Green Synthesis of Flower-Shaped Copper Oxide and Nickel Oxide Nanoparticles via Capparis decidua Leaf Extract for Synergic Adsorption-Photocatalytic Degradation of Pesticides
Abstract
1. Introduction
2. Results and Discussion
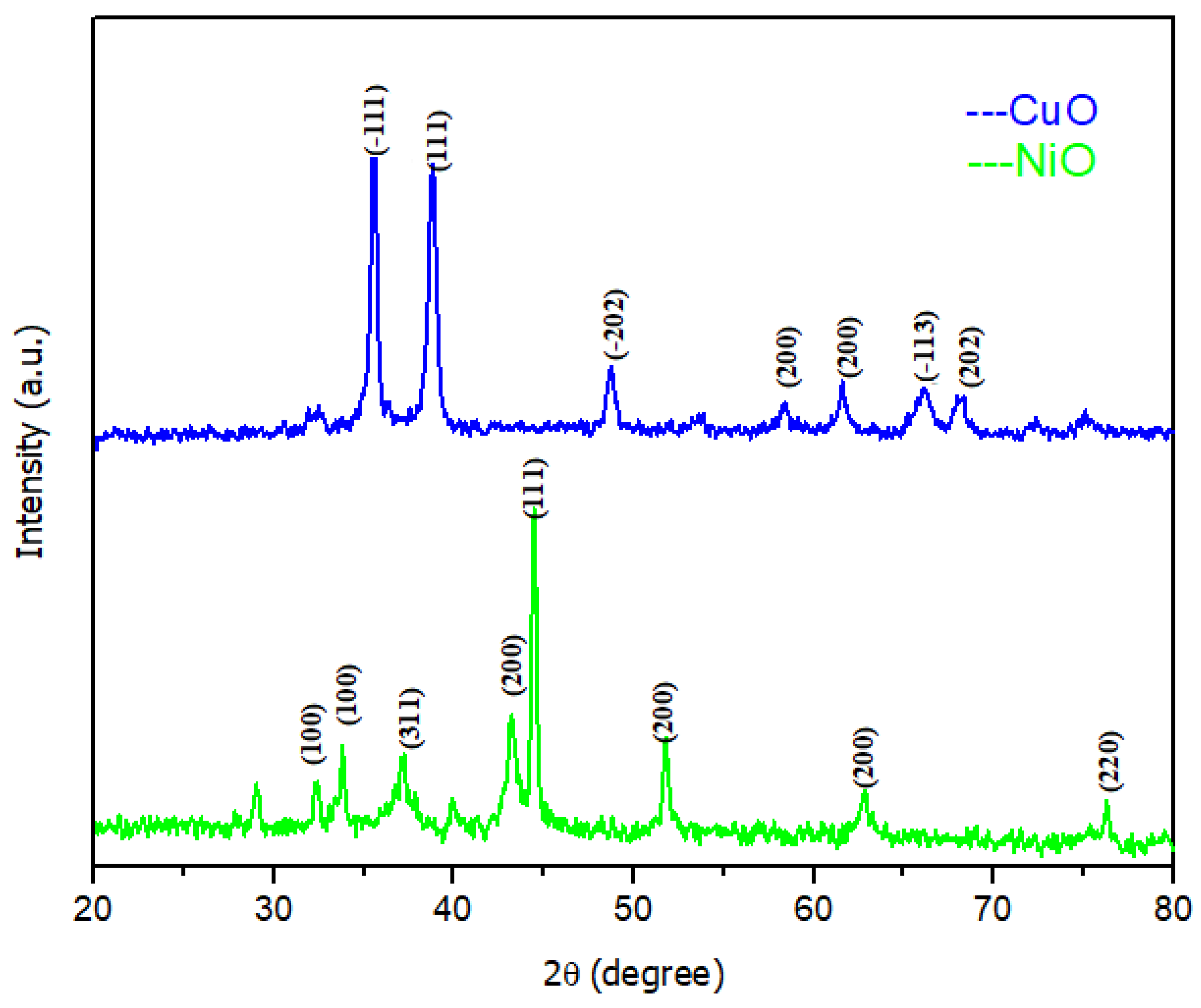
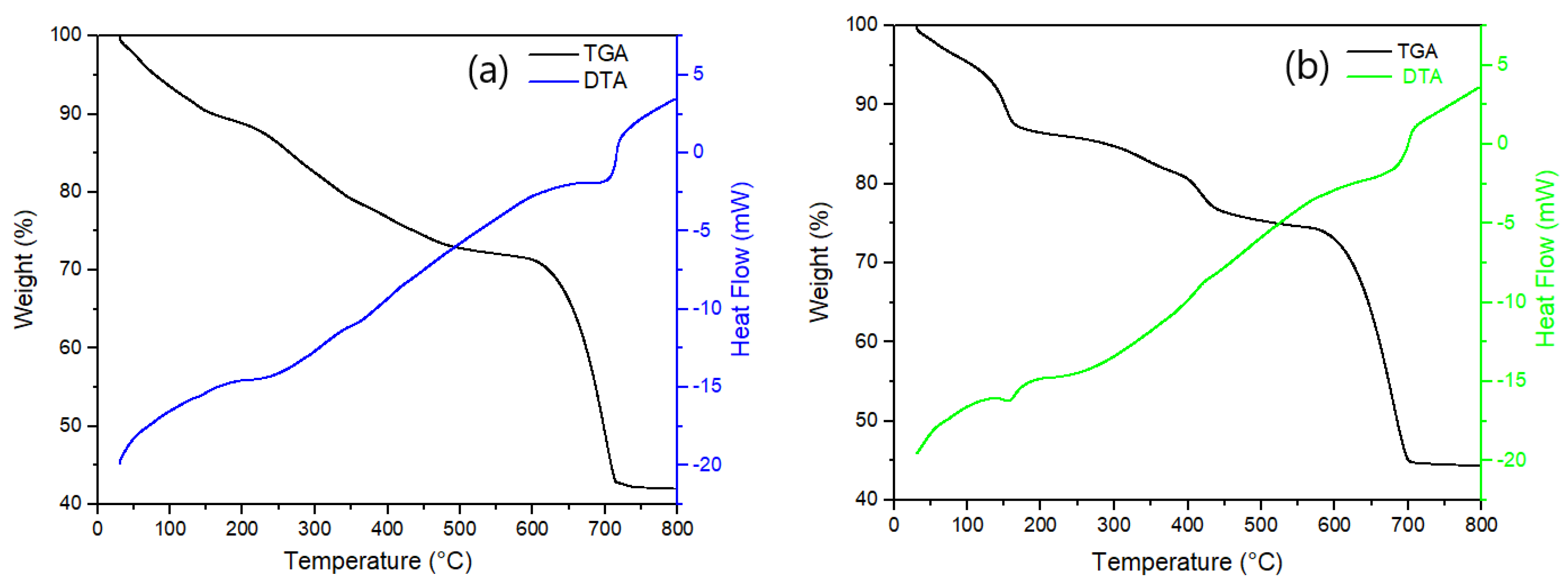
2.1. Photocatalytic Nanoparticles Synthesis and Characterization
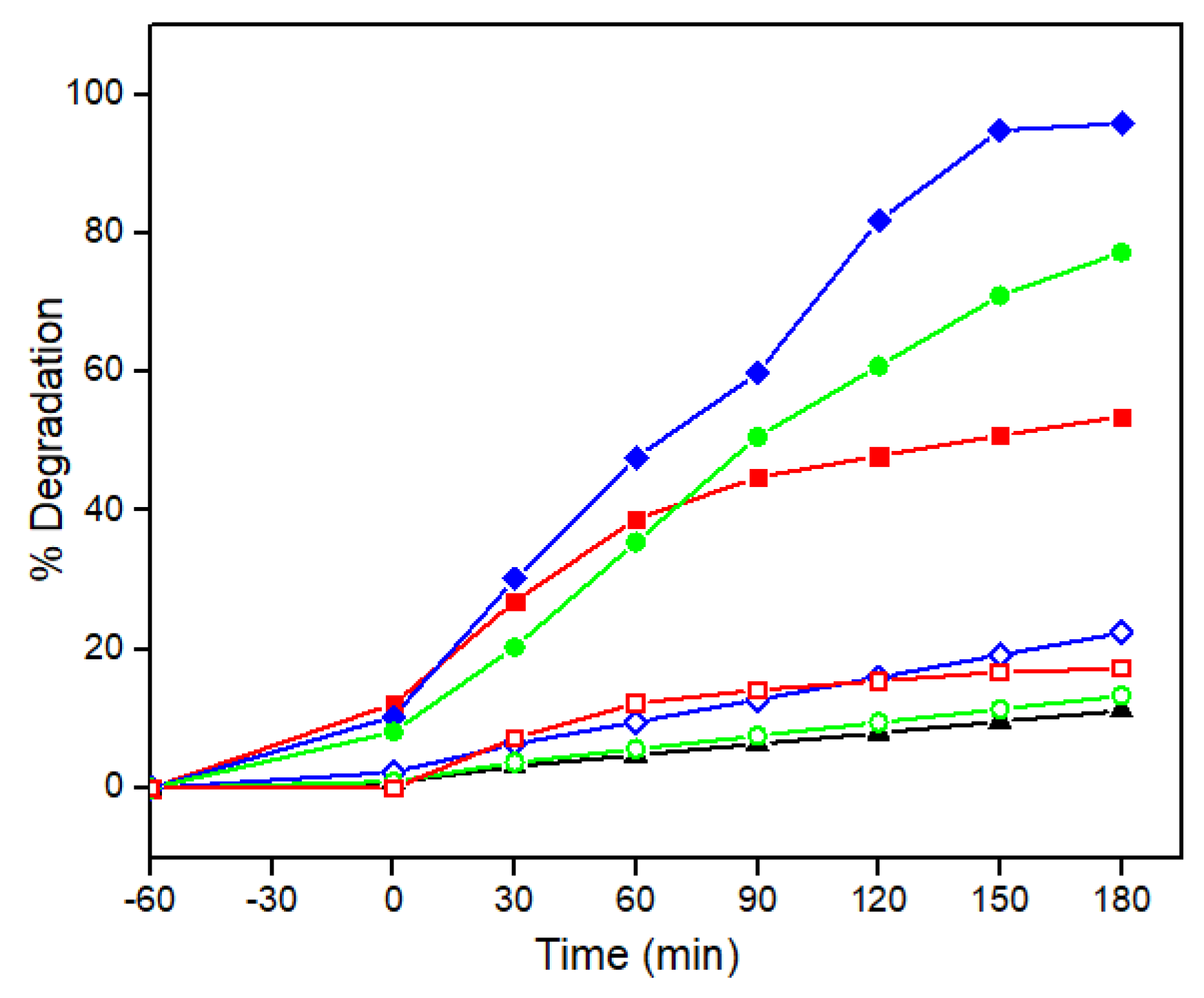
2.2. Evaluating the Photocatalytic Activity of Phyto-Synthesized Nanoparticles
2.3. Understanding the Impact of Operational Variables on Pesticide Removal
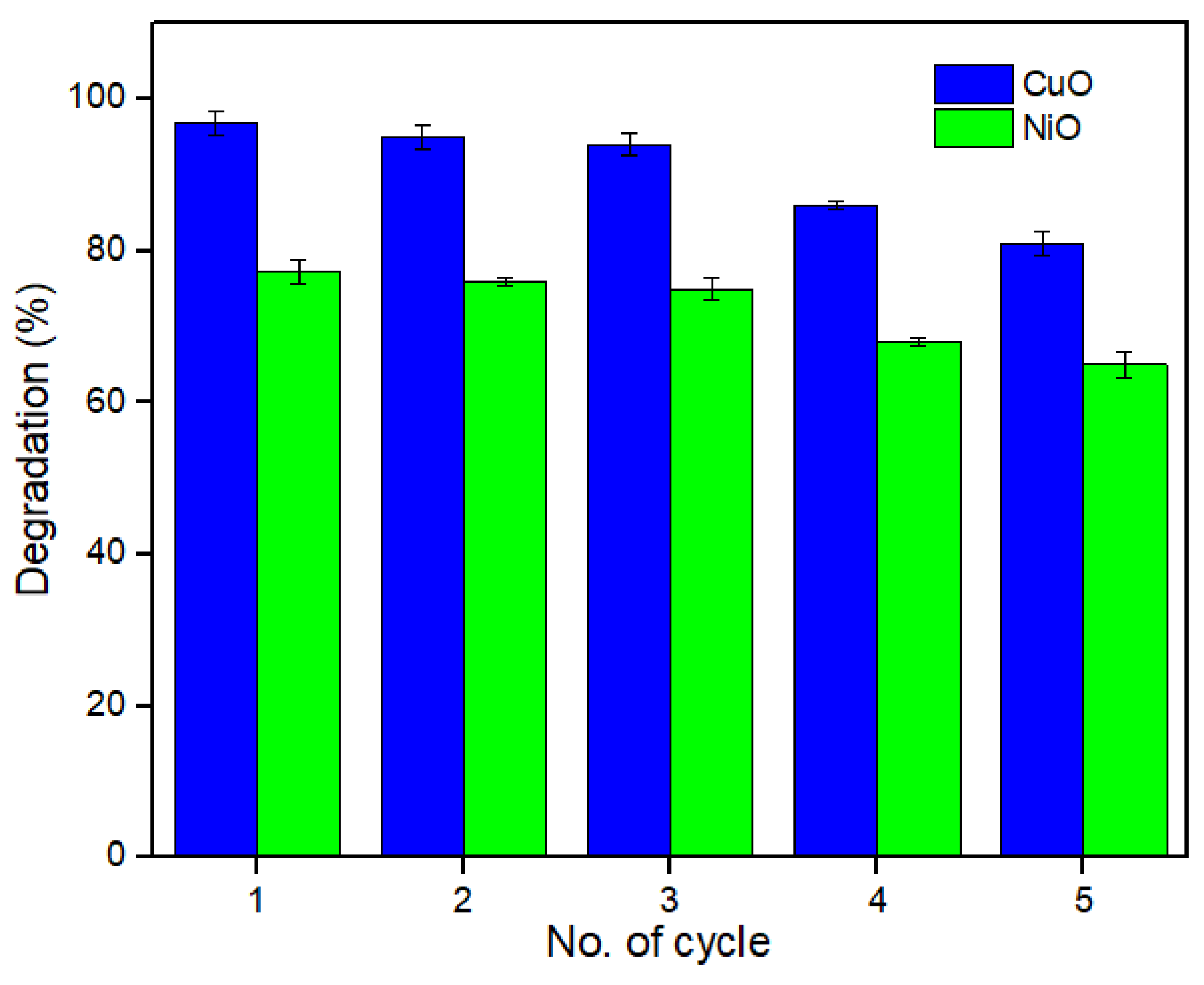
2.4. Photocatalytic Stability of CuO and NiO Photocatalyst for Removal of L-CHT for Five Times
3. Material and Methods
3.1. Chemicals
3.2. Preparation of Plant Extract
3.3. Green synthesis of CuO and NiO Nanoparticles
3.4. Photocatalytic Degradation Experiments
3.5. Characterization Methods and Instruments
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bolzonella, C.; Lucchetta, M.; Teo, G.; Boatto, V.; Zanella, A. Is there a way to rate insecticides that is less detrimental to human and environmental health? Glob. Ecol. Conserv. 2019, 20, e00699. [Google Scholar] [CrossRef]
- Wang, Q.; Rao, P.; Li, G.; Dong, L.; Zhang, X.; Shao, Y.; Gao, N.; Chu, W.; Xu, B.; An, N.; et al. Degradation of imidacloprid by UV-activated persulfate and peroxymonosulfate processes: Kinetics, impact of key factors and degradation pathway. Ecotoxicol. Environ. Saf. 2020, 187, 109779. [Google Scholar] [CrossRef] [PubMed]
- Heck, K.N.; Garcia-Segura, S.; Westerhoff, P.; Wong, M.S. Catalytic Converters for Water Treatment. Accounts Chem. Res. 2019, 52, 906–915. [Google Scholar] [CrossRef] [PubMed]
- Tudi, M.; Ruan, H.D.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. Integrated, Decentralized Wastewater Management for Resource Recovery in Rural and Peri-Urban Areas. Resour. 2017, 6, 22. [Google Scholar] [CrossRef]
- Zulfikar, M.; Nadeem, R.; Javed, T.; Jilani, M.I.; Javed, I. Green synthesis of Fe nanoparticles by using Mangifera indica extract and its application in photo-catalytic degradation of dyes. Water Sci. Technol. 2021, 83, 1739–1752. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial Degradation of Pesticide Residues and an Emphasis on the Degradation of Cypermethrin and 3-phenoxy Benzoic Acid: A Review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Dominguez, S.; Bringas, E.; Rivero, M.J.; Ortiz, I.; Dionysiou, D. Review and perspectives on the use of magnetic nanophotocatalysts (MNPCs) in water treatment. Chem. Eng. J. 2017, 310, 407–427. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Venieri, D.; Mantzavinos, D.; Binas, V. Solar Photocatalysis for Emerging Micro-Pollutants Abatement and Water Disinfection: A Mini-Review. Sustainability 2020, 12, 10047. [Google Scholar] [CrossRef]
- Oliveros, A.N.; Pimentel, J.A.I.; de Luna, M.D.G.; Garcia-Segura, S.; Abarca, R.R.M.; Doong, R.-A. Visible-light photocatalytic diclofenac removal by tunable vanadium pentoxide/boron-doped graphitic carbon nitride composite. Chem. Eng. J. 2021, 403, 126213. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2020, 12, 102. [Google Scholar] [CrossRef]
- Tugaoen, H.O.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Compact light-emitting diode optical fiber immobilized TiO2 reactor for photocatalytic water treatment. Sci. Total Environ. 2018, 613–614, 1331–1338. [Google Scholar] [CrossRef]
- Fagan, R.; McCormack, D.E.; Dionysiou, D.D.; Pillai, S.C. A review of solar and visible light active TiO2 photocatalysis for treating bacteria, cyanotoxins and contaminants of emerging concern. Mater. Sci. Semicond. Process. 2016, 42, 2–14. [Google Scholar] [CrossRef]
- Monteiro, R.A.; Miranda, S.; Vilar, V.; Pastrana-Martínez, L.M.; Tavares, P.; Boaventura, R.A.; Faria, J.L.; Pinto, E.; Silva, A.M. N-modified TiO 2 photocatalytic activity towards diphenhydramine degradation and Escherichia coli inactivation in aqueous solutions. Appl. Catal. B Environ. 2015, 162, 66–74. [Google Scholar] [CrossRef]
- Villaluz, F.J.A.; de Luna, M.D.G.; Colades, J.I.; Garcia-Segura, S.; Lu, M.-C. Removal of 4-chlorophenol by visible-light photocatalysis using ammonium iron(II) sulfate-doped nano-titania. Process. Saf. Environ. Prot. 2019, 125, 121–128. [Google Scholar] [CrossRef]
- EL-Sheshtawy, H.S.; El-Hosainy, H.M.; Shoueir, K.R.; El-Mehasseb, I.M.; El-Kemary, M. Facile immobilization of Ag nano-particles on g-C3N4/V2O5 surface for enhancement of post-illumination, catalytic, and photocatalytic activity removal of organic and inorganic pollutants. Appl. Surf. Sci. 2019, 467–468, 268–276. [Google Scholar] [CrossRef]
- Chen, J.; Loeb, S.; Kim, J.-H. LED revolution: Fundamentals and prospects for UV disinfection applications. Environ. Sci. Water Res. Technol. 2017, 3, 188–202. [Google Scholar] [CrossRef]
- Brillas, E.; Serrà, A.; Garcia-Segura, S. Biomimicry designs for photoelectrochemical systems: Strategies to improve light delivery efficiency. Curr. Opin. Electrochem. 2021, 26, 100660. [Google Scholar] [CrossRef]
- Batista, L.M.B.; dos Santos, A.J.; da Silva, D.R.; Alves, A.P.D.M.; Garcia-Segura, S.; Martínez-Huitle, C.A. Solar photocatalytic application of NbO 2 OH as alternative photocatalyst for water treatment. Sci. Total Environ. 2017, 596–597, 79–86. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Batista, L.M.B.; Martínez-Huitle, C.A.; Alves, A.P.D.M.; Garcia-Segura, S. Niobium Oxide Catalysts as Emerging Material for Textile Wastewater Reuse: Photocatalytic Decolorization of Azo Dyes. Catalysts 2019, 9, 1070. [Google Scholar] [CrossRef]
- Sabouri, Z.; Akbari, A.; Hosseini, H.A.; Darroudi, M. Facile green synthesis of NiO nanoparticles and investigation of dye degradation and cytotoxicity effects. J. Mol. Struct. 2018, 1173, 931–936. [Google Scholar] [CrossRef]
- Yu, C.; Wen, M.; Tong, Z.; Li, S.; Yin, Y.; Liu, X.; Li, Y.; Liang, T.; Wu, Z.; Dionysiou, D.D. Synthesis and enhanced photo-catalytic performance of 0D/2D CuO/tourmaline composite photocatalysts. Beilstein J. Nanotechnol. 2020, 11, 407–416. [Google Scholar] [CrossRef]
- Yu, X.; Zhang, J.; Zhao, Z.; Guo, W.; Qiu, J.; Mou, X.; Li, A.; Claverie, J.P.; Liu, H. NiO-TiO2 p-n heterostructured nanocables bridged by zero-bandgap rGO for highly efficient photocatalytic water splitting. Nano Energy 2015, 16, 207–217. [Google Scholar] [CrossRef]
- Dhineshbabu, N.R.; Rajendran, V.; Nithyavathy, N.; Vetumperumal, R. Study of structural and optical properties of cupric oxide nanoparticles. Appl. Nanosci. 2015, 6, 933–939. [Google Scholar] [CrossRef]
- Serrà, A.; Philippe, L.; Perreault, F.; Garcia-Segura, S. Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Res. 2021, 188, 116543. [Google Scholar] [CrossRef]
- Gonçalves, R.; Toledo, R.; Joshi, N.; Berengue, O. Green Synthesis and Applications of ZnO and TiO2 Nanostructures. Molecules 2021, 26, 2236. [Google Scholar] [CrossRef] [PubMed]
- Mondejar, M.E.; Avtar, R.; Diaz, H.L.B.; Dubey, R.K.; Esteban, J.; Gomez-Morales, A.; Hallam, B.; Mbungi, N.T.; Okolo, C.C.; Prasad, K.A.; et al. Digitalization to achieve sustainable development goals: Steps towards a Smart Green Plane. Sci. Total Environ. 2021, 148539. [Google Scholar] [CrossRef]
- Tugaoen, H.; Garcia-Segura, S.; Hristovski, K.; Westerhoff, P. Challenges in photocatalytic reduction of nitrate as a water treatment technology. Sci. Total Environ. 2017, 599–600, 1524–1551. [Google Scholar] [CrossRef]
- Akel, S.; Dillert, R.; Balayeva, N.O.; Boughaled, R.; Koch, J.; El Azzouzi, M.; Bahnemann, D.W. Ag/Ag2O as a Co-Catalyst in TiO2 Photocatalysis: Effect of the Co-Catalyst/Photocatalyst Mass Ratio. Catalysts 2018, 8, 647. [Google Scholar] [CrossRef]
- Aljabali, A.A.A.; Akkam, Y.; Al Zoubi, M.S.; Al-Batayneh, K.M.; Al-Trad, B.; Alrob, O.A.; Alkilany, A.M.; Benamara, M.; Evans, D.J. Synthesis of Gold Nanoparticles Using Leaf Extract of Ziziphus zizyphus and their Antimicrobial Activity. Nanomaterials 2018, 8, 174. [Google Scholar] [CrossRef]
- Khan, S.H.; Pathak, B. Zinc oxide based photocatalytic degradation of persistent pesticides: A comprehensive review. Environ. Nanotechnol. Monit. Manag. 2020, 13, 100290. [Google Scholar] [CrossRef]
- Serrà, A.; Gómez, E.; Philippe, L. Bioinspired ZnO-based solar photocatalysts for the efficient decontamination of persistent organic pollutants and hexavalent chromium in wastewater. Catalysts 2019, 9, 974. [Google Scholar] [CrossRef]
- Wang, Y.; O’Connor, D.; Shen, Z.; Lo, I.M.; Tsang, D.; Pehkonen, S.; Pu, S.; Hou, D. Green synthesis of nanoparticles for the remediation of contaminated waters and soils: Constituents, synthesizing methods, and influencing factors. J. Clean. Prod. 2019, 226, 540–549. [Google Scholar] [CrossRef]
- Varghese, J.; Zikalala, N.; Sakho, E.H.M.; Oluwafemi, O.S. Green Synthesis Protocol on Metal Oxide Nanoparticles Using Plant Extracts; Elsevier BV, 2020; pp. 67–82. Available online: https://www.sciencedirect.com/science/article/pii/B9780128133576000061?via%3Dihub (accessed on 22 June 2021).
- Yahia, Y.; Benabderrahim, M.A.; Tlili, N.; Bagues, M.; Nagaz, K. Bioactive compounds, antioxidant and antimicrobial activities of extracts from different plant parts of two Ziziphus Mill. species. PLoS ONE 2020, 15, e0232599. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Bhardwaj, K.; Kuča, K.; Kalia, A.; Nepovimova, E.; Verma, R.; Kumar, D. Flower-Based Green Synthesis of Metallic Nanoparticles: Applications beyond Fragrance. Nanomaterials 2020, 10, 766. [Google Scholar] [CrossRef]
- Sarkar, J.; Chakraborty, N.; Chatterjee, A.; Bhattacharjee, A.; Dasgupta, D.; Acharya, K. Green Synthesized Copper Oxide Nanoparticles Ameliorate Defence and Antioxidant Enzymes in Lens culinaris. Nanomaterials 2020, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Khan, A.; Rashid, H. Green synthesis of CuO nanoparticles using Lantana camara flower extract and their potential catalytic activity towards the aza-Michael reaction. RSC Adv. 2020, 10, 14374–14385. [Google Scholar] [CrossRef]
- Sukumar, S.; Rudrasenan, A.; Padmanabhan Nambiar, D. Green-aynthesized rice-shaped copper oxide nanoparticles using Caesalpinia bonducella seed extract and their applications. ACS Omega 2020, 5, 1040–1051. [Google Scholar] [CrossRef] [PubMed]
- Olajire, A.; Mohammed, A. Green synthesis of nickel oxide nanoparticles and studies of their photocatalytic activity in degradation of polyethylene films. Adv. Powder Technol. 2020, 31, 211–218. [Google Scholar] [CrossRef]
- Zhang, Y.; Mahdavi, B.; Mohammadhosseini, M.; Rezaei-Seresht, E.; Paydarfard, S.; Qorbani, M.; Karimian, M.; Abbasi, N.; Ghaneialvar, H.; Karimi, E. Green synthesis of NiO nanoparticles using Calendula officinalis extract: Chemical charactrization, antioxidant, cytotoxicity, and anti-esophageal carcinoma properties. Arab. J. Chem. 2021, 14, 103105. [Google Scholar] [CrossRef]
- Boudiaf, M.; Messai, Y.; Bentouhami, E.; Schmutz, M.; Blanck, C.; Ruhlmann, L.; Bezzi, H.; Tairi, L.; Mekki, D.E. Green synthesis of NiO nanoparticles using Nigella sativa extract and their enhanced electro-catalytic activity for the 4-nitrophenol degradation. J. Phys. Chem. Solids 2021, 153, 110020. [Google Scholar] [CrossRef]
- Dhakad, P.K.; Sharma, P.K.; Kumar, S. A Review on Ethnobiological & Medicinal Potential of Capparaceae Family Plant: Capparis decidua (Forssk.) Edgew. Adv. Pharmacol. Pharm. 2016, 4, 27–39. [Google Scholar] [CrossRef]
- Nazar, S.; Hussain, M.A.; Khan, A.; Muhammad, G.; Tahir, M.N. Capparis decidua Edgew (Forssk.): A comprehensive review of its traditional uses, phytochemistry, pharmacology and nutrapharmaceutical potential. Arab. J. Chem. 2020, 13, 1901–1916. [Google Scholar] [CrossRef]
- Iqbal, A.; Anwar, F.; Nadeem, R.; Sultana, B.; Mushtaq, M. Proximate Composition and Minerals Profile of Fruit and Flower of Karir (Capparis decidua) from Different Regions of Punjab (Pakistan). Asian J. Chem. 2014, 26, 360–364. [Google Scholar] [CrossRef]
- Raza, M.A.; Younas, M.; Schlecht, E. In vitro efficacy of selected medicinal plants from Cholistan desert, Pakistan, against gastrointestinal helminths of sheep and goats. J. Agric. Rural Dev. Trop. Subtrop. 2016, 117, 211–224. [Google Scholar]
- Khan, M.I.; Shoukat, M.A.; Cheema, S.A.; Arif, H.N.; Niazi, N.K.; Azam, M.; Bashir, S.; Ashraf, I.; Qadri, R. Use, contami-nation and exposure of pesticides in pakistan: A review. Pak. J. Agric. Sci. 2020, 57, 131–149. [Google Scholar]
- Ghumro, W.A.; Phulpoto, A.H.; Qazi, M.A.; Mangi, S.; Pirzada, T.; Ahmed, S.; Kanhar, N.A. Pesticide Lambda-Cyhalothrin degradation using Mesorhizobium sp. (S1b) and Bartonella sp. (S2b) strains isolated from cotton crop. Pak. J. Anal. Environ. Chem. 2017, 18, 112–119. [Google Scholar] [CrossRef][Green Version]
- Saleem, U.; Ejaz, S.; Ashraf, M.; Omer, M.O.; Altaf, I.; Batool, Z.; Fatima, R.; Afzal, M. Mutagenic and cytotoxic potential of Endosulfan and Lambda-cyhalothrin—In vitro study describing individual and combined effects of pesticides. J. Environ. Sci. 2014, 26, 1471–1479. [Google Scholar] [CrossRef]
- Nieradko-Iwanicka, B.; Konopelko, M. Effect of Lambdacyhalothrin on Locomotor Activity, Memory, Selected Biochemical Parameters, Tumor Necrosis Factor α, and Interleukin 1ß in a Mouse Model. Int. J. Environ. Res. Public Health 2020, 17, 9240. [Google Scholar] [CrossRef]
- Bownik, A.; Kowalczyk, M.; Bańczerowski, J. Lambda-cyhalothrin affects swimming activity and physiological responses of Daphnia magna. Chemosphere 2019, 216, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.K.; Rai, J.; Bhatt, C.; Rai, M.K.; Goswami, J.; Sahu, A.K.; Singh, T.V.; Nirmala, M.; Mundeja, K.W.A.P. UV-Visible Spectrophotometric Determination of Lambda-Cyhalothrin Insecticide in Vegetables, Soil and Water Samples. J. Ravishankar Univ. (Part-B) 2018, 31, 1–9. [Google Scholar] [CrossRef]
- Shahzadi, N.; Imran, M.; Sarwar, M.; Hashmi, A.S. Identification of pesticides residues in different samples of milk. J. Agroaliment. Process. Technol. 2013, 19, 167–172. [Google Scholar]
- Li, H.; Fang, Y.; Ni, C.; Chen, X.; Mo, J.; Lv, Y.; Chen, Y.; Chen, X.; Lian, Q.; Ge, R.-S. Lambda-cyhalothrin delays pubertal Leydig cell development in rats. Environ. Pollut. 2018, 242, 709–717. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Lou, B.; Xu, C.; Yang, G.; Yu, R.; Wang, X.; Li, X.; Wang, Q.; Wang, Y. Lethal toxicity and gene expression changes in embryonic zebrafish upon exposure to individual and mixture of malathion, chlorpyrifos and lambda-cyhalothrin. Chemosphere 2020, 239, 124802. [Google Scholar] [CrossRef] [PubMed]
- Al-Amoudi, W.M. Toxic effects of Lambda-cyhalothrin, on the rat thyroid: Involvement of oxidative stress and ameliorative effect of ginger extract. Toxicol. Rep. 2018, 5, 728–736. [Google Scholar] [CrossRef]
- Liao, C.H.; He, X.J.; Wang, Z.L.; Barron, A.B.; Zhang, B.; Zeng, Z.J.; Wu, X.B. Short-Term Exposure to Lamb-da-Cyhalothrin Negatively Affects the Survival and Memory-Related Characteristics of Worker Bees Apis mellifera. Arch. Environ. Contam. Toxicol. 2018, 75, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Ezenwosu, S.U.; Nnamonu, E.I.; Odo, G.E.; Ikele, B.C.; Ani, O.C. Evaluation of lambda-cyhalothrin oxidative stress and gonad histoarchitecture toxicity potency in Clarias gariepinus. J. Basic Appl. Zool. 2021, 82, 1–11. [Google Scholar] [CrossRef]
- Fetoui, H.; Makni, M.; Garoui, E.M.; Zeghal, N. Toxic effects of lambda-cyhalothrin, a synthetic pyrethroid pesticide, on the rat kidney: Involvement of oxidative stress and protective role of ascorbic acid. Exp. Toxicol. Pathol. 2010, 62, 593–599. [Google Scholar] [CrossRef]
- Verma, J.P.; Jaiswal, D.K.; Sagar, R. Pesticide relevance and their microbial degradation: A-state-of-art. Rev. Environ. Sci. Bio/Technol. 2014, 13, 429–466. [Google Scholar] [CrossRef]
- Al-Sarar, A.S.; Abobakr, Y.; Bayoumi, A.E.; Hussein, H.I.; Al-Ghothemi, M. Reproductive toxicity and histopathological changes induced by lambda-cyhalothrin in male mice. Environ. Toxicol. 2012, 29, 750–762. [Google Scholar] [CrossRef]
- Ministry of the Environment of Canada. Class 9 Pesticide Ingredients Banned for Cosmetic Use as Classified by the Ontario, Canada, Ministry of the Environment. 2013. Available online: https://www.beyondpesticides.org/assets/media/documents/lawn/documents/Class9PesticideIngredientsBannedforCosmeticUseasClassifiedbytheOntario-FINAL.pdf (accessed on 29 June 2021).
- EPA Lambda-Cyhalothrin; Receipt of Application for Emergency Exemption, Solicitation of Public Comment; Environmental Protection Agency: Washington, DC, USA, 2020; Volume 85.
- Chaudhary, S.; Kaur, Y.; Jayee, B.; Chaudhary, G.R.; Umar, A. NiO nanodisks: Highly efficient visible-light driven photocatalyst, potential scaffold for seed germination of Vigna Radiata and antibacterial properties. J. Clean. Prod. 2018, 190, 563–576. [Google Scholar] [CrossRef]
- Saravanan, S.; Sivasankar, T. Effect of ultrasound power and calcination temperature on the sonochemical synthesis of copper oxide nanoparticles for textile dyes treatment. Environ. Prog. Sustain. Energy 2016, 35, 669–679. [Google Scholar] [CrossRef]
- Bharathi, D.; Preethi, S.; Abarna, K.; Nithyasri, M.; Kishore, P.; Deepika, K. Bio-inspired synthesis of flower shaped iron oxide nanoparticles (FeONPs) using phytochemicals of Solanum lycopersicum leaf extract for biomedical applications. Biocatal. Agric. Biotechnol. 2020, 27, 101698. [Google Scholar] [CrossRef]
- Lalithambika, K.C.; Thayumanavan, A.; Ravichandran, K.; Sriram, S. Photocatalytic and antibacterial activities of eco-friendly green synthesized ZnO and NiO nanoparticles. J. Mater. Sci. Mater. Electron. 2017, 28, 2062–2068. [Google Scholar] [CrossRef]
- Arya, A.; Gupta, K.; Chundawat, T.S.; Vaya, D. Biogenic synthesis of copper and silver nanoparticles using green alga Bot-ryococcus braunii and its antimicrobial activity. Bioinorg. Chem. Appl. 2018, 2018, 789403. [Google Scholar] [CrossRef] [PubMed]
- Shashanka, R. Investigation of optical and thermal properties of CuO and ZnO nanoparticles prepared by Crocus Sativus (Saffron) flower extract. J. Iran. Chem. Soc. 2021, 18, 415–427. [Google Scholar] [CrossRef]
- Mayedwa, N.; Mongwaketsi, N.; Khamlich, S.; Kaviyarasu, K.; Matinise, N.; Maaza, M. Green synthesis of nickel oxide, pal-ladium and palladium oxide synthesized via Aspalathus linearis natural extracts: Physical properties & mechanism of for-mation. Appl. Surf. Sci. 2018, 446, 266–272. [Google Scholar]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Maaza, M.; Ayeshamariam, A.; Kennedy, L.J. Green synthesis of NiO nanopar-ticles using Moringa oleifera extract and their biomedical applications: Cytotoxicity effect of nanoparticles against HT-29 cancer cells. J. Photochem. Photobiol. B Biol. 2016, 164, 352–360. [Google Scholar] [CrossRef]
- Philip, D. Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 42, 1417–1424. [Google Scholar] [CrossRef]
- Narayan, H.; Alemu, H.; Jaybhaye, S. Copper Oxide Nanoparticles: Synthesis and Characterization. In Proceedings of the AATMC-2018, Kalyan, India, 28 February 2018; pp. 43–47. [Google Scholar]
- Sorbiun, M.; Shayegan Mehr, E.; Ramazani, A.; Taghavi Fardood, S. Green synthesis of zinc oxide and copper oxide nano-particles using aqueous extract of oak fruit hull (Jaft) and comparing their photocatalytic degradation of Basic Violet 3. Int. J. Environ. Res. 2018, 12, 29–37. [Google Scholar] [CrossRef]
- Sudhasree, S.; Banu, A.S.; Brindha, P.; Kurian, G.A. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol. Environ. Chem. 2014, 96, 743–754. [Google Scholar] [CrossRef]
- Prabhu, V.G.; Shajira, P.; Lakshmi, N.; Bushiri, M.J. Magnetic properties of Ni/NiO nanocomposites synthesized by one step solution combustion method. J. Phys. Chem. Solids 2015, 87, 238–243. [Google Scholar] [CrossRef]
- Pillai, S.C.; Štangar, U.L.; Byrne, J.A.; Pérez-Larios, A.; Dionysiou, D.D. Photocatalysis for disinfection and removal of con-taminants of emerging concern. Chem. Eng. J. 2015, 261, 1–2. [Google Scholar] [CrossRef]
- Marcelino, R.; Amorim, C.C. Towards visible-light photocatalysis for environmental applications: Band-gap engineering versus photons absorption—A review. Environ. Sci. Pollut. Res. 2018, 26, 4155–4170. [Google Scholar] [CrossRef] [PubMed]
- Koppenol, W.H.; Stanbury, D.M.; Bounds, P.L. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free. Radic. Biol. Med. 2010, 49, 317–322. [Google Scholar] [CrossRef]
- Deng, F.; Zhang, Q.; Yang, L.; Luo, X.; Wang, A.; Luo, S.; Dionysiou, D. Visible-light-responsive graphene-functionalized Bi-bridge Z-scheme black BiOCl/Bi2O3 heterojunction with oxygen vacancy and multiple charge transfer channels for efficient photocatalytic degradation of 2-nitrophenol and industrial wastewater treatment. Appl. Catal. B Environ. 2018, 238, 61–69. [Google Scholar] [CrossRef]
- Zangeneh, H.; Zinatizadeh, A.A.; Feyzi, M.; Zinadini, S.; Bahnemann, D.W. Photomineralization of recalcitrant wastewaters by a novel magnetically recyclable boron doped-TiO2-SiO2 cobalt ferrite nanocomposite as a visible-driven heterogeneous photocatalyst. J. Environ. Chem. Eng. 2018, 6, 6370–6381. [Google Scholar] [CrossRef]
- Marinho, B.A.; Cristóvão, R.O.; Djellabi, R.; Caseiro, A.; Miranda, S.M.; Loureiro, J.M.; Boaventura, R.A.; Dias, M.; Lopes, J.C.B.; Vilar, V.J. Strategies to reduce mass and photons transfer limitations in heterogeneous photocatalytic processes: Hexavalent chromium reduction studies. J. Environ. Manag. 2018, 217, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Khedr, T.M.; El-Sheikh, S.M.; Hakki, A.; Ismail, A.A.; Badawy, W.A.; Bahnemann, D.W. Highly active non-metals doped mixed-phase TiO 2 for photocatalytic oxidation of ibuprofen under visible light. J. Photochem. Photobiol. A Chem. 2017, 346, 530–540. [Google Scholar] [CrossRef]
- Khan, S.; Han, C.; Sayed, M.; Sohail, M.; Jan, S.; Sultana, S.; Khan, H.M.; Dionysiou, D.D. Exhaustive Photocatalytic Lindane Degradation by Combined Simulated Solar Light-Activated Nanocrystalline TiO2 and Inorganic Oxidants. Catalysts 2019, 9, 425. [Google Scholar] [CrossRef]
- Díez, A.M.; Moreira, F.C.; Marinho, B.A.; Espindola, J.; Paulista, L.O.; Sanromán, M.; Pazos, M.; Boaventura, R.A.; Vilar, V.J. A step forward in heterogeneous photocatalysis: Process intensification by using a static mixer as catalyst support. Chem. Eng. J. 2018, 343, 597–606. [Google Scholar] [CrossRef]
- Premalatha, N.; Miranda, L.R. Surfactant modified ZnO–Bi2O3 nanocomposite for degradation of lambda- cyhalothrin pesticide in visible light: A study of reaction kinetics and intermediates. J. Environ. Manag. 2019, 246, 259–266. [Google Scholar] [CrossRef]
- Jonidi-Jafari, A.; Shirzad-Siboni, M.; Yang, J.K.; Naimi-Joubani, M.; Farrokhi, M. Photocatalytic degradation of diazinon with illuminated ZnO-TiO2 composite. J. Taiwan Inst. Chem. Eng. 2015, 50, 100–107. [Google Scholar] [CrossRef]




 ) 4 mg L−1, and (◆) 5 mg L−1) on the photocatalytic degradation of L-CHT using (a) CuO, (b) NiO photocatalyst (Photocatalyst dosage = 1–5 mg L−1, L-CHT = 20 mg L−1, pH = 7).
) 4 mg L−1, and (◆) 5 mg L−1) on the photocatalytic degradation of L-CHT using (a) CuO, (b) NiO photocatalyst (Photocatalyst dosage = 1–5 mg L−1, L-CHT = 20 mg L−1, pH = 7).
 ) 4 mg L−1, and (◆) 5 mg L−1) on the photocatalytic degradation of L-CHT using (a) CuO, (b) NiO photocatalyst (Photocatalyst dosage = 1–5 mg L−1, L-CHT = 20 mg L−1, pH = 7).
) 4 mg L−1, and (◆) 5 mg L−1) on the photocatalytic degradation of L-CHT using (a) CuO, (b) NiO photocatalyst (Photocatalyst dosage = 1–5 mg L−1, L-CHT = 20 mg L−1, pH = 7).
 ) 40 mg L−1, (◆) 50 mg L−1, (▶) 60 mg L−1, and (▲) 70 mg L−1 on the photocatalytic degradation of L-CHT using (a) CuO, (b) NiO photocatalyst (Photocatalyst dosage = 2 mg L−1, L-CHT = 10–70 mg L−1, pH = 7).
) 40 mg L−1, (◆) 50 mg L−1, (▶) 60 mg L−1, and (▲) 70 mg L−1 on the photocatalytic degradation of L-CHT using (a) CuO, (b) NiO photocatalyst (Photocatalyst dosage = 2 mg L−1, L-CHT = 10–70 mg L−1, pH = 7).
 ) 40 mg L−1, (◆) 50 mg L−1, (▶) 60 mg L−1, and (▲) 70 mg L−1 on the photocatalytic degradation of L-CHT using (a) CuO, (b) NiO photocatalyst (Photocatalyst dosage = 2 mg L−1, L-CHT = 10–70 mg L−1, pH = 7).
) 40 mg L−1, (◆) 50 mg L−1, (▶) 60 mg L−1, and (▲) 70 mg L−1 on the photocatalytic degradation of L-CHT using (a) CuO, (b) NiO photocatalyst (Photocatalyst dosage = 2 mg L−1, L-CHT = 10–70 mg L−1, pH = 7).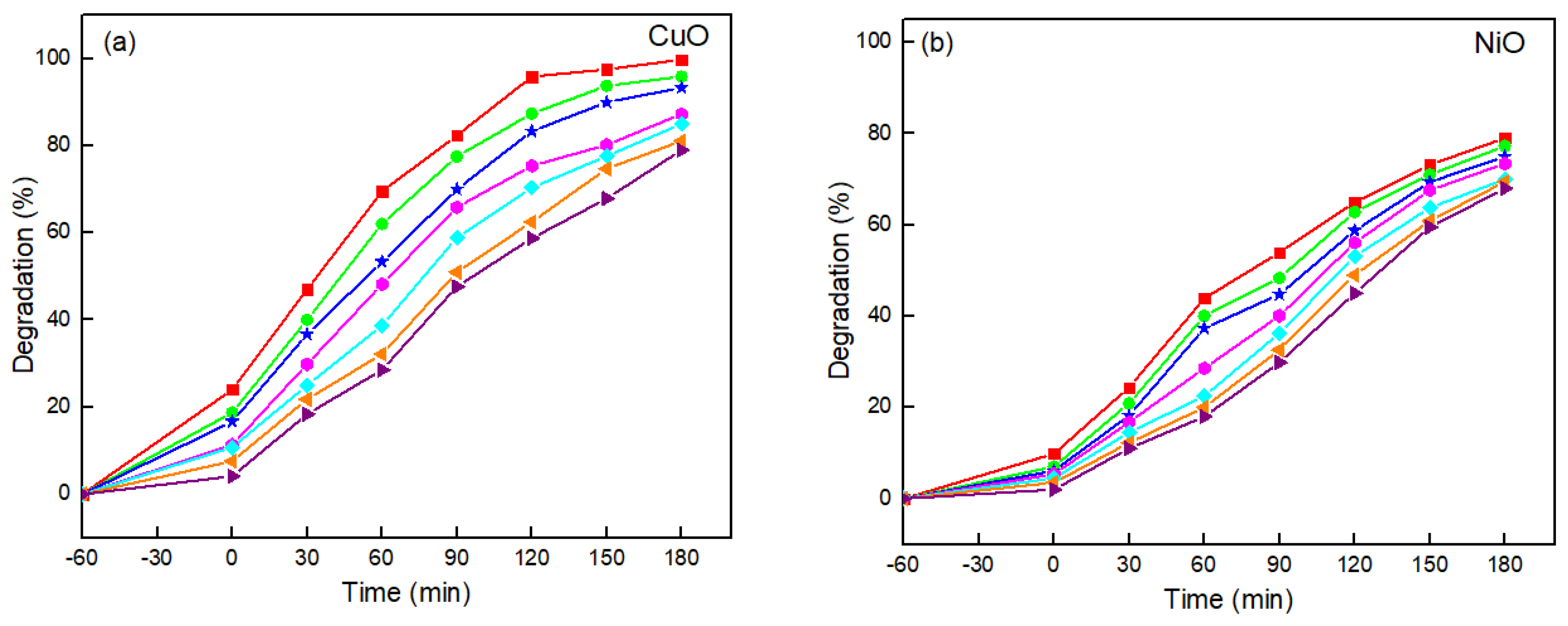


| Pesticide | CAS Number | Molecular Weight | Formula | Structure |
|---|---|---|---|---|
| L-CHT | 91465-08-6 | 449.9 g mol−1 | C23H19ClF3NO3 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iqbal, A.; Haq, A.u.; Cerrón-Calle, G.A.; Naqvi, S.A.R.; Westerhoff, P.; Garcia-Segura, S. Green Synthesis of Flower-Shaped Copper Oxide and Nickel Oxide Nanoparticles via Capparis decidua Leaf Extract for Synergic Adsorption-Photocatalytic Degradation of Pesticides. Catalysts 2021, 11, 806. https://doi.org/10.3390/catal11070806
Iqbal A, Haq Au, Cerrón-Calle GA, Naqvi SAR, Westerhoff P, Garcia-Segura S. Green Synthesis of Flower-Shaped Copper Oxide and Nickel Oxide Nanoparticles via Capparis decidua Leaf Extract for Synergic Adsorption-Photocatalytic Degradation of Pesticides. Catalysts. 2021; 11(7):806. https://doi.org/10.3390/catal11070806
Chicago/Turabian StyleIqbal, Amna, Atta ul Haq, Gabriel Antonio Cerrón-Calle, Syed Ali Raza Naqvi, Paul Westerhoff, and Sergi Garcia-Segura. 2021. "Green Synthesis of Flower-Shaped Copper Oxide and Nickel Oxide Nanoparticles via Capparis decidua Leaf Extract for Synergic Adsorption-Photocatalytic Degradation of Pesticides" Catalysts 11, no. 7: 806. https://doi.org/10.3390/catal11070806
APA StyleIqbal, A., Haq, A. u., Cerrón-Calle, G. A., Naqvi, S. A. R., Westerhoff, P., & Garcia-Segura, S. (2021). Green Synthesis of Flower-Shaped Copper Oxide and Nickel Oxide Nanoparticles via Capparis decidua Leaf Extract for Synergic Adsorption-Photocatalytic Degradation of Pesticides. Catalysts, 11(7), 806. https://doi.org/10.3390/catal11070806








