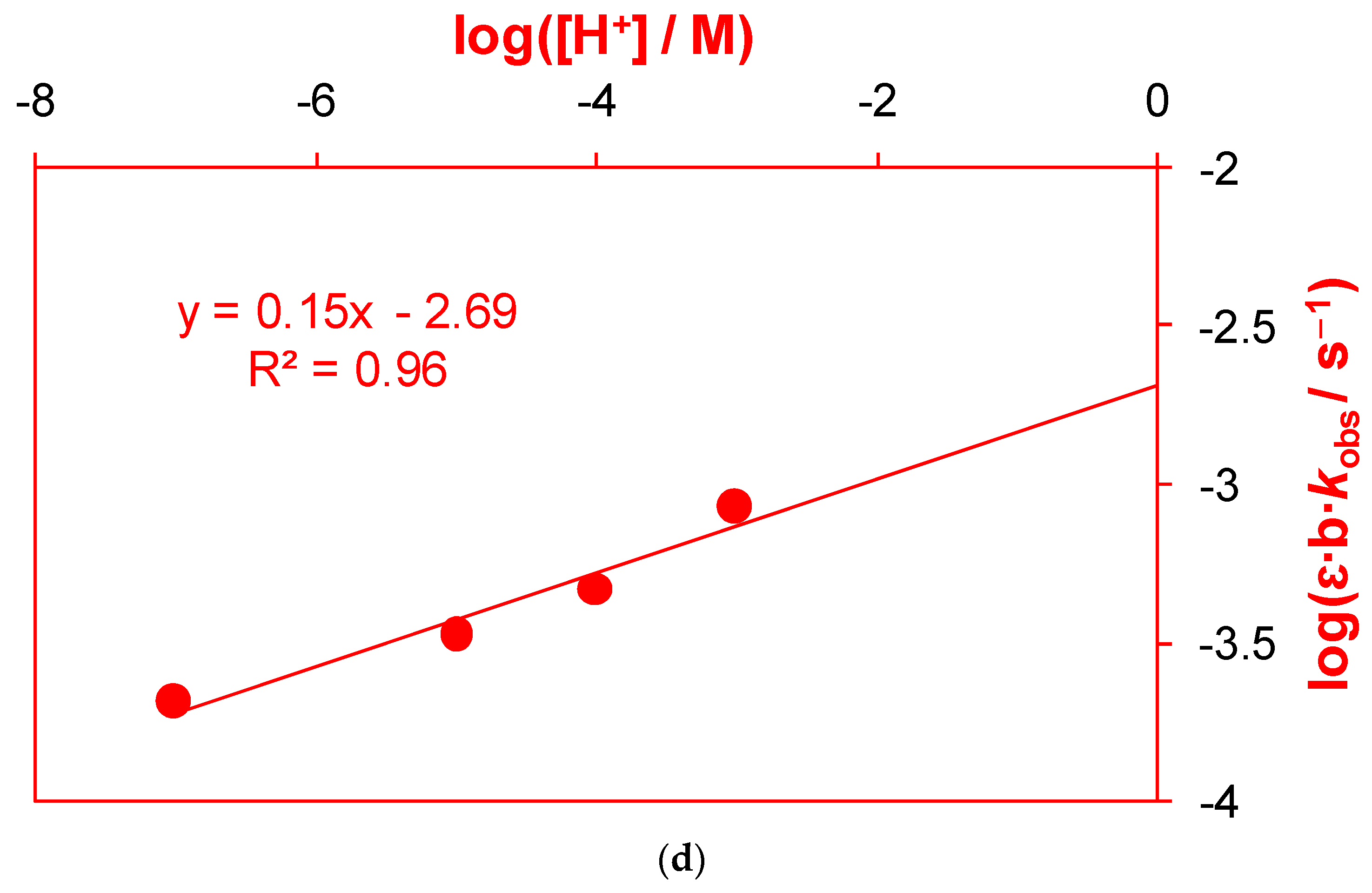
2.1.2. Active Reactants of the Rate-Determining Step of Uncatalyzed Reaction
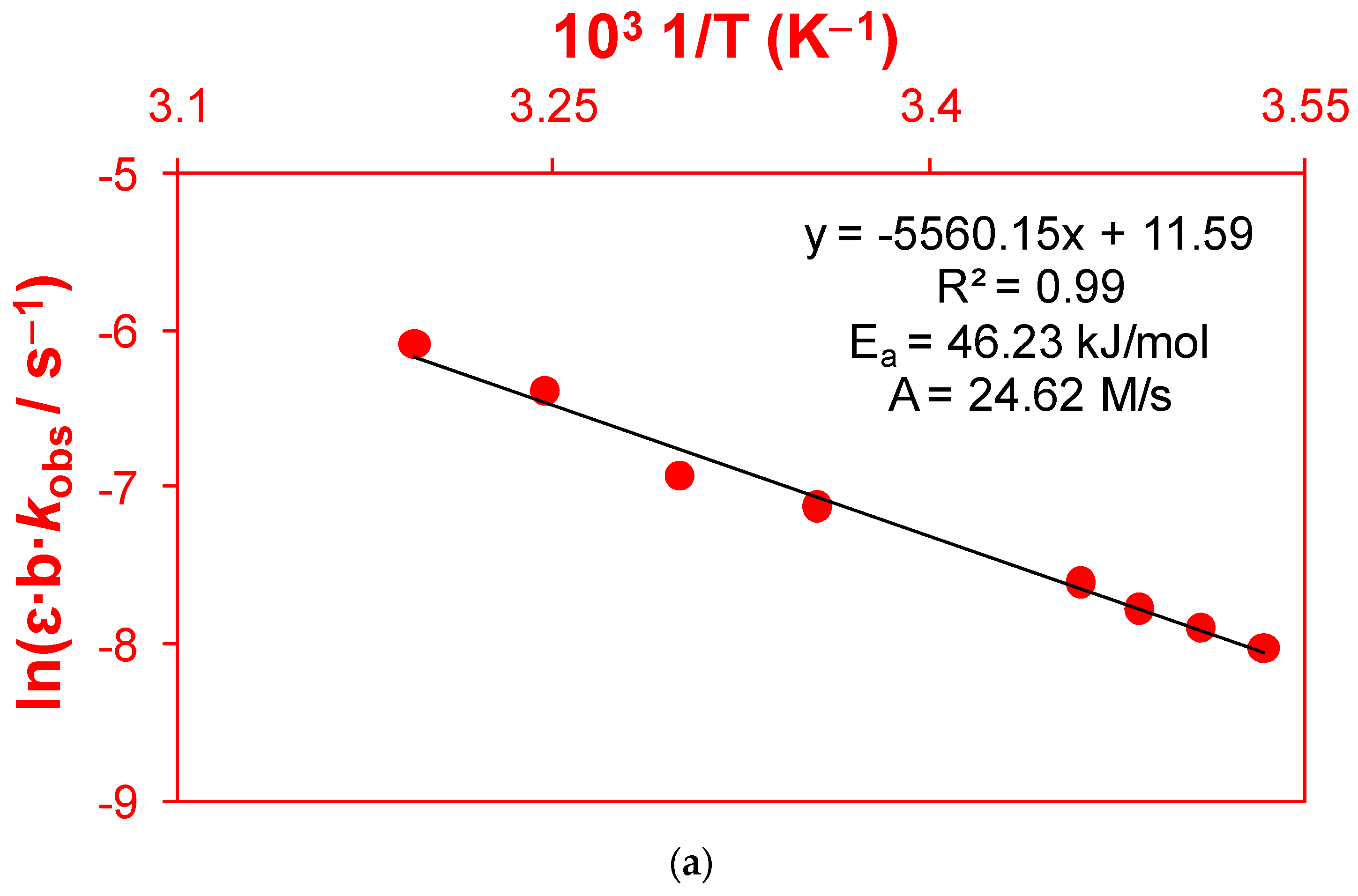
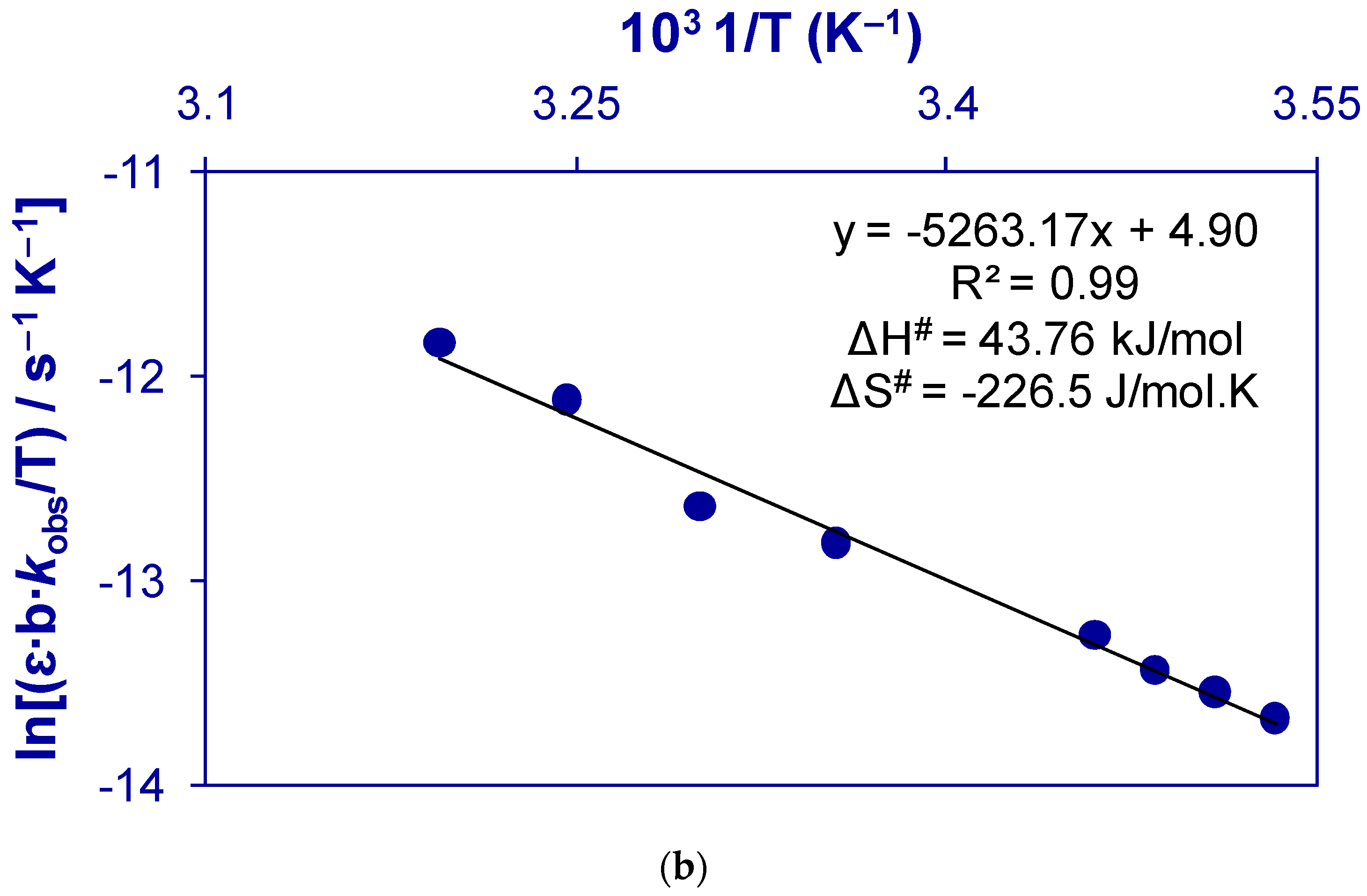
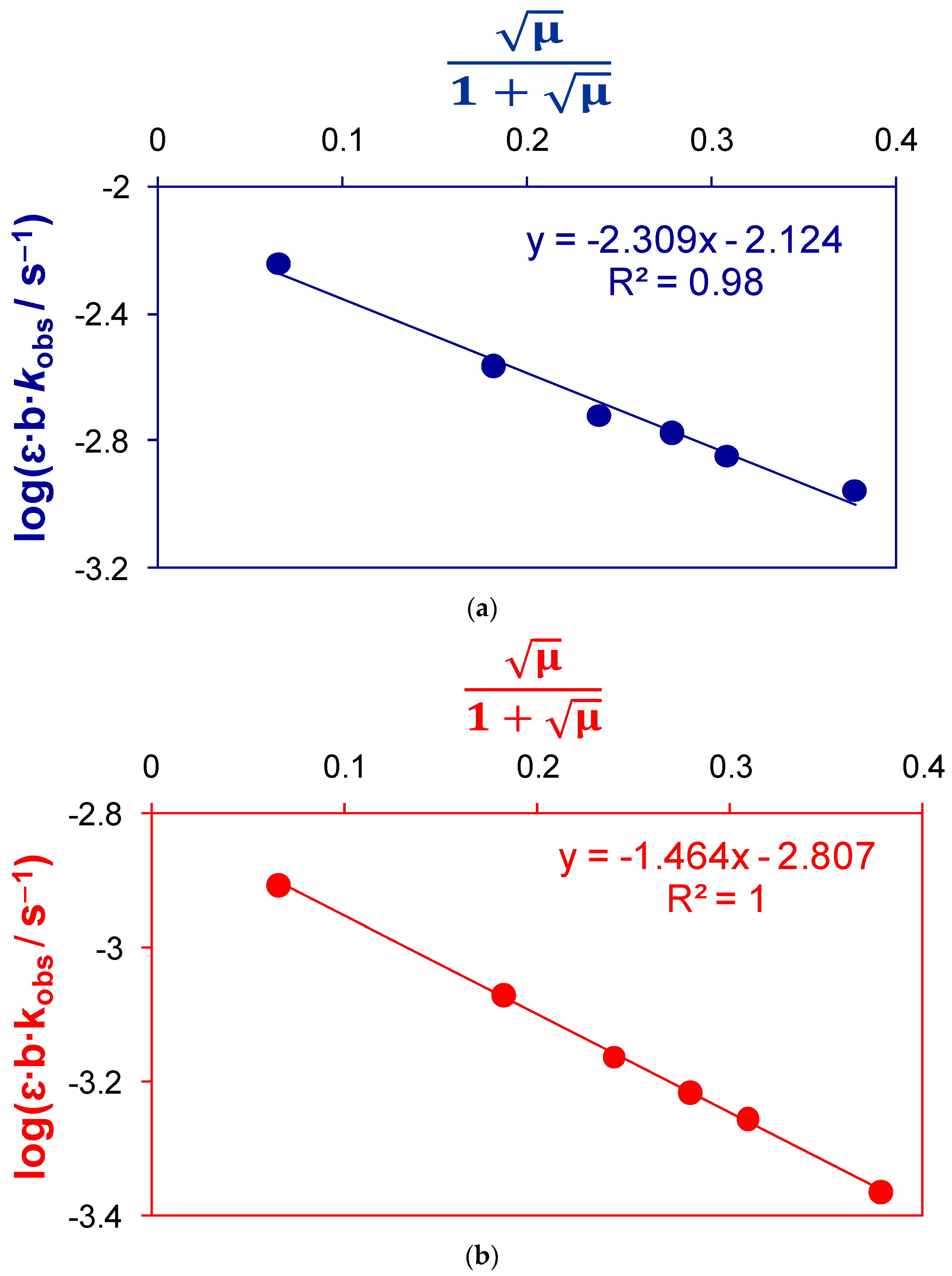
2.1.3. Thermodynamic Parameters of Activation
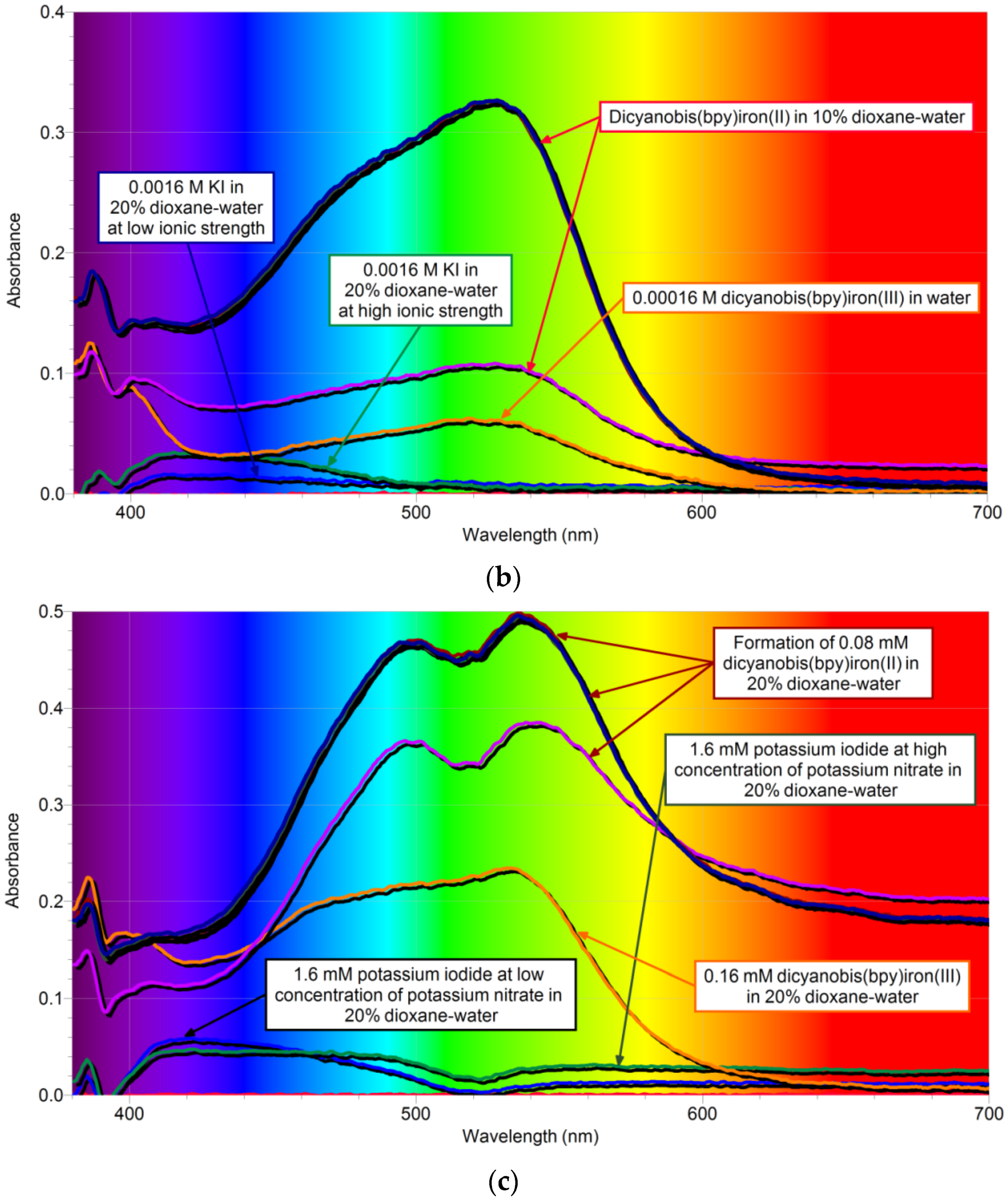
2.2. Kinetic Parameters of 1,4-Dioxane Catalyzed Reaction
2.2.1. Overall Order of Reaction and the Overall Rate Constant of Catalyzed Reaction
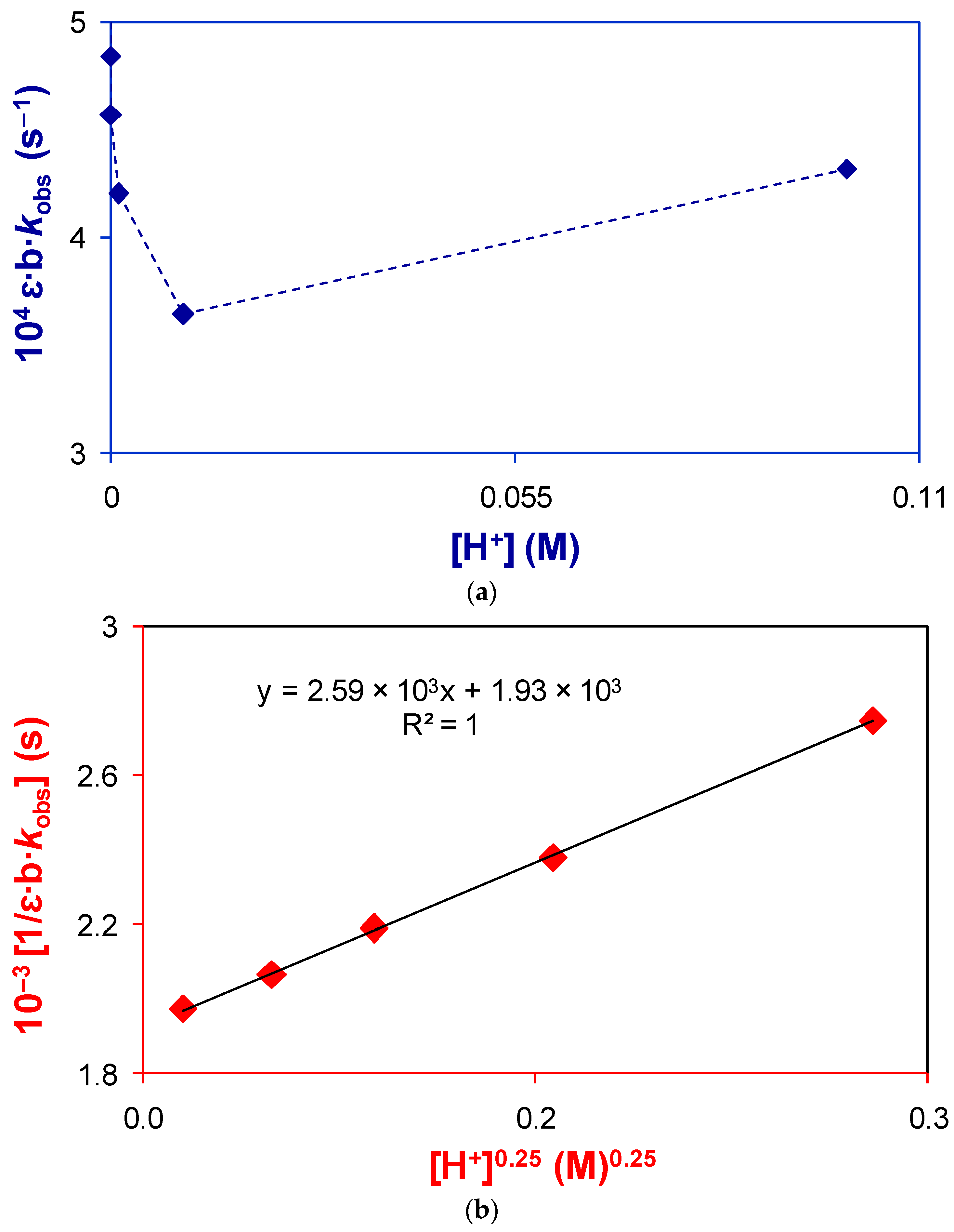
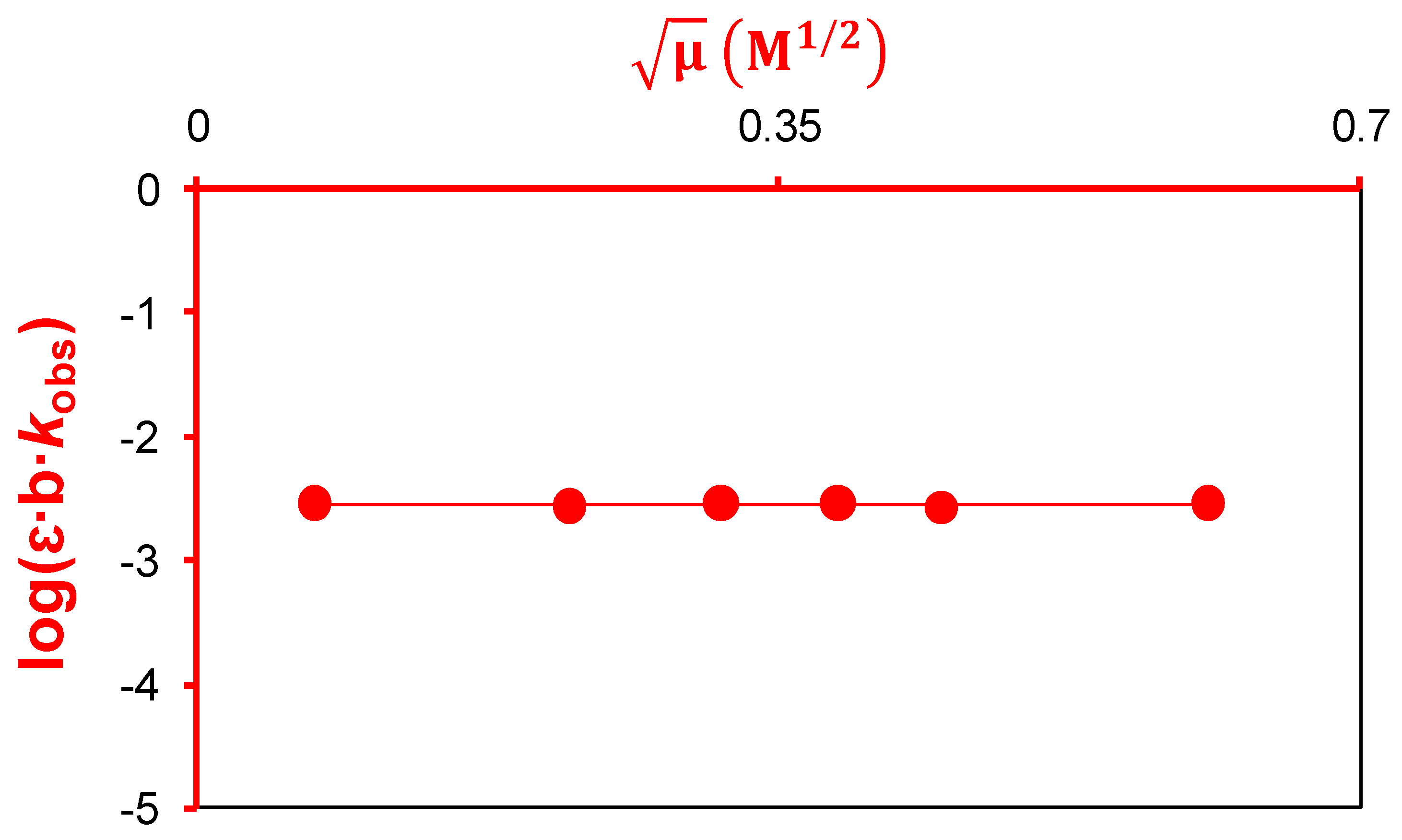
2.2.2. Active Reactants of the Rate-Determining Step of Catalyzed Reaction
3. Materials and Methods
3.1. Materials and Instrumentation
3.2. Kinetic Measurements
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kokkonen, M.; Talebi, P.; Zhou, J.; Asgari, S.; Soomro, S.A.; Elsehrawy, F.; Halme, J.; Ahmad, S.; Hagfeldt, A.; Hashmi, S.G. Advanced research trends in dye-sensitized solar cells. J. Mater. Chem. A 2021, 9, 10527–10545. [Google Scholar] [CrossRef]
- Venkatraman, V.; Raju, R.; Oikonomopoulos, S.P.; Alsberg, B.K. The dye-sensitized solar cell database. J. Cheminformatics 2018, 10, 1–9. [Google Scholar] [CrossRef]
- Sharma, K.; Sharma, V.; Sharma, S.S. Dye-Sensitized Solar Cells: Fundamentals and Current Status. Nanoscale Res. Lett. 2018, 13, 1–46. [Google Scholar] [CrossRef]
- Karthick, S.; Hemalatha, K.; Balasingam, S.K.; Clinton, F.M.; Akshaya, S.; Kim, H. Dye-Sensitized Solar Cells: History, Components, Configuration, and Working Principle. In Interfacial Engineering in Functional Materials for Dye-Sensitized Solar Cells; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 1–16. [Google Scholar]
- Ung, M.C.; Sipaut, C.S.; Dayou, J.; Liow, K.S.; Kulip, J.; Mansa, R.F. Fruit based Dye Sensitized Solar Cells. IOP Conf. Ser. Mater. Sci. Eng. 2017, 217, 12003. [Google Scholar] [CrossRef] [Green Version]
- Cavallo, C.; Di Pascasio, F.; Latini, A.; Bonomo, M.; Dini, D. Nanostructured Semiconductor Materials for Dye-Sensitized Solar Cells. J. Nanomater. 2017, 2017, 5323164. [Google Scholar] [CrossRef]
- Trihutomo, P.; Soeparman, S.; Widhiyanuriyawan, D.; Yuliati, L. Performance Improvement of Dye-Sensitized Solar Cell- (DSSC-) Based Natural Dyes by Clathrin Protein. Int. J. Photoenergy 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huaulmé, Q.; Mwalukuku, V.M.; Joly, D.; Liotier, J.; Kervella, Y.; Maldivi, P.; Narbey, S.; Oswald, F.; Riquelme, A.J.; Anta, J.A.; et al. Photochromic dye-sensitized solar cells with light-driven adjustable optical transmission and power conversion efficiency. Nat. Energy 2020, 5, 468–477. [Google Scholar] [CrossRef]
- Rondan-Gomez, V.; De Los Santos, I.M.; Seuret-Jiménez, D.; Ayala-Mató, F.; Zamudio-Lara, A.; Robles-Bonilla, T.; Courel, M. Recentadvances in dye-sensitizedsolarcells. Appl. Phys. A 2019, 125, 836. [Google Scholar] [CrossRef]
- Bignozzi, C.A.; Argazzi, R.; Boaretto, R.; Busatto, E.; Carli, S.; Ronconi, F.; Caramori, S. The role of transition metal complexes in dye sensitized solar devices. Coord. Chem. Rev. 2013, 257, 1472–1492. [Google Scholar] [CrossRef]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. C Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-Sensitized Solar Cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Grätzel, M. Perspectives for dye-sensitized nanocrystalline solar cells. Prog. Photovolt. Res. Appl. 2000, 8, 171–185. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Grätzel, M. Molecular Photovoltaics. Acc. Chem. Res. 2000, 33, 269–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazeeruddin, K.; Pechy, P.; Gratzel, M. Efficient panchromatic sensitization of nanocrystalline TiO2 films by a black dye based on a trithiocyanato–ruthenium complex. Chem. Commun. 1997, 18, 1705–1706. [Google Scholar] [CrossRef]
- Saadmim, F.; Forhad, T.; Sikder, A.; Ghann, W.; Ali, M.M.; Sitther, V.; Ahammad, A.J.S.; Subhan, A.; Uddin, J. Enhancing the Performance of Dye Sensitized Solar Cells Using Silver Nanoparticles Modified Photoanode. Molecules 2020, 25, 4021. [Google Scholar] [CrossRef]
- Tang, B.; Yu, H.; Peng, H.; Wang, Z.; Li, S.; Ma, T.; Huang, W. Graphene based photoanode for DSSCs with high performances. RSC Adv. 2018, 8, 29220–29227. [Google Scholar] [CrossRef] [Green Version]
- Song, W.; Gong, Y.; Tian, J.; Cao, G.; Zhao, H.; Sun, C. Novel Photoanode for Dye-Sensitized Solar Cells with Enhanced Light-Harvesting and Electron-Collection Efficiency. ACS Appl. Mater. Interfaces 2016, 8, 13418–13425. [Google Scholar] [CrossRef]
- Yan, L.-T.; Wu, F.-L.; Peng, L.; Zhang, L.-J.; Li, P.-J.; Dou, S.-Y.; Li, T.-X. Photoanode of Dye-Sensitized Solar Cells Based on a ZnO/TiO2 Composite Film. Int. J. Photoenergy 2012, 2012, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharjee, R.; Hung, I.-M. A SnO2 and ZnO Nanocomposite Photoanodes in Dye-Sensitized Solar Cells. ECS Solid State Lett. 2013, 2, Q101–Q104. [Google Scholar] [CrossRef]
- Zhang, S.; Jin, J.; Li, D.; Fu, Z.; Gao, S.; Cheng, S.; Yu, X.; Xiong, Y. Increased power conversion efficiency of dye-sensitized solar cells with counter electrodes based on carbon materials. RSC Adv. 2019, 9, 22092–22100. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Wei, W.; Hu, Y.H. NiO as an Efficient Counter Electrode Catalyst for Dye-Sensitized Solar Cells. Top. Catal. 2013, 57, 607–611. [Google Scholar] [CrossRef]
- Meyer, E.; Mbese, J.; Mutukwa, D.; Zingwe, N. Structural, Morphological and Electrochemical Characterization of Hydrothermally Fabricated PdNiCo and PdNiCo-rGO Alloys for Use as Counter Electrode Catalysts in DSSC. Mater. Basel 2019, 12, 3256. [Google Scholar] [CrossRef] [Green Version]
- Xu, T.; Kong, D.; Tang, H.; Qin, X.; Li, X.; Gurung, A.; Kou, K.; Chen, L.; Qiao, Q.; Huang, W. Transparent MoS2/PEDOT Composite Counter Electrodes for Bifacial Dye-Sensitized Solar Cells. ACS Omega 2020, 5, 8687–8696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.-Y.; Li, C.-T.; Yeh, M.-H.; Huang, K.-C.; Chen, P.-W.; Vittal, R.; Ho, K.-C. Graphite with Different Structures as Catalysts for Counter Electrodes in Dye-sensitized Solar Cells. Electrochim. Acta 2015, 179, 211–219. [Google Scholar] [CrossRef]
- Mathew, A.; Rao, G.M.; Munichandraiah, N. Dye sensitized solar cell based on platinum decorated multiwall carbon nanotubes as catalytic layer on the counter electrode. Mater. Res. Bull. 2011, 46, 2045–2049. [Google Scholar] [CrossRef]
- Peng, J.-D.; Wu, Y.-T.; Yeh, M.-H.; Kuo, F.-Y.; Vittal, R.; Ho, K.-C. Transparent Cobalt Selenide/Graphene Counter Electrode for Efficient Dye-Sensitized Solar Cells with Co2+/3+-Based Redox Couple. ACS Appl. Mater. Interfaces 2020, 12, 44597–44607. [Google Scholar] [CrossRef]
- Khattak, R.; Khan, M.S.; Ullah, R.; Ali, M.; Rahman, W.; Hakeem, F.; Ayaz, K.; Bibi, Z. Effect of the ionic strength on the redox reaction of dicyanobis(bipyridine)iron(III)-iodide in binary and ternary solvent systems. Int. J. Chem. Kinet. 2021, 53, 16–26. [Google Scholar] [CrossRef]
- Boschloo, G.; Hagfeldt, A. Characteristics of the Iodide/Triiodide Redox Mediator in Dye-Sensitized Solar Cells. Acc. Chem. Res. 2009, 42, 1819–1826. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.C.; Chou, J.C.; Lai, C.H.; Kuo, P.Y.; Nien, Y.H.; Chang, J.X.; Hu, G.M.; Yong, Z.R. Dye-Sensitized Solar Cell Using TiO2/AgNWs Film: Application under Low Illumination. In Proceedings of the 2020 IEEE Eurasia Conference on IOT, Communication and Engineering (ECICE), Yunlin, Taiwan, 23–25 October 2020; pp. 353–354. [Google Scholar]
- Boschloo, G. Improving the Performance of Dye-Sensitized Solar Cells. Front. Chem. 2019, 7, 77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaignan, G.P.; Kang, M.-S.; Kang, Y.S. Effects of compositions on properties of PEO–KI–I2 salts polymer electrolytes for DSSC. Solid State Ion. 2006, 177, 1091–1097. [Google Scholar] [CrossRef]
- Rowley, J.G.; Farnum, B.H.; Ardo, S.; Meyer, G.J. Iodide Chemistry in Dye-Sensitized Solar Cells: Making and Breaking I−I Bonds for Solar Energy Conversion. J. Phys. Chem. Lett. 2010, 1, 3132–3140. [Google Scholar] [CrossRef]
- Wang, T.-H.; Huang, T.-W.; Liao, C.-S.; Tsai, Y.-C.; Chang, Y.-W. A photoluminescent layer for improving the performance of dye-sensitized solar cells. Chem. Commun. 2015, 51, 7253–7256. [Google Scholar] [CrossRef]
- Pradhan, S.C.; Hagfeldt, A.; Soman, S. Resurgence of DSCs with copper electrolyte: A detailed investigation of interfacial charge dynamics with cobalt and iodine based electrolytes. J. Mater. Chem. A 2018, 6, 22204–22214. [Google Scholar] [CrossRef]
- Khattak, R.; Khan, M.S.; Summer, S.; Ullah, R.; Afridi, H.; Rehman, Z.; Masood, S.; Noreen, H.; Qazi, R.A.; Begum, B. Kinetics of the oxidation of iodide by dicyanobis(phenanthroline)iron(III) in a binary solvent system. Int. J. Chem. Kinet. 2021, 53, 230–241. [Google Scholar] [CrossRef]
- Ammar, A.M.; Mohamed, H.; Yousef, M.M.K.; Abdel-Hafez, G.M.; Hassanien, A.S.; Khalil, A.S.G. Dye-Sensitized Solar Cells (DSSCs) Based on Extracted Natural Dyes. J. Nanomater. 2019, 2019, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mathew, S.; Yella, A.; Gao, P.; Humphry-Baker, R.; Curchod, B.F.E.; Ashari-Astani, N.; Tavernelli, I.; Rothlisberger, U.; Nazeeruddin, K.; Graetzel, M. Dye-Sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nat. Chem. 2014, 6, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Kakiage, K.; Aoyama, Y.; Yano, T.; Oya, K.; Fujisawa, J.-I.; Hanaya, M. Highly-Efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun. 2015, 51, 15894–15897. [Google Scholar] [CrossRef]
- Mukherjee, S.; Bowman, D.N.; Jakubikova, E. Cyclometalated Fe(II) Complexes as Sensitizers in Dye-Sensitized Solar Cells. Inorg. Chem. 2015, 54, 560–569. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, W.-C.; Zhang, X.-X.; Ghadari, R.; Fang, X.-Q.; Yu, T.; Kong, F.-T. Ruthenium complexes as sensitizers with phenyl-based bipyridine anchoring ligands for efficient dye-sensitized solar cells. J. Mater. Chem. C 2018, 6, 9445–9452. [Google Scholar] [CrossRef]
- Gao, S.; Fan, R.Q.; Wang, X.M.; Qiang, L.S.; Wei, L.G.; Wang, P.; Yang, Y.L.; Wang, Y.L. Advanced CdII complexes as high efficiency co-sensitizers for enhanced dye-sensitized solar cell performance. Dalton Trans. 2015, 44, 18187–18195. [Google Scholar] [CrossRef] [Green Version]
- Gondane, V.; Bhargava, P. Acetylacetone: A promising electrolyte solvent for dye sensitized solar cells. RSC Adv. 2016, 6, 37167–37172. [Google Scholar] [CrossRef]
- Fukui, A.; Komiya, R.; Yamanaka, R.; Islam, A.; Han, L. Effect of a redox electrolyte in mixed solvents on the photovoltaic performance of a dye-sensitized solar cell. Sol. Energy Mater. Sol. Cells 2006, 90, 649–658. [Google Scholar] [CrossRef]
- Gu, P.; Yang, D.; Zhu, X.; Sun, H.; Wangyang, P.; Li, J.; Tian, H. Influence of electrolyte proportion on the performance of dye-sensitized solar cells. AIP Adv. 2017, 7, 105219. [Google Scholar] [CrossRef]
- Abu Talip, R.; Yahya, W.; Bustam, M. Ionic Liquids Roles and Perspectives in Electrolyte for Dye-Sensitized Solar Cells. Sustainability 2020, 12, 7598. [Google Scholar] [CrossRef]
- Shi, L.-Y.; Chen, T.-L.; Chen, C.-H.; Cho, K.-C. Synthesis and Characterization of a Gel-Type Electrolyte with Ionic Liquid Added for Dye-Sensitized Solar Cells. Int. J. Photoenergy 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Wu, J.; Lan, Z.; Lin, J.; Huang, M.; Li, P. Effect of solvents in liquid electrolyte on the photovoltaic performance of dye-sensitized solar cells. J. Power Sources 2007, 173, 585–591. [Google Scholar] [CrossRef]
- Boldrini, C.L.; Manfredi, N.; Perna, F.M.; Capriati, V.; Abbotto, A. Eco-Friendly Sugar-Based Natural Deep Eutectic Solvents as Effective Electrolyte Solutions for Dye-Sensitized Solar Cells. ChemElectroChem 2020, 7, 1707–1712. [Google Scholar] [CrossRef]
- Subramania, A.; Vijayakumar, E.; Sivasankar, N.; Priya, A.R.S.; Kim, K.-J. Effect of different compositions of ethylene carbonate and propylene carbonate containing iodide/triiodide redox electrolyte on the photovoltaic performance of DSSC. Ionics 2013, 19, 1649–1653. [Google Scholar] [CrossRef]
- Srivishnu, K.S.; Prasanthkumar, S.; Giribabu, L. Cu(ii/i) redox couples: Potential alternatives to traditional electrolytes for dye-sensitized solar cells. Mater. Adv. 2021, 2, 1229–1247. [Google Scholar] [CrossRef]
- Summer, S.; Shamim, A.; Khattak, R.; Qamar, N.; Naqvi, I.I. An Insight into the Kinetics and Mechanism of Oxidation of Tris(4,4′-dimethyl-2,2′-bipyridine)iron(II) by Bromate. Russ. J. Phys. Chem. A 2020, 94, 544–550. [Google Scholar] [CrossRef]
- Summer, S.; Naqvi, I.I.; Khattak, R.; Gulzar, S.; Reyaz, F. Kinetics and Mechanism of [Fe (bipy)3]2+ and [BrO3–] System in Aqueous Acidic Medium. J. Chem. Soc. Pak. 2016, 38, 384–389. [Google Scholar]
- Khattak, R.; Naqvi, I.I.; Farrukh, M.A. Kinetics and mechanism of the oxidation of a ferrous complex with an α,α′-diimine chelate ligand by ceric sulfate in aqueous acidic medium by UV-Vis absorption spectroscopy. J. Iran. Chem. Soc. 2008, 5, 631–640. [Google Scholar] [CrossRef]
- Khattak, R.; Khan, M.S.; Naqvi, I.I. Mechanism of the electron-exchange reactions between mixed ligand Fe(III) complexes and cyano complex of Fe(II). Bulg. Chem. Commun. 2018, 50, 38–44. [Google Scholar]
- Khattak, R.; Naqvi, I.I.; Summer, S.; Sayed, M. Mechanism of the oxidation of 1-(ferrocenyl)-ethanone/ethanol by dicyanobis(phenanthroline)iron(III). Arab. J. Chem. 2019, 12, 4240–4250. [Google Scholar] [CrossRef] [Green Version]
- Khattak, R.; Naqvi, I.I. Study of the effect of structure on the kinetics and mechanism of the redox reactions of Fe(III)/Fe(II) complexes. Bulg. Chem. Commun. 2019, 51, 129–134. [Google Scholar]
- Khattak, R.; Nazir, M.; Summer, S.; Sayed, M.; Minhaz, A.; Naqvi, I.I. Thermodynamic aspect: Kinetics of the reduction of dicyanobis(phen)iron(III) by acetylferrocene and methylferrocenemethanol. Chem. Pap. 2018, 72, 883–893. [Google Scholar] [CrossRef]
- Khattak, R. Comparative Kinetic Study for the Electron Transfer Reactions of Some Iron Complexes. Ph.D. Thesis, University of Karachi, Karachi, Pakistan, 2011. [Google Scholar]
- Khattak, R.; Naqvi, I.I. Identification, preparation and characterization of some iron complexes. J. Res. Sci. 2008, 19, 17–35. [Google Scholar]
- Khattak, R.; Sayed, M.; Khan, M.S.; Noreen, H. Introductory Chapter: Redox—An Overview. In Redox; Khattak, R., Ed.; IntechOpen: London, UK, 2020; pp. 1–10. [Google Scholar]
- Khattak, R.; Naqvi, I.I. Addition product of iron(II) complex of aromatic diimine with sulphuric acid. J. Res. Sci. 2007, 18, 219–235. [Google Scholar]
- Wang, X.; Stanbury, D.M. Copper Catalysis of the Oxidation of Iodide by [FeIII(bpy)2(CN)2]+ in Acetonitrile. J. Phys. Chem. A 2004, 108, 7637–7638. [Google Scholar] [CrossRef]
- Wang, X.; Stanbury, D.M. Direct oxidation of l-cysteine by [FeIII(bpy)2(CN)2]+ and [FeIII(bpy)(CN)4]−. Inorg. Chem. 2008, 47, 1224–1236. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Stanbury, D.M. Oxidation of Iodide by a Series of Fe(III) Complexes in Acetonitrile. Inorg. Chem. 2006, 45, 3415–3423. [Google Scholar] [CrossRef] [PubMed]
- Bella, F.; Gerbaldi, C.; Barolo, C.; Grätzel, M. Aqueous dye-sensitized solar cells. Chem. Soc. Rev. 2015, 44, 3431–3473. [Google Scholar] [CrossRef] [Green Version]
- Tsukaue, Y.; Takimoto, Y.; Nakao, G.; Yoshida, K. Effect of NOx on Oxidation of Triiodide Ions. J. Nucl. Sci. Technol. 1994, 31, 813–820. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khattak, R.; Khan, M.S.; Iqbal, Z.; Ullah, R.; Khan, A.; Summer, S.; Noreen, H.; Zahoor, M.; El-Bahy, S.M.; Batiha, G.E.-S. Catalytic Effect of 1,4-Dioxane on the Kinetics of the Oxidation of Iodide by Dicyanobis(bipyridine)iron(III) in Water. Catalysts 2021, 11, 840. https://doi.org/10.3390/catal11070840
Khattak R, Khan MS, Iqbal Z, Ullah R, Khan A, Summer S, Noreen H, Zahoor M, El-Bahy SM, Batiha GE-S. Catalytic Effect of 1,4-Dioxane on the Kinetics of the Oxidation of Iodide by Dicyanobis(bipyridine)iron(III) in Water. Catalysts. 2021; 11(7):840. https://doi.org/10.3390/catal11070840
Chicago/Turabian StyleKhattak, Rozina, Muhammad Sufaid Khan, Zahoor Iqbal, Rizwan Ullah, Abbas Khan, Shazia Summer, Hamsa Noreen, Muhammad Zahoor, Salah M. El-Bahy, and Gaber El-Saber Batiha. 2021. "Catalytic Effect of 1,4-Dioxane on the Kinetics of the Oxidation of Iodide by Dicyanobis(bipyridine)iron(III) in Water" Catalysts 11, no. 7: 840. https://doi.org/10.3390/catal11070840
APA StyleKhattak, R., Khan, M. S., Iqbal, Z., Ullah, R., Khan, A., Summer, S., Noreen, H., Zahoor, M., El-Bahy, S. M., & Batiha, G. E.-S. (2021). Catalytic Effect of 1,4-Dioxane on the Kinetics of the Oxidation of Iodide by Dicyanobis(bipyridine)iron(III) in Water. Catalysts, 11(7), 840. https://doi.org/10.3390/catal11070840






