Efficient Advanced Oxidation Process (AOP) for Photocatalytic Contaminant Degradation Using Exfoliated Metal-Free Graphitic Carbon Nitride and Visible Light-Emitting Diodes
Abstract
1. Introduction
2. Results and Discussion
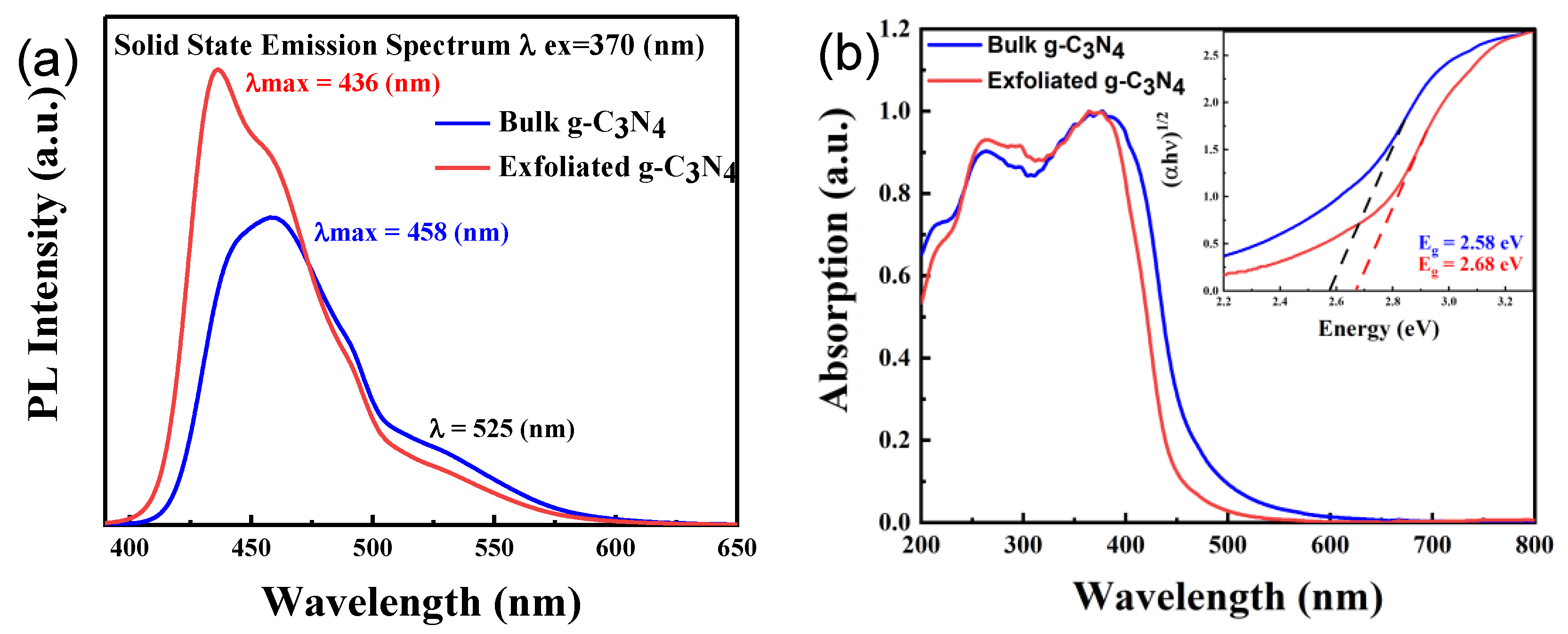
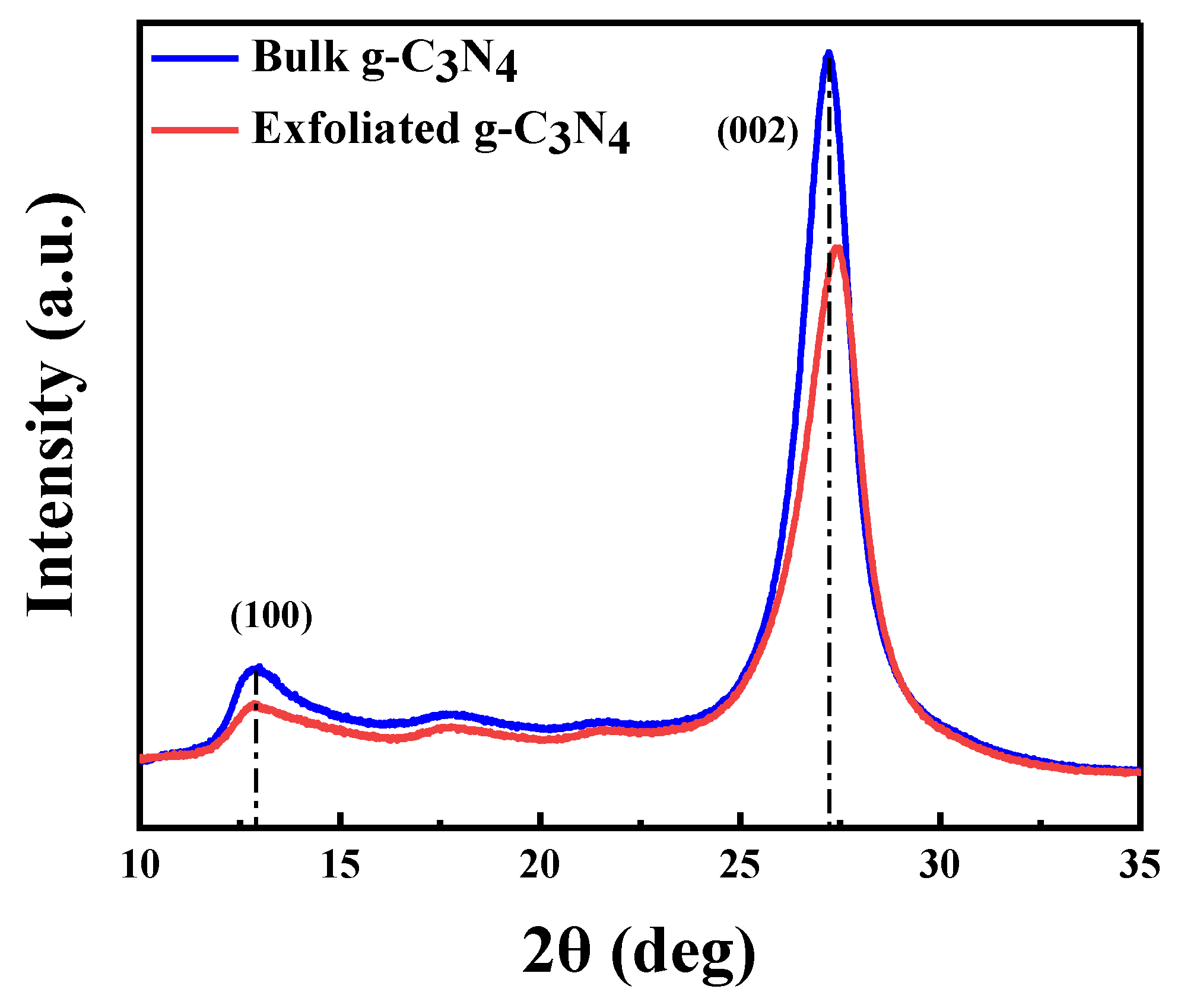
2.1. Photocatalyst Characterization
2.2. Photocatalytic Degradation of Phenol
2.2.1. Effect of Exfoliation
2.2.2. Effect of the Light Source
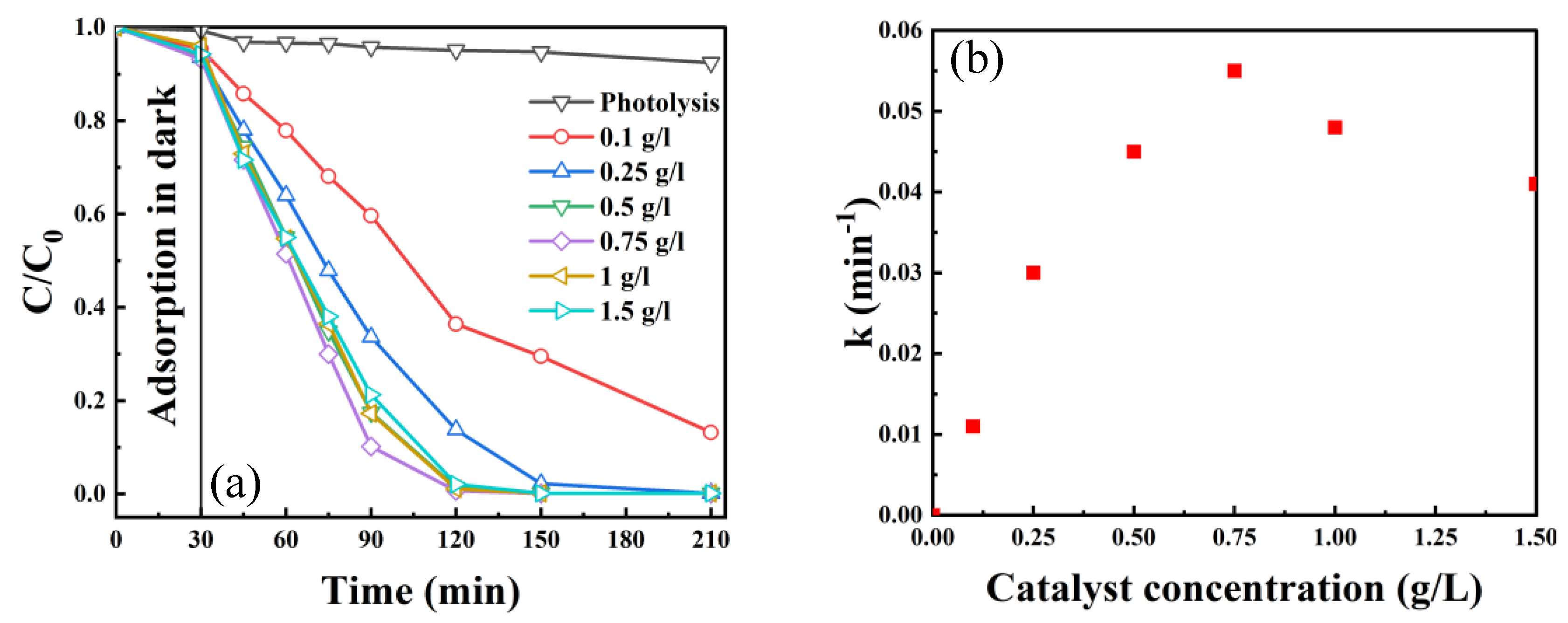
2.2.3. Effect of Catalyst Amount
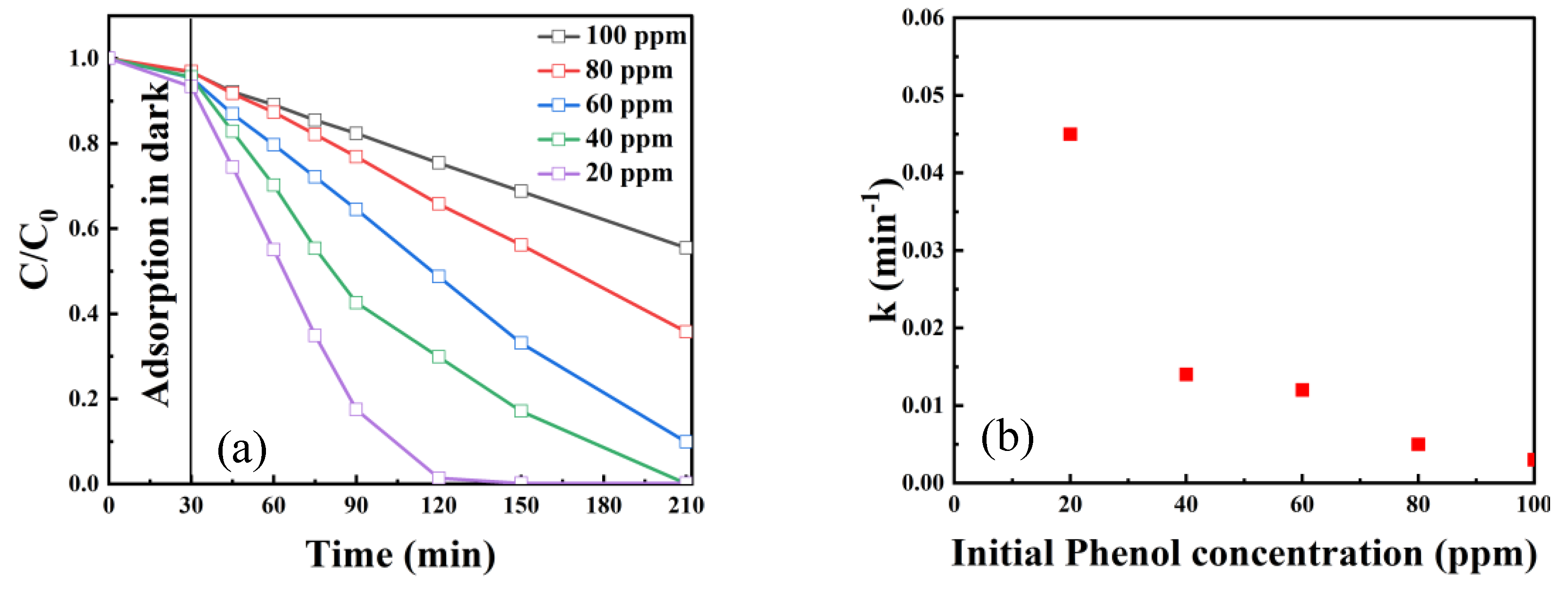
2.2.4. Effect of Pollutant Concentration
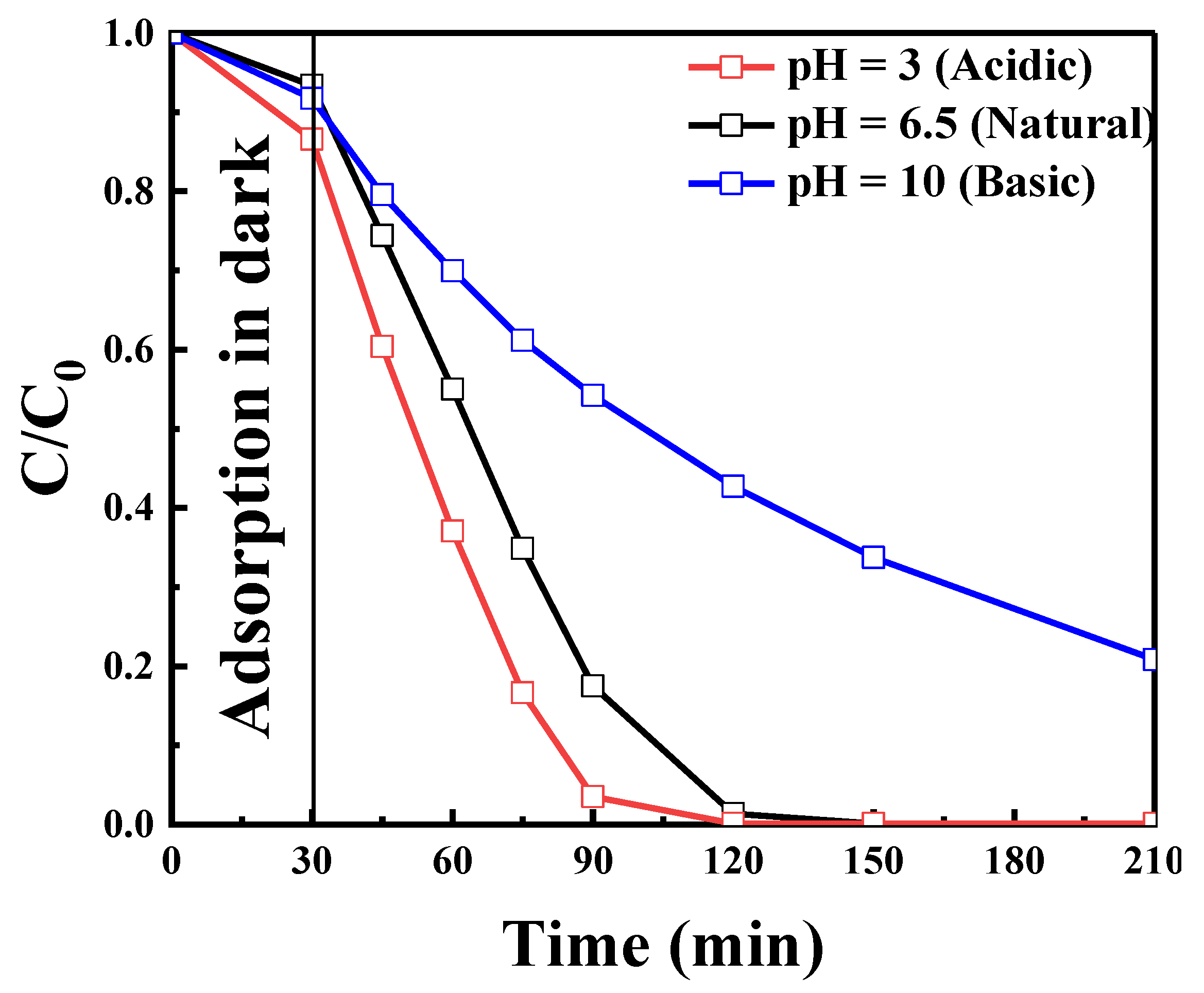
2.2.5. Effect of Initial pH
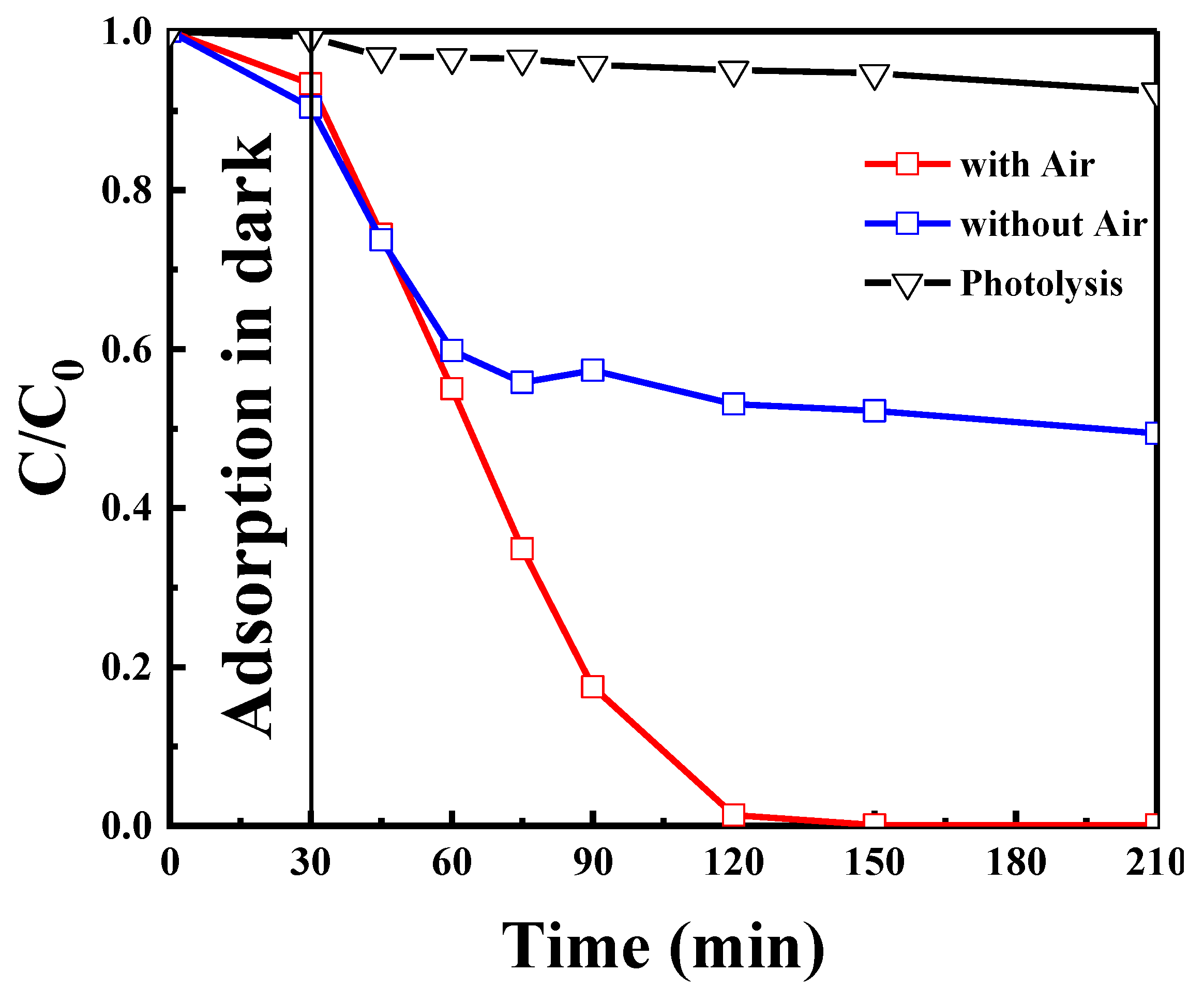
2.2.6. Effect of Dissolved Oxygen
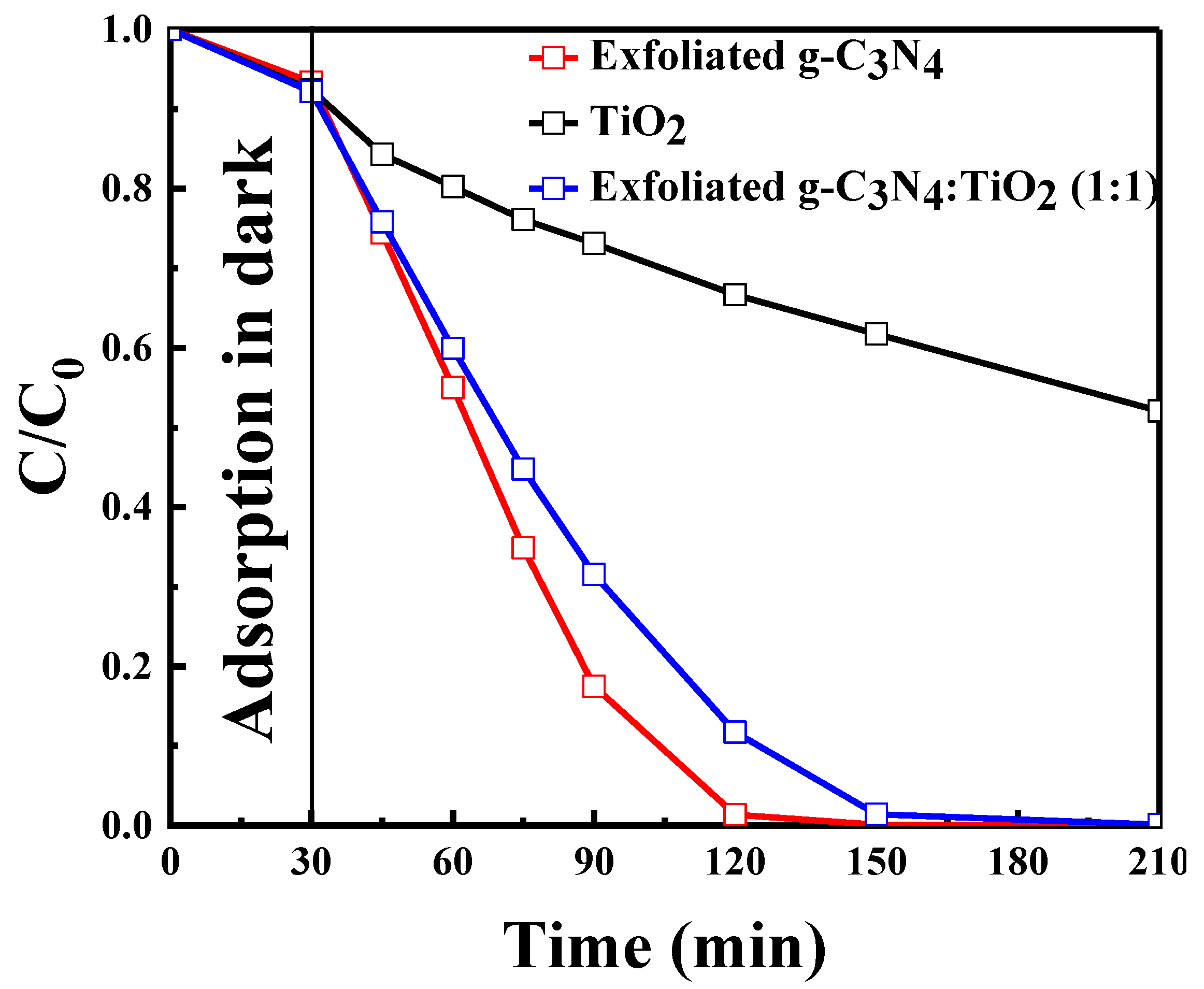
2.2.7. Effect of TiO2 and its Composite
2.2.8. Catalyst Reutilization Tests
2.2.9. Photocatalytic Efficiency for the Degradation of a Mixture of Organic Pollutants
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis and Characterization of the Catalysts
3.3. Photocatalytic Experimentation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dewidar, H.; Nosier, S.; El-Shazly, A. Photocatalytic degradation of phenol solution using Zinc Oxide/UV. J. Chem. Heal. Saf. 2018, 25, 2–11. [Google Scholar] [CrossRef]
- Zarin, S.; Aslam, Z.; Zahir, A.; Kamal, M.S.; Rana, A.G.; Ahmad, W.; Ahmed, S. Synthesis of bimetallic/carbon nanocomposite and its application for phenol removal. J. Iran. Chem. Soc. 2018, 15, 2689–2701. [Google Scholar] [CrossRef]
- Hararah, M.A.; Ibrahim, K.A.; Al-Muhtaseb, A.H.; Yousef, R.I.; Abu-Surrah, A.; Qatatsheh, A. Removal of phenol from aqueous solutions by adsorption onto polymeric adsorbents. J. Appl. Polym. Sci. 2010, 117, 1908–1913. [Google Scholar] [CrossRef]
- Aslam, Z.; Qaiser, M.; Ali, R.; Abbas, A.; Ihsanullah; Zarin, S. Al2O3/MnO2/CNTs nanocomposite: Synthesis, characterization and phenol adsorption. Full Nanotub. Carbon Nanostruct. 2019, 27, 591–600. [Google Scholar] [CrossRef]
- Shen, W.; Ge, Q.; Gu, K.; Nie, Y.; Jiao, L.; Zhu, Z.; Fang, Y. Stable Organic Titanium Catalysts and Reactive Distillation Used for the Transesterification of Dimethyl Carbonate with Phenol. Chem. Eng. Technol. 2020, 43, 2359–2364. [Google Scholar] [CrossRef]
- Huang, C.-H.; Liou, R.-M.; Chen, S.-H.; Hung, M.-Y.; Lai, C.-L.; Lai, J.-Y. Microbial degradation of phenol in a modified three-stage airlift packing-bed reactor. Water Environ. Res. 2010, 82, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, Y.; Koparal, A.S.; Öğütveren, Ü.B. Phenol Removal through Chemical Oxidation using Fenton Reagent. Chem. Eng. Technol. 2007, 30, 583–586. [Google Scholar] [CrossRef]
- Tasbihi, M.; Acharjya, A.; Thomas, A.; Reli, M.; Ambrožová, N.; Kočcí, K.; Schomäcker, R. Photocatalytic CO2 Reduction by Mesoporous Polymeric Carbon Nitride Photocatalysts. J. Nanosci. Nanotechnol. 2018, 18, 5636–5644. [Google Scholar] [CrossRef]
- Chowdhury, P.; Nag, S.; Ray, A.K. Degradation of Phenolic Compounds Through UV and Visible- Light-Driven Photocatalysis: Technical and Economic Aspects. In Phenolic Compounds—Natural Sources, Importance and Applications; IntechOpen: London, UK, 2017. [Google Scholar]
- Torres-Pinto, A.; Sampaio, M.J.; Silva, C.G.; Faria, J.L.; Silva, A.M. Metal-free carbon nitride photocatalysis with in situ hydrogen peroxide generation for the degradation of aromatic compounds. Appl. Catal. B Environ. 2019, 252, 128–137. [Google Scholar] [CrossRef]
- Kumar, S.; Karthikeyan, S.; Lee, A.F. g-C3N4-Based Nanomaterials for Visible Light-Driven Photocatalysis. Catalysts 2018, 8, 74. [Google Scholar] [CrossRef]
- Rana, A.G.; Ahmad, W.; Al-Matar, A.; Shawabkeh, R.; Aslam, Z. Synthesis and characterization of Cu–Zn/TiO2 for the photocatalytic conversion of CO2 to methane. Environ. Technol. 2016, 38, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Rosli, N.I.M.; Lam, S.-M.; Sin, J.-C.; Satoshi, I.; Mohamed, A.R. Photocatalytic Performance of ZnO/g-C3N4 for Removal of Phenol under Simulated Sunlight Irradiation. J. Environ. Eng. 2018, 144, 04017091. [Google Scholar] [CrossRef]
- Moradi, V.; Ahmed, F.; Jun, M.B.; Blackburn, A.; Herring, R.A. Acid-treated Fe-doped TiO2 as a high performance photocatalyst used for degradation of phenol under visible light irradiation. J. Environ. Sci. 2019, 83, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.J.; Silva, A.M.; Silva, C.G.; Faria, J.L. Graphitic carbon nitride modified by thermal, chemical and mechanical processes as metal-free photocatalyst for the selective synthesis of benzaldehyde from benzyl alcohol. J. Catal. 2017, 353, 44–53. [Google Scholar] [CrossRef]
- Nobijari, L.A.; Schwarze, M.; Tasbihi, M. Photocatalytic Degradation of Phenol Using Photodeposited Pt Nanoparticles on Titania. J. Nanosci. Nanotechnol. 2020, 20, 1056–1065. [Google Scholar] [CrossRef]
- Al-Kandari, H.; Abdullah, A.M.; Ahmad, Y.H.; AlQaradawi, S.Y.; Mohamed, A.M. An efficient eco advanced oxidation process for phenol mineralization using a 2D/3D nanocomposite photocatalyst and visible light irradiations. Sci. Rep. 2017, 7, 9898. [Google Scholar] [CrossRef]
- Ren, H.-T.; Jia, S.-Y.; Wu, Y.; Wu, S.-H.; Zhang, T.-H.; Han, X. Improved Photochemical Reactivities of Ag2O/g-C3N4 in Phenol Degradation under UV and Visible Light. Ind. Eng. Chem. Res. 2014, 53, 17645–17653. [Google Scholar] [CrossRef]
- Lee, S.C.; Lintang, H.O.; Yuliati, L. A Urea Precursor to Synthesize Carbon Nitride with Mesoporosity for Enhanced Activity in the Photocatalytic Removal of Phenol. Chem. Asian J. 2012, 7, 2139–2144. [Google Scholar] [CrossRef]
- Hu, J.-Y.; Tian, K.; Jiang, H. Improvement of phenol photodegradation efficiency by a combined g-C3N4/Fe(III)/persulfate system. Chemosphere 2016, 148, 34–40. [Google Scholar] [CrossRef]
- Deng, P.; Gan, M.; Zhang, X.; Li, Z.; Hou, Y. Non-noble-metal Ni nanoparticles modified N-doped g-C3N4 for efficient photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2019, 44, 30084–30092. [Google Scholar] [CrossRef]
- Huang, Z.; Li, F.; Chen, B.; Lu, T.; Yuan, Y.; Yuan, G. Well-dispersed g-C3N4 nanophases in mesoporous silica channels and their catalytic activity for carbon dioxide activation and conversion. Appl. Catal. B Environ. 2013, 136-137, 269–277. [Google Scholar] [CrossRef]
- Sharma, M.; Vaidya, S.; Ganguli, A.K. Enhanced photocatalytic activity of g-C3N4-TiO2 nanocomposites for degradation of Rhodamine B dye. J. Photochem. Photobiol. A Chem. 2017, 335, 287–293. [Google Scholar] [CrossRef]
- Hernández-Uresti, D.; Vázquez, A.; Sanchez-Martinez, D.; Obregón, S. Performance of the polymeric g-C3N4 photocatalyst through the degradation of pharmaceutical pollutants under UV–vis irradiation. J. Photochem. Photobiol. A Chem. 2016, 324, 47–52. [Google Scholar] [CrossRef]
- Zheng, Q.; Durkin, D.P.; Elenewski, J.E.; Sun, Y.; Banek, N.A.; Hua, L.; Chen, H.; Wagner, M.J.; Zhang, W.; Shuai, D. Visible-Light-Responsive Graphitic Carbon Nitride: Rational Design and Photocatalytic Applications for Water Treatment. Environ. Sci. Technol. 2016, 50, 12938–12948. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-J.; Shen, Z.; Wu, S.; Su, Y.; Pei, L.; Ji, Z.; Ding, M.; Bai, W.; Chen, Y.; Yu, Z.-T.; et al. Liquid exfoliation of g-C3N4 nanosheets to construct 2D-2D MoS2/g-C3N4 photocatalyst for enhanced photocatalytic H2 production activity. Appl. Catal. B Environ. 2019, 246, 120–128. [Google Scholar] [CrossRef]
- Dang, T.T.T.; Le, S.T.T.; Channei, D.; Khanitchaidecha, W.; Nakaruk, A. Photodegradation mechanisms of phenol in the photocatalytic process. Res. Chem. Intermed. 2016, 42, 5961–5974. [Google Scholar] [CrossRef]
- Benisti, I.; Shaik, F.; Xing, Z.; Ben-Refael, A.; Amirav, L.; Paz, Y. The effect of Pt cocatalyst on the performance and transient IR spectrum of photocatalytic g-C3N4 nanospheres. Appl. Surf. Sci. 2021, 542, 148432. [Google Scholar] [CrossRef]
- Yang, Y.; Lei, W.; Xu, Y.; Zhou, T.; Xia, M.; Hao, Q. Determination of trace uric acid in serum using porous graphitic carbon nitride (g-C3N4) as a fluorescent probe. Microchim. Acta 2017, 185, 39. [Google Scholar] [CrossRef]
- Dong, F.; Wang, Z.; Sun, Y.; Ho, W.-K.; Zhang, H. Engineering the nanoarchitecture and texture of polymeric carbon nitride semiconductor for enhanced visible light photocatalytic activity. J. Colloid Interface Sci. 2013, 401, 70–79. [Google Scholar] [CrossRef]
- Papailias, I.; Giannakopoulou, T.; Todorova, N.; Demotikali, D.; Vaimakis, T.; Trapalis, C. Effect of processing temperature on structure and photocatalytic properties of g-C3N4. Appl. Surf. Sci. 2015, 358, 278–286. [Google Scholar] [CrossRef]
- Djoko, S.Y.T.; Bashiri, H.; Njoyim, E.T.; Arabamiri, M.; Djepang, S.; Tamo, A.K.; Laminsi, S.; Tasbihi, M.; Schwarze, M.; Schomäcker, R.; et al. Urea and green tea like precursors for the preparation of g-C3N4 based carbon nanomaterials (CNMs) composites as photocatalysts for photodegradation of pollutants under UV light irradiation. J. Photochem. Photobiol. A Chem. 2020, 398, 112596. [Google Scholar] [CrossRef]
- Stroyuk, A.L.; Panasiuk, Y.V.; Raevskaya, A.E.; Kuchmy, S.Y. Spectral and Luminescent Characteristics of Products from Exfoliation of Graphitic Carbon Nitride Produced at Various Temperatures. Theor. Exp. Chem. 2015, 51, 243–251. [Google Scholar] [CrossRef]
- Jiang, J.; Ou-Yang, L.; Zhu, L.; Zheng, A.; Zou, J.; Yi, X.; Tang, H. Dependence of electronic structure of g-C3N4 on the layer number of its nanosheets: A study by Raman spectroscopy coupled with first-principles calculations. Carbon 2014, 80, 213–221. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced Photoresponsive Ultrathin Graphitic-Phase C3N4 Nanosheets for Bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, E.; Hu, X.; Tang, C.; Wan, J.; Li, J.; Fan, J. A simple process to prepare few-layer g-C3N4 nanosheets with enhanced photocatalytic activities. Appl. Surf. Sci. 2015, 358, 246–251. [Google Scholar] [CrossRef]
- Yu, B.; Meng, F.; Khan, M.W.; Qin, R.; Liu, X. Facile synthesis of AgNPs modified TiO2@g-C3N4 heterojunction composites with enhanced photocatalytic activity under simulated sunlight. Mater. Res. Bull. 2020, 121, 110641. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Rodríguez-Padrón, D.; Puente-Santiago, A.R.; Kubacka, A.; Luque, R.; Fernández-García, M. Sunlight-Driven Hydrogen Production Using an Annular Flow Photoreactor and g-C3N4-Based Catalysts. ChemPhotoChem 2018, 2, 870–877. [Google Scholar] [CrossRef]
- Yuan, X.; Zhou, C.; Jin, Y.; Jing, Q.; Yang, Y.; Shen, X.; Tang, Q.; Mu, Y.; Du, A.-K. Facile synthesis of 3D porous thermally exfoliated g-C3N4 nanosheet with enhanced photocatalytic degradation of organic dye. J. Colloid Interface Sci. 2016, 468, 211–219. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.-Q.; Bao, S.-J.; Lu, S.; Xu, M.; Long, D.; Pu, S. Tuning and thermal exfoliation graphene-like carbon nitride nanosheets for superior photocatalytic activity. Ceram. Int. 2016, 42, 18521–18528. [Google Scholar] [CrossRef]
- Yang, L.; Liu, X.; Liu, Z.; Wang, C.; Liu, G.; Li, Q.; Feng, X. Enhanced photocatalytic activity of g-C3N4 2D nanosheets through thermal exfoliation using dicyandiamide as precursor. Ceram. Int. 2018, 44, 20613–20619. [Google Scholar] [CrossRef]
- Niu, P.; Zhang, L.; Liu, G.; Cheng, H.-M. Graphene-Like Carbon Nitride Nanosheets for Improved Photocatalytic Activities. Adv. Funct. Mater. 2012, 22, 4763–4770. [Google Scholar] [CrossRef]
- Wang, J.; Guo, P.; Dou, M.; Cheng, Y.; Jönsson, P.G.; Zhao, Z. Visible light-driven g-C3N4/m-Ag2Mo2O7 composite photocatalysts: Synthesis, enhanced activity and photocatalytic mechanism. RSC Adv. 2014, 4, 51008–51015. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, W.; Chen, X.; Wang, J.; Zhu, Y. Photocatalytic activity enhancement of core-shell structure g-C3N4@TiO2 via controlled ultrathin g-C3N4 layer. Appl. Catal. B Environ. 2018, 220, 337–347. [Google Scholar] [CrossRef]
- Zhu, B.; Xia, P.; Ho, W.; Yu, J. Isoelectric point and adsorption activity of porous g-C3N4. Appl. Surf. Sci. 2015, 344, 188–195. [Google Scholar] [CrossRef]
- Dong, S.; Zeng, Z.; Cai, W.; Zhou, Z.; Dou, C.; Liu, H.; Xia, J. The zeta potentials of g-C3N4 nanoparticles: Effect of electrolyte, ionic strength, pH, and humic acid. J. Nanoparticle Res. 2019, 21, 233. [Google Scholar] [CrossRef]
- Babu, B.; Shim, J.; Kadam, A.; Yoo, K. Modification of porous g-C3N4 nanosheets for enhanced photocatalytic activity: In-situ synthesis and optimization of NH4Cl quantity. Catal. Commun. 2019, 124, 123–127. [Google Scholar] [CrossRef]
- Ding, W.; Liu, S.; He, Z. One-step synthesis of graphitic carbon nitride nanosheets for efficient catalysis of phenol removal under visible light. Chin. J. Catal. 2017, 38, 1711–1718. [Google Scholar] [CrossRef]
- Sampaio, M.J.; Silva, C.G.; Silva, A.M.T.; Faria, J.L. Kinetic modelling for the photocatalytic degradation of phenol by using TiO2-coated glass raschig rings under simulated solar light. J. Chem. Technol. Biotechnol. 2014, 91, 346–352. [Google Scholar] [CrossRef]
- Herrmann, J.-M. Titania-based true heterogeneous photocatalysis. Environ. Sci. Pollut. Res. 2012, 19, 3655–3665. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, S.P.; Behera, A.; Martha, S.; Acharya, R.; Parida, K. Facile synthesis of exfoliated graphitic carbon nitride for photocatalytic degradation of ciprofloxacin under solar irradiation. J. Mater. Sci. 2019, 54, 5726–5742. [Google Scholar] [CrossRef]
- Daneshvar, N.; Rabbani, M.; Modirshahla, N.; Behnajady, M. Kinetic modeling of photocatalytic degradation of Acid Red 27 in UV/TiO2 process. J. Photochem. Photobiol. A Chem. 2004, 168, 39–45. [Google Scholar] [CrossRef]
- Lam, S.-M.; Sin, J.-C.; Mohamed, A.R. Parameter effect on photocatalytic degradation of phenol using TiO2-P25/activated carbon (AC). Korean J. Chem. Eng. 2010, 27, 1109–1116. [Google Scholar] [CrossRef]
- Malekshoar, G.; Pal, K.; He, Q.; Yu, A.; Ray, A.K. Enhanced Solar Photocatalytic Degradation of Phenol with Coupled Graphene-Based Titanium Dioxide and Zinc Oxide. Ind. Eng. Chem. Res. 2014, 53, 18824–18832. [Google Scholar] [CrossRef]
- Benhebal, H.; Chaib, M.; Salmon, T.; Geens, J.; Leonard, A.; Lambert, S.D.; Crine, M.; Heinrichs, B. Photocatalytic degradation of phenol and benzoic acid using zinc oxide powders prepared by the sol–gel process. Alex. Eng. J. 2013, 52, 517–523. [Google Scholar] [CrossRef]
- Tseng, D.-H.; Juang, L.-C.; Huang, H.-H. Effect of Oxygen and Hydrogen Peroxide on the Photocatalytic Degradation of Monochlorobenzene in Aqueous Suspension. Int. J. Photoenergy 2012, 2012, 328526. [Google Scholar] [CrossRef]
- Escobar, J.A.P.; Moctezuma, E.; Rosales, B.S. Heterojunctions for Photocatalytic Wastewater Treatment: Positive Holes, Hydroxyl Radicals and Activation Mechanism under UV and Visible Light. Int. J. Chem. React. Eng. 2020, 18. [Google Scholar] [CrossRef]
- Riaz, N.; Bustam, M.A.; Chong, F.K.; Man, Z.B.; Khan, M.S.; Shariff, A.M. Photocatalytic Degradation of DIPA Using Bimetallic Cu-Ni/TiO2Photocatalyst under Visible Light Irradiation. Sci. World J. 2014, 2014, 342020. [Google Scholar] [CrossRef]
- Al Hashemi, W.; Maraqa, M.; Rao, M.; Hossain, M. Characterization and removal of phenolic compounds from condensate-oil refinery wastewater. Desalination Water Treat. 2015, 54, 660–671. [Google Scholar] [CrossRef]
- Graham Solomons, T.W.; Fryhle, C.B. Organic Chemistry, 10th ed.; Wiley: Hoboken, NJ, USA, 2011; p. 1164S. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, A.G.; Tasbihi, M.; Schwarze, M.; Minceva, M. Efficient Advanced Oxidation Process (AOP) for Photocatalytic Contaminant Degradation Using Exfoliated Metal-Free Graphitic Carbon Nitride and Visible Light-Emitting Diodes. Catalysts 2021, 11, 662. https://doi.org/10.3390/catal11060662
Rana AG, Tasbihi M, Schwarze M, Minceva M. Efficient Advanced Oxidation Process (AOP) for Photocatalytic Contaminant Degradation Using Exfoliated Metal-Free Graphitic Carbon Nitride and Visible Light-Emitting Diodes. Catalysts. 2021; 11(6):662. https://doi.org/10.3390/catal11060662
Chicago/Turabian StyleRana, Adeem Ghaffar, Minoo Tasbihi, Michael Schwarze, and Mirjana Minceva. 2021. "Efficient Advanced Oxidation Process (AOP) for Photocatalytic Contaminant Degradation Using Exfoliated Metal-Free Graphitic Carbon Nitride and Visible Light-Emitting Diodes" Catalysts 11, no. 6: 662. https://doi.org/10.3390/catal11060662
APA StyleRana, A. G., Tasbihi, M., Schwarze, M., & Minceva, M. (2021). Efficient Advanced Oxidation Process (AOP) for Photocatalytic Contaminant Degradation Using Exfoliated Metal-Free Graphitic Carbon Nitride and Visible Light-Emitting Diodes. Catalysts, 11(6), 662. https://doi.org/10.3390/catal11060662








