Photocatalytic Degradation of Valsartan by MoS2/BiOCl Heterojunctions
Abstract
1. Introduction
2. Results
2.1. Catalyst Characterization
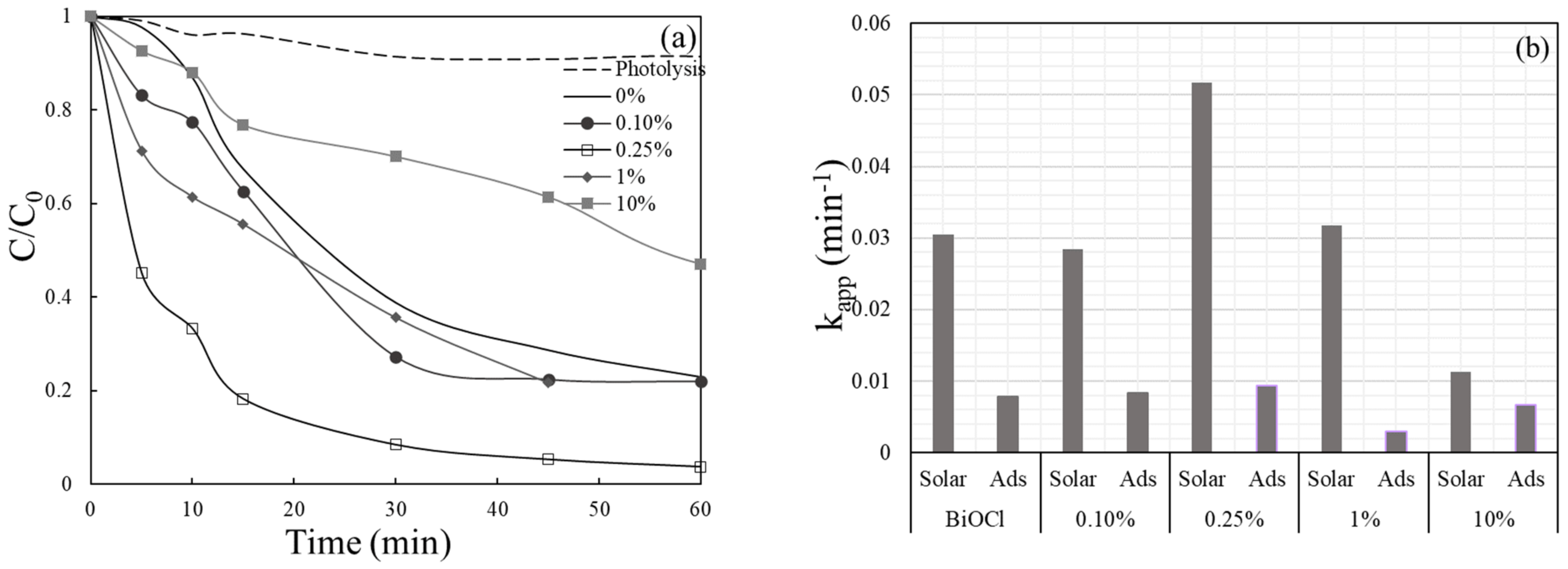
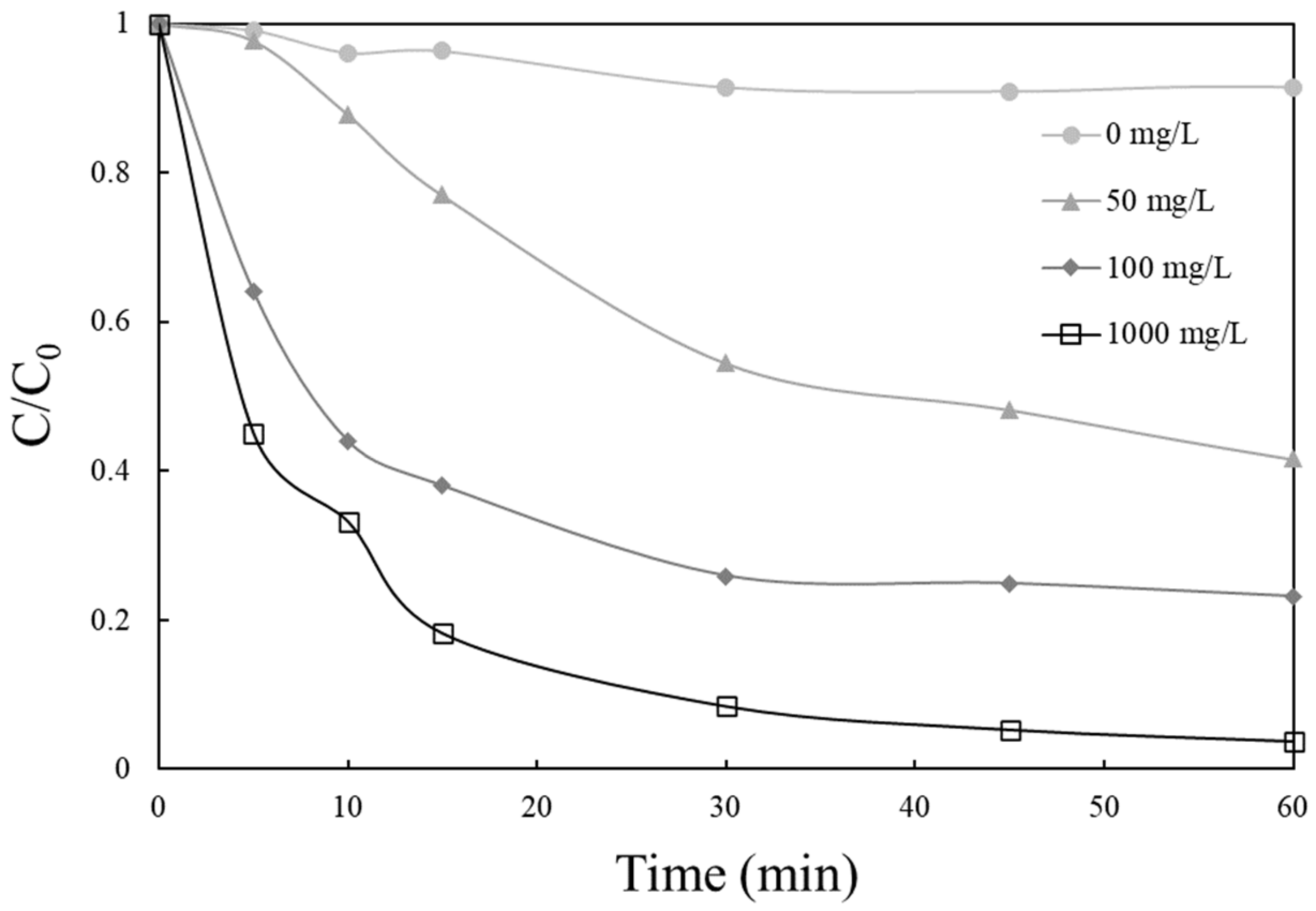
2.2. Catalyst Activity
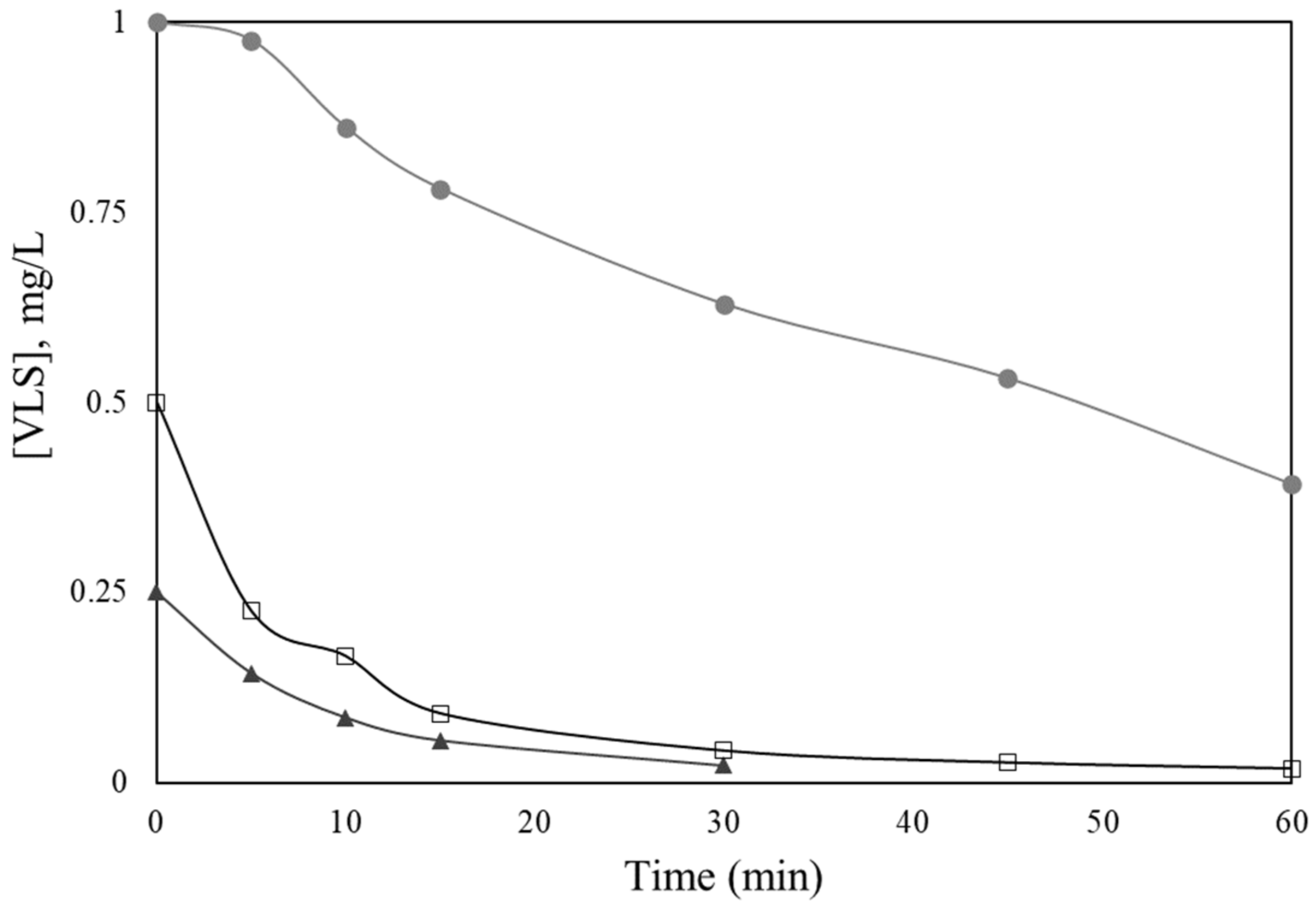
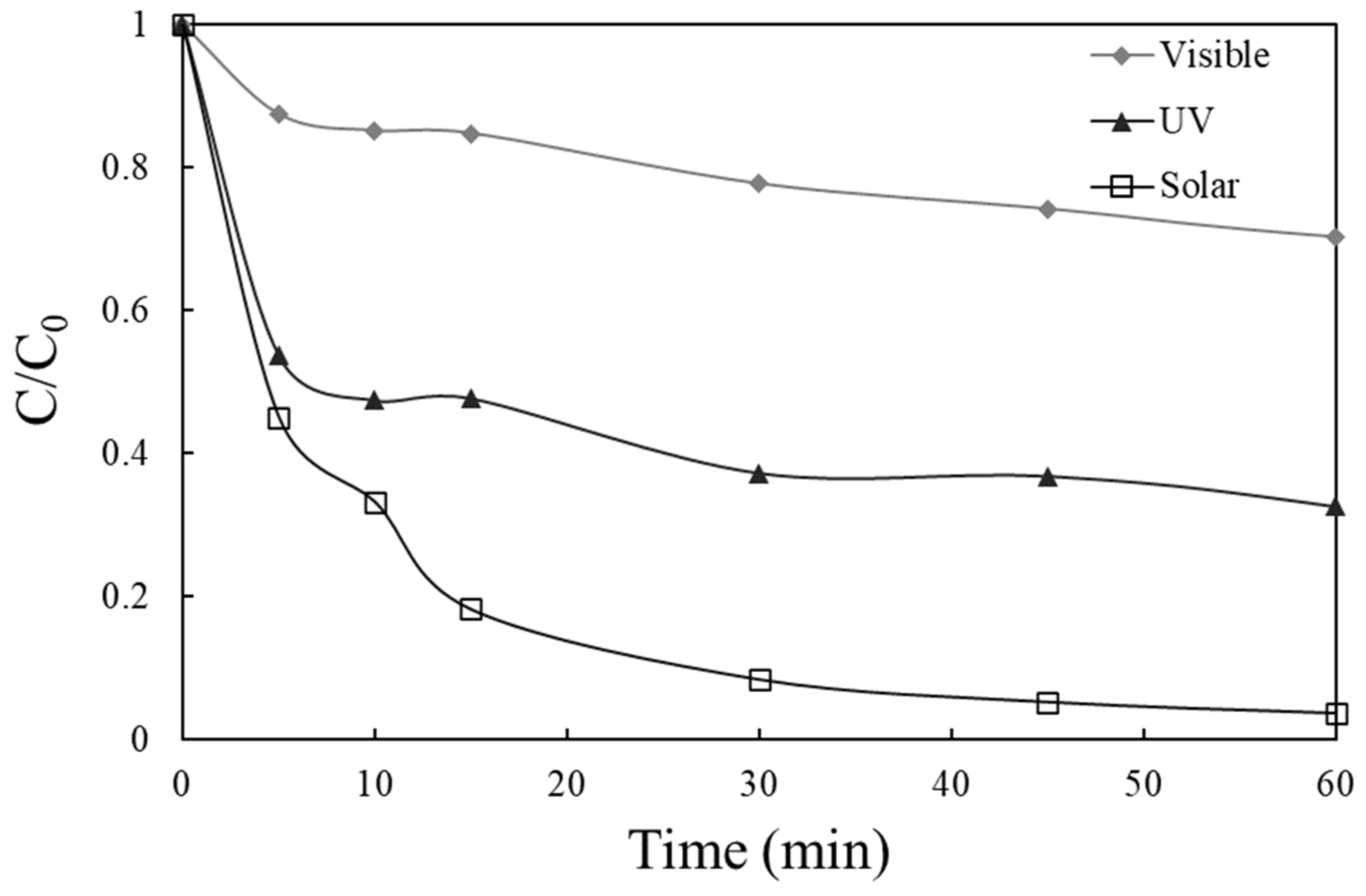
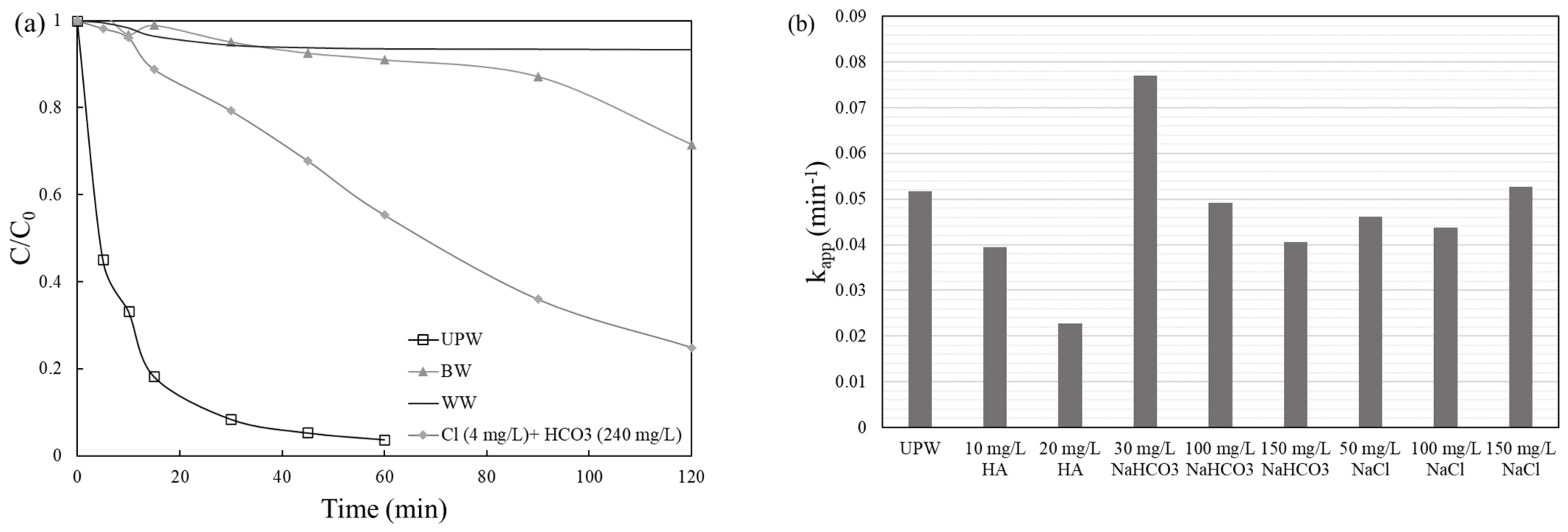
2.3. Effect of Operating Conditions
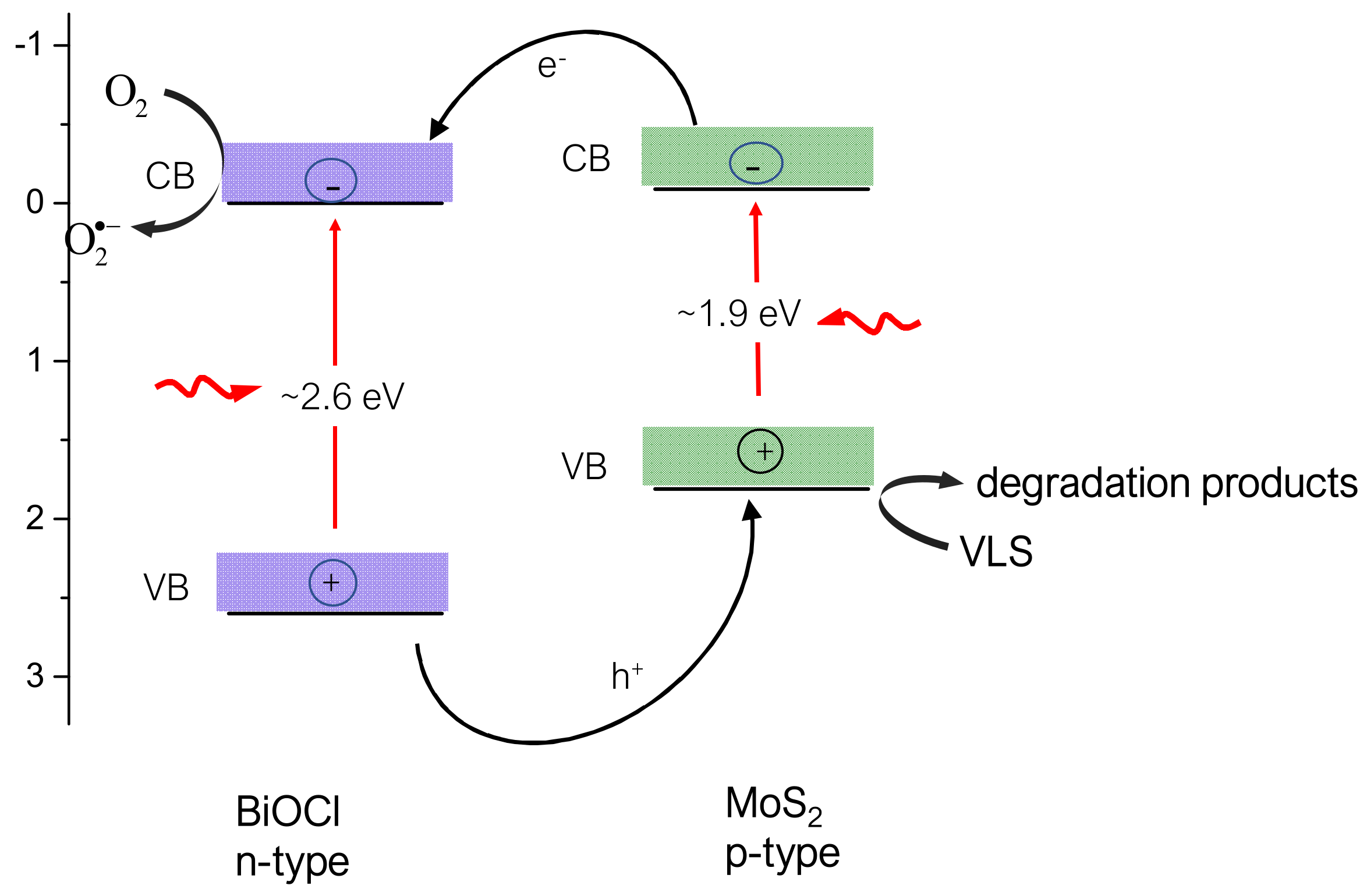
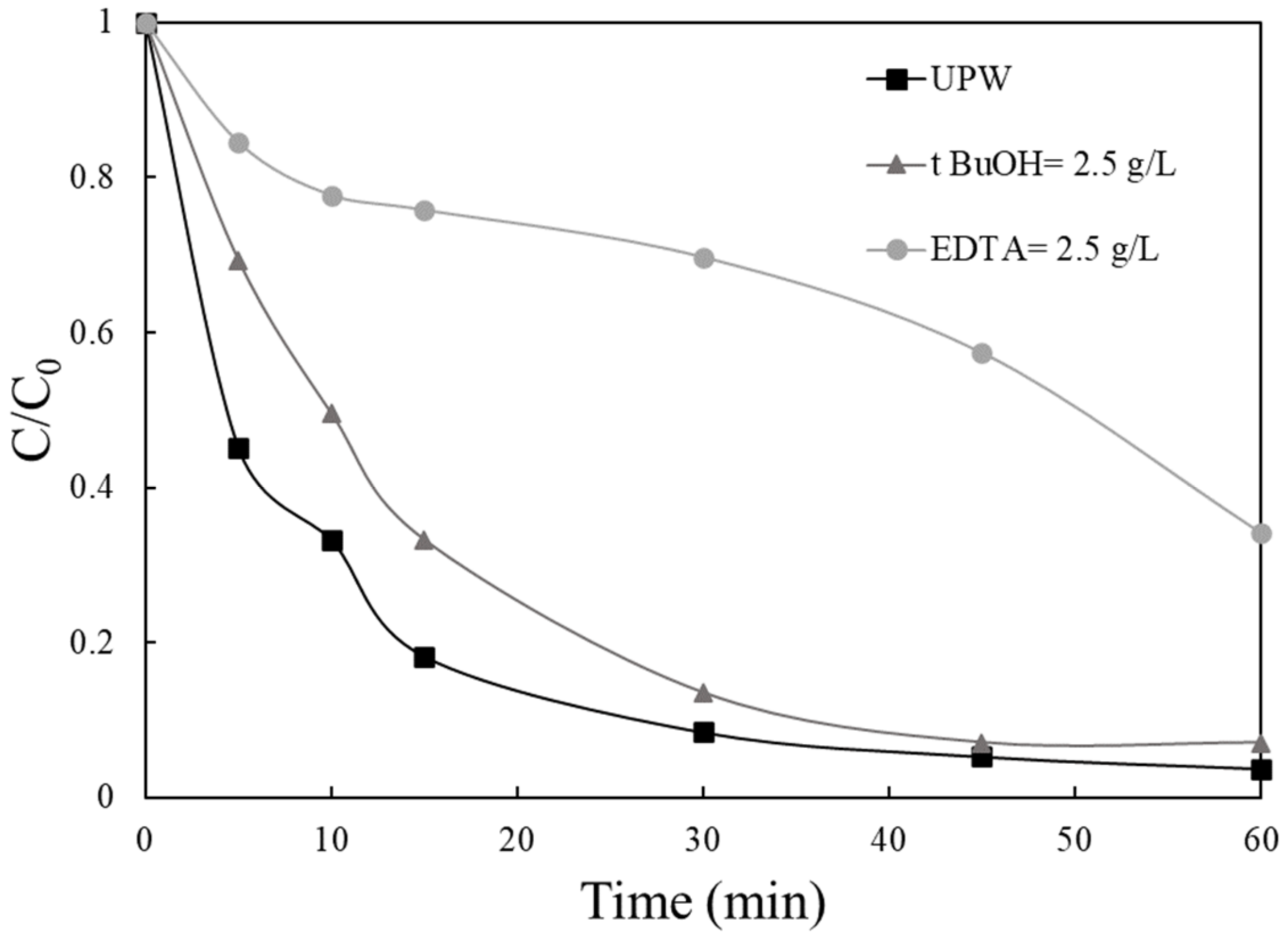
2.4. Mechanism of VLS Degradation and Catalyst Reusability
3. Materials and Methods
3.1. Chemicals and Water Matrices
3.2. Preparation of Photocatalyst
3.3. Catalyst Characterization
3.4. Experimental Procedure
4. Conclusions
- The addition of small amounts of MoS2 on BiOCl enhanced its photocatalytic activity, which is maximized for the 0.25 wt.% MoS2 sample.
- Several operating factors influence VLS degradation kinetics, such catalyst and VLS concentration and the water matrix.
- The organic matter present in real water matrices seem to slow down VLS removal rate.
- The photogenerated holes are proposed to be the primary oxidative species in the system.
- The as-synthesized 0.25 MoS2/BiOCl photocatalyst exhibits excellent performance after repeated use.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kummerer, K. Antibiotics in the aquatic environment—A review—Part I. Chemosphere 2009, 75, 417–434. [Google Scholar] [CrossRef] [PubMed]
- López-Pacheco, I.Y.; Silva-Núñez, A.; Salinas-Salazar, C.; Arévalo-Gallegos, A.; Lizarazo-Holguin, L.A.; Barceló, D.; Iqbal, H.M.N.; Parra-Saldívar, R. Anthropogenic contaminants of high concern: Existence in water resources and their adverse effects. Sci. Total Environ. 2019, 690, 1068–1088. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.H.; Reinhard, M.; Gin, K.Y.-H. Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions—A review. Water Res. 2018, 133, 182–207. [Google Scholar] [CrossRef]
- Martinez-Pachon, D.; Espinosa-Barrera, P.; Rincon-Ortiz, J.; Moncayo-Lasso, A. Treatment of two sartan antihypertensives in water by photo-electro-Fenton using BDD anodes: Degradation kinetics, theoretical analyses, primary transformations and matrix effects. Chemosphere 2021, 270, 129491. [Google Scholar] [CrossRef] [PubMed]
- Rosario Brunettom, M.; Contreras, Y.; Clavijo, S.; Torres, D.; Delgado, Y.; Ovalles, F.; Ayala, C.; Gallignani, M.; Estela, J.J.; Martin, V.C. Determination of losartan, telmisartan, and valsartan by direct injection of human urine into a column-switching liquid chromatographic system with fluorescence detection. J. Pharm. Biomed. Anal. 2009, 50, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Saydam, M.; Takka, S. Bioavailability file: Valsartan. FABAD J. Pharm. Sci. 2007, 32, 185–196. [Google Scholar]
- Hernández, F.; Ibáñez, M.; Gracia-Lor, E.; Sancho, J.V. Retrospective LC-QTOF-MS analysis searching for pharmaceutical metabolites in urban wastewater. J. Sep. Sci. 2011, 34, 3517–3526. [Google Scholar] [CrossRef] [PubMed]
- Klosterhaus, S.L.; Grace, R.; Hamilton, M.C.; Yee, D. Method validation and reconnaissance of pharmaceuticals, personal care products, and alkylphenols in surface waters, sediments, and mussels in an urban estuary. Environ. Int. 2013, 54, 92–99. [Google Scholar] [CrossRef]
- Seabra Pereira, C.D.; Maranho, L.A.; Cortez, F.S.; Pusceddu, F.H.; Santos, A.R.; Ribeiro, D.A.; Cesar, A.; Guimarães, L.L. Occurrence of pharmaceuticals and cocaine in a Brazilian coastal zone. Sci. Total Environ. 2016, 548–549, 148–154. [Google Scholar] [CrossRef]
- Botero-Coy, A.M.; Martínez-Pachón, D.; Boix, C.; Rincón, R.J.; Castillo, N.; Arias-Marín, L.P.; Manrique-Losada, L.; Torres-Palma, R.; Moncayo-Lasso, A.; Hernández, F. An investigation into the occurrence and removal of pharmaceuticals in Colombian wastewater. Sci. Total Environ. 2018, 642, 842–853. [Google Scholar] [CrossRef]
- Klavarioti, M.; Mantzavinos, D.; Kassinos, D. Removal of residual pharmaceuticals from aqueous systems by advanced oxidation processes. Environ. Int. 2009, 35, 402–417. [Google Scholar] [CrossRef]
- Giwa, A.; Yusuf, A.; Balogun, H.A.; Sambudi, N.S.; Bilad, M.R.; Adeyemi, I.; Chakraborty, S.; Curcio, S. Recent advances in advanced oxidation processes for removal of contaminants from water: A comprehensive review. Process Saf. Environ. 2021, 146, 220–256. [Google Scholar] [CrossRef]
- Parsons, S. Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2004. [Google Scholar]
- Petala, A.; Noe, A.; Frontistis, Z.; Drivas, C.; Kennou, S.; Mantzavinos, D.; Kondarides, D.I. Synthesis and characterization of CoOx/BiVO4 photocatalysts for the degradation of propyl paraben. J. Hazard. Mater. 2019, 372, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Hapeshi, E.; Achilleos, A.; Vasquez, M.I.; Michael, C.; Xekoukoulotakis, N.P.; Mantzavinos, D.; Kassinos, D. Drugs degrading photocatalytically: Kinetics and mechanisms of ofloxacin and atenolol removal on titania suspensions. Water Res. 2010, 44, 1737–1746. [Google Scholar] [CrossRef]
- Carp, O.; Huisman, C.L.; Reller, A. Photoinduced Reactivity of Titanium Dioxide. Prog. Solid State Chem. 2004, 32, 33–177. [Google Scholar] [CrossRef]
- Lan, Y.; Lu, Y.; Ren, Z. Mini review on photocatalysis of titanium dioxide nanoparticles and their solar applications. Nano Energy 2013, 2, 1031–1045. [Google Scholar] [CrossRef]
- Meng, X.; Zhang, Z. Bismuth-based photocatalytic semiconductors: Introduction, challenges and possible approaches. J. Mol. Catal. A Chem. 2016, 423, 533–549. [Google Scholar] [CrossRef]
- Saison, T.; Chemin, N.; Chaneac, C.; Durupthy, O.; Mariey, L.; Mauge, F.; Brezova, V.; Jolivet, J.P. New Insights into BiVO4 Properties as Visible Light Photocatalyst. J. Phys. Chem. C 2015, 119, 12967–12977. [Google Scholar] [CrossRef]
- Jiang, H.; Dai, H.; Meng, X.; Zhang, L.; Deng, J.; Liu, Y.; Au, C.T. Hydrothermal fabrication and visible-light-driven photocatalytic properties of bismuth vanadate with multiple morphologies and/or porous structures for Methyl Orange degradation. J. Environ. Sci. 2012, 24, 449–457. [Google Scholar] [CrossRef]
- Nong, L.X.; Nguyen, V.H.; Bach, L.G.; Tran, T.V.; Nguyen, T.D. Photocatalytic activity for degradation of sulfamethoxazole by BiVO4 using visible light. IOP Conf. Ser. Mater. Sci. Eng. 2020, 736, 042016. [Google Scholar] [CrossRef]
- Ioannidi, A.; Petala, A.; Frontistis, Z. Copper phosphide promoted BiVO4 photocatalysts for the degradation of sulfamethoxazole in aqueous media. J. Environ. Chem. Eng. 2020, 8, 104340. [Google Scholar] [CrossRef]
- Park, Y.; McDonald, K.J.; Choi, K.-S. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013, 42, 2321–2337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.L.; Liu, C.M.; Huang, F.Q.; Zheng, C.; Wang, W.D. Study of the electronic structure and photocatalytic activity of the BiOCl photocatalyst. Appl. Catal. B Environ. 2006, 68, 125–129. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, K.; Zhang, L. Visible light photocatalysis of BiOI and its photocatalytic activity enhancement by in situ ionic liquid modification. J. Phys. Chem. C 2011, 115, 14300–14308. [Google Scholar] [CrossRef]
- Wei, Z.; Li, R.; Wang, R. Enhanced visible light photocatalytic activity of BiOBr by in situ reactable ionic liquid modification for pollutant degradation. RSC Adv. 2018, 8, 7956. [Google Scholar] [CrossRef]
- Zhong, X.; Zhang, K.X.; Wu, D.; Ye, X.Y.; Huang, W.; Zhou, B.X. Enhanced photocatalytic degradation of levofloxacin by Fe-doped BiOCl nanosheets under LED light irradiation. Chem. Eng. J. 2020, 383, 132148. [Google Scholar] [CrossRef]
- Ahmed, K.E.; Kuo, D.H.; Duresa, L.W. Synthesis and characterizations of BiOCl nanosheets with controlled particle growth for efficient organic dyes degradation. J. Ind. Eng. Chem. 2020, 83, 200–207. [Google Scholar] [CrossRef]
- Chen, F.; Liu, H.; Bagwasi, S.; Shen, X.; Zhang, J. Photocatalytic study of BiOCl for degradation of organic pollutants under UV irradiation. J. Photochem. Photobiol. A Chem. 2010, 215, 76–80. [Google Scholar] [CrossRef]
- Shen, T.; Shi, X.; Guo, J.; Li, J.; Yuan, S. Photocatalytic removal of NO by light-driven Mn3O4/BiOCl heterojunction photocatalyst: Optimization and mechanism. Chem. Eng. J. 2021, 408, 128014. [Google Scholar] [CrossRef]
- Zhang, D.; Tan, G.; Wang, M.; Li, B.; Dang, M.; Wang, Y.; Zhang, B.; Ren, H.; Xia, A. The enhanced photocatalytic activity of Ag-OVs-(0 0 1) BiOCl by separating secondary excitons under double SPR effects. Appl. Surf. Sci. 2020, 530, 147228. [Google Scholar] [CrossRef]
- Tao, S.; Sun, S.; Zhao, T.; Cui, J.; Yang, M.; Yu, X.; Yang, Q.; Zhang, X.; Liang, S. One-pot construction of Ta-doped BiOCl/Bi heterostructures toward simultaneously promoting visible light harvesting and charge separation for highly enhanced photocatalytic activity. Appl. Surf. Sci. 2021, 543, 148798. [Google Scholar] [CrossRef]
- Liu, W.; Wang, S.; Zhao, Y.; Sun, C.; Xu, H.; Zhao, J. PVP-induced Bi2S3/BiOCl photocatalyst with open hollow structures for the removal of ciprofloxacin under visible-light irradiation. J. Alloys Compd. 2020, 157995. [Google Scholar] [CrossRef]
- Bao, S.; Liang, H.; Li, C.; Bai, J. A heterostructure BiOCl nanosheets/TiO2 hollow-tubes composite for visible light-driven efficient photodegradation antibiotic. J. Photochem. Photobiol. A Chem. 2020, 397, 112590. [Google Scholar] [CrossRef]
- Wu, D.; Wang, X.; Wang, H.; Wang, F.; Wang, D.; Gao, Z.; Wang, X.; Wu, F.; Jiang, K. Ultrasonic-assisted synthesis of two dimensional BiOCl/MoS2 with tunable band gap and fast charge separation for enhanced photocatalytic performance under visible light. J. Colloid Interface Sci. 2019, 533, 539–547. [Google Scholar] [CrossRef]
- Qiao, X.; Hu, F.; Hou, D.; Li, D. PEG assisted hydrothermal synthesis of hierarchical MoS2 microspheres with excellent adsorption behavior. Mater. Lett. 2016, 169, 241–245. [Google Scholar] [CrossRef]
- Jeong, N.; Kim, H.; Kim, W.; Choi, J.Y.; Hwang, K.; Nam, J.; Han, J.; Jwa, E.; Jeong, Y.C. Direct synthesis, characterization, and reverse electrodialysis applications of MoS2 thin film on aluminum foil. Mater. Charact. 2020, 164, 110361. [Google Scholar] [CrossRef]
- Guan, Y.; Wu, J.; Liu, Q.; Gu, M.; Lin, Y.; Qi, Y.; Jia, T.; Pan, W.; He, P.; Li, Q. Fabrication of BiOI/MoS2 heterojunction photocatalyst with different treatment methods for enhancing photocatalytic performance under visible-light. Mater. Res. Bull. 2019, 120, 110579. [Google Scholar] [CrossRef]
- Hou, J.; Dai, D.; Wei, R.; Wu, X.; Wang, X.; Tahir, M.; Zou, J.J. Narrowing the Band Gap of BiOCl for the Hydroxyl Radical Generation of Photocatalysis under Visible Light. ACS Sustain. Chem. Eng. 2019, 7, 16569–16576. [Google Scholar] [CrossRef]
- Abellan, M.N.; Gimenez, J.; Esplugas, S. Photocatalytic degradation of antibiotics: The case of sulfamethoxazole and trimethoprim. Catal. Today 2009, 144, 131–136. [Google Scholar] [CrossRef]
- Yan, H.; Liu, L.; Wang, R.; Zhu, W.; Ren, X.; Luo, L.; Zhang, X.; Luo, S.; Ai, X.; Wang, J. Binary composite MoS2/TiO2 nanotube arrays as a recyclable and efficient photocatalyst for solar water disinfection. Chem. Eng. J. 2020, 401, 126052. [Google Scholar] [CrossRef]
- Guo, L.; Yang, Z.; Marcus, K.; Li, Z.; Luo, B.; Zhou, L.; Wang, X.; Du, Y.; Yang, Y. MoS2/TiO2 heterostructures as nonmetal plasmonic photocatalysts for highly efficient hydrogen evolution. Energy Environ. Sci. 2018, 11, 106–114. [Google Scholar] [CrossRef]
- Teng, W.; Wang, Y.; Huang, H.; Li, X.; Tang, Y. Enhanced photoelectrochemical performance of MoS2 nanobelts-loaded TiO2 nanotube arrays by photo-assisted electrodeposition. Appl. Surf. Sci. 2017, 425, 507–517. [Google Scholar] [CrossRef]
- Medinas, D.B.; Cerchiaro, G.; Trindade, D.F.; Augusto, O. The carbonate radical and related oxidants derived from bicarbonate buffer. IUBMB Life 2007, 59, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Flanders, P.M.; Miller, P.L.; Strathmann, T.J. Oxidation of sulfamethoxazole and related antimicrobial agents by TiO2 photocatalysis. Water Res. 2007, 41, 2612–2626. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Lu, X.; Fan, J.; Cai, X.; Gao, B.; Miao, R.; Wang, J.; Lv, Y. Comparative study of the photocatalytic performance for the degradation of different dyes by ZnIn2S4: Adsorption, active species and pathways. RSC Adv. 2017, 7, 12292–12300. [Google Scholar] [CrossRef]
- Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Kinetics of ethyl paraben degradation by simulated solar radiation in the presence of N-doped TiO2 catalysts. Water Res. 2015, 81, 157–166. [Google Scholar] [CrossRef]
- Wang, F.; Li, G.; Zheng, J.; Ma, J.; Yang, C.; Wang, Q. Hydrothermal synthesis of flower-like molybdenum disulfide microspheres and their application in electrochemical supercapacitors. RSC Adv. 2018, 8, 38945. [Google Scholar] [CrossRef]
- Cullity, B.D.; Weymouth, J.W. Elements of X-ray diffraction. Am. J. Phys. 1957, 25, 394–395. [Google Scholar] [CrossRef]

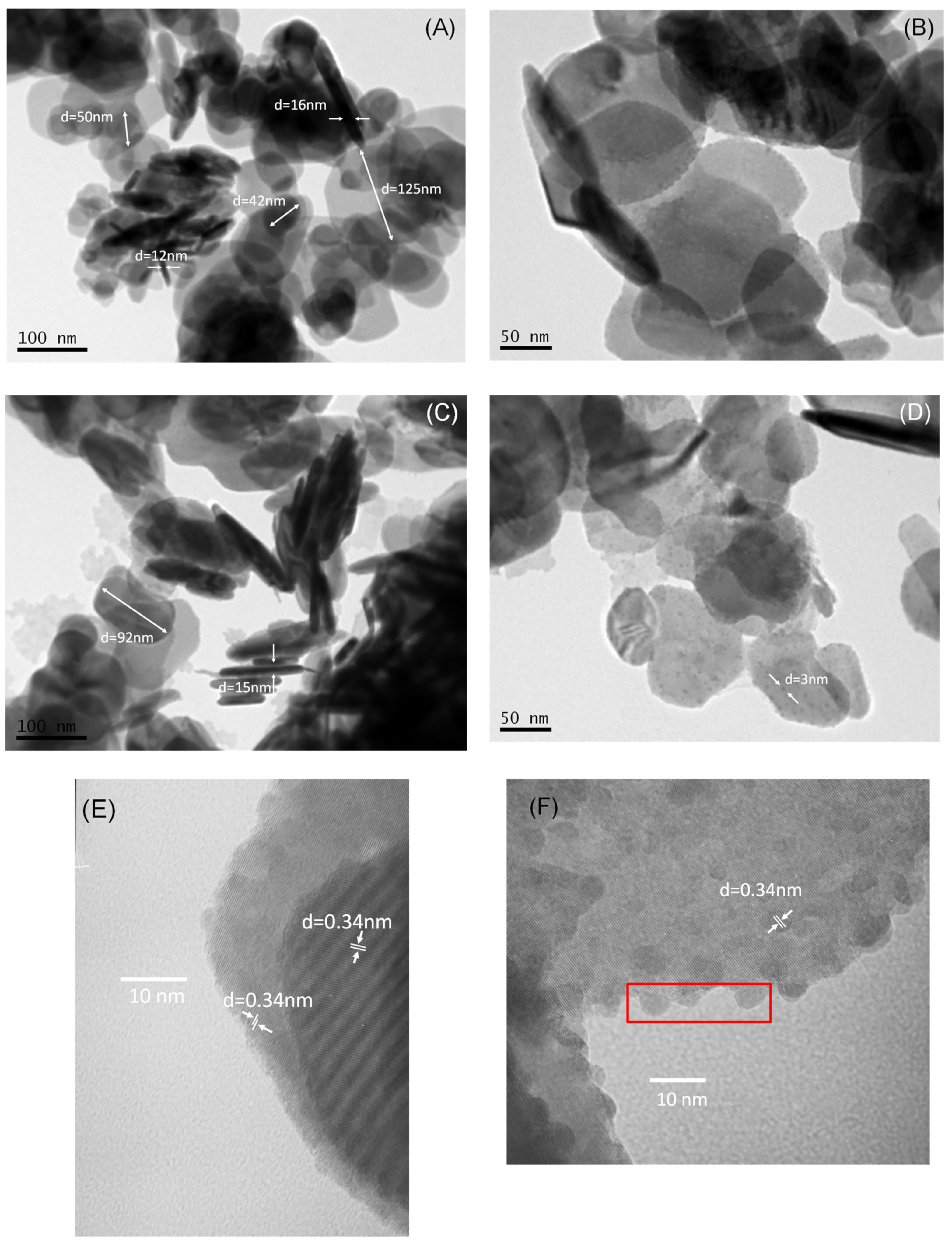
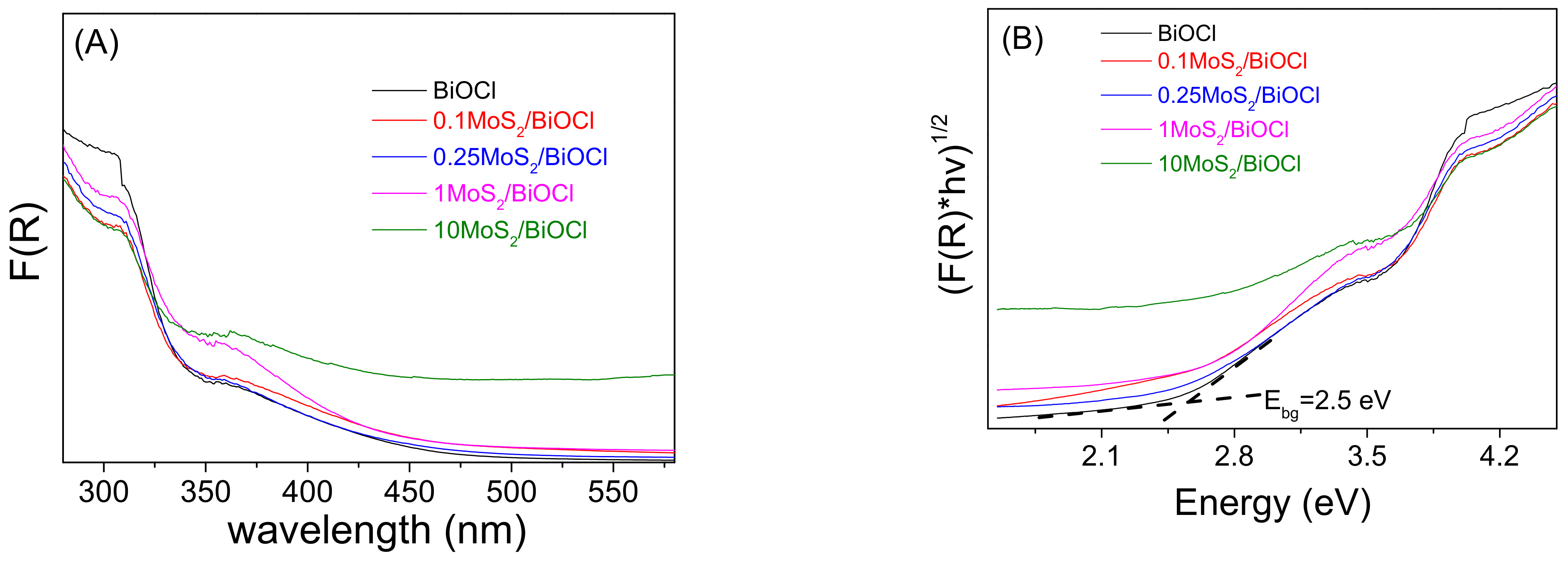


| Samples | Primary Crystallize Size, nm | SSA, m2/g |
|---|---|---|
| 10 wt.% MoS2/BiOCl | 10 | 38 |
| 1% wt.% MoS2/BiOCl | 19 | 27 |
| 0.25% wt.% MoS2/BiOCl | 26 | 20 |
| 0.1% wt.% MoS2/BiOCl | 26 | 18 |
| BiOCl | 26 | 21 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grilla, E.; Kagialari, M.N.; Petala, A.; Frontistis, Z.; Mantzavinos, D. Photocatalytic Degradation of Valsartan by MoS2/BiOCl Heterojunctions. Catalysts 2021, 11, 650. https://doi.org/10.3390/catal11060650
Grilla E, Kagialari MN, Petala A, Frontistis Z, Mantzavinos D. Photocatalytic Degradation of Valsartan by MoS2/BiOCl Heterojunctions. Catalysts. 2021; 11(6):650. https://doi.org/10.3390/catal11060650
Chicago/Turabian StyleGrilla, Eleni, Maria Nefeli Kagialari, Athanasia Petala, Zacharias Frontistis, and Dionissios Mantzavinos. 2021. "Photocatalytic Degradation of Valsartan by MoS2/BiOCl Heterojunctions" Catalysts 11, no. 6: 650. https://doi.org/10.3390/catal11060650
APA StyleGrilla, E., Kagialari, M. N., Petala, A., Frontistis, Z., & Mantzavinos, D. (2021). Photocatalytic Degradation of Valsartan by MoS2/BiOCl Heterojunctions. Catalysts, 11(6), 650. https://doi.org/10.3390/catal11060650









