High Catalytic Performance and Sustainability of Zr Modified Aluminophosphate for Vapor-Phase Selective O-Methylation of Catechol with Methanol
Abstract
1. Introduction
2. Results and Discussion
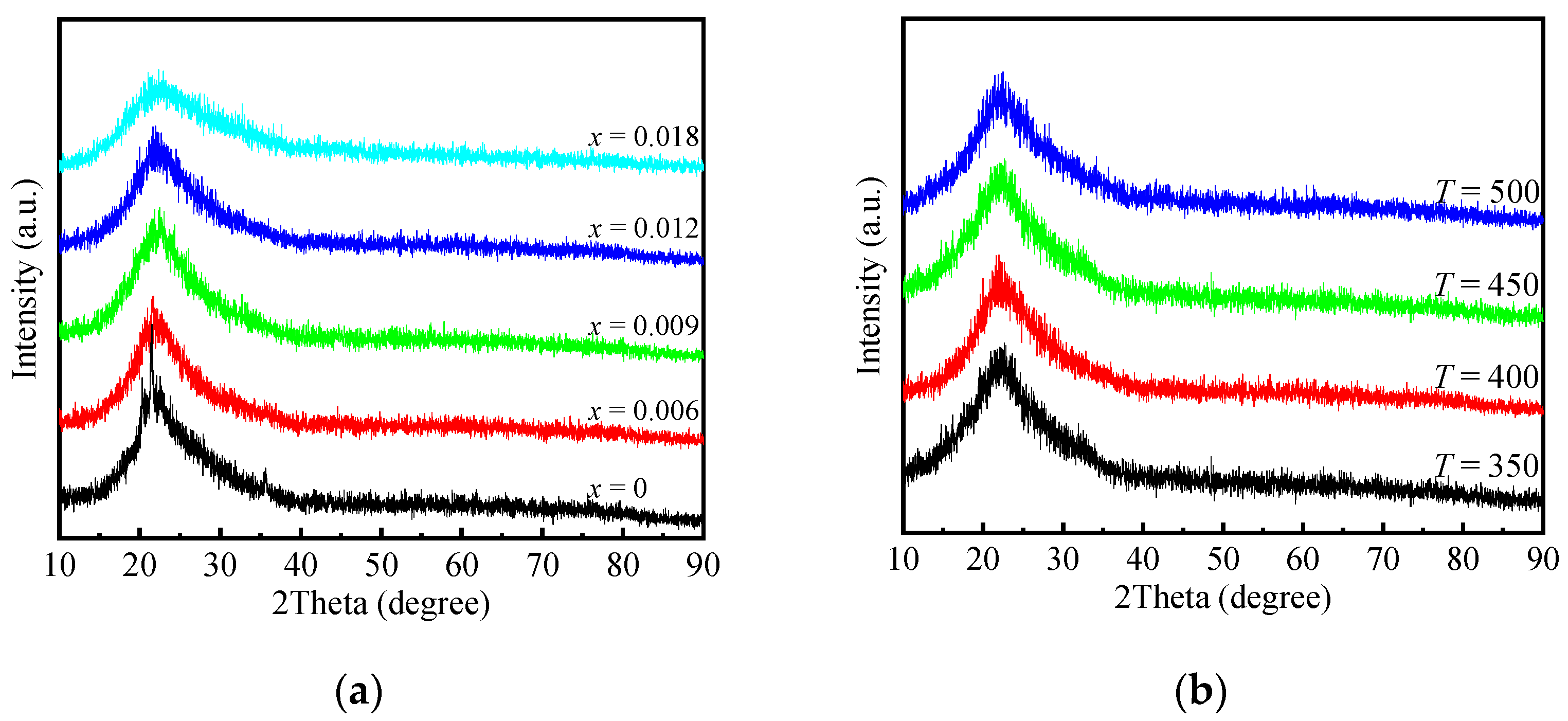
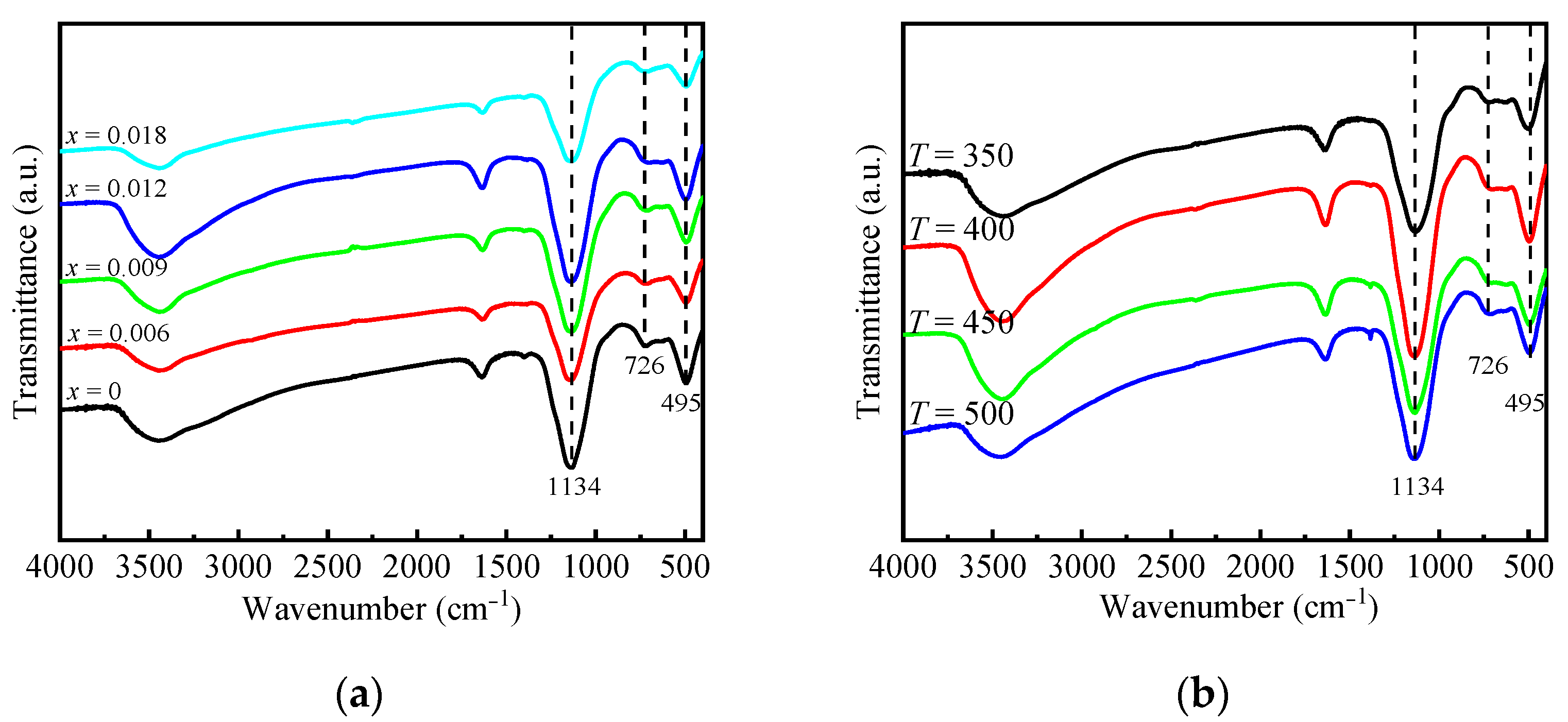
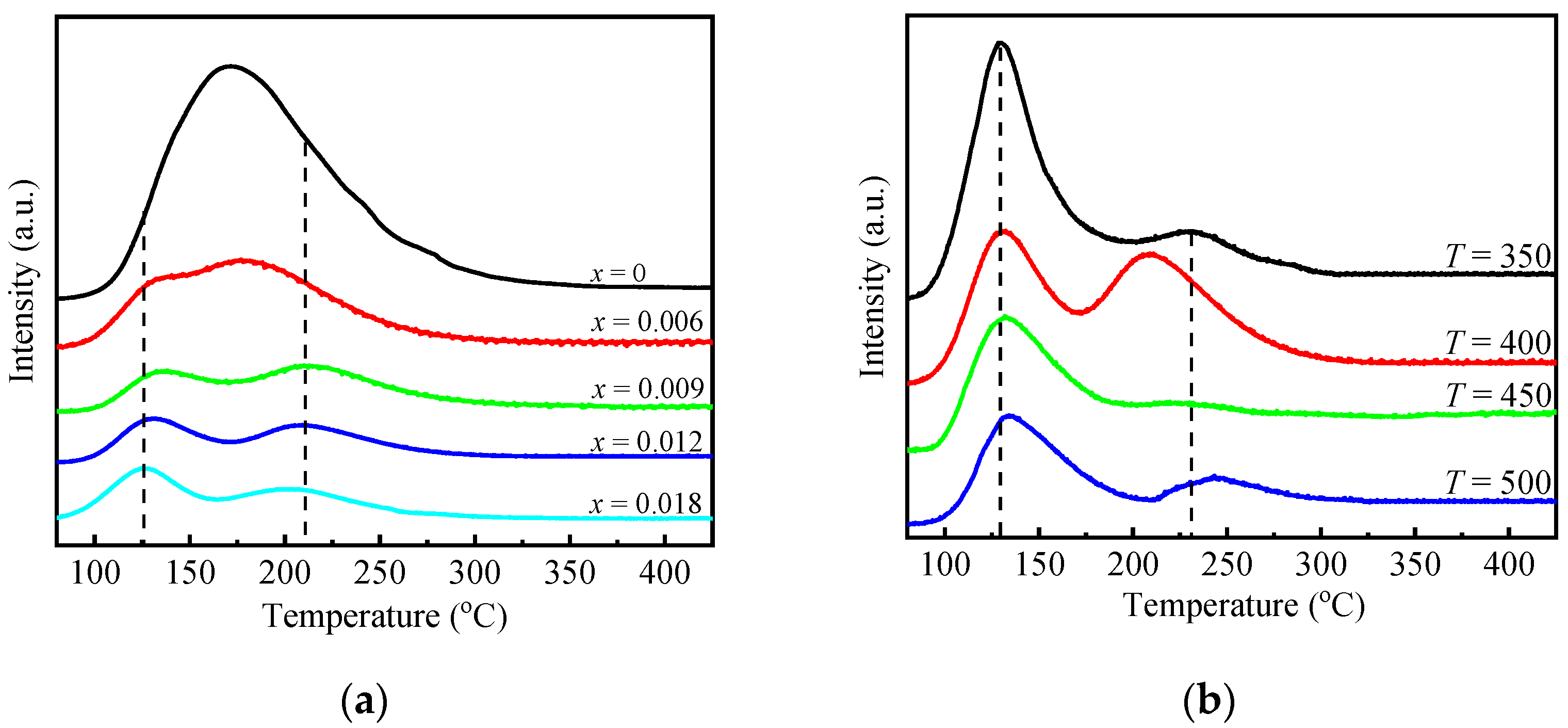
2.1. Physicochemical Properties of AlP1.1Zrx-T
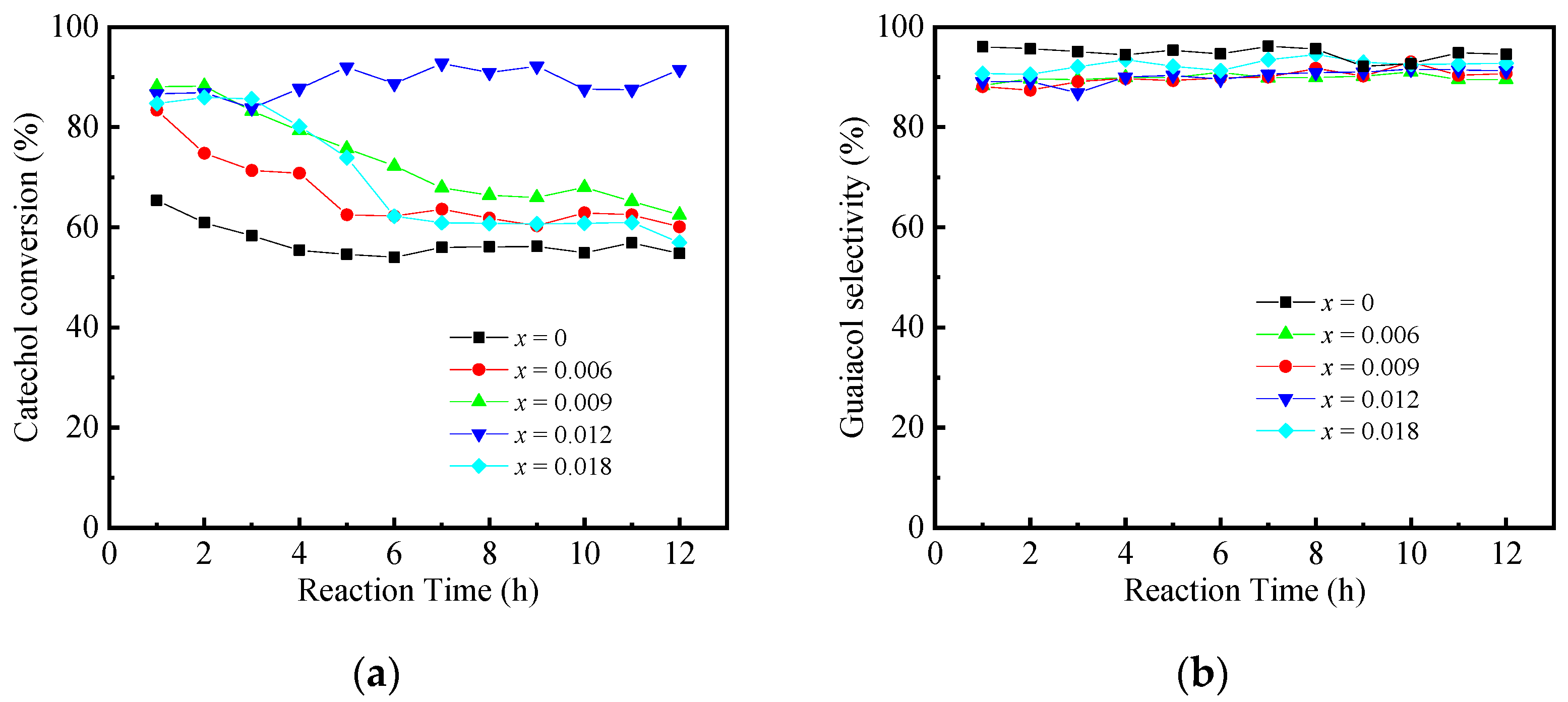
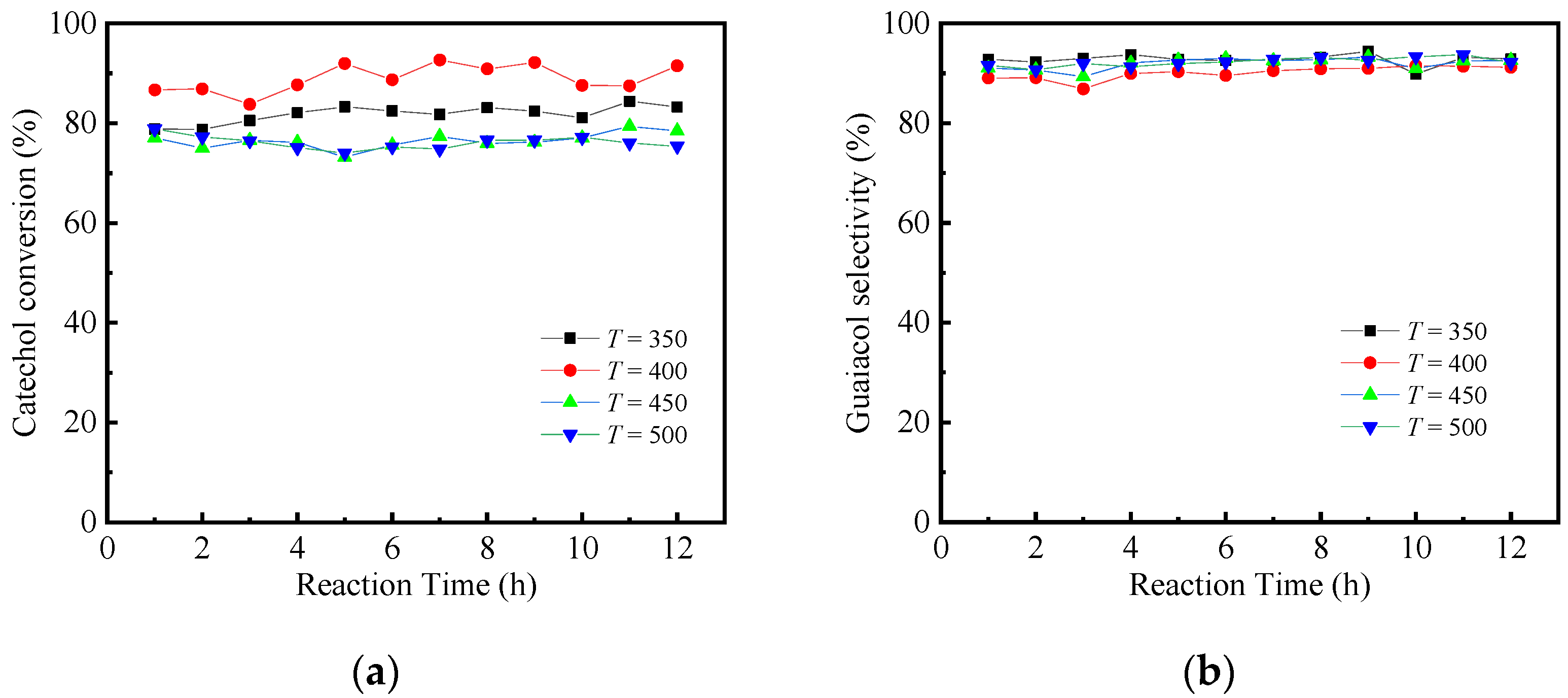
2.2. Catalysis Reaction
3. Materials and Methods
3.1. Material Preparation
3.2. Material Characterization
3.3. Catalytic Test
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Llevot, A.; Grau, E.; Carlotti, S.; Grelier, S.; Cramail, H. From Lignin-derived Aromatic Compounds to Novel Biobased Polymers. Macromol. Rapid Commun. 2016, 37, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.B.; Zhou, J.X.; Xia, Z.; Wang, Z.G.; Xu, Z.W.; Xu, W.J.; Yan, P.F.; Liu, K.R.; Guo, X.W.; Zhang, Z.C. Anatase TiO2 activated by gold nanoparticles for selective hydrodeoxygenation of guaiacol to phenolics. ACS Catal. 2017, 7, 695–705. [Google Scholar] [CrossRef]
- Kakhlon, O.; Ferreira, I.; Solmesky, L.; Weil, M.; Senderowitz, H.; Lossos, A.; Alvarez, R.; Pampou, S.; Escriba, P.; Yue, W. Guaiacol can be a drug-candidate for treating adult polyglucosan body disease. Neurology 2018, 17, e99694. [Google Scholar]
- Lui, M.Y.; Lokare, K.S.; Hemming, E.; Stanley, J.N.G.; Perosa, A.; Selva, M.; Masters, A.F.; Maschmeyer, T. Microwave-assisted methylation of dihydroxybenzene derivatives with dimethyl carbonate. RSC Adv. 2016, 6, 58443–58451. [Google Scholar] [CrossRef]
- Kabra, S.K.; Huuhtanen, M.; Keiski, R.L.; Yadav, G.D. Selectivity engineering of O-methylation of hydroxybenzenes with dimethyl carbonate using ionic liquid as catalyst. React. Chem. Eng. 2016, 1, 330–339. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Balakrishna, G.; Rajesh, B.; Jayasri, V.; Sikhwivhilu, L.M.; Coville, N.J. Alkylation of catechol with methanol to guaiacol over sulphate-modified zirconia solid acid catalysts. React. Kinet. Catal. Lett. 2007, 92, 311–317. [Google Scholar] [CrossRef]
- Vishwanathan, V.; Balakrishna, G.; Rajesh, B.; Jayasri, V.; Sikhwivhilu, L.M.; Coville, N.J. Alkylation of catechol with methanol to give guaiacol over sulphate-modified zirconia solid acid catalysts: The influence of structural modification of zirconia on catalytic performance. Catal. Commun. 2008, 9, 2422–2427. [Google Scholar] [CrossRef]
- Fu, Z.H.; Yu, Y.; Yin, D.L.; Xu, Y.Z.; Liu, H.P.; Liao, H.Y.; Xu, Q.; Tan, F.Q.; Wang, J. Vapor-phase highly selective O-methylation of catechol with methanol over ZnCl2 modified-Al2O3 catalysts. J. Mol. Catal. A Chem. 2005, 232, 69–75. [Google Scholar] [CrossRef]
- Zou, X.J.; Zhu, X.M.; Li, X.M.; Wang, Z.L.; Liu, G.; Jia, M.J.; Zhang, W.X. Vapour-phase O-methylation of catechol with methanol on SiO2-supported ammonium metatungstate catalysts. Chin. J. Catal. 2008, 29, 671–676. [Google Scholar]
- Jafari, A.A.; Khodadadi, A.; Mortazavi, Y. Vapor-phase selective O-alkylation of catechol with methanol over lanthanum phosphate and its modified catalysts with Ti and Cs. J. Mol. Catal. A Chem. 2013, 372, 79–83. [Google Scholar] [CrossRef]
- Liao, X.Z.; Zhou, Z.; Wang, Z.L.; Zou, X.J.; Liu, G.; Jia, M.J.; Zhang, W.X. Preformed precursor of microporous aluminophosphate coating on mesoporous SBA-15: Synthesis, characterization, and catalytic property for selective O-methylation of catechol. J. Colloid Interface Sci. 2007, 308, 176–181. [Google Scholar] [CrossRef]
- Liu, G.; Yang, L.X.; Wu, S.J.; Jia, M.J.; Zhang, W.X. Influence of acid-base properties of K-loaded aluminophosphate catalysts on their catalytic behavior in the O-methylation of catechol. Acta Phys. Chim. Sin. 2014, 30, 1163–1168. [Google Scholar]
- Zhu, X.M.; Liu, X.M.; Jia, M.J.; Liu, G.; Zhang, W.X.; Jiang, D.Z. Vapour-phase selective O-methylation of catechol with methanol over Ti-containing aluminium phosphate catalysts. Appl. Catal. A Gen. 2005, 282, 155–161. [Google Scholar] [CrossRef]
- Mao, H.F.; Li, X.L.; Xu, F.; Xiao, Z.B.; Zhang, W.X.; Meng, T. Vapour-phase selective O-Methylation of catechol with methanol over metal phosphate catalysts. Catalysts 2021, 11, 531. [Google Scholar] [CrossRef]
- Liu, G.; Wang, Z.L.; Jia, M.J.; Zou, X.J.; Zhu, X.M.; Zhang, W.X.; Jiang, D.Z. Thermally stable amorphous mesoporous aluminophosphates with controllable P/Al ratio: Synthesis, characterization, and catalytic performance for selective O-methylation of catechol. J. Phys. Chem. B. 2006, 110, 16953–16960. [Google Scholar] [CrossRef]
- Liu, G.; Jia, M.J.; Zhou, Z.; Zhang, W.X.; Wu, T.H.; Jiang, D.Z. Synthesis of amorphous mesoporous aluminophosphate materials with high thermal stability using a citric acid route. Chem. Commun. 2004, 10, 1660–1661. [Google Scholar] [CrossRef]
- Dai, W.L.; Kong, W.B.; Wu, G.J.; Li, N.; Li, L.D.; Guan, N.J. Catalytic dehydration of methanol to dimethyl ether over aluminophosphate and silico-aluminophosphate molecular sieves. Catal. Commun. 2011, 12, 535–538. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, Y.Y.; Liu, W.R.; Cai, H.H.; Lv, J.H.; Liu, J.D. Amorphous magnesium substituted mesoporous aluminophosphate: An acid-base sites synergistic catalysis for transesterification of diethyl carbonate and dimethyl carbonate in fixed-bed reactor. Micropor. Mesopor. Mat. 2020, 292, 109757. [Google Scholar] [CrossRef]
- Hamza, A.; Nagaraju, N. Amorphous metal-aluminophosphate catalysts for aldol condensation of n-heptanal and benzaldehyde to jasminaldehyde. Chin. J. Catal. 2015, 36, 209–215. [Google Scholar] [CrossRef]
- Sun, D.L.; Mai, J.J.; Deng, J.R.; Idem, R.; Liang, Z.W. One-Pot synthesis of dialkyl hexane-1,6-dicarbamate from 1,6-hexanediamine, urea, and alcohol over zinc-incorporated berlinite (ZnAlPO4) catalyst. Catalysts 2016, 6, 28. [Google Scholar] [CrossRef]
- Luo, Y.S.; Liang, X.H.; Li, X.Q.; Wang, S.F.; Gao, X.N.; Zhang, Z.G.; Fang, Y.T. Iron doped aluminophosphate molecular sieve with improved adsorption capacity for water vapor. Adsorption 2018, 24, 551–561. [Google Scholar] [CrossRef]
- Vijayasankar, A.V.; Nagaraju, N. Preparation and characterisation of amorphous mesoporous aluminophosphate and metal aluminophosphate as an efficient heterogeneous catalyst for transesterification reaction. Cr. Chim. 2011, 14, 1109–1116. [Google Scholar] [CrossRef]
- Yan, X.; Qiu, Y.C.; Han, Y.D.; Sun, Q.M.; Ge, R.; Song, X.W. Microwave-assisted synthesis of a thermally stable Zn-containing aluminophosphate with ERI-zeotype structure templated by diquaternary alkylammonium. RSC Adv. 2014, 4, 49846–49849. [Google Scholar]
- Vijayasankar, A.V.; Deepa, S.; Venugopal, B.R.; Nagaraju, N. Amorphous mesoporous iron aluminophosphate catalyst for the synthesis of 1,5-benzodiazepines. Chin. J. Catal. 2010, 31, 1321–1327. [Google Scholar] [CrossRef]
- Kustov, L.M.; Kustov, A.L.; Kazansky, V.B. Spectroscopic investigation of redox and acidic properties of Co-substituted aluminophosphate CoAPO-11. Mendeleev Commun. 2018, 28, 354–356. [Google Scholar] [CrossRef]
- Kumar, A.; Sarmah, B.; Srivastava, R. C-N bond formation by the activation of alkenes and alkynes using Cu present in the framework and extra-framework of aluminophosphate. Catal. Commun. 2018, 109, 43–49. [Google Scholar] [CrossRef]
- Harish, N.; Kathyayini, N.; Nagaraju, N. Studies on the catalytic activity of mesoporous aluminaaluminophosphate (Al2O3–AlPO4) materials in the synthesis of N.N’-diphenyl urea. React. Kinet. Mech. Catal. 2018, 125, 937–949. [Google Scholar] [CrossRef]
- Shyamprasad, K.; Shamshuddin, S.Z.M.; Shyamsundar, M.; Thimmaraju, N. Modified mesoporous aluminophosphate as an efficient solid acid catalyst for the synthesis of novel O- and N-acetylated compounds: Solvent free condition. J. Porous Mat. 2016, 23, 1095–1105. [Google Scholar] [CrossRef]
- Shi, J.H.; Liu, G.; Fan, Z.Q.; Nie, L.Y.; Zhang, Z.H.; Zhang, W.X.; Huo, Q.S.; Yan, W.F.; Jia, M.J. Amorphous mesoporous aluminophosphate as highly efficient heterogeneous catalysts for transesterification of diethyl carbonate with dimethyl carbonate. Catal. Commun. 2011, 12, 721–725. [Google Scholar] [CrossRef]
- Dawaymeh, F.; Elmutasim, O.; Gaber, D.; Gaber, S.; Reddy, K.S.K.; Basina, G.; Polychronopoulou, K.; Al Wahedi, Y.; Karanikolos, G.N. Metal substitution effects of aluminophosphate AlPO4-5 as solid acid catalyst for esterification of acetic acid with ethanol. Mol. Catal. 2021, 501, 111371. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, Q. Synthesis, characterization, and base–catalytic performance of ordered mesoporous aluminophosphate oxynitride materials. J. Mater. Res. 2007, 22, 3330–3337. [Google Scholar] [CrossRef]
- Akri, M.; El Kasmi, A.; Batiot-Dupeyrat, C.; Qiao, B.T. Highly active and carbon-resistant nickel single-atom catalysts for methane dry reforming. Catalysts 2020, 10, 630. [Google Scholar] [CrossRef]



| Catalysts | SBET (m2 g−1) | Vp (cm3·g−1) | Da (nm) |
|---|---|---|---|
| AlP1.1-400 | 126 | 0.83 | 23.5 |
| AlP1.1Zr0.006-400 | 141 | 0.50 | 19.0 |
| AlP1.1Zr0.009-400 | 163 | 0.84 | 17.2 |
| AlP1.1Zr0.012-400 | 183 | 0.83 | 18.8 |
| AlP1.1Zr0.018-400 | 195 | 0.68 | 15.0 |
| AlP1.1Zr0.012-350 | 183 | 0.83 | 19.1 |
| AlP1.1Zr0.012-450 | 181 | 0.82 | 18.8 |
| AlP1.1Zr0.012-500 | 180 | 0.82 | 18.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Xu, D.; Yin, Y.; Zou, X.; Wang, Y.; Shang, X.; Wang, X. High Catalytic Performance and Sustainability of Zr Modified Aluminophosphate for Vapor-Phase Selective O-Methylation of Catechol with Methanol. Catalysts 2021, 11, 740. https://doi.org/10.3390/catal11060740
Ren X, Xu D, Yin Y, Zou X, Wang Y, Shang X, Wang X. High Catalytic Performance and Sustainability of Zr Modified Aluminophosphate for Vapor-Phase Selective O-Methylation of Catechol with Methanol. Catalysts. 2021; 11(6):740. https://doi.org/10.3390/catal11060740
Chicago/Turabian StyleRen, Xinfeng, Dongfei Xu, Yuchen Yin, Xiujing Zou, Yankai Wang, Xingfu Shang, and Xueguang Wang. 2021. "High Catalytic Performance and Sustainability of Zr Modified Aluminophosphate for Vapor-Phase Selective O-Methylation of Catechol with Methanol" Catalysts 11, no. 6: 740. https://doi.org/10.3390/catal11060740
APA StyleRen, X., Xu, D., Yin, Y., Zou, X., Wang, Y., Shang, X., & Wang, X. (2021). High Catalytic Performance and Sustainability of Zr Modified Aluminophosphate for Vapor-Phase Selective O-Methylation of Catechol with Methanol. Catalysts, 11(6), 740. https://doi.org/10.3390/catal11060740





