TiO2 Inverse Opals Modified by Ag Nanoparticles: A Synergic Effect of Enhanced Visible-Light Absorption and Efficient Charge Separation for Visible-Light Photocatalysis
Abstract
:1. Introduction
2. Results and Discussion
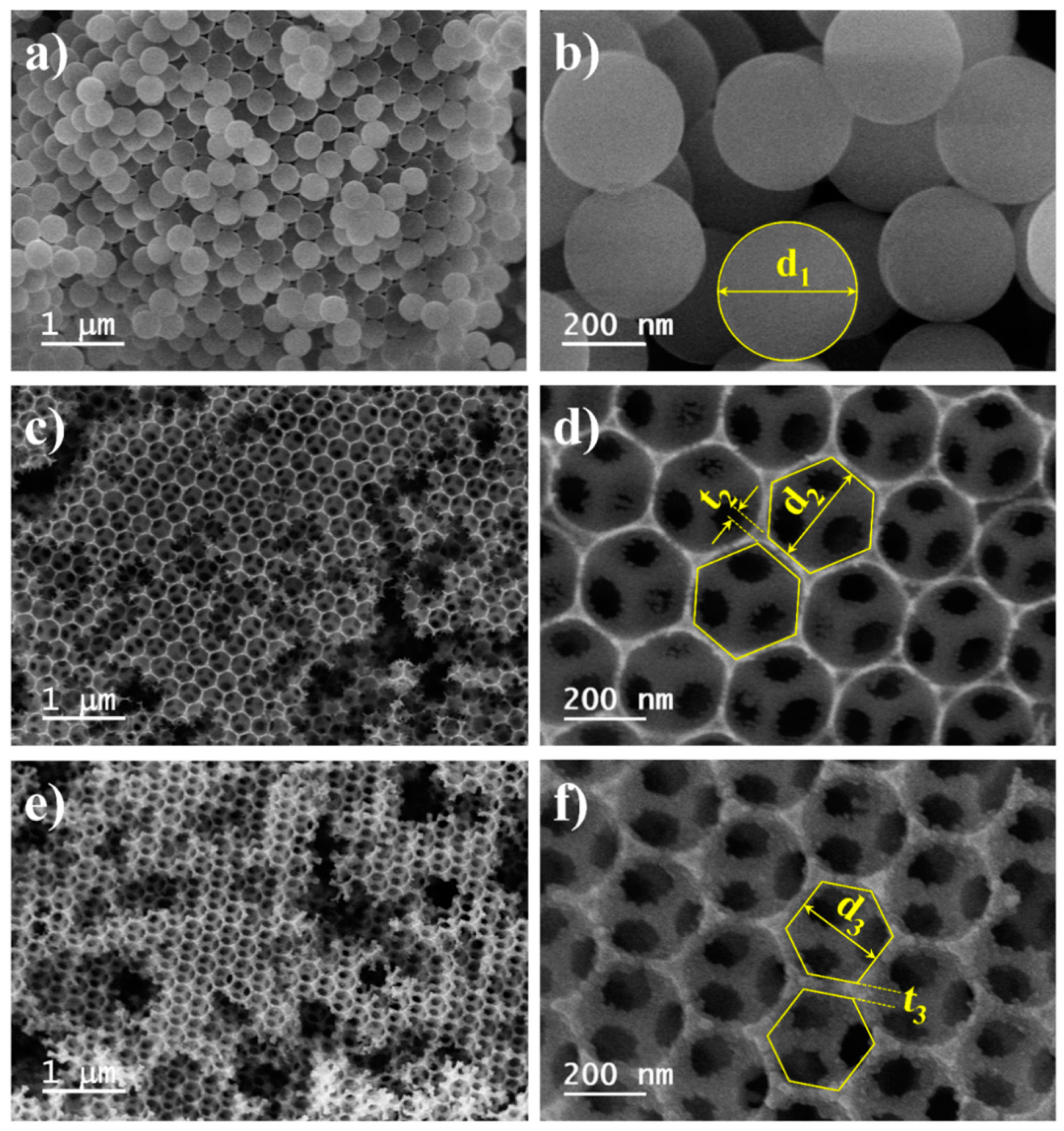
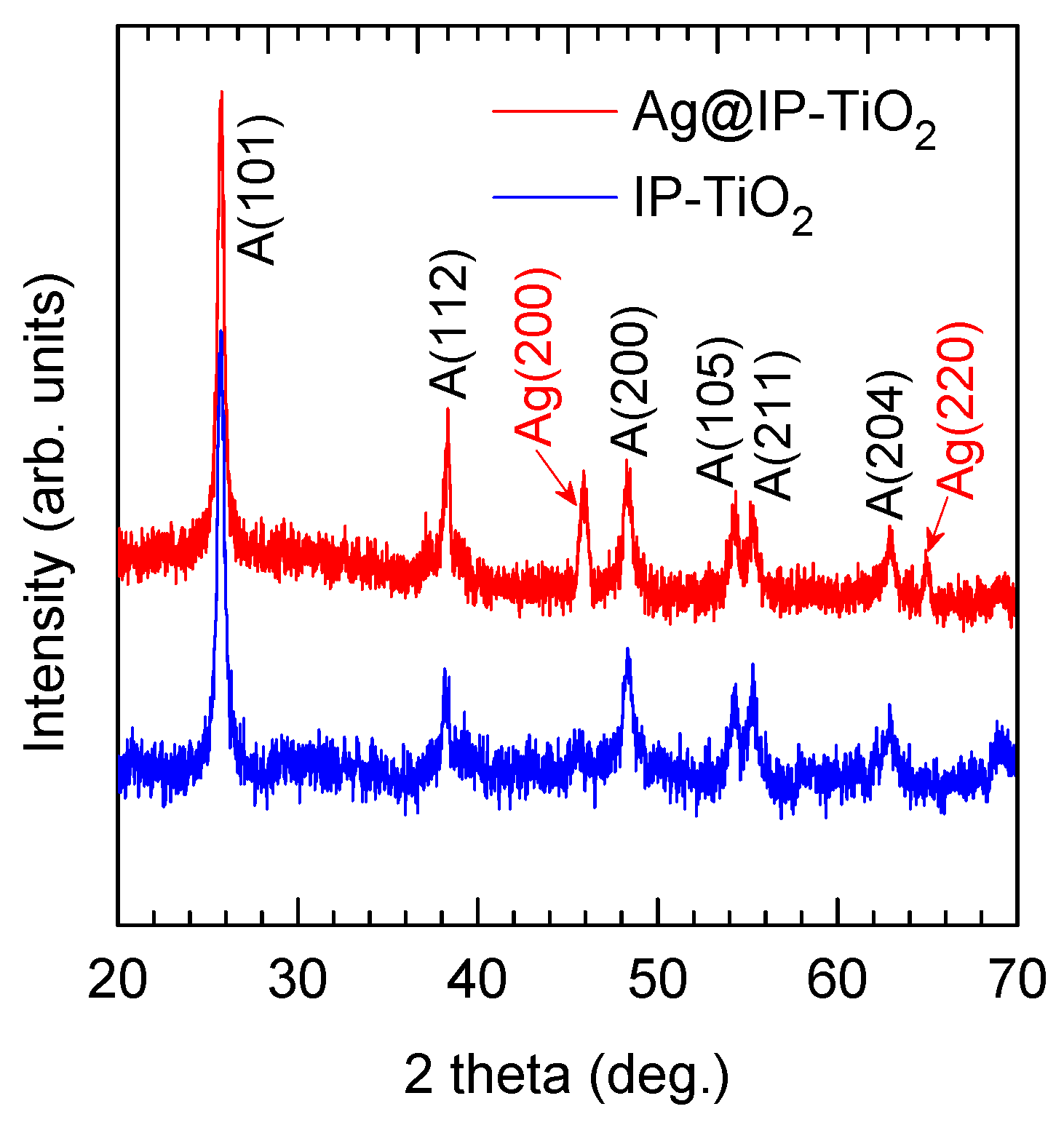
2.1. Morphology and Crystalline Structure of IP-TiO2 and Ag@IP-TiO2
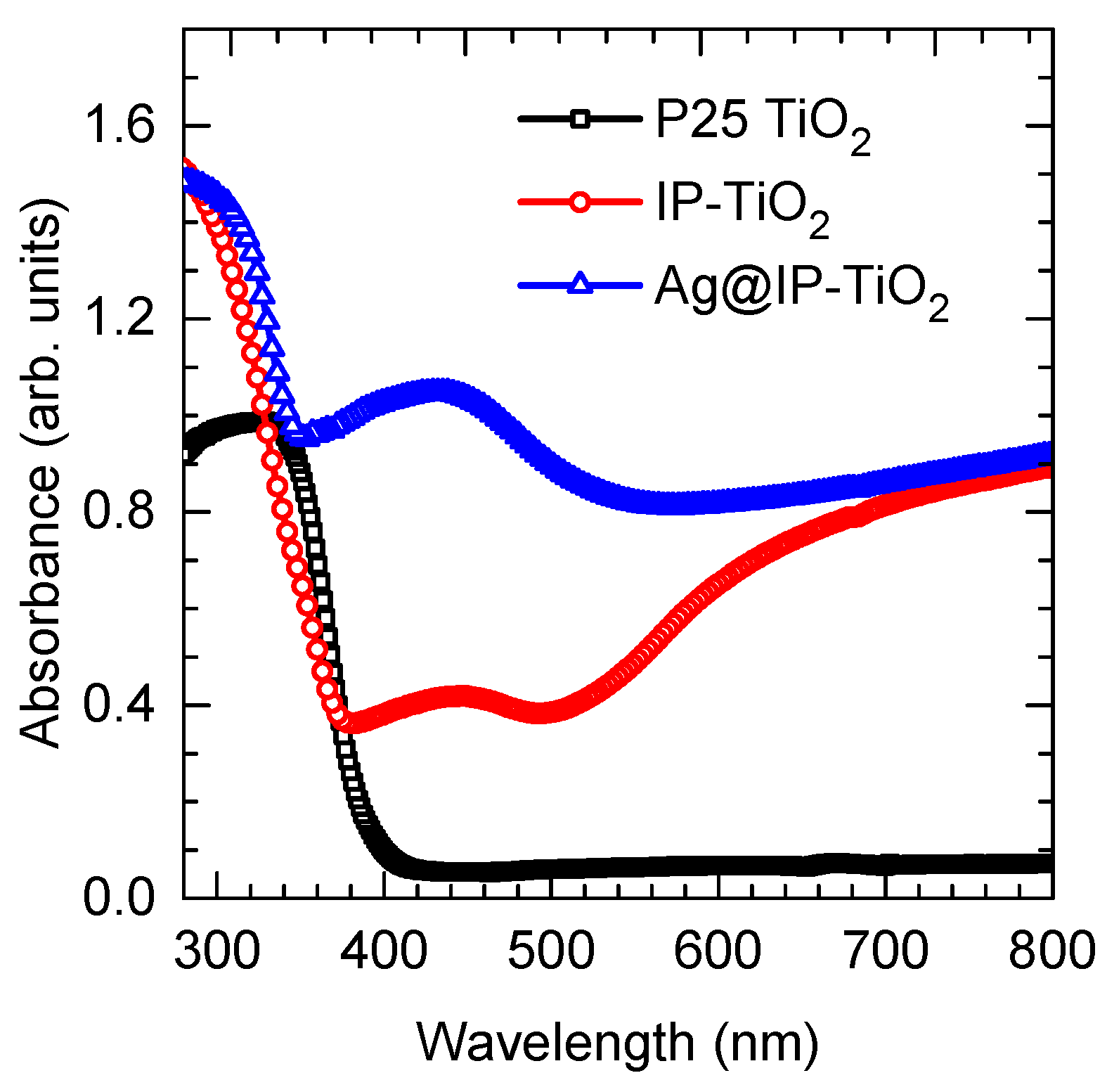
2.2. Optical Properties of IP-TiO2 and Ag@IP-TiO2
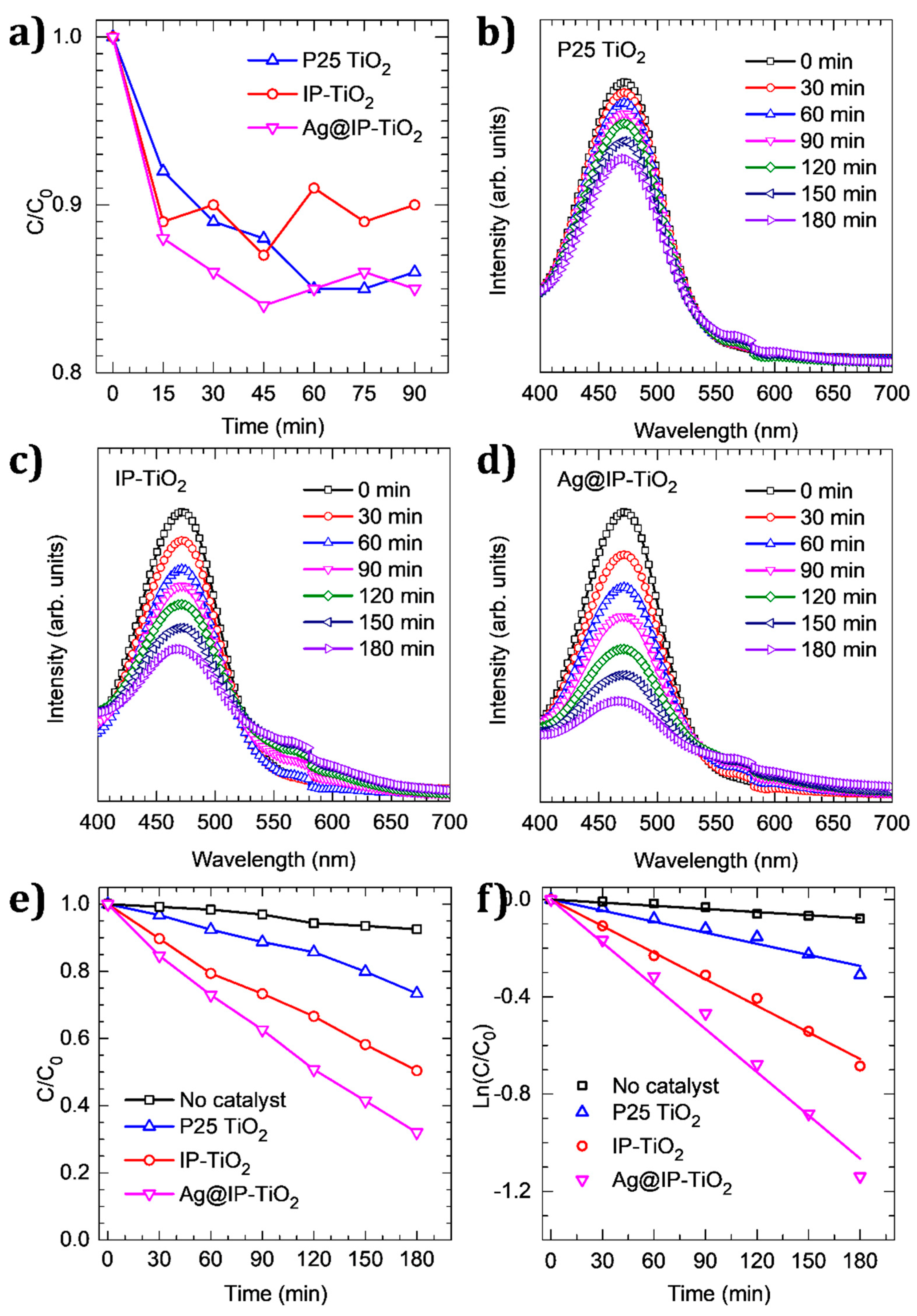
2.3. Photocatalytic Activity of IP-TiO2 and Ag@IP-TiO2
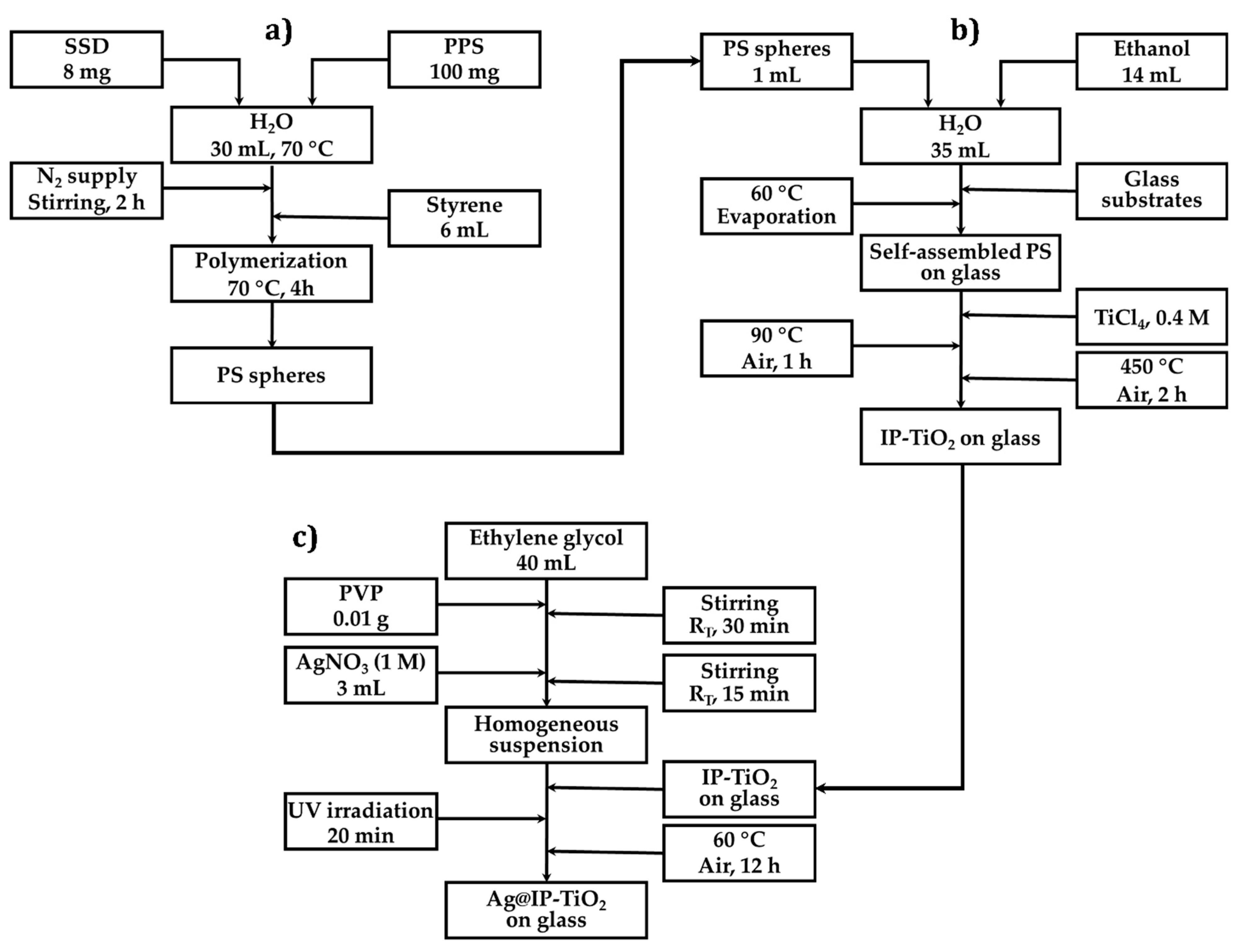
3. Materials and Methods
3.1. Materials
3.2. Methods
3.2.1. Synthesis of Polystyrene Spheres
3.2.2. Synthesis of IP-TiO2
3.2.3. Synthesis of Ag@IP-TiO2
3.2.4. Material Characterizations
3.2.5. Photocatalytic Study
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gothwal, R.; Shashidhar, T. Antibiotic Pollution in the Environment: A Review. Clean Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Kraemer, S.A.; Ramachandran, A.; Perron, G.G. Antibiotic pollution in the environment: From microbial ecology to public policy. Microorganisms 2019, 7, 180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Zhang, T. Biodegradation and adsorption of antibiotics in the activated sludge process. Environ. Sci. Technol. 2010, 44, 3468–3473. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yu, J.; Xiang, Q.; Cheng, B. Enhanced photocatalytic activity of hierarchical macro/mesoporous TiO2–graphene composites for photodegradation of acetone in air. Appl. Catal. B Environ. 2012, 119–120, 109–116. [Google Scholar] [CrossRef]
- Schneider, J.; Matsuoka, M.; Takeuchi, M.; Zhang, J.; Horiuchi, Y.; Anpo, M.; Bahnemann, D.W. Understanding TiO2 Photocatalysis: Mechanisms and Materials. Chem. Rev. 2014, 114, 9919–9986. [Google Scholar] [CrossRef]
- Bhanvase, B.A.; Shende, T.P.; Sonawane, S.H. A review on graphene–TiO2 and doped graphene–TiO2 nanocomposite photocatalyst for water and wastewater treatment. Environ. Technol. Rev. 2017, 6, 1–14. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, H.; Wang, W.; Zhang, L. TiO2/graphene porous composite and its photocatalytic degradation of methylene blue. JMADE 2016, 108, 632–639. [Google Scholar] [CrossRef]
- Senthil, R.A.; Theerthagiri, J.; Selvi, A.; Madhavan, J. Synthesis and characterization of low-cost g-C3N4/TiO2 composite with enhanced photocatalytic performance under visible-light irradiation. Opt. Mater. 2017, 64, 533–539. [Google Scholar] [CrossRef]
- Devi, L.G.; Kavitha, R. A review on non metal ion doped titania for the photocatalytic degradation of organic pollutants under UV/solar light: Role of photogenerated charge carrier dynamics in enhancing the activity. Appl. Catal. B Environ. 2013, 140–141, 559–587. [Google Scholar] [CrossRef]
- Park, H.; Park, Y.; Kim, W.; Choi, W. Surface modification of TiO2 photocatalyst for environmental applications. J. Photochem. Photobiol. C Photochem. Rev. 2013, 15, 1–20. [Google Scholar] [CrossRef]
- Asahi, R.; Morikawa, T.; Ohwaki, T.; Aoki, K.; Taga, Y. Visible-light photocatalysis in nitrogen-doped titanium oxides. Science 2001, 293, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Asahi, R.; Morikawa, T.; Irie, H.; Ohwaki, T. Nitrogen-Doped Titanium Dioxide as Visible-Light-Sensitive Photocatalyst: Designs, Developments, and Prospects. Chem. Rev. 2014, 114, 9824–9852. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, L.; Yu, P.Y.; Mao, S.S. Increasing Solar Absorption for Photocatalysis with Black Hydrogenated Titanium Dioxide Nonocrystals. Science 2011, 331, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Naldoni, A.; Allieta, M.; Santangelo, S.; Marelli, M.; Fabbri, F.; Cappelli, S.; Bianchi, C.L.; Psaro, R.; Dal Santo, V. Effect of nature and location of defects on bandgap narrowing in black TiO2 nanoparticles. J. Am. Chem. Soc. 2012, 134, 7600–7603. [Google Scholar] [CrossRef]
- Chen, X.; Liu, L.; Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 2015, 44, 1861–1885. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, T.S.; Parikh, S.P.; Gandhi, V.G. Black TiO2: A review of its properties and conflicting trends. Chem. Eng. J. 2020, 389, 123918. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, B.; Xu, J.; Fu, Z.; Wu, M.; Li, F. Facile synthesis of Ag nanoparticles supported on TiO2 inverse opal with enhanced visible-light photocatalytic activity. Thin Solid Film. 2012, 520, 3515–3522. [Google Scholar] [CrossRef]
- Wu, M.; Jin, J.; Liu, J.; Deng, Z.; Li, Y.; Deparis, O.; Su, B.L. High photocatalytic activity enhancement of titania inverse opal films by slow photon effect induced strong light absorption. J. Mater. Chem. A 2013, 1, 15491–15500. [Google Scholar] [CrossRef]
- Lee, S.S.; Bai, H.; Liu, Z.; Sun, D.D. Optimization and an insightful properties-Activity study of electrospun TiO2/CuO composite nanofibers for efficient photocatalytic H2 generation. Appl. Catal. B Environ. 2013, 140–141, 68–81. [Google Scholar] [CrossRef]
- Sordello, F.; Minero, C. Photocatalytic hydrogen production on Pt-loaded TiO2 inverse opals. Appl. Catal. B Environ. 2015, 163, 452–458. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Jiao, J.; Zhao, Z.; Liu, J.; Li, J.; Jiang, G.; Wang, Y.; Duan, A. Fabrication of inverse opal TiO2-supported Au@CdS core-shell nanoparticles for efficient photocatalytic CO2 conversion. Appl. Catal. B Environ. 2015, 179, 422–432. [Google Scholar] [CrossRef]
- Yu, J.; Lei, J.; Wang, L.; Zhang, J.; Liu, Y. TiO2 inverse opal photonic crystals: Synthesis, modification, and applications—A review. J. Alloys Compd. 2018, 769, 740–757. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, J.; Wang, X.; Xu, H.; Yuan, S.; Zhang, Q.; Zhang, M. Preparation of inverse opal titanium dioxide for photocatalytic performance research. Opt. Mater. 2019, 96, 109287. [Google Scholar] [CrossRef]
- Fiorenza, R.; Bellardita, M.; Scirè, S.; Palmisano, L. Photocatalytic H2 production over inverse opal TiO2 catalysts. Catal. Today 2019, 113–119. [Google Scholar] [CrossRef] [Green Version]
- Temerov, F.; Ankudze, B.; Saarinen, J.J. TiO2 inverse opal structures with facile decoration of precious metal nanoparticles for enhanced photocatalytic activity. Mater. Chem. Phys. 2020, 242. [Google Scholar] [CrossRef]
- Zul, A.; Temerov, F.; Saarinen, J.J. Multilayer TiO2 Inverse Opal with Gold Nanoparticles for Enhanced Photocatalytic Activity. ACS Omega 2020, 5, 11595–11604. [Google Scholar] [CrossRef]
- Li, Y.; Piret, F.; Léonard, T.; Su, B.-L. Rutile TiO2 inverse opal with photonic bandgap in the UV–visible range. J. Colloid Interface Sci. 2010, 348, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Temerov, F.; Pham, K.; Juuti, P.; Mäkelä, J.M.; Grachova, E.V.; Kumar, S.; Eslava, S.; Saarinen, J.J. Silver-Decorated TiO2 Inverse Opal Structure for Visible Light-Induced Photocatalytic Degradation of Organic Pollutants and Hydrogen Evolution. ACS Appl. Mater. Interfaces 2020, 12, 41200–41210. [Google Scholar] [CrossRef]
- Ye, J.; He, J.; Wang, S.; Zhou, X.; Zhang, Y.; Liu, G.; Yang, Y. Nickel-loaded black TiO2 with inverse opal structure for photocatalytic reduction of CO2 under visible light. Sep. Purif. Technol. 2019, 220, 8–15. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Z.; Wang, H.; Cheng, W.; Chen, M.; Shangguan, W.; Ding, G. TiO2-graphene nanocomposites for photocatalytic hydrogen production from splitting water. Int. J. Hydrogen Energy 2012, 37, 2224–2230. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, L.; Dong, W.; Zheng, F.; Shen, M.; Wang, J. Inverse opal structured Ag/TiO2 plasmonic photocatalyst prepared by pulsed current deposition and its enhanced visible light photocatalytic activity. J. Mater. Chem. A 2014, 2, 824–832. [Google Scholar] [CrossRef]
- Orilall, M.C.; Abrams, N.M.; Lee, J.; DiSalvo, F.J.; Wiesner, U. Highly crystalline inverse opal transition metal oxides via a combined assembly of soft and hard chemistries. J. Am. Chem. Soc. 2008, 130, 8882–8883. [Google Scholar] [CrossRef]
- Kang, J.; Kim, J.; Lee, S.; Wi, S.; Kim, C.; Hyun, S.; Nam, S.; Park, Y.; Park, B. Breathable Carbon-Free Electrode: Black TiO2 with Hierarchically Ordered Porous Structure for Stable Li–O2 Battery. Adv. Energy Mater. 2017, 7, 1700814. [Google Scholar] [CrossRef]
- Reddy, K.M.; Manorama, S.V.; Reddy, A.R. Bandgap studies on anatase titanium dioxide nanoparticles. Mater. Chem. Phys. 2003, 78, 239–245. [Google Scholar] [CrossRef]
- Curti, M.; Mendive, C.B.; Grela, M.A.; Bahnemann, D.W. Stopband tuning of TiO2 inverse opals for slow photon absorption. Mater. Res. Bull. 2017, 91, 155–165. [Google Scholar] [CrossRef]
- Liqiang, J.; Yichun, Q.; Baiqi, W.; Shudan, L.; Baojiang, J.; Libin, Y.; Wei, F.; Honggang, F.; Jiazhong, S. Review of photoluminescence performance of nano-sized semiconductor materials and its relationships with photocatalytic activity. Sol. Energy Mater. Sol. Cells 2006, 90, 1773–1787. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, C.; Liu, X.; Gu, F.; Jiang, H.; Shao, W.; Zhang, L.; He, Y. Synthesis and optical properties of TiO2 nanoparticles. Mater. Lett. 2007, 61, 79–83. [Google Scholar] [CrossRef]
- Xu, J.; Li, L.; Yan, Y.; Wang, H.; Wang, X.; Fu, X.; Li, G. Synthesis and photoluminescence of well-dispersible anatase TiO2 nanoparticles. J. Colloid Interface Sci. 2008, 318, 29–34. [Google Scholar] [CrossRef]
- Guo, J.; Benz, D.; Doan Nguyen, T.T.; Nguyen, P.H.; Thi Le, T.L.; Nguyen, H.H.; La Zara, D.; Liang, B.; Hintzen, H.T.; van Ommen, J.R.; et al. Tuning the photocatalytic activity of TiO2 nanoparticles by ultrathin SiO2 films grown by low-temperature atmospheric pressure atomic layer deposition. Appl. Surf. Sci. 2020, 530, 147244. [Google Scholar] [CrossRef]
- Pallotti, D.K.; Passoni, L.; Maddalena, P.; Di Fonzo, F.; Lettieri, S. Photoluminescence Mechanisms in Anatase and Rutile TiO2. J. Phys. Chem. C 2017, 121, 9011–9021. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, P.; Liu, J.; Yu, J. New understanding of the difference of photocatalytic activity among anatase, rutile and brookite TiO2. Phys. Chem. Chem. Phys. 2014, 16, 20382–20386. [Google Scholar] [CrossRef]
- Hirakawa, T.; Kamat, P.V. Charge separation and catalytic activity of Ag@TiO2 core-shell composite clusters under UV-irradiation. J. Am. Chem. Soc. 2005, 127, 3928–3934. [Google Scholar] [CrossRef]
- Guo, J.; Yuan, S.; Yu, Y.; Van Ommen, J.R.; Van Bui, H.; Liang, B. Room-temperature pulsed CVD-grown SiO2 protective layer on TiO2 particles for photocatalytic activity suppression. RSC Adv. 2017, 7, 4547–4554. [Google Scholar] [CrossRef] [Green Version]
- Stets, S.; do Amaral, B.; Schneider, J.T.; de Barros, I.R.; de Liz, M.V.; Ribeiro, R.R.; Nagata, N.; Peralta-Zamora, P. Antituberculosis drugs degradation by UV-based advanced oxidation processes. J. Photochem. Photobiol. A Chem. 2018, 353, 26–33. [Google Scholar] [CrossRef]
- Singh, J.; Tripathi, N.; Mohapatra, S. Synthesis of Ag–TiO2 hybrid nanoparticles with enhanced photocatalytic activity by a facile wet chemical method. Nano Struct. Nano Objects 2019, 18, 100266. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, L. Silver nanoparticles doped TiO2 catalyzed Suzuki-coupling of bromoaryl with phenylboronic acid under visible light. J. Photochem. Photobiol. B Biol. 2020, 205, 111807. [Google Scholar] [CrossRef]
- Awazu, K.; Fujimaki, M.; Rockstuhl, C.; Tominaga, J.; Murakami, H.; Ohki, Y.; Yoshida, N.; Watanabe, T. A plasmonic photocatalyst consisting of silver nanoparticles embedded in titanium dioxide. J. Am. Chem. Soc. 2008, 130, 1676–1680. [Google Scholar] [CrossRef]
- Cho, S.A.; Jang, Y.J.; Lim, H.D.; Lee, J.E.; Jang, Y.H.; Nguyen, T.T.H.; Mota, F.M.; Fenning, D.P.; Kang, K.; Shao-Horn, Y.; et al. Hierarchical Porous Carbonized Co3O4 Inverse Opals via Combined Block Copolymer and Colloid Templating as Bifunctional Electrocatalysts in Li–O2 Battery. Adv. Energy Mater. 2017, 7, 1700391. [Google Scholar] [CrossRef]
- Nédez, C.; Ray, J.L. Efficient removal of polymerization inhibitors by adsorption on the surface of an optimized alumina. Langmuir 1999, 15, 5932–5936. [Google Scholar] [CrossRef]
- Sofianou, M.V.; Boukos, N.; Vaimakis, T.; Trapalis, C. Decoration of TiO2 anatase nanoplates with silver nanoparticles on the {101} crystal facets and their photocatalytic behaviour. Appl. Catal. B Environ. 2014, 158–159, 91–95. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, T.-H.T.; Bui, T.-T.; Van Bui, H.; Dao, V.-D.; Le Thi Ngoc, L. TiO2 Inverse Opals Modified by Ag Nanoparticles: A Synergic Effect of Enhanced Visible-Light Absorption and Efficient Charge Separation for Visible-Light Photocatalysis. Catalysts 2021, 11, 761. https://doi.org/10.3390/catal11070761
Le T-HT, Bui T-T, Van Bui H, Dao V-D, Le Thi Ngoc L. TiO2 Inverse Opals Modified by Ag Nanoparticles: A Synergic Effect of Enhanced Visible-Light Absorption and Efficient Charge Separation for Visible-Light Photocatalysis. Catalysts. 2021; 11(7):761. https://doi.org/10.3390/catal11070761
Chicago/Turabian StyleLe, Thanh-Hiep Thi, Thanh-Trang Bui, Hao Van Bui, Van-Duong Dao, and Loan Le Thi Ngoc. 2021. "TiO2 Inverse Opals Modified by Ag Nanoparticles: A Synergic Effect of Enhanced Visible-Light Absorption and Efficient Charge Separation for Visible-Light Photocatalysis" Catalysts 11, no. 7: 761. https://doi.org/10.3390/catal11070761
APA StyleLe, T.-H. T., Bui, T.-T., Van Bui, H., Dao, V.-D., & Le Thi Ngoc, L. (2021). TiO2 Inverse Opals Modified by Ag Nanoparticles: A Synergic Effect of Enhanced Visible-Light Absorption and Efficient Charge Separation for Visible-Light Photocatalysis. Catalysts, 11(7), 761. https://doi.org/10.3390/catal11070761







