In Vitro Digestibility and Antioxidant Activity of Plant Protein Isolate and Milk Protein Concentrate Blends
Abstract
:1. Introduction
2. Results and Discussion
2.1. Reverse-Phase Ultra-Performance Liquid Chromatography (RP-UPLC)
2.2. Estimation of PDCASS
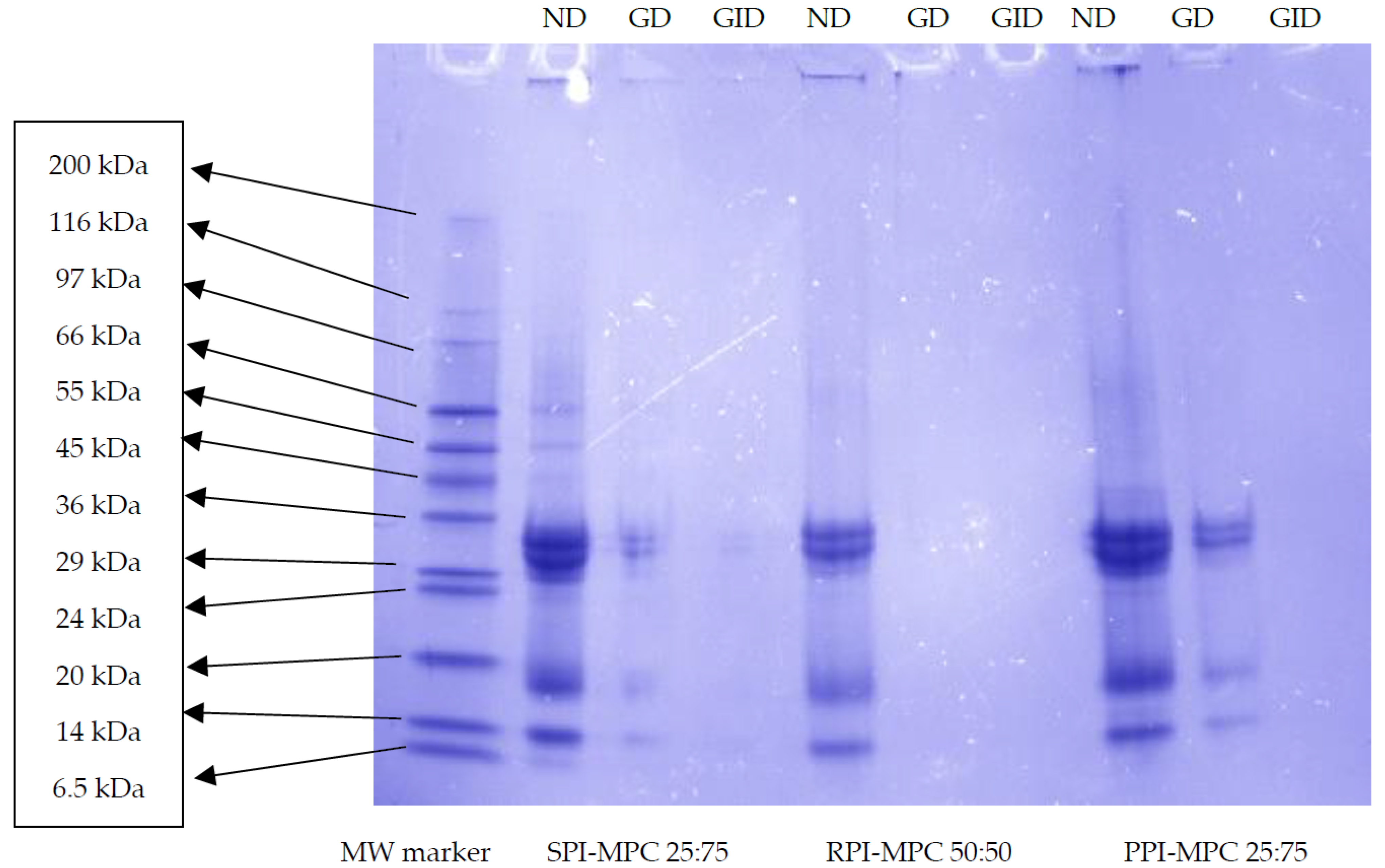
2.3. SDS-PAGE Analysis of Simulated Gastro-Intestinal Digestion (SGID)-Treated Blends
2.4. Degree of Hydrolysis (DH)
2.5. Molecular Mass Distribution
2.6. ABTS Radical (ABTS●) Scavenging Activity
3. Materials and Methods
3.1. Materials
3.2. Blending of Plant Protein Samples with MPC
3.3. Reverse-Phase Ultra-Performance Liquid Chromatography (RP-UPLC)
3.4. Protein Digestibility Corrected Amino Acid Score (PDCAAS)
3.5. Simulated Gastro-Intestinal Digestion (SGID) of the Plant Isolates and Their Associated Blends
3.6. SDS-PAGE Analysis
3.7. Degree of Hydrolysis (DH)
3.8. Gel Permeation–High Performance Liquid Chromatography (GP-HPLC)
3.9. ABTS Radical (ABTS•) Scavenging Assay
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Abbreviation | Description |
| ABTS | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| α-la | α-lactalbumin |
| β-lg | β-lactoglobulin |
| ACN | acetonitrile |
| AA | amino acid |
| ANF | antinutritional factor |
| BCAA | branched chain amino acid |
| DH | degree of hydrolysis |
| DIAS | digestible indispensable amino acid score |
| EAA | essential amino acid |
| GP-HPLC | gel permeation - high performance liquid chromatography |
| EC50 | half maximal effective concentration |
| MPC | milk protein concentrate |
| MW | molecular weight |
| NEAA | non-essential amino acid |
| PPC | pea protein concentrate |
| PPI | pea protein isolate |
| PBS | phosphate-buffered saline |
| PAGE | polyacrylamide gel electrophoresis |
| PDCAAS | protein digestibility corrected amino acid score |
| RP-UPLC | reverse-phase ultra-performance liquid chromatography |
| RPC | rice protein concentrate |
| RPI | rice protein isolate |
| SGID | simulated gastro-intestinal digestion |
| SDS | sodium dodecyl sulfate |
| SD | standard deviation |
| SPI | soy protein isolate |
| TCA | trichloroacetic acid |
| TFA | trifluoroacetic acid |
| TNBS | trinitrobenzenesulfonic acid |
| WP | whey protein |
References
- Anderson, C.A.M.; Bradley, R. The potential of novel plant protein foods to improve dietary patterns and markers of cardiovascular health. Am. J. Clin. Nutr. 2020, 112, 1151–1152. [Google Scholar] [CrossRef] [PubMed]
- Loveday, S.M. Plant protein ingredients with food functionality potential. Nutr. Bull. 2020, 45, 321–327. [Google Scholar] [CrossRef]
- Foegeding, E.A.; Davis, J.P. Food protein functionality: A comprehensive approach. Food Hydrocolloid 2011, 25, 1853–1864. [Google Scholar] [CrossRef]
- Boye, J.; Zare, F.; Pletch, A. Pulse proteins: Processing, characterization, functional properties and applications in food and feed. Food Res. Int. 2010, 43, 414–431. [Google Scholar] [CrossRef]
- Alves, A.C.; Tavares, G.M. Mixing animal and plant proteins: Is this a way to improve protein techno-functionalities? Food Hydrocolloid 2019, 97, 105171. [Google Scholar] [CrossRef]
- Silva, J.V.C.; Jacquette, B.; Amagliani, L.; Schmitt, C.; Nicolai, T.; Chassenieux, C. Heat-induced gelation of micellar casein/plant protein oil-in-water emulsions. Colloids Surf. A 2019, 569, 85–92. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Fry, C.S.; Borack, M.S.; Cope, M.B.; Mukherjea, R.; et al. Proteinblend ingestion following resistance exercise promoteshuman muscle protein synthesis. J. Nutr. 2013, 143, 410–416. [Google Scholar] [CrossRef]
- Reidy, P.T.; Walker, D.K.; Dickinson, J.M.; Gundermann, D.M.; Drummond, M.J.; Timmerman, K.L.; Cope, M.B.; Mukherjea, R.; Jennings, K.; Volpi, E.; et al. Soy-dairy protein blend and whey protein ingestion after resistance exercise increases amino acid transport and transporter expression in human skeletal muscle. J. Appl. Physiol. 2014, 116, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Butteiger, D.N.; Cope, M.; Liua, P.; Mukherjea, R.; Volpi, E.; Rasmussen, B.B.; Krul, E.S. A soy, whey and caseinate blend extends postprandial skeletal muscle protein synthesis in rats. Clin. Nutr. 2013, 32, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Klebach, M.; Visser, M.; Hofman, Z. Amino acid availability of a dairy and vegetable protein blend compared to single casein, whey, soy, and pea proteins: A double-blind, cross-over trial. Nutrients 2019, 11, 2613. [Google Scholar] [CrossRef] [Green Version]
- Khalesi, M.; FitzGerald, R.J. Insolubility in milk protein concentrates: Potential causes and strategies to minimize its occurrence. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Mathai, J.K.; Liu, Y.; Stein, H.H. Values for digestible indispensable amino acid scores (DIAAS) for some dairy and plant proteins may better describe protein quality than values calculated using the concept for protein digestibility-corrected amino acid scores (PDCAAS). Br. J. Nutr. 2017, 117, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Rutherfurd, S.M.; Fanning, A.C.; Miller, B.J.; Moughan, P.J. Protein digestibility-corrected amino acid scores and digestible indispensable amino acid scores differentially describe protein quality in growing male rats. J. Nutr. 2015, 145, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Gorissen, S.H.M.; Witard, O.C. Characterising the muscle anabolic potential of dairy, meat and plant-based protein sources in older adults. Proc. Nutr. Soc. 2018, 77, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Sarwar, G. The protein digestibility–corrected amino acid score method overestimates quality of proteins containing antinutritional factors and of poorly digestible proteins supplemented with limiting amino acids in rats. J. Nutr. 1997, 127, 758–764. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; Zhu, B.; Liu, Y.; Xiong, Y. Interfacial structural role of pH-shifting processed pea protein in the oxidative stability of oil/water emulsions. J. Agric. Food Chem. 2014, 62, 1683–1691. [Google Scholar] [CrossRef]
- Dickinson, E. Milk protein interfacial layers and the relationship to emulsion stability and rheology. Colloids Surf. B 2001, 20, 197–210. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Y.; Li, L.; Qi, B.; Ju, M.; Xu, Y.; Zhang, Y.; Sui, X. Covalent conjugates of anthocyanins to soy protein: Unravelling their structure features and in vitro gastrointestinal digestion fate. Food Res. Int. 2019, 120, 603–609. [Google Scholar] [CrossRef]
- Stojadinovic, M.; Radosavljevic, J.; Ognjenovic, J.; Vesic, J.; Prodic, I.; Stanic-Vucinic, D.; Velickovic, T.C. Binding affinity between dietary polyphenols and β-lactoglobulin negatively correlates with the protein susceptibility to digestion and total antioxidant activity of complexes formed. Food Chem. 2013, 136, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Gilani, G.S.; Xiao, C.W.; Cockell, K.A. Impact of antinutritional factors in food proteins on the digestibility of protein and the bioavailability of amino acids and on protein quality. Br. J. Nutr. 2012, 108, 315–332. [Google Scholar] [CrossRef]
- Paul, G.L. The rationale for consuming protein blends in sports nutrition. J. Am. Coll. Nutr. 2009, 28, 464–472. [Google Scholar] [CrossRef]
- Nongonierma, A.B.; FitzGerald, R.J. Investigation of the potential of hemp, pea, rice and soy protein hydrolysates as a source of dipeptidyl peptidase IV (DPP-IV) inhibitory peptides. Food Digest. Res. Curr. Opin. 2015, 6, 19–29. [Google Scholar] [CrossRef]
- Xu, Z.; Mao, T.M.; Huang, L.; Yu, Z.C.; Yin, B.; Chen, M.L.; Cheng, Y.H. Purification and identification immunomodulatory peptide from rice bran protein hydrolysates. Food Agric. Immunol. 2019, 30, 150–162. [Google Scholar] [CrossRef] [Green Version]
- Nwachukwu, I.D.; Aluko, R.E. Structural and functional properties of food protein-derived antioxidant peptides. J. Food Biochem. 2019, 43, e12761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, H.; Baik, B.-K. Antioxidant activity and phenolic content of lentils (Lens culinaris), chickpeas (Cicer arietinum L.), peas (Pisum sativum L.) and soybeans (Glycine max), and their quantitative changes during processing. Int. J. Food Sci. Technol. 2008, 43, 1971–1978. [Google Scholar] [CrossRef]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef]
- Peñta-Ramos, E.A.; Xiong, Y.L. Antioxidant activity of soy protein hydrolysates in a liposomal system. J. Food Sci. 2002, 67, 2952–2956. [Google Scholar] [CrossRef]
- Cervato, G.; Cazzola, R.; Cestaro, B. Studies on the antioxidant activity of milk caseins. Int. J. Food Sci. Nutr. 1999, 50, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Gad, A.S.; Khadrawy, Y.A.; El-Nekeety, A.A.; Mohamed, S.R.; Hassan, N.S.; Abdel-Wahhab, M.A. Antioxidant activity and hepatoprotective effects of whey protein and Spirulina in rats. Nutrition 2011, 27, 582–589. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, M. Physical and chemical modification of SPI as a potential means to enhance small peptide contents and antioxidant activity found in hydrolysates. Innov. Food Sci. Emerg. 2010, 11, 677–683. [Google Scholar] [CrossRef]
- Beermann, C.; Euler, M.; Herzberg, J.; Stahl, B. Anti-oxidative capacity of enzymatically released peptides from soybean protein isolate. Eur. Food Res. Technol. 2009, 229, 637–644. [Google Scholar] [CrossRef]
- Guan, H.; Diao, X.; Jiang, F.; Han, J.; Kong, B. The enzymatic hydrolysis of soy protein isolate by Corolase PP under high hydrostatic pressure and its effect on bioactivity and characteristics of hydrolysates. Food Chem. 2018, 245, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Ciulu, M.; Cádiz-Gurrea, M.L.; Segura-Carretero, A. Extraction and analysis of phenolic compounds in rice: A review. Molecules 2018, 23, 2890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fathi, P.; Moosavi-Nasab, M.; Mirzapour-Kouhdasht, A.; Khalesi, M. Generation of hydrolysates from rice bran proteins using a combined ultrasonication-Alcalase hydrolysis treatment. Food Biosci. 2021. [Google Scholar] [CrossRef]
- Pownall, T.L.; Udenigwe, C.C.; Aluko, R.E. Amino acid composition and antioxidant properties of pea seed (Pisum sativum L.) enzymatic protein hydrolysate fractions. J. Agric. Food Chem. 2010, 58, 4712–4718. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Liang, R.; Yang, Y.; Sun, N.; Lina, S. Optimization of pea protein hydrolysate preparation and purification of antioxidant peptides based on an in silico analytical approach. LWT Food Sci. Technol. 2020, 123, 109126. [Google Scholar] [CrossRef]
- Awad, S.; El-Sayed, M.; Wahba, A.; El Attar, A.; Yousef, M.; Zedan, M. Antioxidant activity of milk protein hydrolysate in alloxan-induced diabetic rats. J. Dairy Sci. 2016, 99, 8499–8510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cermeño, M.; Connolly, A.; O’Keeffe, M.B.; Flynn, C.; Alashi, A.M.; Aluko, R.E.; FitzGerald, R.J. Identification of bioactive peptides from brewers’ spent grain and contribution of Leu/Ile to bioactive potency. J. Funct. Foods 2019, 60, 103455–103463. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO). Protein Quality Evaluation: Report of the Joint FAO/WHO Expert Consultation; Food and Agriculture Organization of the United Nations: Auckland, New Zealand, 2011. [Google Scholar]
- Walsh, D.J.; Bernard, H.; Murray, B.A.; MacDonald, J.; Pentzien, A.K.; Wright, G.A.; Wal, J.M.; Struthers, A.D.; Meisel, H.; FitzGerald, R.J. In vitro generation and stability of the lactokinin β-Lactoglobulin fragment (142–148). J. Dairy Sci. 2004, 87, 3845–3857. [Google Scholar] [CrossRef] [Green Version]
- Le Maux, S.; Nongonierma, A.B.; Barre, C.; FitzGerald, R.J. Enzymatic generation of whey protein hydrolysates under pH-controlled and non pH-controlled conditions: Impact on physicochemical and bioactive properties. Food Chem. 2016, 199, 246–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spellman, D.; O’Cuinn, G.; FitzGerald, R.J. Physicochemical and sensory characteristics of whey protein hydrolysates generated at different total solids levels. J. Dairy Res. 2005, 72, 138–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amigo-Benavent, M.; Khalesi, M.; Thapa, G.; FitzGerald, R.J. Methodologies for bioactivity assay: Biochemical study. In Biologically Active Peptides; Elsevier: Amsterdam, The Netherlands, 2021; pp. 103–153. [Google Scholar] [CrossRef]


| Amino Acid (g/100 g Protein) | MPC | SPI | PPI | RPI | SPI–MPC 25:75 (Protein Basis) | PPI–MPC 25:75 (Protein Basis) | RPI–MPC 50:50 (Protein Basis) |
|---|---|---|---|---|---|---|---|
| L-Cysteine | 0.54 | 1.30 | 0.80 | 1.50 | 0.73 | 0.60 | 1.02 |
| L-Methionine | 2.02 | 1.30 | 0.90 | 2.30 | 1.84 | 1.74 | 2.16 |
| L-Tryptophan | 1.08 | 0.90 | 0.80 | 0.70 | 1.03 | 1.01 | 0.89 |
| L-Aspartic acid + L-Asparagine | 6.28 | 10.90 | 9.20 | 7.30 | 7.43 | 7.01 | 6.79 |
| L-Threonine | 3.72 | 3.20 | 3.10 | 3.10 | 3.59 | 3.56 | 3.41 |
| L-Serine | 4.89 | 5.10 | 4.20 | 4.40 | 4.94 | 4.72 | 4.65 |
| L-Glutamic acid + L-Glutamine | 17.10 | 18.40 | 13.40 | 15.50 | 17.42 | 16.17 | 16.30 |
| L-Proline | 8.20 | 4.80 | 3.60 | 3.20 | 7.35 | 7.05 | 5.70 |
| L-Glycine | 1.50 | 3.80 | 3.30 | 3.50 | 2.07 | 1.95 | 2.50 |
| L-Alanine | 2.65 | 3.80 | 3.40 | 4.70 | 2.94 | 2.84 | 3.68 |
| L-Valine | 5.33 | 4.10 | 4.00 | 4.60 | 5.02 | 5.00 | 4.97 |
| L-Isoleucine | 4.27 | 3.90 | 3.60 | 4.10 | 4.18 | 4.10 | 4.19 |
| L-Leucine | 7.66 | 7.00 | 6.70 | 8.50 | 7.50 | 7.42 | 8.08 |
| L-Tyrosine | 4.27 | 3.70 | 3.00 | 4.40 | 4.13 | 3.95 | 4.34 |
| L-Phenylalanine | 4.09 | 4.90 | 4.40 | 5.10 | 4.29 | 4.17 | 4.60 |
| L-Lysine | 7.01 | 5.90 | 5.80 | 3.00 | 6.73 | 6.71 | 5.01 |
| L-Histidine | 1.93 | 2.40 | 2.00 | 1.90 | 2.05 | 1.95 | 1.92 |
| L-Arginine | 2.77 | 7.20 | 7.00 | 7.10 | 3.88 | 3.83 | 4.94 |
| Ratio of BCAAs (%) | 20.03 | 16.20 | 18.06 | 20.26 | 19.17 | 19.72 | 20.15 |
| First limiting EAA | Trp | Trp | Cys + Met | Lys | Trp | Trp | Trp |
| Ratio of NEAAs (%) | 43.01 | 49.35 | 46.09 | 46.88 | 44.69 | 43.74 | 44.94 |
| In vitro digestibility (%) | 94 | 106 | 108 | 107 | 98 | 102 | 102 |
| PDCAAS | 1.09 | 1.08 | 1.03 | 0.70 | 1.13 | 1.32 | 1.03 |
| Sample | DH (%), Gastric Digestion | DH (%), Gastric Followed by Intestinal Digestion | EC50 (mg/mL), Undigested | EC50 (mg/mL), Gastric Digestion | EC50 (mg/mL), Gastric Followed by Intestinal Digestion |
|---|---|---|---|---|---|
| SPI | 2.44 ± 0.32 a,* | 8.64 ± 0.41 a | 3.05 ± 0.23 a | 0.31 ± 0.03 b | 0.19 ± 0.01 b |
| RPI | 2.15 ± 0.03 a | 7.58 ± 0.96 a | 6.42 ± 0.35 d | 2.27 ± 0.24 e | 1.89 ± 0.27 d |
| PPI | 3.95 ± 0.24 b | 8.90 ± 0.60 a | 5.21 ± 0.20 c | 0.94 ± 0.07 d | 0.49 ± 0.03 c |
| SPI–MPC (25:75) | 6.58 ± 0.12 d | 12.65 ± 0.40 b | 3.25 ± 0.21 a | 0.11 ± 0.01 a | 0.10 ± 0.01 a |
| RPI–MPC (50:50) | 4.41 ± 0.24 b,c | 16.99 ± 0.62 d | 4.12 ± 0.28 c | 2.15 ± 0.26 e | 1.04 ± 0.15 e |
| PPI–MPC (25:75) | 4.66 ± 0.25 c | 15.08 ± 0.37 c | 3.76 ± 0.19 b | 0.76 ± 0.01 c | 0.26 ± 0.05 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalesi, M.; FitzGerald, R.J. In Vitro Digestibility and Antioxidant Activity of Plant Protein Isolate and Milk Protein Concentrate Blends. Catalysts 2021, 11, 787. https://doi.org/10.3390/catal11070787
Khalesi M, FitzGerald RJ. In Vitro Digestibility and Antioxidant Activity of Plant Protein Isolate and Milk Protein Concentrate Blends. Catalysts. 2021; 11(7):787. https://doi.org/10.3390/catal11070787
Chicago/Turabian StyleKhalesi, Mohammadreza, and Richard J. FitzGerald. 2021. "In Vitro Digestibility and Antioxidant Activity of Plant Protein Isolate and Milk Protein Concentrate Blends" Catalysts 11, no. 7: 787. https://doi.org/10.3390/catal11070787
APA StyleKhalesi, M., & FitzGerald, R. J. (2021). In Vitro Digestibility and Antioxidant Activity of Plant Protein Isolate and Milk Protein Concentrate Blends. Catalysts, 11(7), 787. https://doi.org/10.3390/catal11070787







