Insight into Platinum Poisoning Effect on Cu-SSZ-13 in Selective Catalytic Reduction of NOx with NH3
Abstract
:1. Introduction
2. Results and Discussion
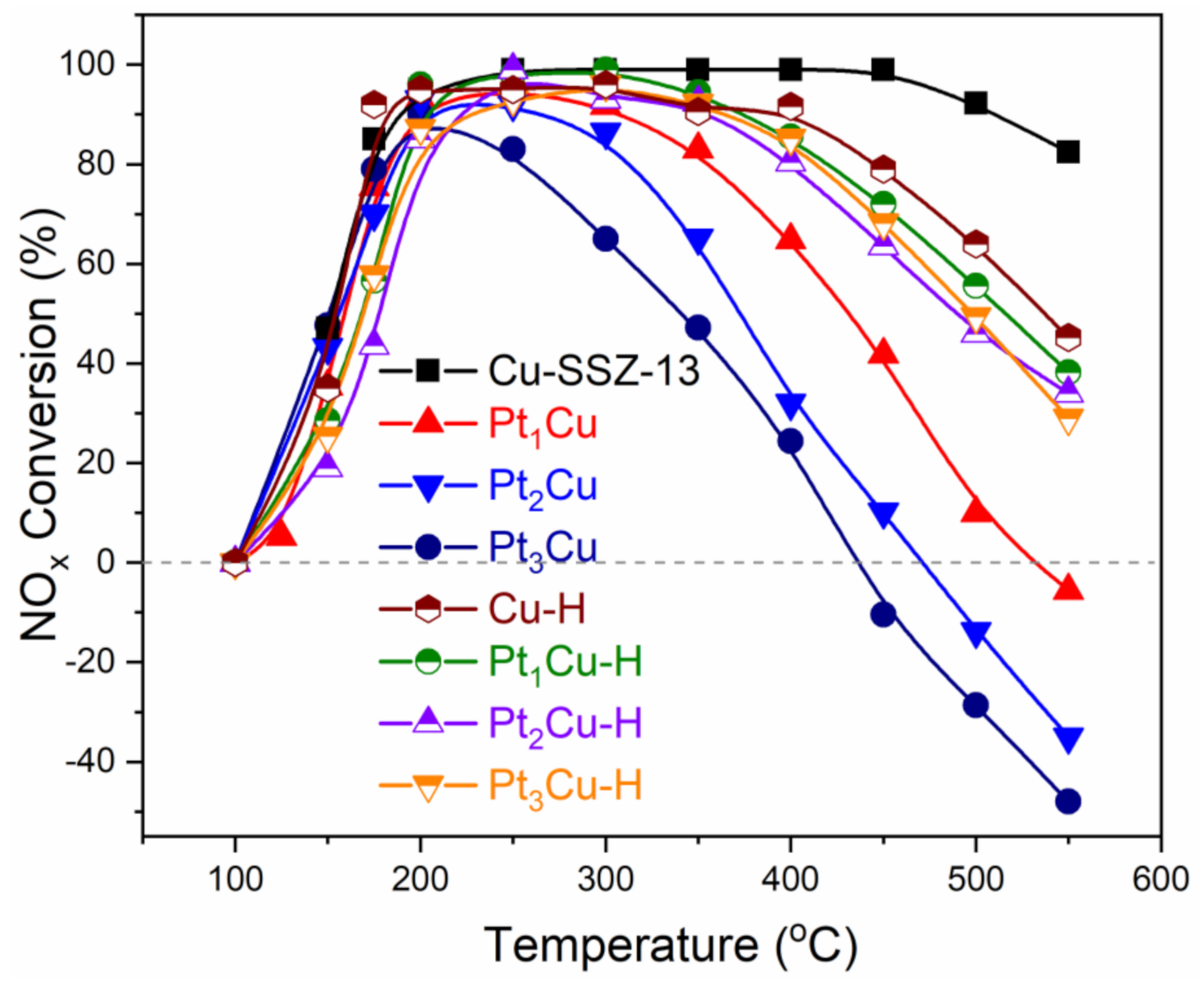
2.1. Influence of Pt Poisoning on the Catalytic Performance of Cu-SSZ-13
2.2. Transient Reactions
2.3. Pt Poisoning Effect on the Texture and Structure
2.4. Pt Poisoning Effect on the Catalytic Active Sites
2.4.1. H2-TPR
2.4.2. X-ray Photoelectron Spectroscopy
3. Experimental
3.1. Catalyst Preparation
3.2. Catalytic Performance Test
3.3. Transient Reaction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef]
- Nova, I.; Tronconi, E. Urea-SCR Technology for deNOx After Treatment of Diesel Exhausts; Springer: New York, NY, USA, 2014. [Google Scholar]
- Shen, M.; Zhang, Y.; Wang, J.; Wang, C.; Wang, J. Nature of SO3 poisoning on Cu/SAPO-34 SCR catalysts. Catalysts 2018, 358, 277–286. [Google Scholar] [CrossRef]
- Kumar, A.; Smith, M.A.; Kamasamudram, K.; Currier, N.W.; An, H.; Yezerets, A. Identification of two types of Cu sites in Cu/SSZ-13 and their unique responses to hydrothermal aging and sulfur poisoning. Catal. Today 2014, 231, 75–82. [Google Scholar] [CrossRef]
- Gao, F.; Szanyi, J. On the hydrothermal stability of Cu/SSZ-13 SCR catalysts. Appl. Catal. A Gen. 2018, 560, 185–194. [Google Scholar] [CrossRef]
- Lezcano-Gonzalez, I.; Deka, U.; van der Bij, H.E.; Paalanen, P.; Arstad, B.; Weckhuysen, B.M.; Beale, A.M. Chemical deactivation of Cu-SSZ-13 ammonia selective catalytic reduction (NH3-SCR) systems. Appl. Catal. B Environ. 2014, 339–349, 154–155. [Google Scholar] [CrossRef]
- Hammershøi, P.S.; Jangjou, Y.; Epling, W.S.; Jensen, A.D.; Janssens, T.V.W. Reversible and irreversible deactivation of Cu-CHA NH3 -SCR catalysts by SO2 and SO3. Appl. Catal. B Environ. 2018, 226, 38–45. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Wang, J.; Wang, J.; Yu, T.; Shen, M.; Wang, W.; Li, W. The effect of sulfate species on the activity of NH3–SCR over Cu/SAPO-34. Appl. Catal. B Environ. 2017, 204, 239–249. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.K.; Min, K.M.; Hong, S.B.; Nam, I.-S.; Cho, B.K. Hydrothermal stability of CuSSZ13 for reducing NOx by NH3. J. Catal. 2014, 311, 447–457. [Google Scholar] [CrossRef]
- Ma, J.; Si, Z.C.; Weng, D.; Wu, X.D.; Ma, Y. Potassium poisoning on Cu-SAPO-34 catalyst for selective catalytic reduction of NOx with ammonia. Chem. Eng. J. 2015, 267, 191–200. [Google Scholar] [CrossRef]
- Fan, C.; Chen, Z.; Pang, L.; Ming, S.; Dong, C.; Albert, K.B.; Liu, P.; Wang, J.; Zhu, D.; Chen, H.; et al. Steam and alkali resistant Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx in diesel exhaust. Chem. Eng. J. 2018, 334, 344–354. [Google Scholar] [CrossRef]
- Shwan, S.; Jansson, J.; Olsson, L.; Skoglundh, M. Chemical deactivation of Fe-BEA as NH3-SCR catalyst—Effect of phosphorous. Appl. Catal. B Environ. 2014, 147, 111–123. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, H.; Haller, G.; Li, Y. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-Chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Liu, M.; Li, X.; Ma, Y.; Yong, X.; Chen, H.; Li, Y. Phosphorus modification to improve the hydrothermal stability of a Cu-SSZ-13 catalyst for selective reduction of NOx with NH3. Appl. Catal. B Environ. 2019, 252, 230–239. [Google Scholar] [CrossRef]
- Gu, Y.; Marino, S.; Cortés-Reyes, M.; Pieta, I.S.; Pihl, J.A.; Epling, W.S. Integration of an Oxidation Catalyst with Pd/Zeolite-Based Passive NOx Adsorbers: Impacts on Degradation Resistance and Desorption Characteristics. Ind. Eng. Chem. Res. 2020, 60, 6455–6464. [Google Scholar] [CrossRef]
- Chen, X.; Currier, N.; Yezerets, A.; Kamasamudram, K. Axially Resolved Performance of Cu-Zeolite SCR Catalysts. SAE Int. J. Engines 2013, 6, 856–861. [Google Scholar] [CrossRef]
- Girard, J.W.; Montreuil, C.; Kim, J.; Cavataio, G.; Lambert, C. Origin and Effect of Ultra-Low Platinum Contamination on Diesel-SCR Catalysts. SAE Int. J. Fuels Lubr. 2008, 1, 1553–1559. [Google Scholar]
- Yu, T.; Xu, M.; Huang, Y.; Wang, J.; Wang, J.; Lv, L.; Qi, G.; Li, W.; Shen, M. Insight of platinum poisoning Cu/SAPO-34 during NH3-SCR and its promotion on catalysts regeneration after hydrothermal treatment. Appl. Catal. B Environ. 2017, 204, 525–536. [Google Scholar] [CrossRef]
- Cavataio, G.; Jen, H.-W.; Girard, J.W.; Dobson, D.; Warner, J.R.; Lambert, C.K. Laboratory Study of Soot, Propylene, and Diesel Fuel Impact on Zeolite-Based SCR Filter Catalysts. SAE Int. J. Fuels Lubr. 2009, 2, 204–216. [Google Scholar] [CrossRef]
- Lezcano-Gonzalez, I.; Wragg, D.S.; Slawinski, W.A.; Hemelsoet, K.; Deyne, A.; Waroquier, M.; van Speybroeck, V.; Beale, A.M. Determination of the Nature of the Cu Coordination Complexes Formed in the Presence of NO and NH3 within SSZ-13. J. Phys. Chem. C 2015, 119, 24393–24403. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Cui, H.; Dong, X.; Zhao, H.; Wang, Y.; Chen, H.; Yao, M.; Li, Y. N2O formation in the selective catalytic reduction of NOx with NH3ona CeMoOx catalyst. Appl. Catal. A Gen. 2015, 505, 8–15. [Google Scholar] [CrossRef]
- Dong, X.; Wang, J.; Zhao, H.; Li, Y. The promotion effect of CeOx on Cu-SAPO-34 catalyst for selective catalytic reduction of NOx with ammonia. Catal. Today 2015, 258, 28–34. [Google Scholar] [CrossRef]
- Zhao, H.; Li, H.; Li, X.; Liu, M.; Li, Y. The promotion effect of Fe to Cu-SAPO-34 for selective catalytic reduction of NOx with NH3. Catal. Today 2017, 297, 84–91. [Google Scholar] [CrossRef]
- Zhao, H.; Zhao, Y.; Ma, Y.; Yong, X.; Wei, M.; Chen, H.; Zhang, C.; Li, Y. Enhanced hydrothermal stability of a Cu-SSZ-13 catalyst for the selective reduction of NOx by NH3 synthesized with SAPO-34 micro-crystallite as seed. J. Catal. 2019, 377, 218–223. [Google Scholar] [CrossRef]
- Shan, Y.; Shi, X.; Yan, Z.; Liu, J.; Yu, Y.; He, H. Deactivation of Cu-SSZ-13 in the presence of SO2 during hydrothermal aging. Catal. Today 2019, 320, 84–90. [Google Scholar] [CrossRef]
- Shan, Y.; Du, J.; Yu, Y.; Shan, W.; Shi, X.; He, H. Precise control of post-treatment significantly increases hydrothermal stability of in-situ synthesized cu-zeolites for NH3-SCR reaction. Appl. Catal. B Environ. 2020, 266, 118655. [Google Scholar] [CrossRef]
- Shan, Y.; Sun, Y.; Du, J.; Zhang, Y.; Shi, X.; Yu, Y.; Shan, W.; He, H.H. Hydrothermal aging alleviates the inhibition effects of NO2 on Cu-SSZ-13 for NH3-SCR. Appl. Catal. B Environ. 2020, 275, 119105. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure–activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Kwak, J.H.; Zhu, H.; Lee, J.H.; Peden, C.H.F.; Szanyi, J. Two different cationic positions in Cu-SSZ-13? Chem. Commun. 2012, 48, 4758–4760. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Y.; Zhao, H.; Liu, M.; Ma, Y.; Yong, X.; Chen, H.; Li, Y. The Cu migration of Cu-SAPO-34 catalyst for ammonia selective catalytic reduction of NOx during high temperature hydrothermal aging treatment. Catal. Today 2019, 237, 126–133. [Google Scholar] [CrossRef]
- Cordatos, H.; Gorte, R.J. CO, NO, and H2 Adsorption on Ceria-Supported Pd. J. Catal. 1996, 159, 112–118. [Google Scholar] [CrossRef]
- Şen, F.; Gökağaç, G.; Şen, S. High performance Pt nanoparticles prepared by new surfactants for C1 to C3 alcohol oxidation reactions. J. Nanopart. Res. 2013, 15, 1979. [Google Scholar] [CrossRef]
- Borges, L.R.; Lopez-Castillo, A.; Meira, D.M.; Gallo, J.M.R.; Zanchet, D.; Bueno, J.M.C. Effect of the Pt Precursor and Loading on the Structural Parameters and Catalytic Properties of Pt/Al2O3. ChemCatChem 2019, 11, 3064–3074. [Google Scholar] [CrossRef]

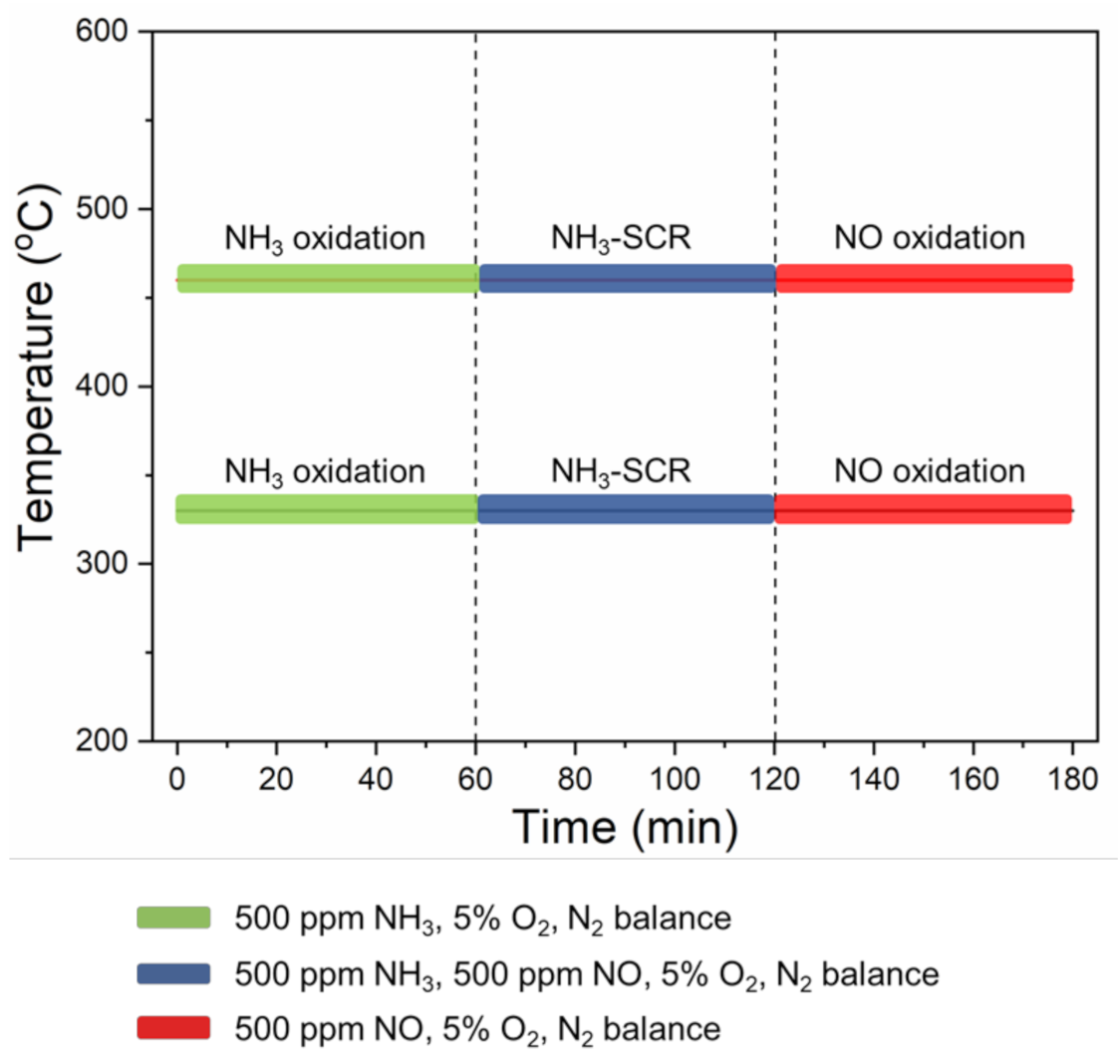


| Sample T (°C) | Reaction | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NH3 Oxidation | SCR Reaction | NO Oxidation | ||||||||||
| NO | NH3 | N2O | NO2 | NO | NH3 | N2O | NO2 | NO | N2O | NO2 | ||
| (ppm) | (ppm) | (ppm) | ||||||||||
| Cu-SSZ-13 | 330 | 0 | 59 | 4 | 0 | 0 | 0 | 4 | 0 | 485 | 1 | 5 |
| 460 | 0 | 0 | 1 | 0 | 0 | 0 | 5 | 0 | 479 | 1 | 13 | |
| Pt1Cu | 330 | 0 | 0 | 7 | 0 | 85 | 0 | 18 | 11 | 465 | 1 | 12 |
| 460 | 48 | 0 | 4 | 3 | 306 | 0 | 8 | 44 | 437 | 1 | 56 | |
| Pt2Cu | 330 | 0 | 0 | 12 | 0 | 152 | 0 | 39 | 14 | 435 | 2 | 23 |
| 460 | 98 | 0 | 4 | 23 | 356 | 0 | 8 | 92 | 397 | 1 | 81 | |
| Pt3Cu | 330 | 17 | 0 | 24 | 10 | 232 | 0 | 59 | 29 | 424 | 1 | 35 |
| 460 | 160 | 0 | 5 | 35 | 488 | 0 | 12 | 118 | 404 | 1 | 90 | |
| Sample | Cu Content a (wt. %) | Pt Content a wt. % | Surface Area b (m2/g) | Pore Volume b (cm3/g) |
|---|---|---|---|---|
| Cu-SSZ-13 | 4.11 | NDe | 498 | 0.21 |
| Pt1Cu | 4.13 | 0.009 | 412 | 0.2 |
| Pt2Cu | 4.08 | 0.051 | 390 | 0.19 |
| Pt3Cu | 4.14 | 0.1 | 382 | 0.19 |
| Cu-H | 4.06 | ND | 406 | 0.17 |
| Pt1Cu-H | 4.10 | 0.01 | 355 | 0.17 |
| Pt2Cu-H | 4.10 | 0.048 | 342 | 0.16 |
| Pt3Cu-H | 4.09 | 0.1 | 335 | 0.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Han, L.; Wang, Y.; Zheng, J. Insight into Platinum Poisoning Effect on Cu-SSZ-13 in Selective Catalytic Reduction of NOx with NH3. Catalysts 2021, 11, 796. https://doi.org/10.3390/catal11070796
Zhao H, Han L, Wang Y, Zheng J. Insight into Platinum Poisoning Effect on Cu-SSZ-13 in Selective Catalytic Reduction of NOx with NH3. Catalysts. 2021; 11(7):796. https://doi.org/10.3390/catal11070796
Chicago/Turabian StyleZhao, Huawang, Lei Han, Yujie Wang, and Jiandong Zheng. 2021. "Insight into Platinum Poisoning Effect on Cu-SSZ-13 in Selective Catalytic Reduction of NOx with NH3" Catalysts 11, no. 7: 796. https://doi.org/10.3390/catal11070796
APA StyleZhao, H., Han, L., Wang, Y., & Zheng, J. (2021). Insight into Platinum Poisoning Effect on Cu-SSZ-13 in Selective Catalytic Reduction of NOx with NH3. Catalysts, 11(7), 796. https://doi.org/10.3390/catal11070796





