Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution
Abstract
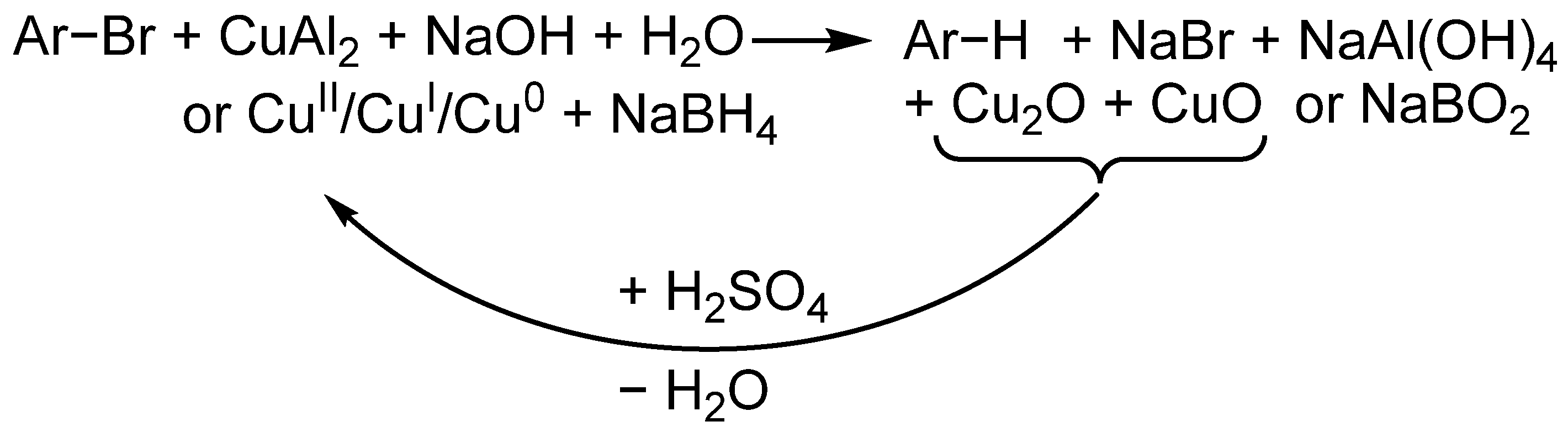
1. Introduction
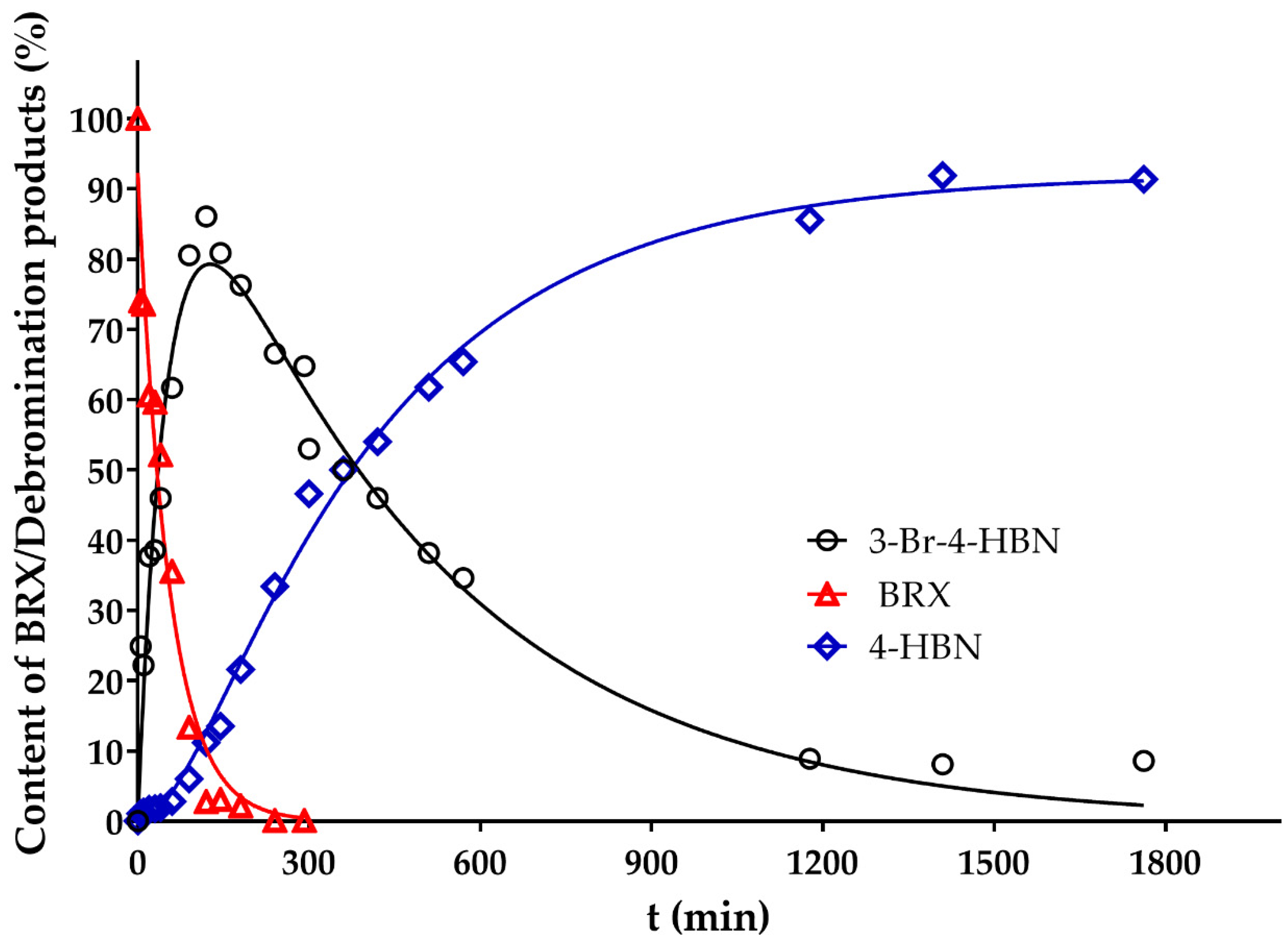
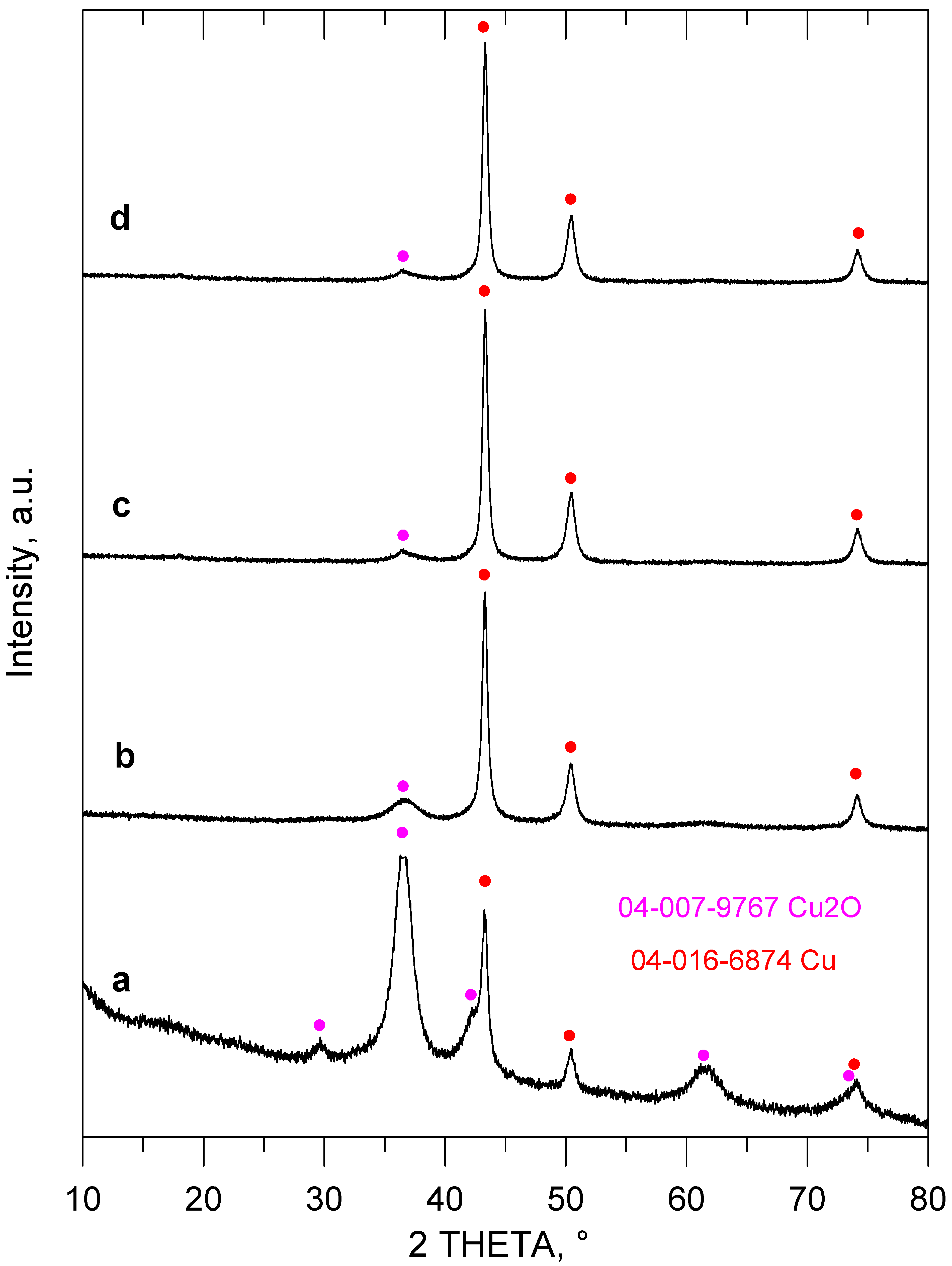
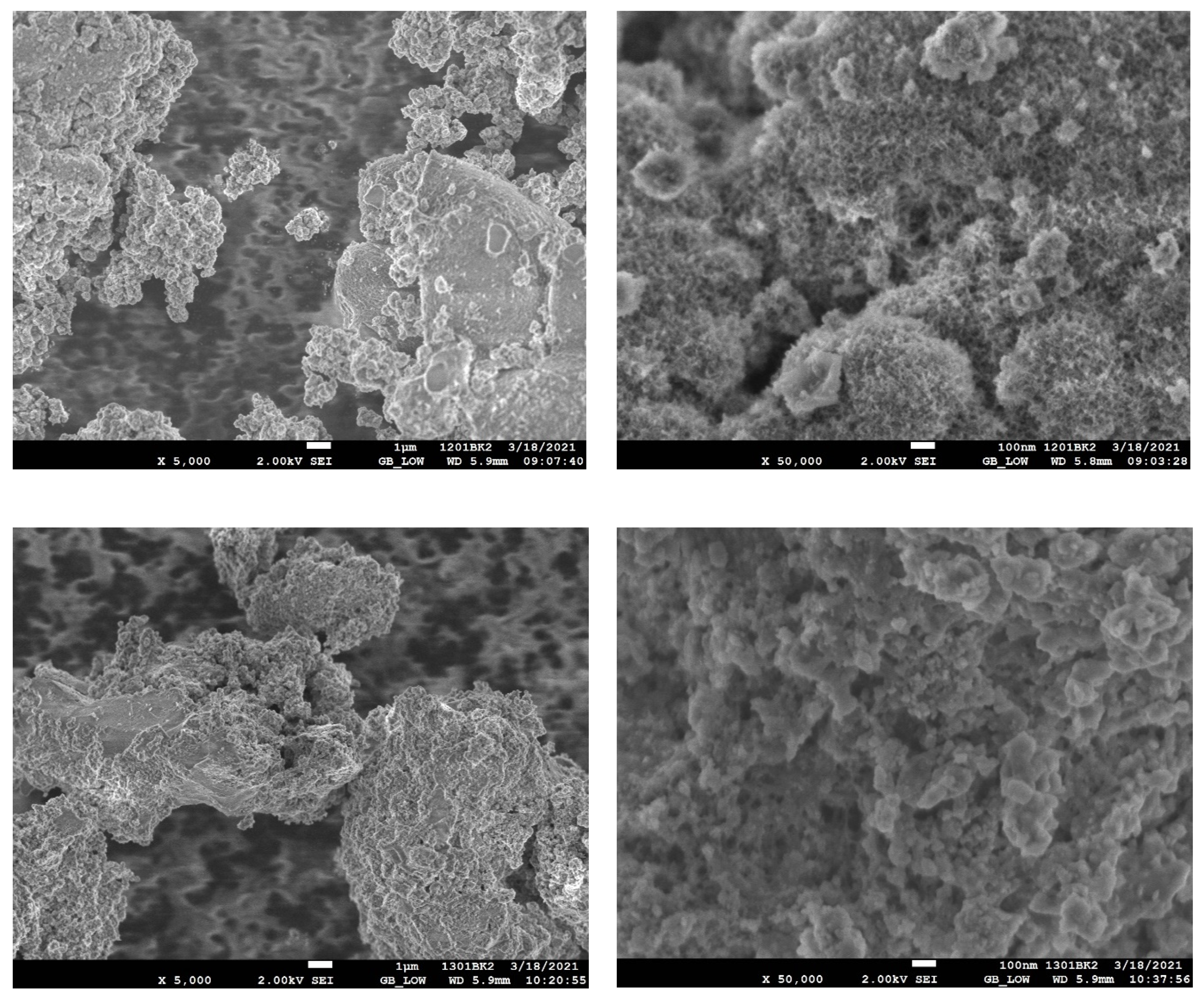
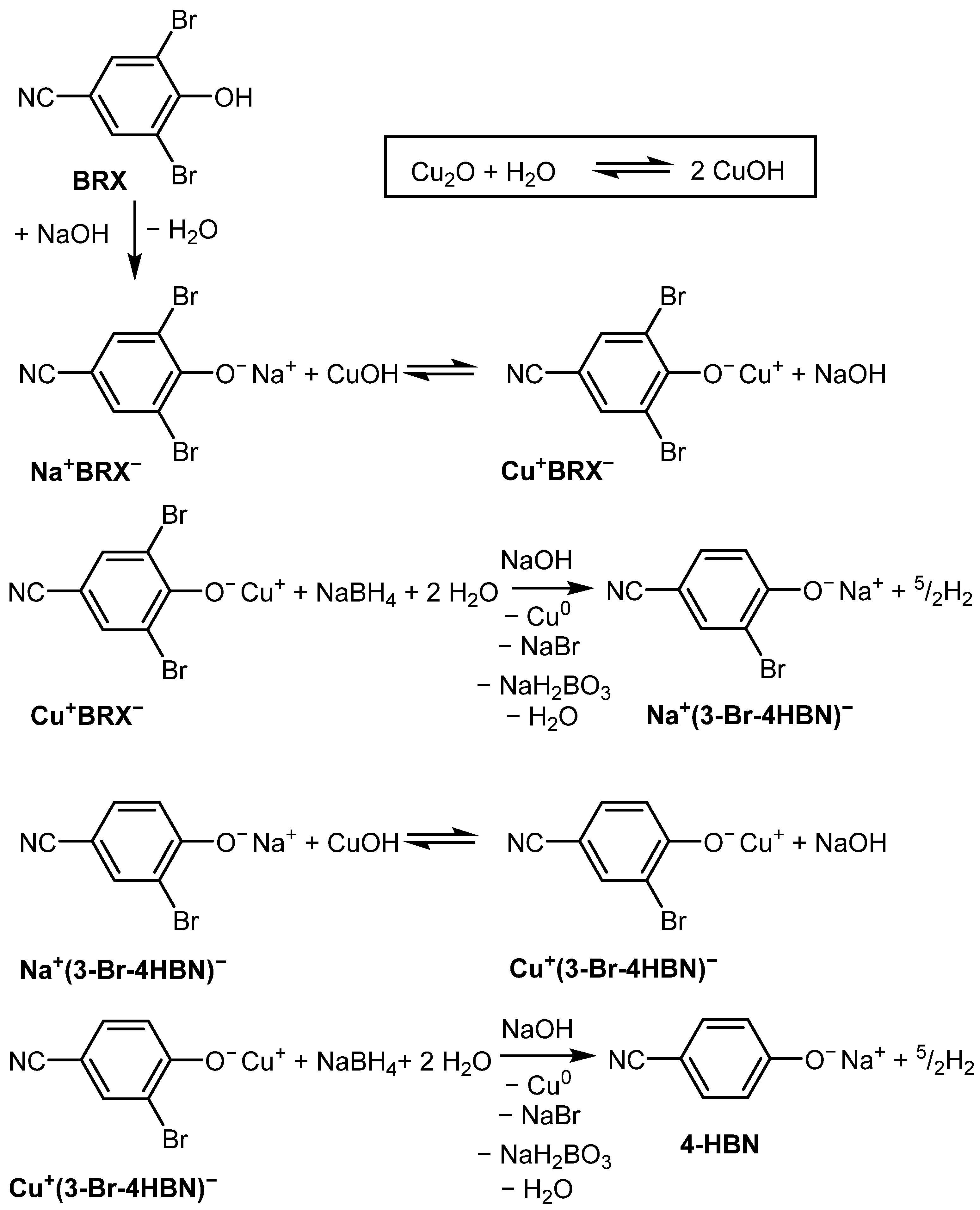
2. Results and Discussion
3. Materials and Methods
3.1. Chemical Analysis
3.2. HDB Procedures
3.2.1. HDB of Brominated Phenols Using Studied Al Alloys, Electropositive Metals or Cu-Based Catalysts and NaBH4
3.2.2. HDB of Brominated Phenols Using In Situ Generated Cu/Cu2O
3.2.3. HDB Kinetic Experiments
3.3. Evaluation of Kinetics of the HDB of BRX)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hao, W.; Liu, Y. C–H bond halogenation catalyzed or mediated by copper: An overview. Beilstein J. Org. Chem. 2015, 11, 2132–2144. [Google Scholar] [CrossRef] [PubMed]
- Petrone, D.A.; Ye, J.; Lautens, M. Modern transition-metal-catalyzed carbon–halogen bond formation. Chem. Rev. 2016, 116, 8003–8104. [Google Scholar] [CrossRef]
- Yasamut, K.; Jongcharoenkamol, J.; Ruchirawat, S.; Ploypradith, P. A modified Cu(0)-Cu(I)-mediated Caryl-Caryl Ullmann coupling for the synthesis of biaryls. Tetrahedron 2016, 72, 5994–6000. [Google Scholar] [CrossRef]
- Li, Y.; Han, D.; Arai, Y.; Fu, X.; Li, X.; Huang, W. Kinetics and mechanisms of debromination of tetrabromobisphenol A by Cu coated nano zerovalent iron. Chem. Eng. J. 2019, 373, 95–103. [Google Scholar] [CrossRef]
- Weidlich, T. The influence of copper on halogenation/dehalogenation reactions of aromatic compounds and its role in the destruction of polyhalogenated aromatic contaminants. Catalysts 2021, 11, 378. [Google Scholar] [CrossRef]
- Alaee, M.; Arias, P.; Sjodin, A.; Bergman, A. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef]
- Müller, F.; Applebyki, A.P. Weed Control, 2. Individual Herbicides. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-WCH: Weinheim, Germany, 2012; Volume 39, pp. 209–385. [Google Scholar]
- Shaw, S.D.; Blum, A.; Weber, R.; Kannan, K.; Rich, D.; Lucas, D.; Koshland, C.P.; Dobraca, D.; Hanson, S.; Birnbaum, L.S. Halogenated flame retardants: Do the fire safety benefits justify the risks? Rev. Environ. Health 2010, 25, 261–305. [Google Scholar] [CrossRef]
- Arena, M.; Auteri, D.; Barmaz, S.; Bellisai, G.; Brancato, A.; Brocca, D.; Bura, L.; Byers, H.; Chiusolo, A.; Marques, D.C.; et al. Peer review of the pesticide risk assessment of the active substance bromoxynil (variant evaluated bromoxynil octanoate). J. EFSA 2017, 15, 24. [Google Scholar]
- Holtze, M.S.; Sørensen, S.R.; Sørensen, J.; Aamand, J. Microbial degradation of benzonitrile herbicides dichlobenil, bromoxynil and ioxynil in soil and subsurface environments–insights into degradation pathways, persistent metabolites and involved degrader organisms. Environ. Pollut. 2008, 154, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Knossow, N.; Siebner, H.; Bernstein, A. Isotope analysis method for the herbicide bromoxynil and its application to study photo-degradation processes. J. Hazard. Mater. 2020, 388, 122036. [Google Scholar] [CrossRef]
- Palma, P.; Kuster, M.; Alvarenga, P.; Palma, V.L.; Fernandes, R.M.; Soares, A.M.V.M.; de Alda, M.J.L.; Barcelo, D.; Barbosa, I.R. Risk assessment of representative and priority pesticides, in surface water of the Alqueva reservoir (South of Portugal) using on-line solid phase extraction–liquid chromatography–tandem mass spectrometry. Environ. Int. 2009, 35, 545–551. [Google Scholar] [CrossRef]
- Maddila, S.; Lavanya, P.; Jonnalagadda, S.B. Degradation, mineralization of bromoxynil pesticide by heterogeneous photocatalytic ozonation. J. Ind. Eng. Chem. 2015, 24, 333–341. [Google Scholar] [CrossRef]
- Huang, Z.; Deng, D.; Qiao, J.; Ju, Y.; Chen, Y.; Dionysiou, D.D. New insight into the cosolvent effect on the degradation of tetrabromobisphenol A (TBBPA) over millimeter-scale palladised sponge iron (Pd-s-Fe0) particles. Chem. Eng. J. 2019, 361, 1423–1436. [Google Scholar] [CrossRef]
- Iordache, I.; Wilson, S.; Lundanes, E.; Iordache, M.; Pavel, V.L.; Aelenei, N. The Fenton and sono-Fenton processes applied for pesticide degradation. Environ. Eng. Manag. J. 2010, 9, 519–525. [Google Scholar] [CrossRef]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W. Degradation of bromoxynil and trifluralin in natural water by direct photolysis and UV plus H2O2 advanced oxidation process. Water Res. 2010, 44, 2221–2228. [Google Scholar] [CrossRef]
- Chelme-Ayala, P.; El-Din, M.G.; Smith, D.W. Kinetics and mechanism of the degradation of two pesticides in aqueous solutions by ozonation. Chemosphere 2010, 78, 557–562. [Google Scholar] [CrossRef]
- Bououden, Z.; Halladja, S.; Sleiman, M.; Leremboure, M.; Richard, C. Mechanism of bromoxynil phototransformation: Effect of medium and surfactant. J. Photochem. Photobiol. A Chem. 2018, 365, 151–156. [Google Scholar] [CrossRef]
- Horikoshi, S.; Miura, T.; Kajitani, M.; Horikoshi, N.; Serpone, N. Photodegradation of tetrahalobisphenol-A (X=Cl, Br) flame retardants and delineation of factors affecting the process. Appl. Catal. B 2008, 84, 797–802. [Google Scholar] [CrossRef]
- Zhong, Y.; Liang, X.; Zhong, Y.; Zhu, J.; Zhu, S.; Yuan, P.; He, H.; Zhang, J. Heterogeneous UV/Fenton degradation of TBBPA catalyzed by titanomagnetite: Catalyst characterization, performance and degradation products. Water Res. 2012, 46, 4633–4644. [Google Scholar] [CrossRef] [PubMed]
- Sandhiya, L.; Senthilkumar, K. Reaction mechanism and kinetics of the degradation of bromoxynil initiated by OH radical. RSC Adv. 2014, 4, 7749. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Shen, J.; Wang, M.; Yang, Q.; Zheng, M. Environmental characteristics and formations of polybrominated dibenzo-p-dioxins and dibenzofurans. Environ. Int. 2021, 152, 106450. [Google Scholar] [CrossRef] [PubMed]
- Schouttete, K.V.K.M.; Hennebel, T.; Dheere, E.; Bertelkamp, C.; De Ridder, D.J.; Maes, S.; Chys, M.; Van Hulle, S.W.H.; Vanden Bussche, J.; Vanhaecke, L.; et al. Effect of oxidation and catalytic reduction of trace organic contaminants on their activated carbon adsorption. Chemosphere 2016, 165, 191–201. [Google Scholar] [CrossRef]
- Ma, X.X.; Liu, Y.; Li, X.Q.; Xu, J.G.; Gu, G.D.; Xia, C.H. Water: The most effective solvent for liquid-phase hydrodechlorination of chlorophenols over Raney Ni catalyst. Appl. Catal. B 2015, 165, 351–359. [Google Scholar] [CrossRef]
- Weidlich, T.; Prokes, L.; Pospisilova, D. Debromination of 2,4,6-tribromophenol coupled with biodegradation. Centr. Eur. J. Chem. 2013, 11, 979–987. [Google Scholar] [CrossRef]
- Weidlich, T.; Krejčová, A.; Prokeš, L. Hydrodebromination of 2,4,6-tribromophenol in aqueous solution using Devarda’s alloy. Monatsh. Chem. 2013, 144, 155–162. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; Wang, R.; Huang, K.; Luo, W.; Tao, X.; Dang, Z.; Yin, H.; Guo, C.; Lu, G. Debromination of 2,2′,4,4′-tetrabromodiphenyl ether (BDE-47) by synthetic Pd/Fe0 and Cu/Fe0 in different protic solvents. Chemosphere 2018, 212, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Tang, T.; Lu, G.; Zheng, Z.; Huang, K.; Li, H.; Tao, X.; Yin, H.; Shi, Z.; Lin, Z.; et al. Mechanisms and pathways of debromination of polybrominated diphenyl ethers (PBDEs) in various nano-zerovalent iron-based bimetallic systems. Sci. Total Environ. 2019, 661, 18–26. [Google Scholar] [CrossRef]
- Fang, L.; Liu, R.; Xu, L.; Li, J.; Huang, L.-Z.; Li, F. Enhanced debromination of tetrabromobisphenol a by zero-valent copper nanoparticle-modified green rusts. Environ. Sci. Nano 2019, 6, 970–980. [Google Scholar] [CrossRef]
- Durante, C.; Isse, A.A.; Todesco, F.; Gennaro, A. Electrocatalytic Activation of Aromatic Carbon-Bromine Bonds toward Carboxylation at Silver and Copper Cathodes. J. Electrochem. Soc. 2013, 160, G3073–G3079. [Google Scholar] [CrossRef]
- Mouselmani, R.; Hachem, A.; Alaaeddine, A.; Métay, E.; Lemaire, M. Reduction of aromatic nitriles into aldehydes using calcium hypophosphite and a nickel precursor. Org. Biomol. Chem. 2018, 16, 6600–6605. [Google Scholar] [CrossRef] [PubMed]
- Lichtenecker, R.J. Synthesis of aromatic 13C/2H-α-ketoacid precursors to be used in selective phenylalanine and tyrosine protein labelling. Org. Biomol. Chem. 2014, 12, 7551–7560. [Google Scholar] [CrossRef]
- Weidlich, T.; Kamenická, B. Recycling of spent hydrodehalogenation catalysts—Problems dealing with separation of aluminium. Inz. Mineral. J. Pol. Mineral. Eng. Soc. 2019, 1, 177–182. [Google Scholar] [CrossRef]
- Bruker AXS GmbH. Diffracplus Basic Evaluating Package. In EVA, 19th ed.; Bruker AXS GmbH: Karlsruhe, Germany, 2013. [Google Scholar]
- Joint Committee on Powder Diffraction Standards. PDF-4+ Database; International Centre of Diffraction Data: Swarthmore, PA, USA.
- Weidlich, T.; Kamenicka, B.; Melanova, K.; Cicmancova, V.; Komersova, A.; Cermak, J. Hydrodechlorination of different chloroaromatic compounds at room temperature and ambient pressure—Differences in reactivity of Cu- and Ni-based Al alloys in an alkaline aqueous solution. Catalysts 2020, 10, 994. [Google Scholar] [CrossRef]
- Raut, S.S.; Kamble, S.P.; Kulkarni, P.S. Efficacy of zero-valent copper (Cu0) nanoparticles and reducing agent for dechlorination of mono chloroaromatics. Chemosphere 2016, 159, 359–366. [Google Scholar] [CrossRef] [PubMed]
- SDBSWeb. National Institute of Advanced Industrial Science and Technology. Available online: https://sdbs.db.aist.go.jp (accessed on 14 April 2021).
- Pouchert, C.J.; Behnke, J. The Aldrich Library of 13C and 1H FT NMR Spectra, 1st ed.; Aldrich Chemical Company, Inc.: St. Louis, MO, USA, 1993; Volume 1–3. [Google Scholar]
- Reich, H.J. Online NMR Database Accessible Free of Charge. Available online: http://www.chem.wisc.edu/areas/organic/index-chem.htm (accessed on 14 April 2021).
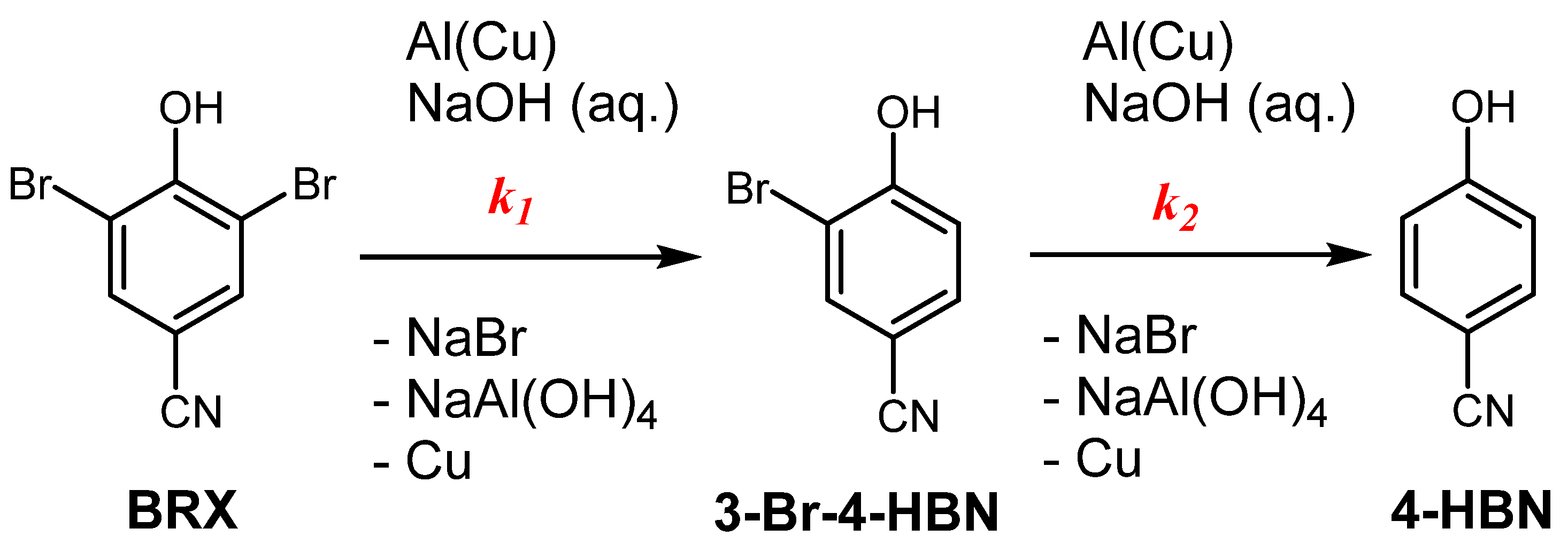
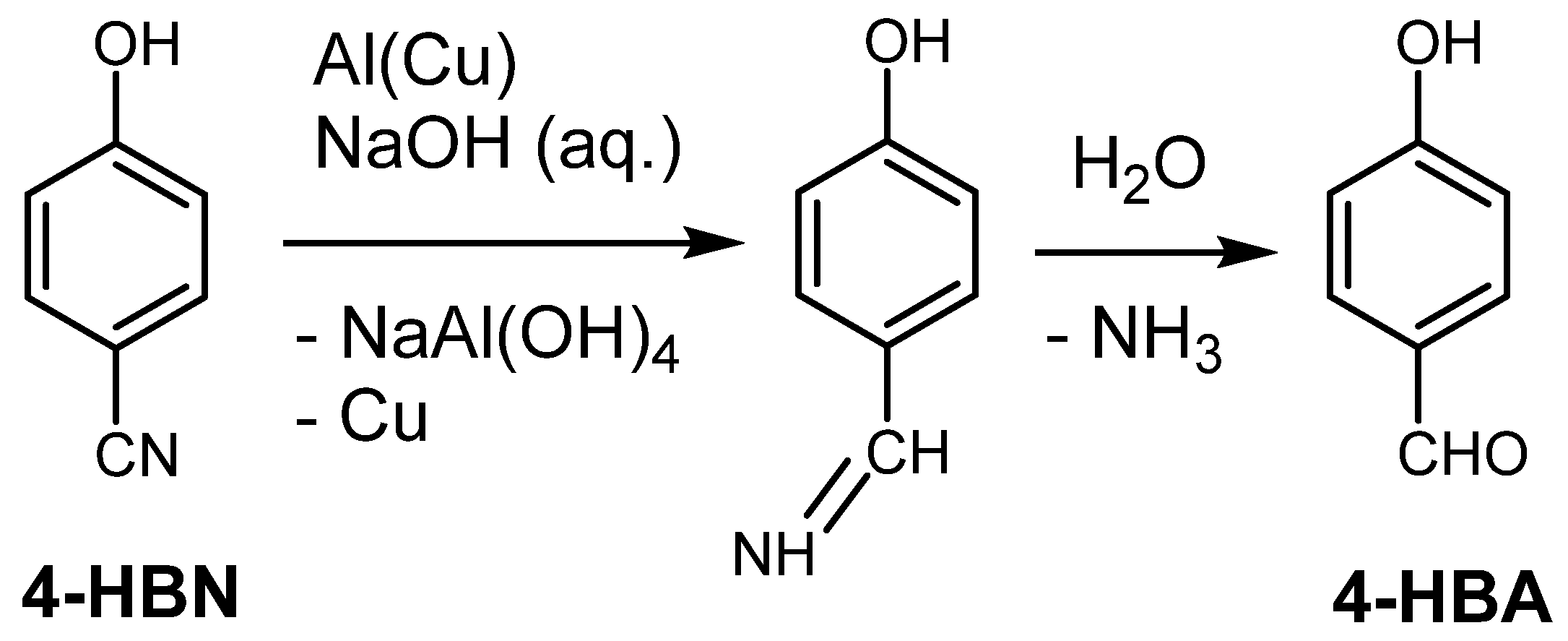
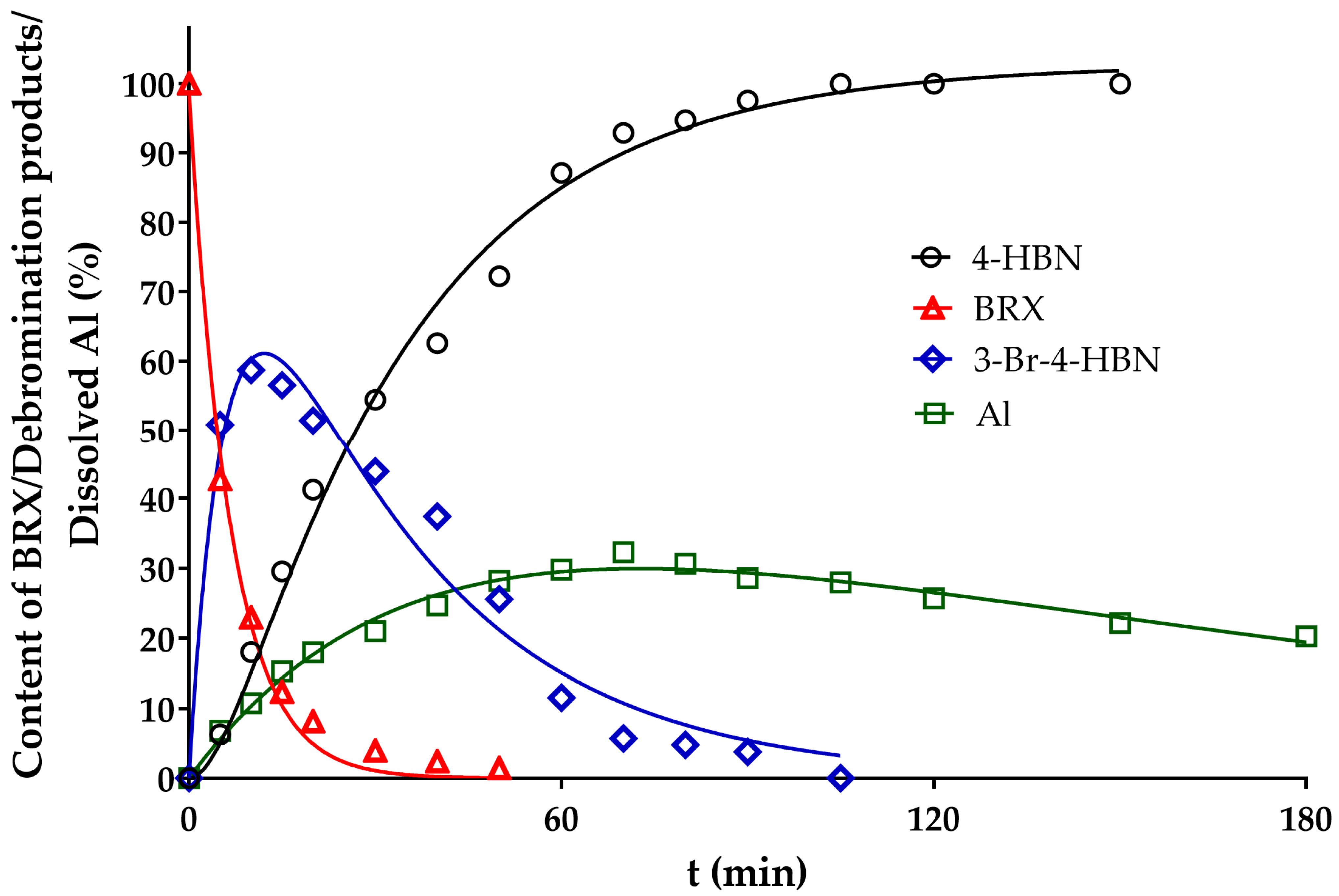
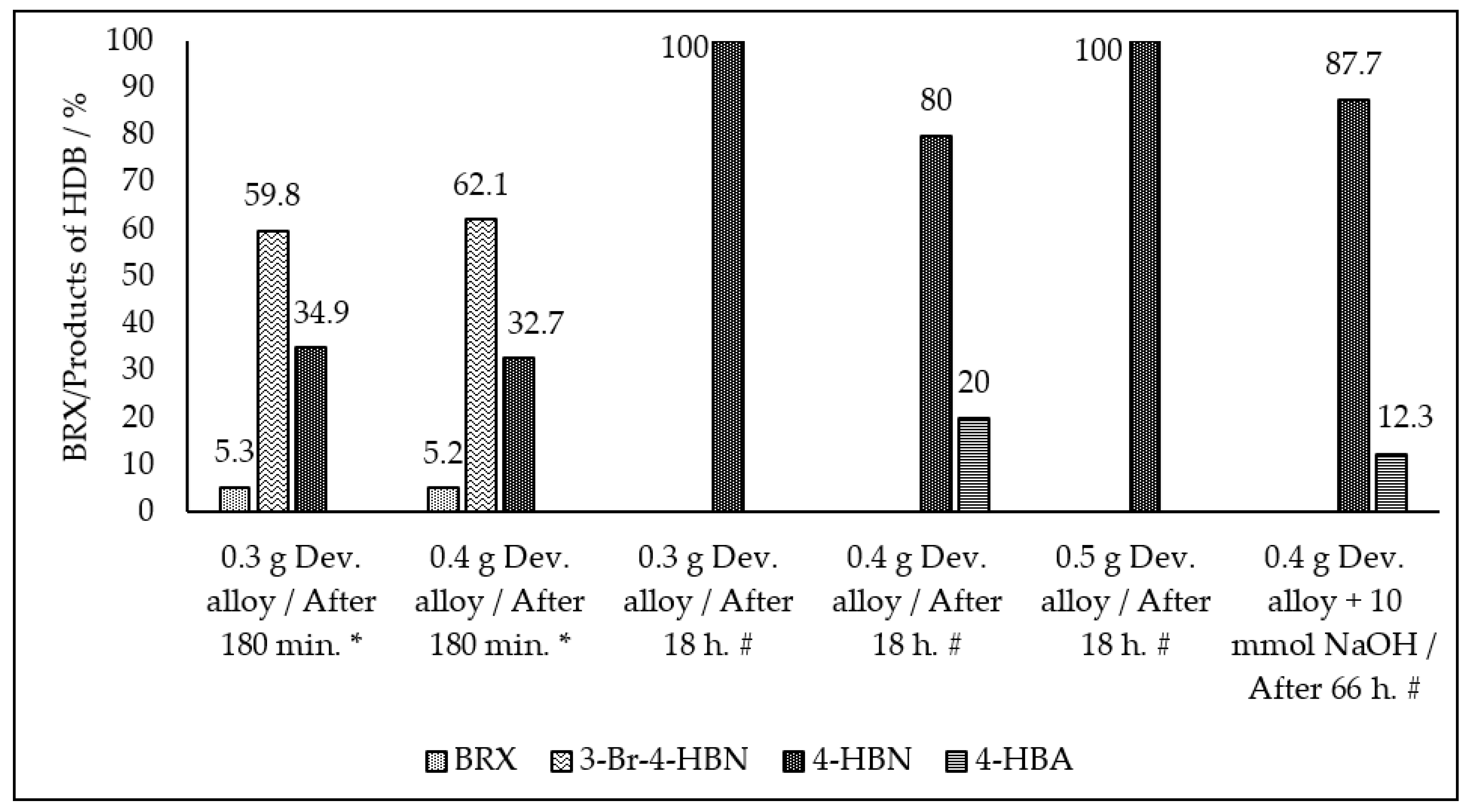


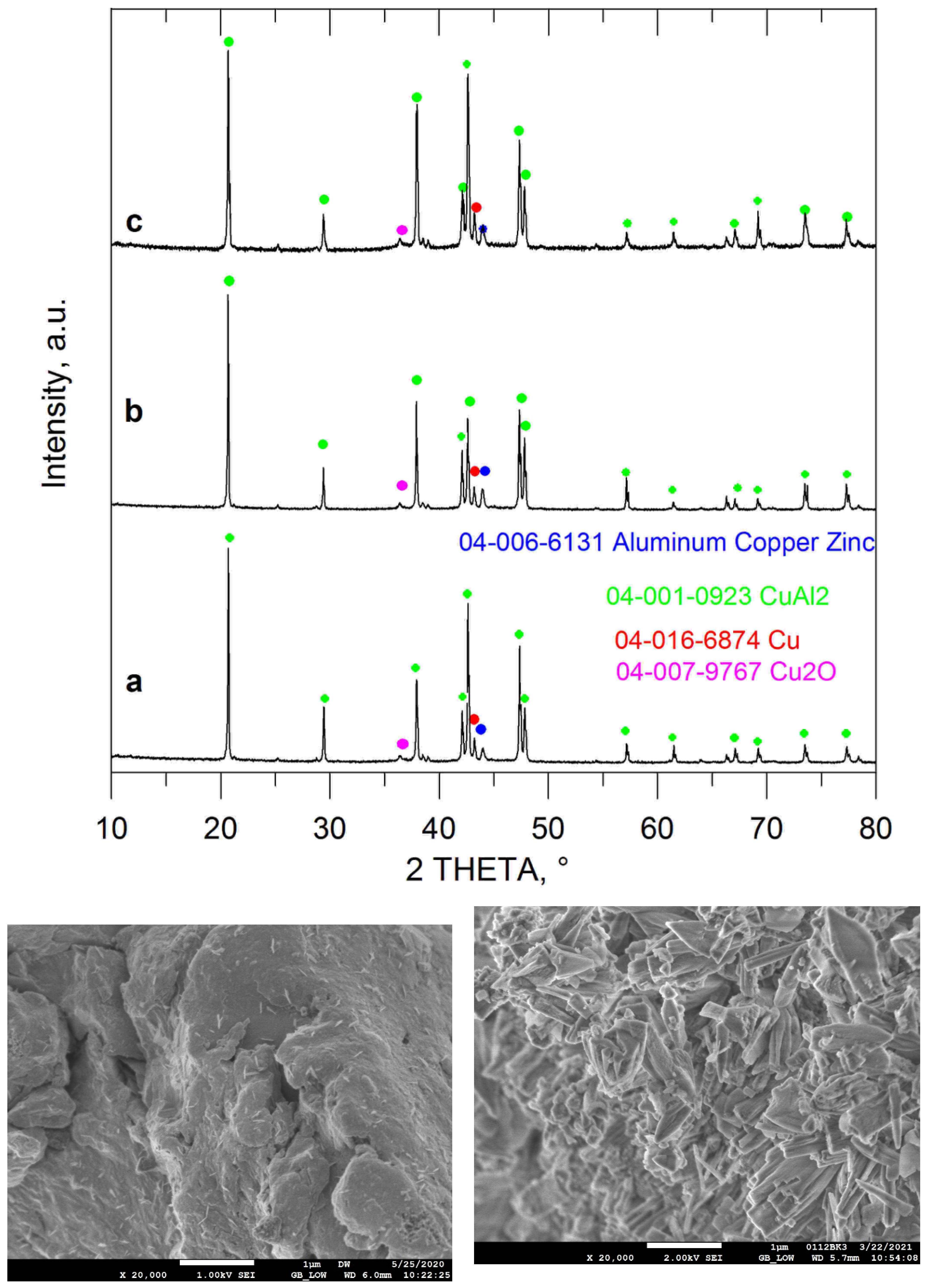
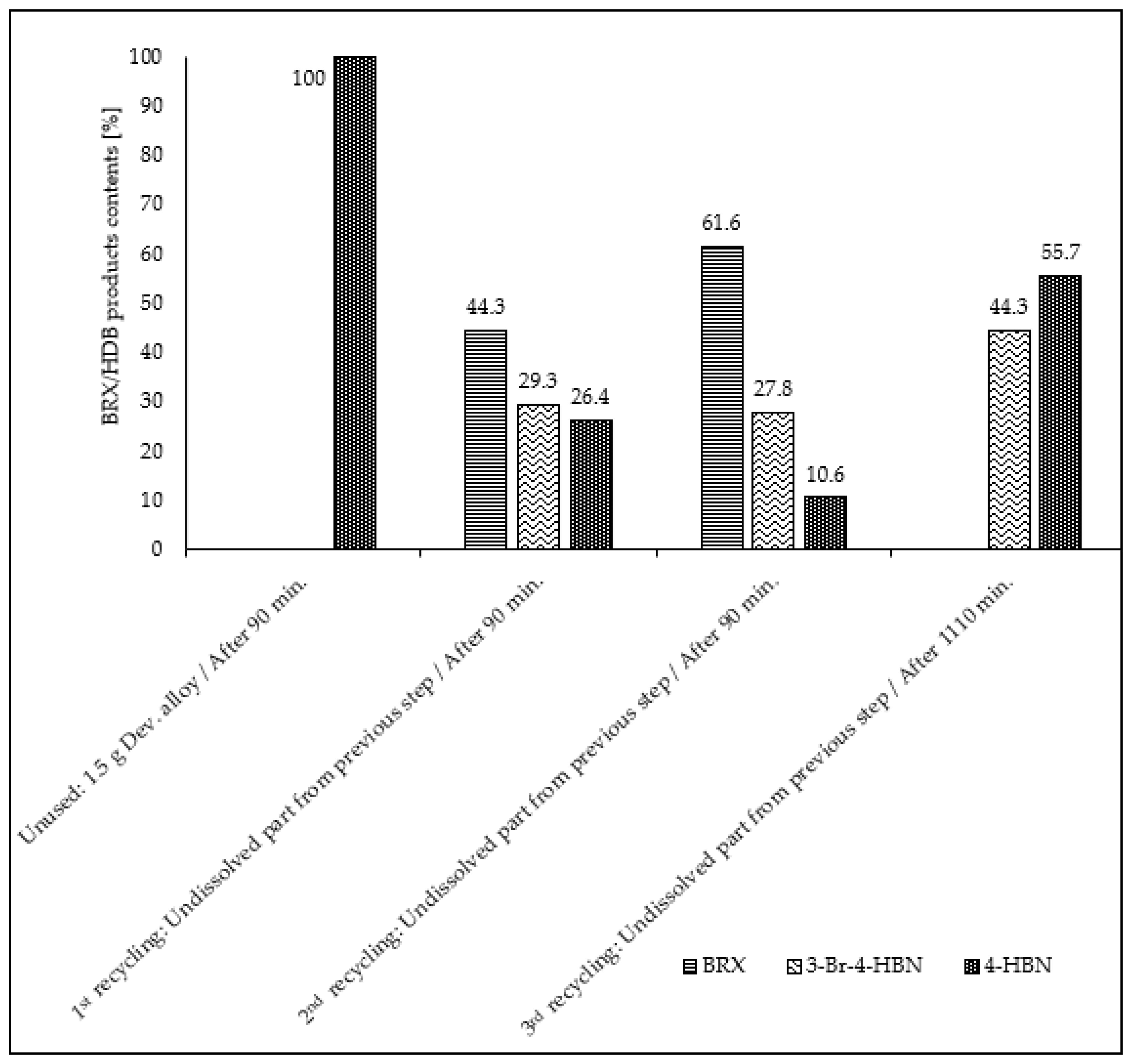
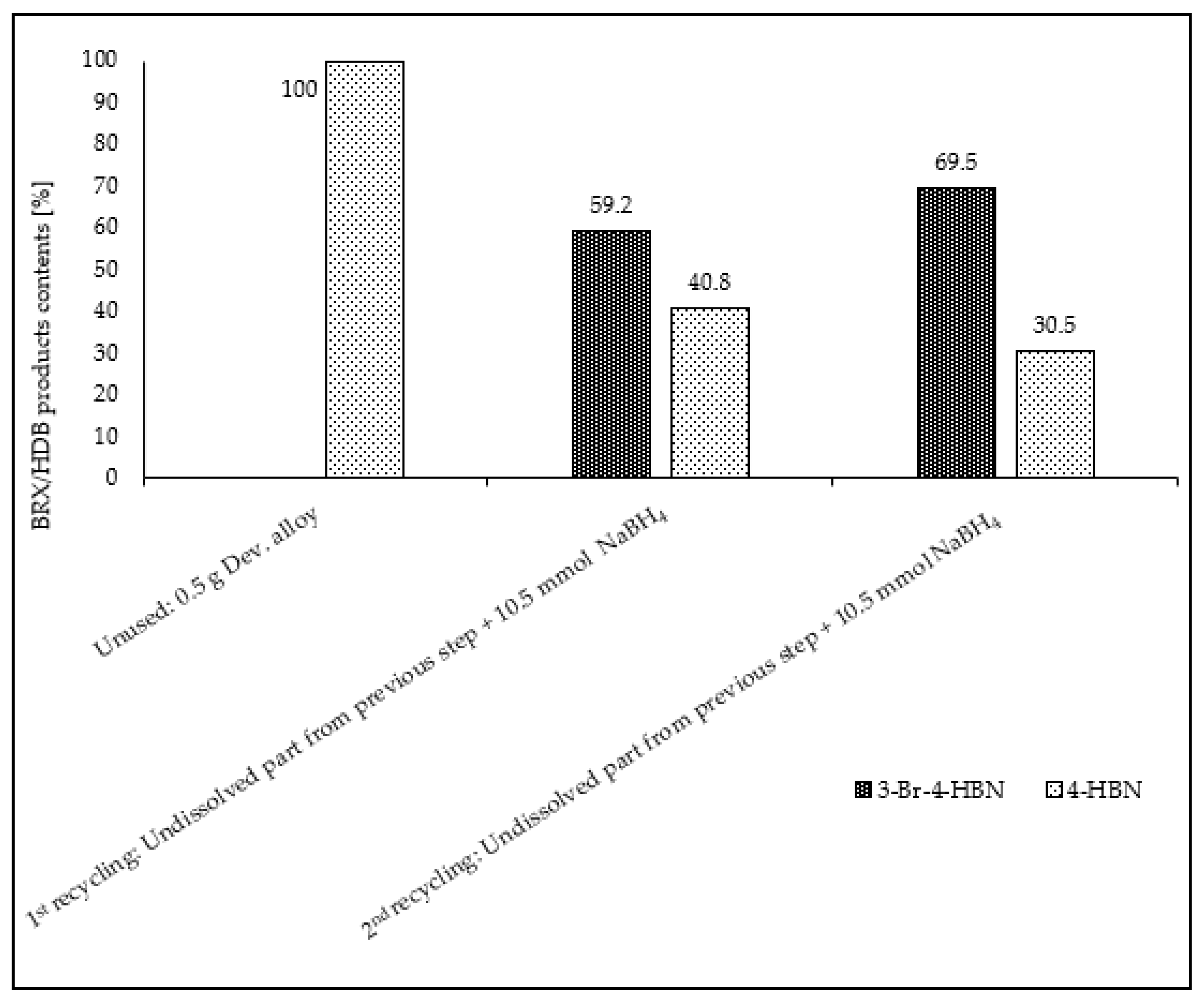
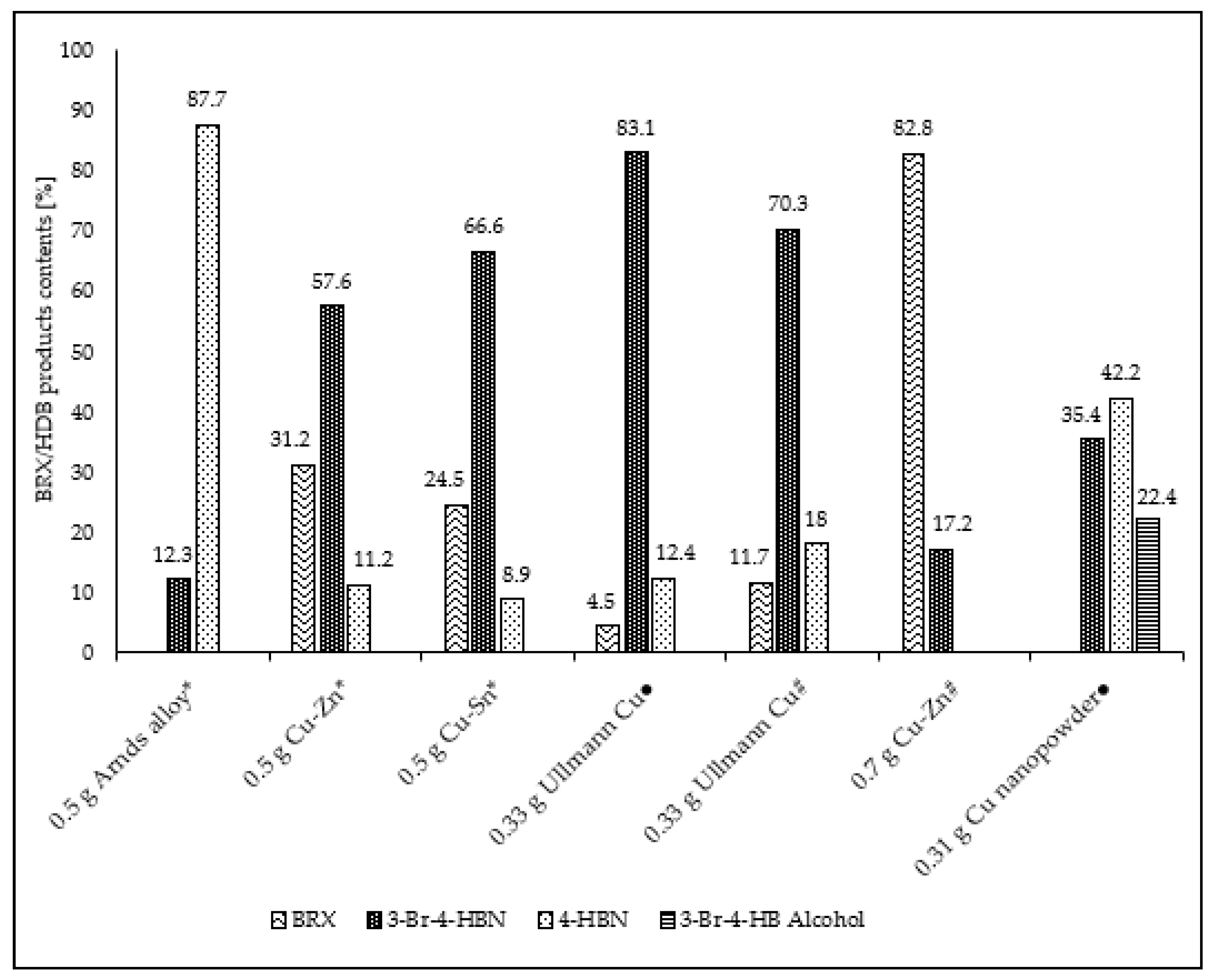





| Entry No. | Reduction Agent Used in aq. Sol of BRX 1 | NaOH Conc. (mmol) | Temperature | Reaction Time | Content of Phenolic Compounds (%) | Content of Dissolved Al (g/L) | Insoluble Matter (g) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| BRX | 4-HBN | 4-HBA | 3-Br-4-HBN | |||||||
| 1 | 0.6 g Dev. alloy | 10 | R.T. | overnight | - | 57.9 | 42.1 | - | 1.565 | 0.3 |
| 2 | 0.6 g Dev. alloy | 10 | R.T. | 66 h | - | 64.5 | 35.5 | - | 1.103 | 0.36 |
| 3 | 0.4 g Dev. alloy | 10 | R.T. | 66 h | - | 80 | 20 | - | 1.525 | 0.14 |
| 4 | 0.4 g Dev. alloy | 20 | R.T. | overnight | - | 87.7 | 12.3 | - | 1.561 | 0.21 |
| 5 | 0.5 g Dev. alloy | 10 | R.T. | overnight | - | 100 | - | - | 0.671 | 0.41 |
| 6 | 0.3 g Dev. alloy | 10 | R.T. | 150 min. | - | 100 | - | - | 0.794 | 0.22 |
| 7 | 0.25 g Dev. alloy | 10 | R.T. | overnight | - | 100 | - | - | n.d. | n.d. |
| 8 | 0.3 g Dev. alloy | 2 | R.T. | 180 min. | 5.3 | 34.9 | - | 59.8 | n.d. | n.d. |
| 9 | 1 g Cu-Zn alloy | 10 | 1 h at 70 °C + 92 h at R.T. | 93 h | 100 | - | - | - | - | n.d. |
| 10 | 0.91 g silver brazing alloy Ag:Cu:Zn | 10 | 1 h at 70 °C + 92 h at R.T. | 93 h | 100 | - | - | - | - | n.d. |
| 11 | 0.7 g Cu-Sn alloy | 10 | 1 h at 70 °C + 92 h at R.T. | 93 h | 100 | - | - | - | - | n.d. |
| 12 | 1 g Fe3Al alloy | 10 | R.T. | overnight | 100 | - | - | - | n.d. | n.d. |
| 13 | 0.3 g Al | 10 | R.T. | overnight | 100 | - | - | - | n.d. | 0 |
| 14 | 0.36 g Al foil | 10 | R.T. | overnight | 100 | - | - | - | n.d. | 0 |
| 15 | 0.5 g Zn | 10 | R.T. | overnight | 100 | - | - | - | - | n.d. |
| 16 | 0.8 g NaBH4 | 10 | R.T. | 96 h | 100 | - | - | - | - | 0 |
| 17 | 0.4 g NaBH4 | 10 | 1 h at 70 °C + 92 h at R.T. | 93 h | - | 6.4 | - | 93.6 | - | 0 |
| Cu/NaBH4 | Cu2O/NaBH4 | Dev. Alloy/NaBH4 | Dev. Alloy/NaOH | |
|---|---|---|---|---|
| k1 ± SD (min−1) calculated based on the time dependence of BRX | (8.8 ± 0.4) × 10−4 | 0.0183 ± 0.0018 | 0.07277 ± 0.0116 | 0.150 ± 0.0082 |
| R2 | 0.9960 | 0.9729 | 0.9620 | 0.9942 |
| k1 ± SD (min−1) calculated based on the time dependence of 3-Br-4-HBN | (7.4 ± 0.2) × 10−4 | 0.0192 ± 0.0026 | * 0.06864 ± 0.0241 | 0.1630 ± 0.0343 |
| k2 ± SD (min−1) calculated based on the time dependence of 3-Br-4-HBN | (7.3 ± 0.3) × 10−5 | 0.00225 ± 0.0030 | * 0.03874 ± 0.0163 | 0.0341 ± 0.0047 |
| R2 | 0.9981 | 0.9565 | 0.9842 | 0.9719 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weidlich, T.; Kamenická, B.; Beneš, L.; Čičmancová, V.; Komersová, A.; Čermák, J.; Švec, P. Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution. Catalysts 2021, 11, 699. https://doi.org/10.3390/catal11060699
Weidlich T, Kamenická B, Beneš L, Čičmancová V, Komersová A, Čermák J, Švec P. Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution. Catalysts. 2021; 11(6):699. https://doi.org/10.3390/catal11060699
Chicago/Turabian StyleWeidlich, Tomáš, Barbora Kamenická, Ludvík Beneš, Veronika Čičmancová, Alena Komersová, Jiří Čermák, and Petr Švec. 2021. "Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution" Catalysts 11, no. 6: 699. https://doi.org/10.3390/catal11060699
APA StyleWeidlich, T., Kamenická, B., Beneš, L., Čičmancová, V., Komersová, A., Čermák, J., & Švec, P. (2021). Cu-Catalyzed Hydrodehalogenation of Brominated Aromatic Pollutants in Aqueous Solution. Catalysts, 11(6), 699. https://doi.org/10.3390/catal11060699







