Effects of RhCrOx Cocatalyst Loaded on Different Metal Doped LaFeO3 Perovskites with Photocatalytic Hydrogen Performance under Visible Light Irradiation
Abstract
1. Introduction
2. Results and Discussion
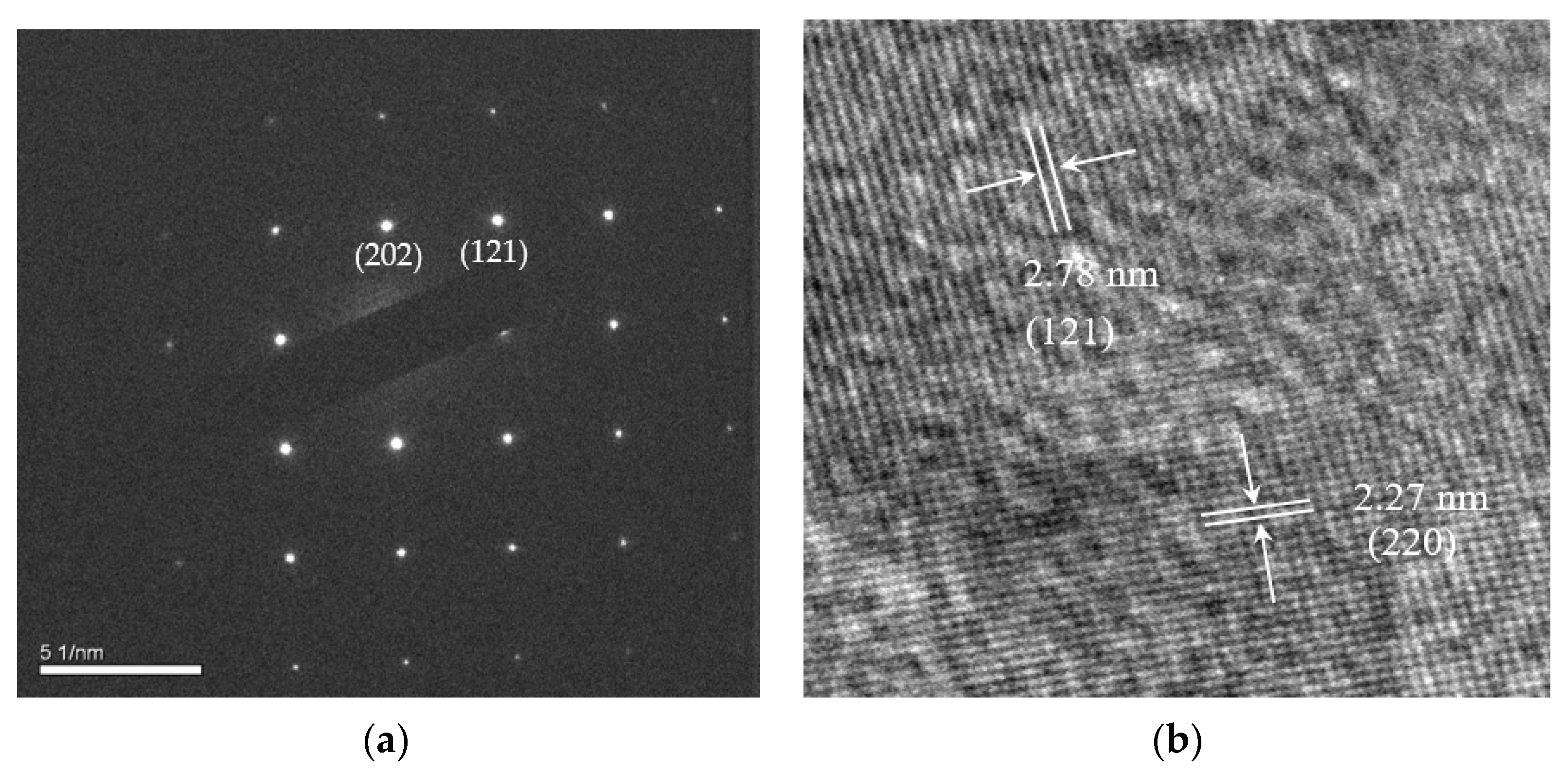
2.1. Different Metal Doping in LaFeO3
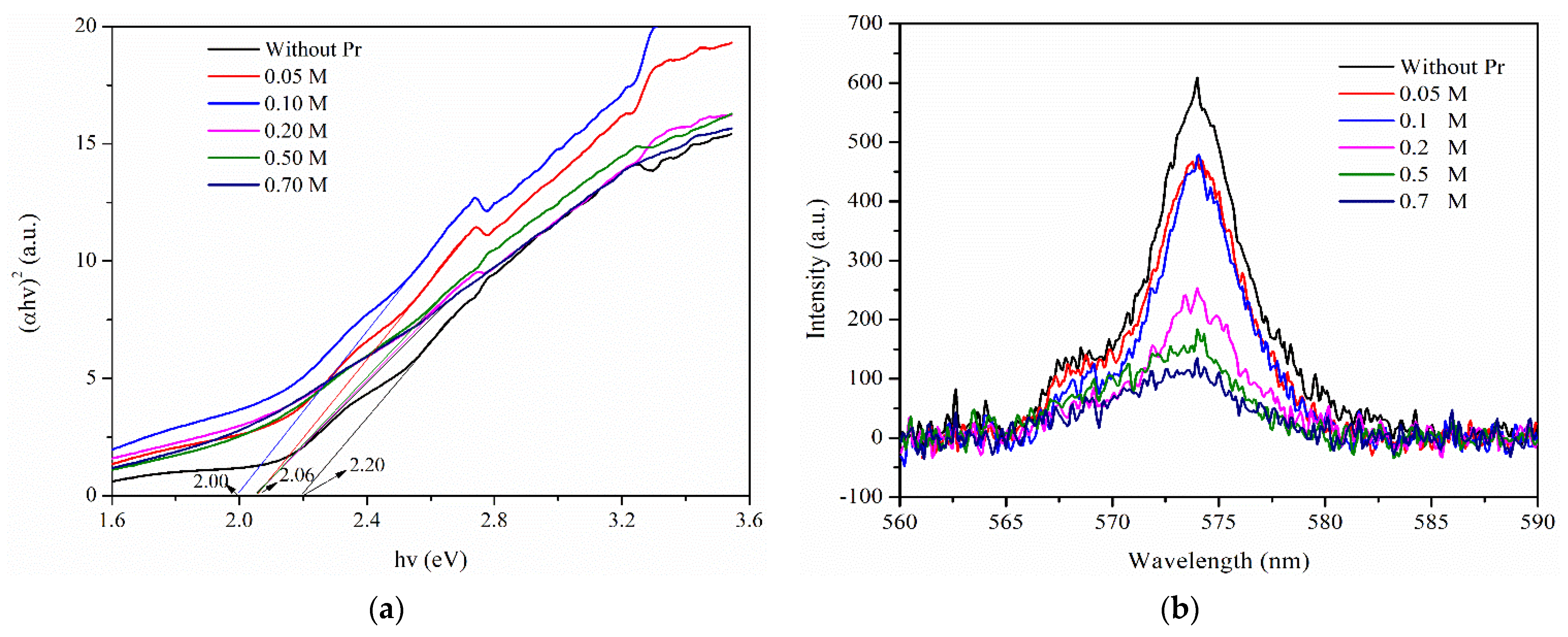
2.2. Photocatalytic Activity of Different Amounts of Pr Doping in LaFeO3
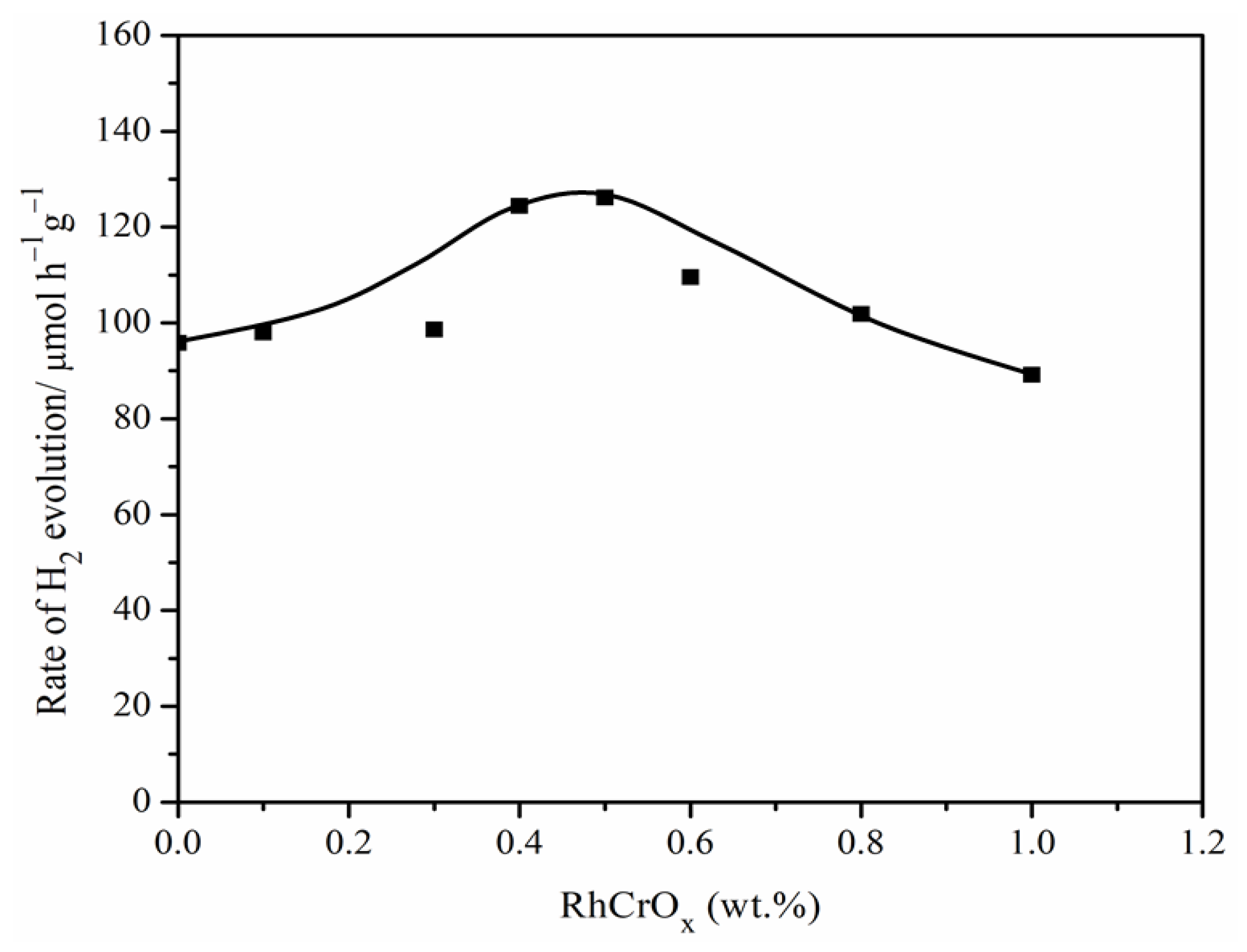
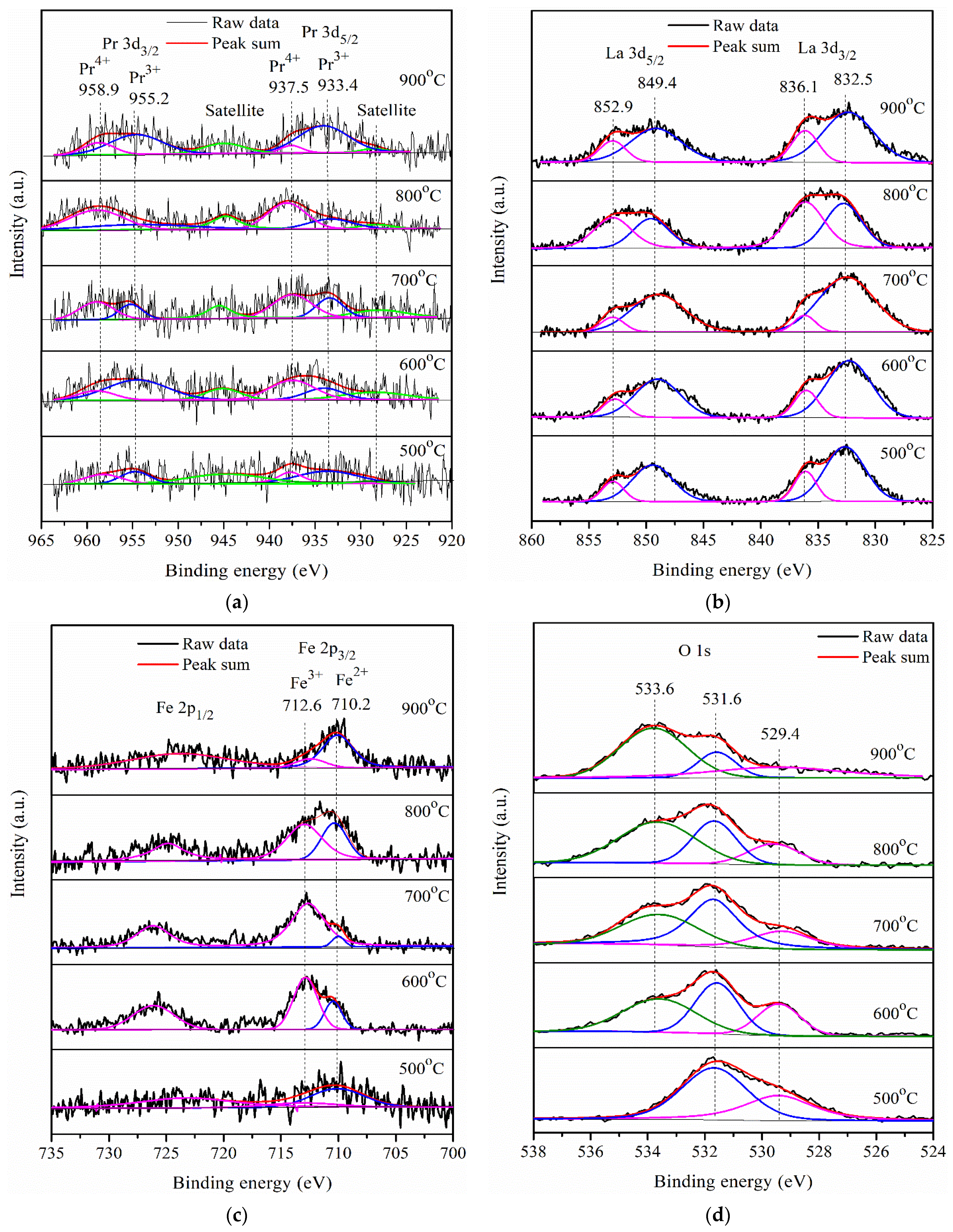
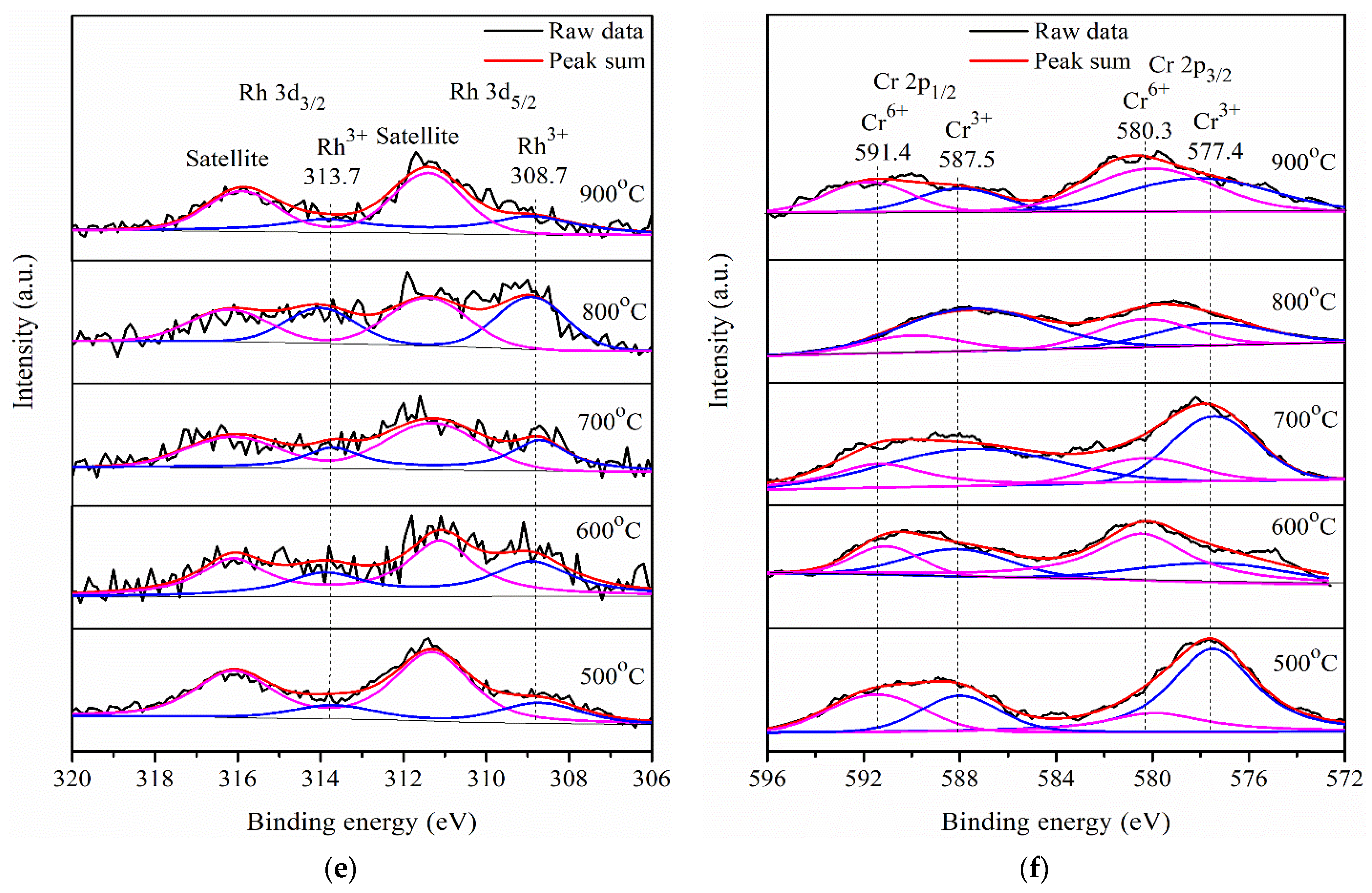
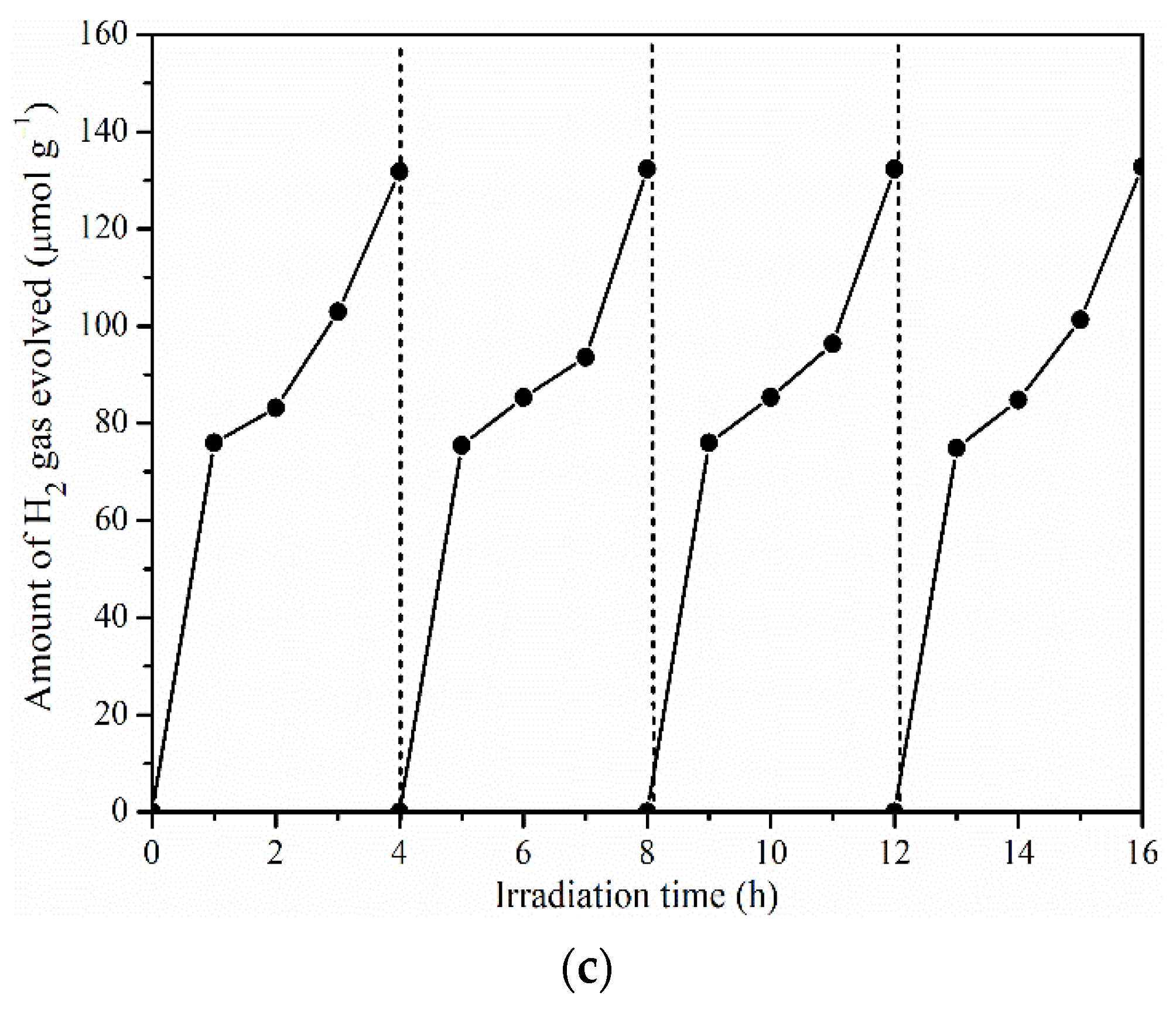
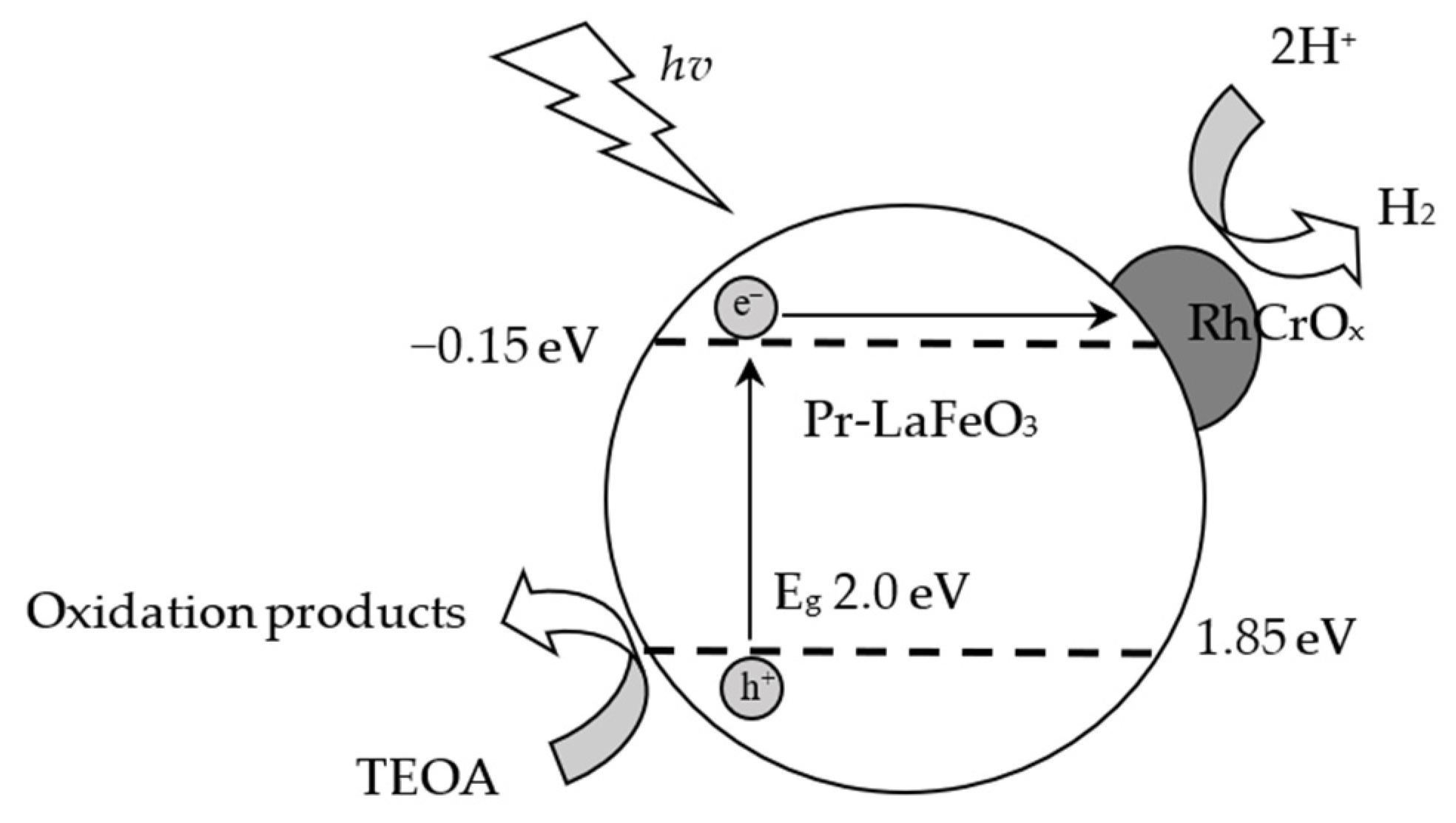
2.3. Photocatalytic Activity of Pr-LaFeO3 with Loading of RhCrOx Cocatalyst
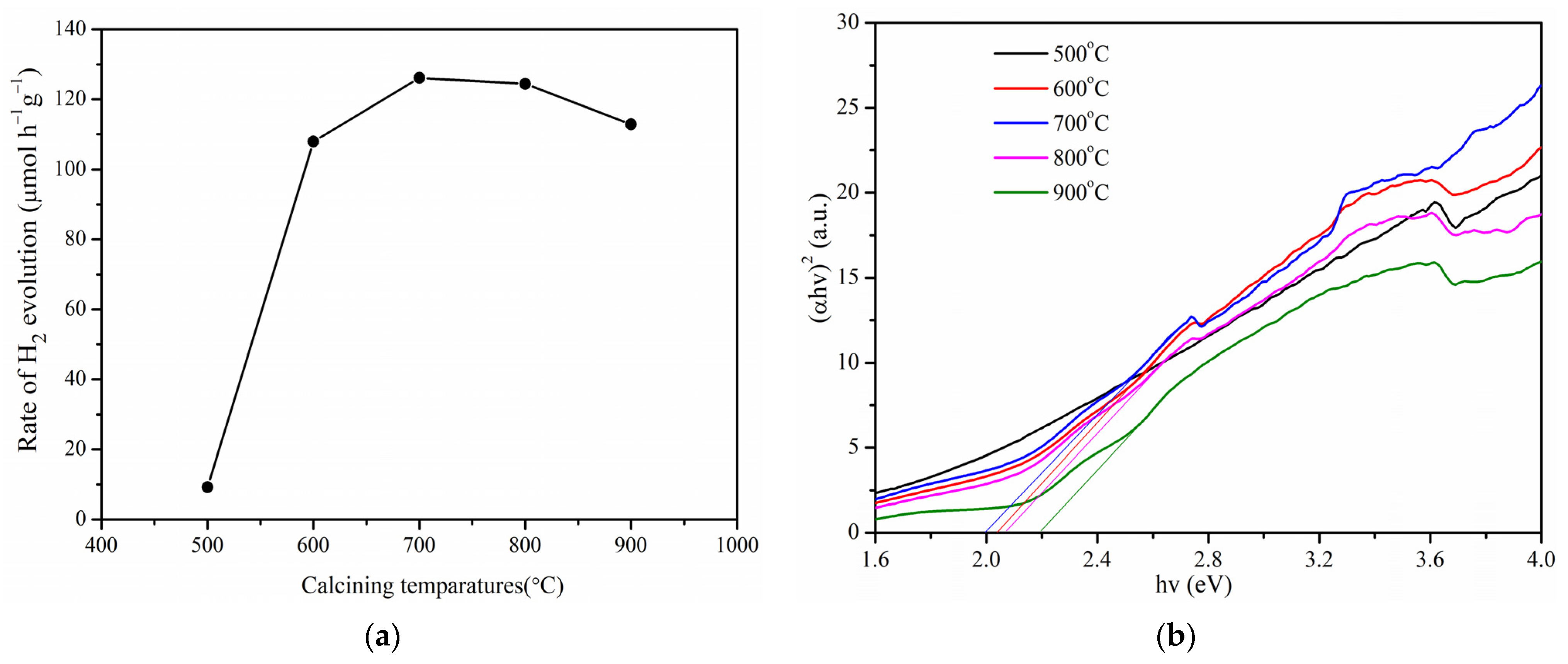
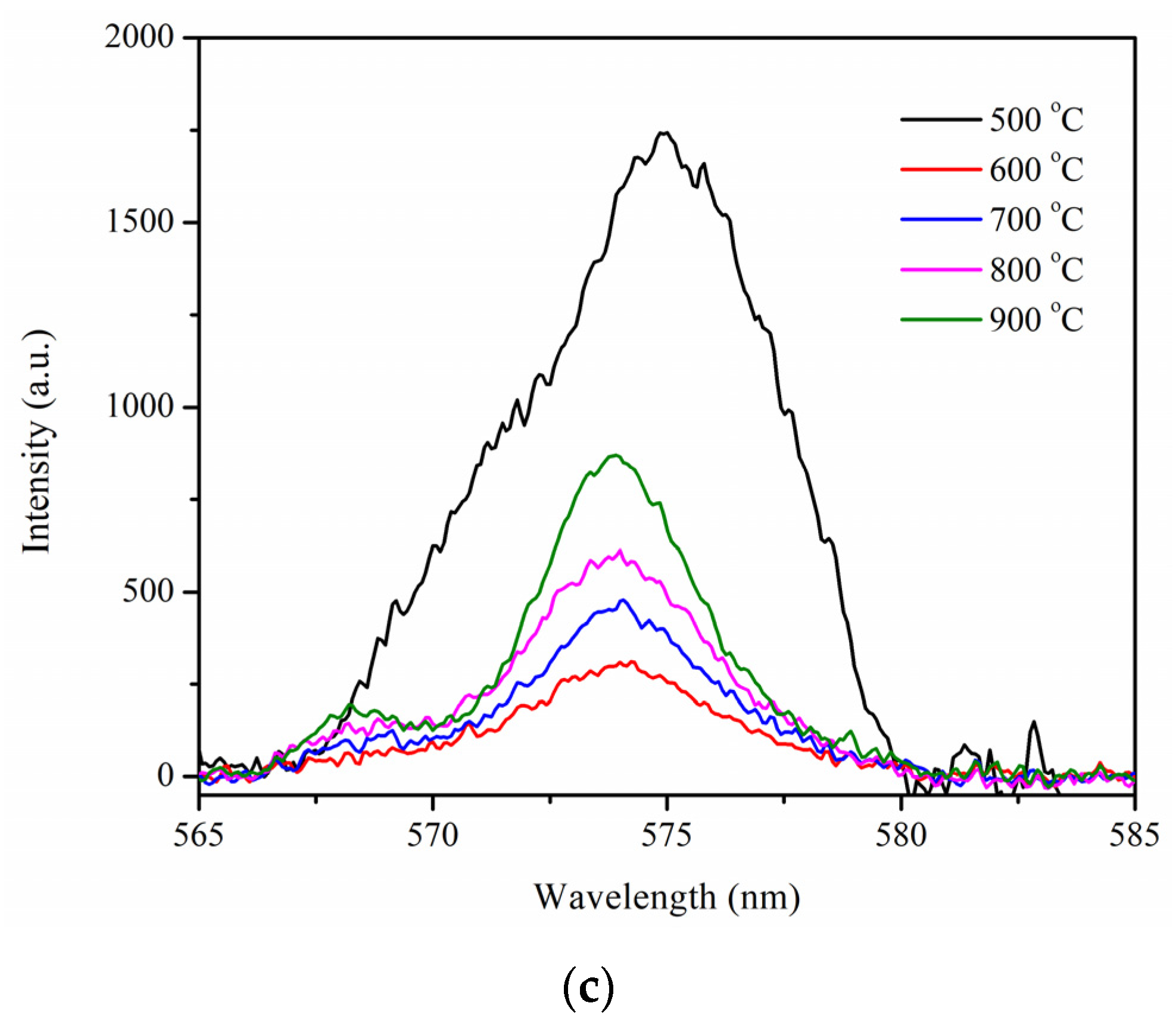
2.4. Photocatalytic Activity of Pr-LaFeO3 Prepared at Different Calcination Temperatures
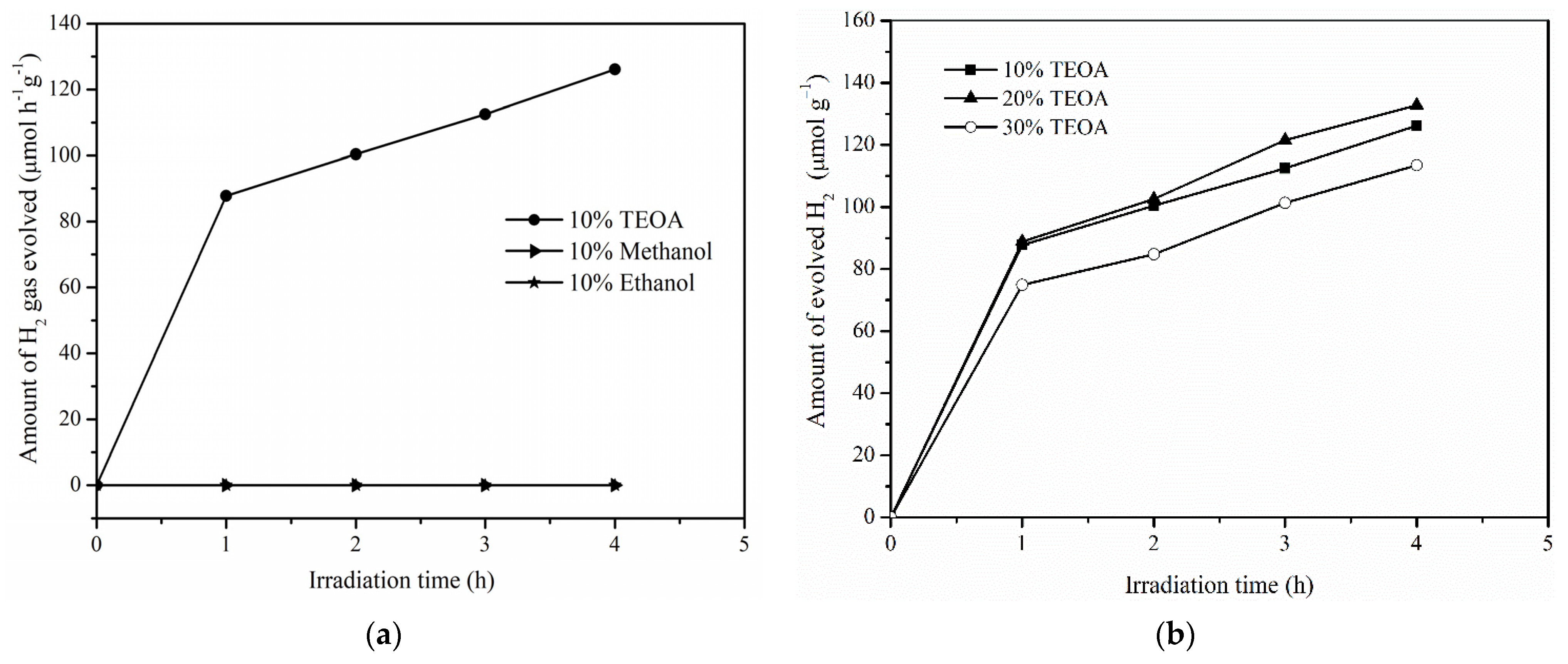
2.5. Photocatalytic Activity of RhCrOx/Pr-LaFeO3 with Different Sacrificial Reagents
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Different Metal Doped LaFeO3 Powders
3.3. RhCrOx/Pr Doped LaFeO3 Preparation
3.4. Characterization of Photocatalysts
3.5. Calibration Curves for Hydrogen
3.6. Photocatalytic H2 Evolution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Acar, C.; Dincer, I.; Naterer, G.F. Review of photocatalytic water-splitting methods for sustainable hydrogen production. Int. J. Energy Res. 2016, 40, 1449–1473. [Google Scholar] [CrossRef]
- Ahmed, K.; Farrok, O.; Rahman, M.M.; Ali, M.S.; Haque, M.M.; Azad, A.K. Proton exchange membrane hydrogen fuel cell as the grid connected power generator. Energies 2020, 13, 6679. [Google Scholar] [CrossRef]
- Barbir, F.; Yazici, S. Status and development of PEM fuel cell technology. Int. J. Energy Res. 2008, 32, 369–378. [Google Scholar] [CrossRef]
- Wu, B.; Matian, M.; Offer, G.J. Hydrogen PEMFC system for automotive applications. Int. J. Low Carbon Technol. 2012, 7, 28–37. [Google Scholar] [CrossRef]
- Li, R. Latest progress in hydrogen production from solar water splitting via photocatalysis, photoelectrochemical, and photovoltaic-photoelectrochemical solutions. Chin. J. Catal. 2017, 38, 512. [Google Scholar] [CrossRef]
- Lin, W.C.; Yang, W.D.; Huang, I.L.; Wu, T.S.; Chung, Z.J. Hydrogen production from methanol/water photocatalytic decomposition using Pt/TiO2-xNxcatalyst. Energy Fuels 2009, 23, 2192–2196. [Google Scholar] [CrossRef]
- Tasleem, S.; Tahir, M. Recent progress in structural development and band engineering of perovskites materials for photocatalytic solar hydrogen production: A review. Int. J. Hydrogen Energy 2020, 47, 19078–19111. [Google Scholar] [CrossRef]
- Tijare, S.N.; Joshi, M.V.; Padole, P.S.; Mangrulkar, P.A.; Rayalu, S.S.; Labhsetwar, N.K. Photocatalytic hydrogen generation through water splitting on nano-crystalline LaFeO3 perovskite. Int. J. Hydrogen Energy 2012, 37, 10451–10456. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, J.; Yang, F.; Yang, X.; He, Y. The influence of Ca substitution on LaFeO3 nanoparticles in terms of structural and magnetic properties. J. Appl. Biomater. Func. Mater. 2018, 16, 17–25. [Google Scholar] [CrossRef]
- Xu, K.; Xu, H.; Feng, G.; Feng, J. Photocatalytic hydrogen evolution performance of NiS cocatalyst modified LaFeO3/g-C3N4 heterojunctions. New J. Chem. 2017, 41, 14602–14609. [Google Scholar] [CrossRef]
- Ismael, M. Highly effective ruthenium-doped TiO2 nanoparticles photocatalyst for visible-light-driven photocatalytic hydrogen production. New J. Chem. 2019, 43, 9596. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Palma, V. Enhanced photocatalytic hydrogen production from glucose aqueousmatrices on Ru-doped LaFeO3. Appl. Catal. B Environ. 2017, 207, 182–194. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Sannino, D. Enhanced photocatalytic hydrogen production from glucose on Rh-doped LaFeO3. Chem. Eng. Trans. 2017, 60, 235–240. [Google Scholar]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401. [Google Scholar] [CrossRef]
- Zhai, Q.; Xie, S.; Fan, W.; Zhang, Q.; Wang, Y.; Deng, W.; Wang, Y. Photocatalytic conversion of carbon dioxide with water into methane: Platinum and copper(I) oxide co-catalysts with a core–shell structure. Angew. Chem. Int. Ed. 2013, 52, 5776. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Shen, S.; Liu, G.; Qi, Y.; Zhang, F.; Li, C. Interface engineering of a CoOx/Ta3N5 photocatalyst for unprecedented water oxidation performance under visible-light-irradiation. Angew. Chem. Int. Ed. 2015, 54, 3047. [Google Scholar] [CrossRef]
- Hoffmann, M.R.; Martin, S.T.; Choi, W.; Bahnemannt, D.W. Environmental applications of semiconductor photocatalysis. Chem. Rev. 1995, 95, 69–96. [Google Scholar] [CrossRef]
- Daskalaki, V.M.; Antoniadou, M.; Puma, G.L.; Kondarides, D.I.; Lianos, P. Solar light-responsive Pt/CdS/TiO2 photocatalysts for hydrogen production and simultaneous degradation of inorganic or organic sacrificial agents in wastewater. Environ. Sci. Technol. 2010, 44, 7200–7205. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhang, X.; Lu, G.; Li, S. Improved quantum yield for photocatalytic hydrogen generation under visible light irradiation over eosin sensitized TiO2-Investigation of different noble metal loading. J. Mol. Catal. A Chem. 2006, 259, 275. [Google Scholar] [CrossRef]
- Sayama, K.; Yase, K.; Arakawa, H.; Asakura, K.; Tanaka, A.; Domen, K.; Onishi, T. Photocatalytic activity and reaction mechanism of Pt-intercalated K4Nb6O17 catalyst on the water splitting in carbonate salt aqueous solution. J. Photochem. Photobiol. A Chem. 1998, 114, 125–135. [Google Scholar] [CrossRef]
- Jang, J.S.; Ham, D.J.; Lakshminarasimhan, N.; Choi, W.Y.; Lee, J.S. Role of platinum-like tungsten carbide as cocatalyst of CdS photocatalyst for hydrogen production under visible light irradiation. Appl. Catal. A Gen. 2008, 346, 149–154. [Google Scholar] [CrossRef]
- Matsumura, M.; Saho, Y.; Tsubomura, H. Photocatalytic hydrogen production from solutions of slufite using platinized cadmium sulfide powder. J. Phys. Chem. 1983, 87, 3807–3808. [Google Scholar] [CrossRef]
- Liu, L.; Ji, Z.; Zou, W.; Gu, X.; Deng, Y.; Gao, F.; Tang, C.; Dong, L. In situ loading transition metal oxide clusters on TiO2 nanosheets as cocatalysts for exceptional high photoacitivity. ACS Catal. 2013, 3, 2052–2061. [Google Scholar] [CrossRef]
- Wang, M.; Shen, S.; Li, L.; Tang, Z.; Yang, J. Effects of sacrificial reagents on photocatalytic hydrogen evolution over different photocatalysts. J. Mater. Sci. 2017, 52, 5155–5164. [Google Scholar] [CrossRef]
- Kawai, T.; Sakata, T. Photocatalytic hydrogen production from liquid methanol and water. J. Chem. Soc. Chem. Commun. 1980, 15, 694–695. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Torres-Martinez, L.M.; Moctezuma, E.; Singh, A.P.; Wickman, B. Green synthesis of earth-abundant metal sulfides (FeS2, CuS, and NiS2) and their use as visible-light active photocatalysts for H2 generation and dye removal. J. Mater. Sci. Mater. Electron. 2018, 29, 11613–11626. [Google Scholar] [CrossRef]
- DeLaive, P.J.; Foreman, T.K.; Giannotti, C.; Whitten, D.G. Photoinduced electron transfer reactions of transition-metal complexes with amines. Mechanistic studies of alternate pathways to back electron transfer. J. Am. Chem. Soc. 1980, 102, 5627–5631. [Google Scholar] [CrossRef]
- Du, C.; Yan, B.; Lin, Z.; Yang, G. Enhanced carrier separation and increased electron density in 2D heavily N-doped ZnIn2S4 for photocatalytic hydrogen production. J. Mater. Chem. A 2020, 8, 207–217. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting-The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Berto, T.F.; Sanwald, K.E.; Byers, J.P.; Browning, N.D.; Gutiérrez, O.Y.; Lercher, J.A. Enabling overall water splitting on photocatalysts by CO-covered noble metal co-catalysts. J. Phys. Chem. Lett. 2016, 7, 4358–4362. [Google Scholar] [CrossRef] [PubMed]
- Ran, J.; Zhang, J.; Yu, J.; Jaroniecc, M.; Qiao, S.Z. Earth-abundant cocatalysts for semiconductorbased photocatalytic water splitting. Chem. Soc. Rev. 2014, 43, 7787. [Google Scholar] [CrossRef]
- Chou, H.L.; Hwang, B.J.; Sund, C.L. Catalysis in fuel cells and hydrogen production. In Catalysis in Fuel Cells and Hydrogen Production in Batteries, Hydrogen Storage and Fuel Cells; Suib, S.L., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 217–270. [Google Scholar]
- Lin, Z.; Du, C.; Yan, B.; Yang, G. Amorphous Fe2O3 for photocatalytic hydrogen evolution. Catal. Sci. Technol. 2019, 9, 5582–5592. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Liu, X.; Li, S.; Zhao, M. XPS study on O(ls) and Fe(2p) for nanocrystalline composite oxide LaFeO3 with the perovskite structure. Mater. Chem. Phys. 1994, 38, 355–362. [Google Scholar] [CrossRef]
- Pagliaro, M.V.; Bellini, M.; Bevilacqua, M.; Filippi, J.; Folliero, M.G.; Marchionni, A.; Miller, H.A.; Oberhauser, W.; Caporali, S.; Innocenti, M.; et al. Carbon supported Rh nanoparticles for the production of hydrogen and chemicals by the electroreforming of biomass-derived alcohols. RSC Adv. 2017, 7, 13971. [Google Scholar] [CrossRef]
- Yu, S.; Xu, S.; Sun, B.; Lu, Y.; Li, L.; Zou, W.; Wang, P.; Gao, F.; Tang, C.; Dong, L. Synthesis of CrOx/C catalysts for low temperature NH3-SCR with enhanced regeneration ability in the presence of SO2. RSC Adv. 2018, 8, 3858. [Google Scholar] [CrossRef]
- Jones, W.; Martin, D.J.; Caravaca, A.; Beale, A.M.; Bowker, M.; Maschmeyer, T.; Hartley, G.; Masters, A. A comparison of photocatalytic reforming reactions of methanol andtriethanolamine with Pd supported on titania and graphitic carbonnitride. Appl. Catal. B Environ. 2019, 240, 373–379. [Google Scholar] [CrossRef]
- Berr, M.J.; Wagner, P.; Fischbach, S.; Vaneski, A.; Schneider, J.; Susha, A.S.; Rogach, A.L.; Jäckel, F.; Feldmann, J. Hole scavenger redox potentials determine quantum efficiency and stability of Pt-decorated CdS nanorods for photocatalytic hydrogen generation. Appl. Phys. Lett. 2012, 100, 223903. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, G.; Xiong, J.; Zhu, J.; Gan, Y.; Zhang, M.; Li, Z.; Dou, S. Synergistic impact of cocatalysts and hole scavenger for promoted photocatalytic H2 evolution in mesoporous TiO2 NiS hybrid. J. Energy Chem. 2019, 32, 45. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, W.; Crittenden, J.; Minakata, D.; Chen, Y.; Wang, P. Effects of inorganic electron donors in photocatalytic hydrogen production over Ru/(CuAg)0.15In0.3Zn1.4S2 under visible light irradiation. J. Renew. Sustain. Energy 2014, 6, 033131. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Ciambelli, P. Production of hydrogen from glucose by LaFeO3 based photocatalytic process during water treatment. Int. J. Hydrogen Energy 2016, 41, 959–966. [Google Scholar] [CrossRef]
- Iervolino, G.; Vaiano, V.; Sannino, D.; Rizzo, L.; Galluzzi, A.; Polichetti, M.; Pepe, G.; Campiglia, P. Hydrogen production from glucose degradation in water and wastewater treated by Ru-LaFeO3/Fe2O3 magnetic particles photocatalysis and heterogeneous. Int. J. Hydrogen Energy 2018, 43, 2184–2196. [Google Scholar] [CrossRef]
- Chen, Z.; Fan, T.; Zhang, Q.; He, J.; Fan, H.; Sun, Y.; Yi, X.; Li, J. Interface engineering: Surface hydrophilic regulation of LaFeO3 towards enhanced visible light photocatalytic hydrogen evolution. J. Colloid Interface Sci. 2019, 536, 105–111. [Google Scholar] [CrossRef]
- Li, J.; Pan, X.; Xu, Y.; Jia, L.; Yi, X.; Fang, W. Synergetic effect of copper species as cocatalyst on LaFeO3 for enhanced visible-light photocatalytic hydrogen evolution. Int. J. Hydrogen Energy 2015, 40, 13918–13925. [Google Scholar] [CrossRef]
- Hisatomi, T.; Takanabe, K.; Domen, K. Photocatalytic water-splitting reaction from catalytic and kinetic perspectives. Catal. Lett. 2015, 145, 95–108. [Google Scholar] [CrossRef]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the Debye–Scherrer equation. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Chrysicopoulou, P.; Davazoglou, D.; Trapalis, C.; Kordas, G. Optical properties of very thin (<100 nm) sol–gel TiO2 films. Thin Solid Films 1998, 323, 188–193. [Google Scholar] [CrossRef]
- Xu, Y.; Schoonen, M.A.A. The absolute energy positions of conduction and valence bands of selected semiconducting minerals. Am. Mineral. 2000, 85, 543–556. [Google Scholar] [CrossRef]
- Mulliken, R.S. A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J. Chem. Phys. 1934, 2, 782–793. [Google Scholar] [CrossRef]
- Butler, M.A.; Ginley, D.S. Rediction of flatband potentials at semiconductor-electrolyte interfaces from atomic electronegativities. J. Electrochem. Soc. 1978, 125, 228–232. [Google Scholar] [CrossRef]
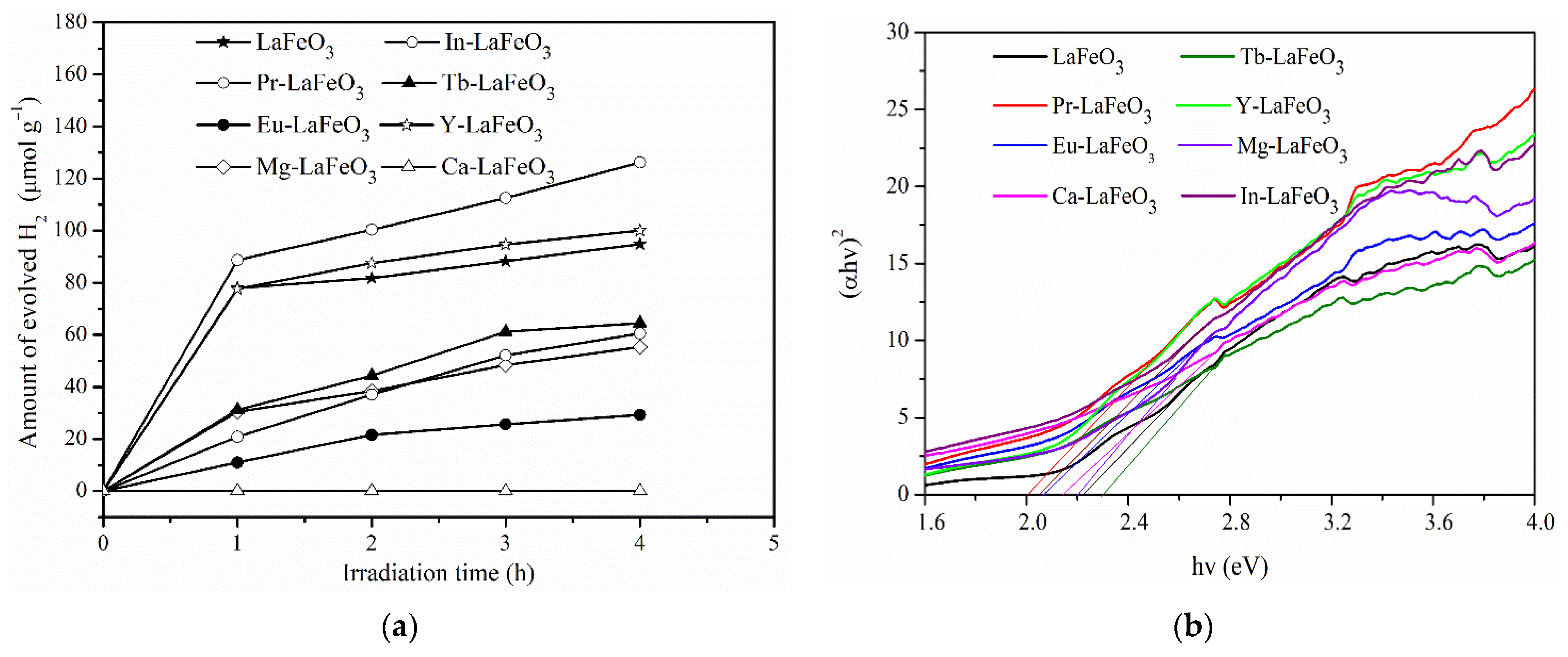

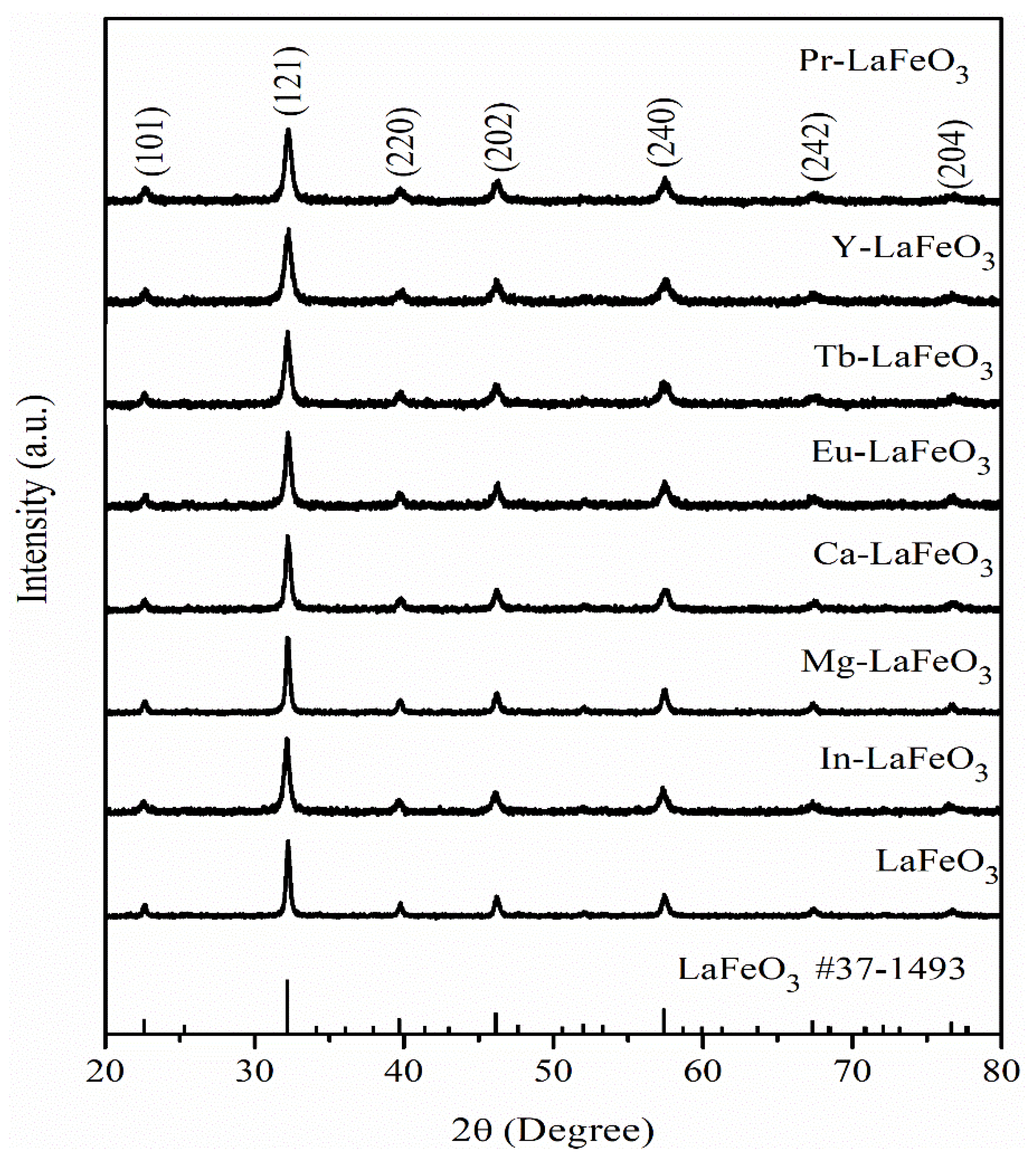
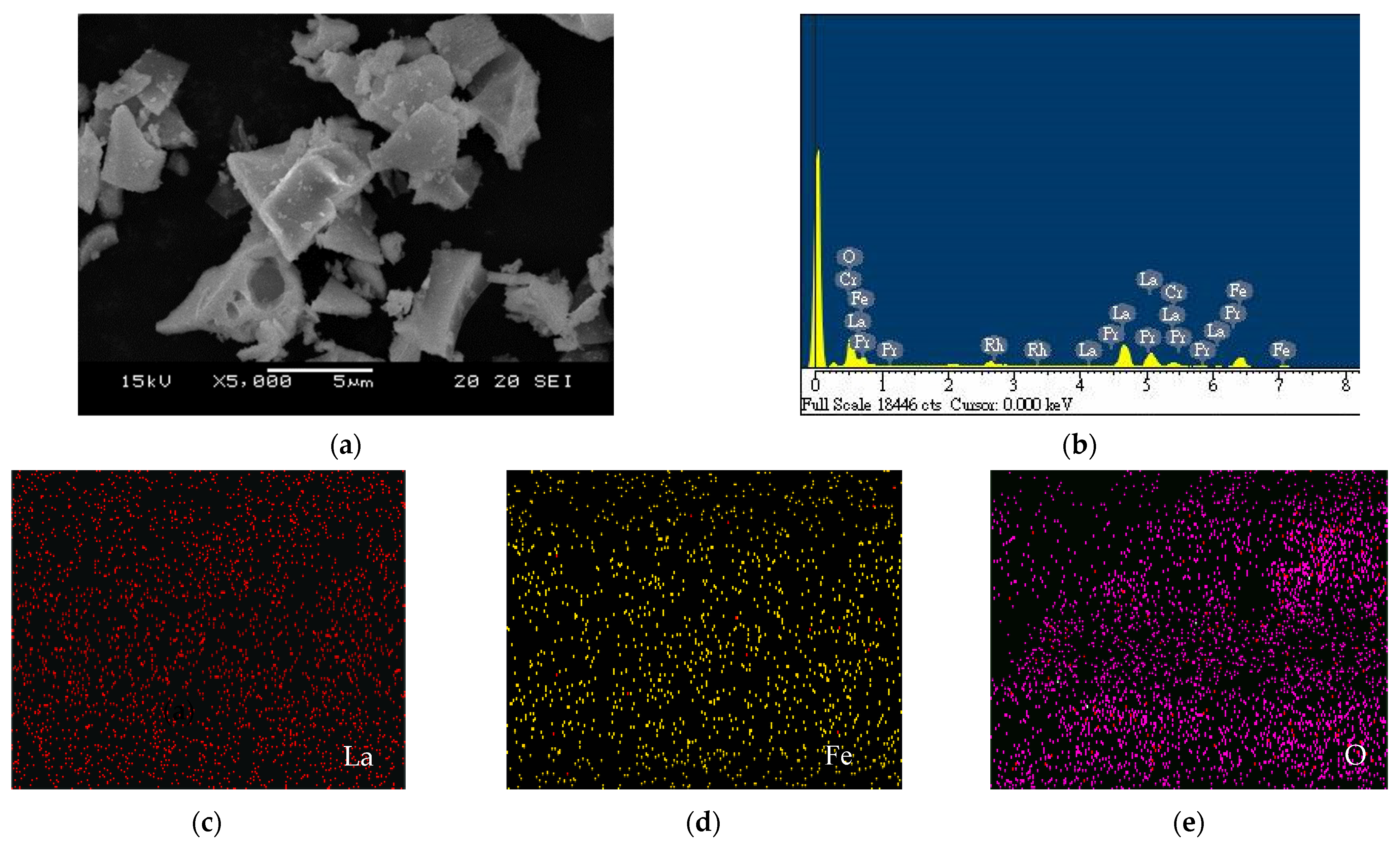


| Phtotcatalysts | Eg (eV) | ECB (eV) | EVC (eV) | Crystallite Sizes (nm) |
|---|---|---|---|---|
| LaFeO3 | 2.31 | −0.11 | 2.20 | 28.79 |
| Eu-LaFeO3 | 2.08 | −0.07 | 2.01 | 19.38 |
| Ca-LaFeO3 | 2.17 | −0.10 | 2.07 | 21.39 |
| Pr-LaFeO3 | 2.00 | −0.01 | 1.99 | 16.04 |
| Mg-LaFeO3 | 2.20 | −0.10 | 2.10 | 27.85 |
| Tb-LaFeO3 | 2.30 | −0.17 | 2.13 | 18.31 |
| In-LaFeO3 | 2.05 | −0.04 | 2.01 | 20.10 |
| Y-LaFeO3 | 2.00 | −0.01 | 1.99 | 15.60 |
| Calcining Temperature (°C) | Fe2+ (Area %) | Fe3+ (Area %) |
|---|---|---|
| 500 | 75.9 | 24.1 |
| 600 | 27.9 | 72.1 |
| 700 | 11.4 | 88.6 |
| 800 | 37.0 | 63.0 |
| 900 | 81.0 | 19.0 |
| Phtotcatalysts | Sacrificial Agents | Cocatalyst | Light Source | H2 Evolution Rate | Reference |
|---|---|---|---|---|---|
| Pr-doped LaFeO3 | 20% TEOA solution | RhCrOx | 350 W Xe lamp | 127 μmol h−1 g−1 | This work |
| Rh-doped LaFeO3 | Glucose | - | UV-LEDs 10 W, light intensity: 57 mW/cm2) 375–380 nm | 3822 μmol h−1 g−1 L−1 | [13] |
| Ru-doped LaFeO3 | Glucose | - | UV-LEDs 10 W, light intensity: 57 mW/cm2) 375–380 nm | 875 μmol h−1 g−1 | [12] |
| LaFeO3 | Glucose | - | UV-LEDs 10 W, light intensity: 57 mW/cm2) 375–380 nm | 400 μmol h−1 g−1 | [41] |
| Ru-LaFeO3/Fe2O3 | Glucose | Visible LEDs, 440 nm | 1000 μmol h−1 g−1 L−1 | [42] | |
| Nano LaFeO3 | Ethanol solution | 19 μL Pt | 400 W tungsten light sources | 3315 μmol h−1 g−1 | [8] |
| Polyaniline-coverd LaFeO3 | 10% TEOA solution | 3% Pt | 300 W Xe lamp | 92.4 μmol h−1 | [43] |
| Ternary LaFe0.8/LaCu0.2 catalysts | 12.5% HCHO solution | - | UV light (cut-off λ < 400 nm) 125 W Xe lamp | 250 μmol h−1 g−1 | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chiang, T.H.; Viswanath, G.; Chen, Y.-S. Effects of RhCrOx Cocatalyst Loaded on Different Metal Doped LaFeO3 Perovskites with Photocatalytic Hydrogen Performance under Visible Light Irradiation. Catalysts 2021, 11, 612. https://doi.org/10.3390/catal11050612
Chiang TH, Viswanath G, Chen Y-S. Effects of RhCrOx Cocatalyst Loaded on Different Metal Doped LaFeO3 Perovskites with Photocatalytic Hydrogen Performance under Visible Light Irradiation. Catalysts. 2021; 11(5):612. https://doi.org/10.3390/catal11050612
Chicago/Turabian StyleChiang, Tzu Hsuan, Gujjula Viswanath, and Yu-Si Chen. 2021. "Effects of RhCrOx Cocatalyst Loaded on Different Metal Doped LaFeO3 Perovskites with Photocatalytic Hydrogen Performance under Visible Light Irradiation" Catalysts 11, no. 5: 612. https://doi.org/10.3390/catal11050612
APA StyleChiang, T. H., Viswanath, G., & Chen, Y.-S. (2021). Effects of RhCrOx Cocatalyst Loaded on Different Metal Doped LaFeO3 Perovskites with Photocatalytic Hydrogen Performance under Visible Light Irradiation. Catalysts, 11(5), 612. https://doi.org/10.3390/catal11050612







