Abstract
Biodiesel is one of the most significant and valuable alternatives to fossil fuels. In the process of transesterification to produce biodiesel from various feedstocks, glycerol is one of the side products obtained, in a high glycerol: biodiesel weight ratio (1:10). Therefore, the growing world demand for biodiesel prompted a glycerol surplus. It is, thus, of interest to find new and added-value paths for the transformation of this abundant chemical. One of the most auspicious glycerol applications is the production of fuel additives, namely cyclic acetals and ketals, from aldehydes and ketones, respectively. In this work, coordination polymers based on nitrile (trimethylphosphonic acid) and Ln3+/Eu3+ are used as catalysts for the acetalization of the bio-renewable glycerol into oxygenated fuel additives. Solketal is the major product obtained from the reaction of glycerol with acetone. This product improves the cold flow properties, lowering the viscosity of biodiesel, improving combustion, and boosting the octane number. The stability of the materials is studied as well as their recovery and reuse.
1. Introduction
Glycerol is one of the side products of the process of transesterification to produce biodiesel, comprising 10 wt.% of the total product. Thus, the increasing demand for biodiesel triggered a glycerol oversupply [1,2,3,4]. Despite its low price, glycerol purification is an expensive process, and researchers seek routes to incorporate crude glycerol into various branches of industry [5]. Glycerol may be converted into valuable chemicals by various catalytic processes, such as etherification, reforming, oxidation, carbonylation, hydrogenolysis, acetalization, dehydration, and esterification. A practical way of dealing with the crude glycerol overflow is by reacting it with aldehydes and ketones to produce fuels or fuel additives, such as cyclic acetals and ketals [1,2,3,4,6]. Glycerol can be upgraded to valuable compounds such as propanediol, acrolein, glycerol carbonate, glyceric acid, tartronic acid, syngas, and solketal, thereby providing opportunities for additional revenue for the biodiesel industry and the agricultural sector. Solketal is of particular interest because it is a 100% bio-based chemical, produced from glycerol conversion with acetone. This reaction proceeds under mild conditions and finds many applications [7]. Moreover, as a fuel additive, solketal can reduce gum formation and increase the octane number. Since solketal is easily hydrolyzed in water it does not contaminate water sources, as so many additives do, and this is the main reason for them being phased out [8,9].
Usually, the acetalization reaction of glycerol with acetone proceeds in the presence of an acid catalyst (Scheme 1) and has been studied under different conditions and catalysts [1,2,3,4,9]. Although homogeneous acid catalytic systems (e.g., p-toluenesulfonic, sulfuric, and phosphoric acids) are suitable for glycerol acetalization, their use is complicated by the need to separate the catalysts from products and because they are not considered environmentally friendly [7]. Moreover, although glycerol and acetone are polar compounds, the two reactants are almost immiscible [10]. Thus, heterogeneous catalysts have been the choice for glycerol acetalization, even if water is formed during the reaction, as a byproduct, inducing a reversible reaction. In short, heterogeneous catalysts are easy to handle, avoid energy-intensive catalyst separation processes, waste generation, and corrosion hazards [11]. Solid materials of acidic character, such as amberlyst (Amberlyst-15, Amberlist-35, Amberlist-36), silica supported heteropoly acids, mesostructured silica, clays and also zeolites of different structure have been tested as catalysts for glycerol acetalization, both in batch and in continuous processes [1,2,3,4,9,12,13,14].
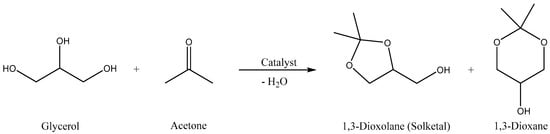
Scheme 1.
Reaction between glycerol and acetone (Gly/Ace) to produce useful cyclic acetals.
Metal-Organic Frameworks (MOFs) and Coordination Polymer (CPs) are hybrid materials, comprising an organic linker self-assembled with metal centres, which have been used extensively as heterogeneous catalysts [11,15,16,17,18,19], including in acid catalysis [11,20]. To our knowledge there are only two reports on the solketal synthesis from glycerol acetalization using MOFs [21,22]. Bakuru et al. have used isostructural UIO-66 (Hf, Zr, Ce) in the synthesis of alkyl levulinate and solketal and obtained one of the highest activities reported for solketal synthesis [21]. Timofeeva et al. used isostructural MIL-100(V, Al, Fe, and Cr), MIL-53(V, Al, Fe, and Cr) and mixed MIL-53(Al,V) (Al/V from 0 to 100) in the synthesis of solketal from glycerol and acetone at 70 °C in acetonitrile [22].
Here, CPs based on nitrilo(trimethylphosphonic acid) and Ln3+/Eu3+ are used as catalysts for the acetalization of the bio-renewable glycerol into oxygenated fuel additives, with solketal being the major product obtained in the reaction of glycerol with acetone. The stability of the materials is also reported as well as their recovery and reuse.
2. Results and Discussion
2.1. Catalysts Synthesis and Characterization
The organic-inorganic hybrid catalysts studies in this work have been previously reported by us [17,23]. The two materials were prepared by reaction of nitrilo(trimethylphosphonic acid (H6nmp) with Eu3+ (UAV-63 [17]) and La3+ (UAV-20 [23]) (Scheme 2). UAV-63 and UAV-20 are a 3D and 1D coordination polymer, respectively, and crystals of both are easily isolated after a few minutes under microwave irradiation and water and sulfuric acid as the solvents. In the case of UAV-63, the acid is also the source of the sulfate anions coordinated to Eu3+. Both materials are isolated as white powders with a plate-like morphology with sizes ranging from 5-15 μm. For the complete characterization, the reader is directed to their previous reported works.
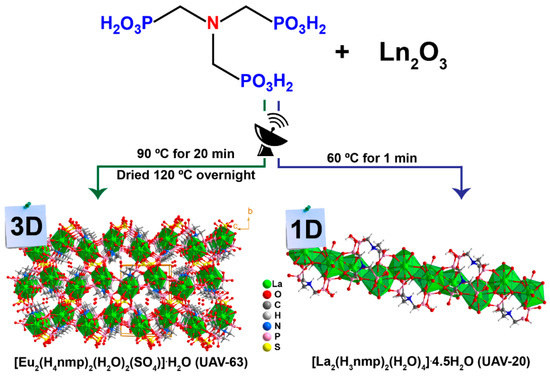
Scheme 2.
Schematic representation of the microwave-assisted synthesis and structural features of [Eu2(H4nmp)2(H2O)2(SO4)]·H2O (UAV-63) and [La2(H3nmp)2(H2O)4]·4.5H2O (UAV-20).
2.2. Reaction of Glycerol with Acetone
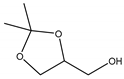
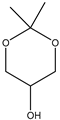
The acetalization reaction of glycerol with acetone in the presence of UAV-20 and UAV-63 was investigated at 40 °C and 55 °C, with different glycerol/acetone (Gly/Ace) molar ratios, without the addition of solvent. The reaction affords (2,2-dimethyl-1,3-dioxolan-4-yl)methanol (1,3-dioxolane for short, a five-membered acetal also known as solketal) and 2,2-dimethyl-1,3-dioxan-5-ol (1,3-dioxane for short, a six-membered acetal) (Scheme 1). The temperature is an important parameter influencing the catalytic activity of these materials during acetalization (please consider Table 1). Increasing temperature from 40 °C to 55 °C (after 6 h) increases the conversion of glycerol from 22% to 84% for UAV-63 and from 8% to 56% for UAV-20. Selectivity to the two cyclic acetals also changes with temperature but solketal is always the major product. For UAV-63, at 55 °C the selectivity for solketal reaches 96%, much higher than at 40 °C (70%). For UAV-20 the selectivity increased from 72% (40 °C) to 90% (55 °C). When a different Gly/Ace molar ratio was studied (1:4), the conversion decreased for the same time of reaction with both catalysts, and the selectivity did not suffer any change for UAV-63 or suffered only a small change in the case of UAV-20.

Table 1.
Influence of temperature and Gly/Ace molar ratio on the acetalization reaction of glycerol and acetone in the presence of UAV-20 and UAV-63, after 6 h 1.
The reaction kinetic profiles of the two catalysts shown in Figure 1a reveal an induction period of 3-4 hours. UAV-63 conversion increases from 40% after 3 h to 84% after 6 h, while UAV-20 conversion increases from 22% (4 h) to 56% (6 h). Figure 1b shows the selectivity towards the acetals. Dioxane is obtained at the beginning of the reaction and decreases with increasing time, while the amount of dioxolane increases with time, particularly after the induction period. UAV-20 selectivity is similar up to 4 h, reaching 73% for solketal and 27% for dioxane. After 4 h of reaction the selectivity for solketal increases.
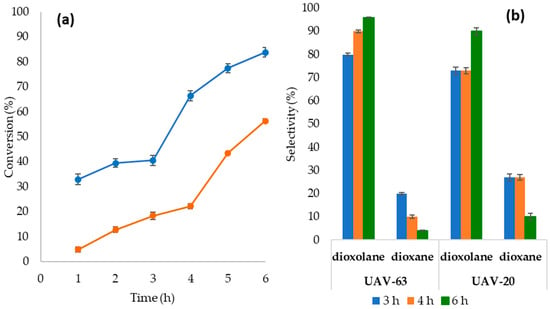
Figure 1.
Kinetic profiles (a) for the Gly/Ace acetalization reactions in the presence of UAV-20 (orange dots) and UAV-63 (blue dots). Selectivity (b) for 1,3-dioxolane (solketal) and 1,3-dioxane for different times of reaction in the presence of UAV-20 and UAV-63. Reaction conditions: 55 °C; 5% (w/w) catalyst; Gly/Ace ratio 1:10.
These results compare well with other recent studies on the acetalization of glycerol with acetone in the presence of MOFs (Table 2). Bakuru et al. reported substantially different conversions of glycerol for three isostructural UiO-66(M) MOFs [M=Zr(IV), Ce(IV) or Hf(IV)]. UiO-66(Hf) yielded 95% conversion and 97% selectivity for solketal at room temperature after 1 h, using 10 wt% catalyst. Under the same conditions, UiO-66(Ce) afforded 70% conversion and 90% selectivity, and UiO-66(Zr) only 2% conversion and 73% selectivity for solketal. The catalytic activity follows the Brønsted acidity of these MOFs, UiO 66(Hf) > UiO-66(Ce) > UiO-66(Zr) [21]. Timofeeva and collaborators have studied the same reaction in the presence of the isostructural MIL 100(M) and MIL- 53(M) (M = V, Al, Fe and Cr), along with the mixed MIL-53(Al,V) (Al/V—100/0, 75/25, 50/50, 25/75 and 0/100 atom/atom) using acetonitrile as solvent. At 70 °C and 20 wt% catalyst in 5 mL of acetonitrile, the highest conversion of glycerol was observed for MIL-100(V) (85.4% with 97.7% selectivity for solketal) and MIL-47(V) (75.9% with 97.5% selectivity for solketal), and the lowest conversion for MIL-100(Cr) and MIL 53(Cr) (4.2% and 1.6%, respectively). At 25 °C and 2.5 wt% catalyst in 15 mL of acetonitrile, lower conversions of glycerol where obtained, although maintaining the high selectivity for solketal [22].

Table 2.
Influence of temperature on the acetalization reaction between glycerol and acetone in the presence of UAV-20 and UAV-63, after 6 h, Gly/Ace ratio 1:10 1.
2.3. Catalyst Stability
The stability of UAV-20 and UAV-63 was studied in three consecutive runs (6 h) of Gly/Ace acetalization reaction, at 55 °C. Catalyst recovery comprised washing with ethanol and drying at 80 °C between runs (experimental Section 3.4). For UAV-63 there was a slight conversion decrease, from 84% to 69%, while for UAV-20 the conversion remained constant in consecutive runs (Figure 2a). No products other than solketal and dioxane were detected but the selectivity slightly changed after the first run (Figure 2b). With both catalysts, dioxane increases in the same order of magnitude from run 1 to run 2, but selectivities remain stable for run 3.
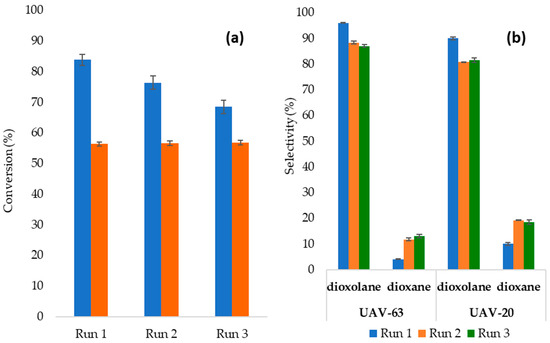
Figure 2.
(a) Conversion of glycerol in the presence of UAV-63 (blue bars) and UAV-20 (orange bars); (b) UAV-63 and UAV-20 selectivity in three consecutive runs for the Gly/Ace acetalization reaction at 55 °C/6h (catalyst: 5 wt.%), after washing and drying.
Leaching tests were also performed in order to establish the real heterogeneous character of the reaction. After three hours of reaction the solid catalyst was separated from the mixture and the reaction allowed to proceed with the remaining filtrate. The results confirmed heterogeneous catalysis as the conversion of glycerol almost immediately stopped after the catalyst’s removal. Indeed, for UAV-63 and after 3 h of reaction we had 40% conversion before removing the catalyst; after removing the catalyst and at 6 h of reaction we had 43% conversion. For UAV-20 and after 3 h of reaction we had 18% conversion before removing the catalyst; after removing the catalyst and at 6 h of reaction we had 20% conversion. These values compare with 84% for UAV-63 and 56% for UAV-20, after 6 h of reaction without removing the catalyst.
The catalytic results of UAV materials were also compared with the free organic linker H6nmp, and Eu2O3 and La2O3, using the same molar amounts present in the hybrid catalysts. With soluble H6nmp, very similar results to UAV-63 were obtained. After 1 h of reaction and 0.02 mmol of H6nmp, 68% conversion was obtained, better than with both UAV materials, and 73% conversion after 6 h. This reaction resulted also in the two acetals, reaching 80% selectivity for solketal after 1 h of reaction, and no major change was observed subsequently. The downside, of course, is that H6nmp acts homogeneously implying a much more difficult separation and reutilization processes than UAV materials. On the other hand, no reaction occurred after 6 h at 55 °C, Gly/Ace 1/10, when Eu2O3 and La2O3 were used.
As depicted in Figure 2, the catalytic conversion over UAV-63 slightly decreases after each cycle while UAV-20 maintains its activity. This is attributed to partial changes in the crystal structure: UAV-63 is isolated by an in situ single-crystal-to-single-crystal transformation [11]. It is stable at ambient conditions but may partially revert to an intermediate crystalline phase in the presence of water (Figure 3). This transformation is accompanied only by a change in dimensionality (from a 3D network to a 2D layer). The incorporation of water (formed in the catalytic reaction) breaks a P‒O‒M coordination bond, leading to the partial transformation of UAV-63. In fact, while no apparent change in crystal morphology is observed (Figure 4a,b), powder X-ray diffraction (Figure 4c) shows that, after each catalytic run, the material consists of a mixture of two crystalline phases, UAV-63 and the intermediate structure denoted UAV-63_r. In contrast, UAV-20 shows no change in activity, crystal morphology (Figure 5a,b), and structural integrity (even if the material is less crystalline after each run) (Figure 5c). These results are also supported by EDS mapping analysis, showing the homogeneous distribution of the elements (Figure 6) with the La:P and Eu:P:S proportion as expected for these materials (1:3 and 1:3:0.5, respectively).
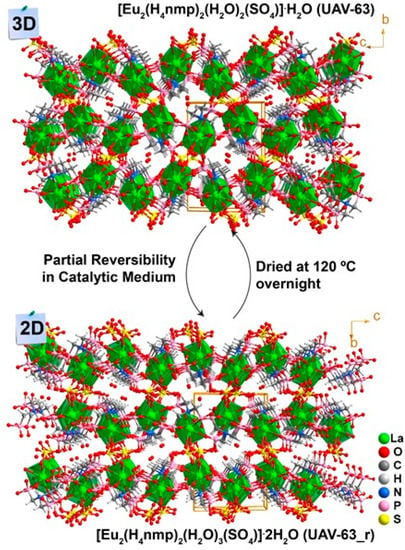
Figure 3.
Schematic representation of the single-crystal to single-crystal transformation of UAV-63 to UAV-63_r in the presence of water formed during the catalytic reaction.
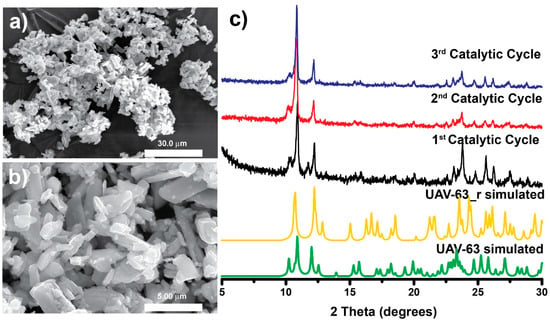
Figure 4.
SEM images of UAV-63 before (a) and after (b) the catalytic acetalization reaction Gly/Ace at 55 °C after 6 h. (c) Powder X-ray diffraction patterns of UAV-63 after each catalytic cycle.
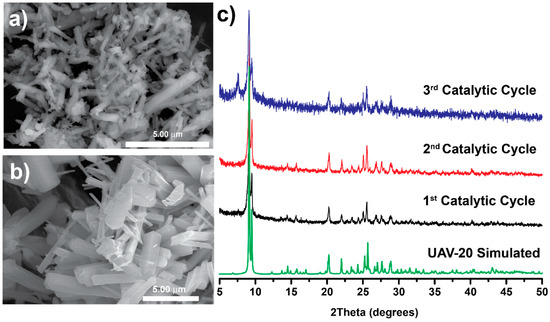
Figure 5.
SEM images of UAV-20 before (a) and after (b) the catalytic acetalization reaction Gly/Ace at 55 °C after 6 h. (c) Powder X-ray diffraction of UAV-20 after each catalytic cycle.
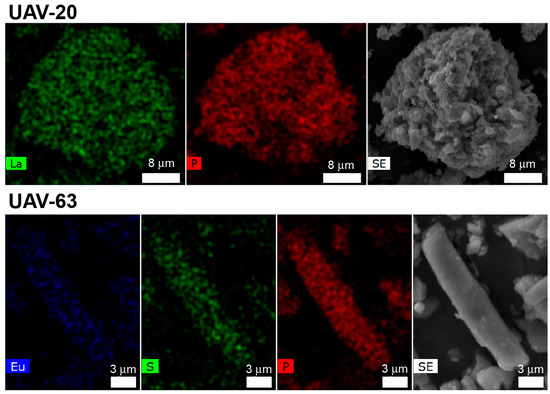
Figure 6.
EDS mapping of UAV-20 and UAV-63 after the 3rd catalytic cycles for the catalytic acetalization reaction Gly/Ace at 55 °C after 6 h.
The FT-IR spectra of UAV materials before and after catalysis are very similar (Figure 7). The typical bands of the coordinated organic linker are observed in the fresh and used solids: a very broad band in the range 3600–3150 cm−1 and a band at 1620 cm−1 due to the ν(O‒H) stretching modes of coordinated water and the deformation δ(H2O) of water molecules, respectively; bands in the range 3200–2800 cm−1 due to (as)symmetric ν(C–H) and ν(N‒H) stretching vibrational modes of the linker. Below 2000 cm−1 the vibrational bands are attributed to the P‒CH2 groups (in the range of 1480 and 1380 cm−1), to the ν(P=O) and ν(SO42−) (for the case of UAV-63) in the 1315 and 1380 cm−1 and to the ν(P‒O) at 1135–860 cm−1. These results indicate that, despite the partial transformation of UAV-63, the overall structural integrity (at least in regard to the functional groups present) is maintained during the catalytic cycles.
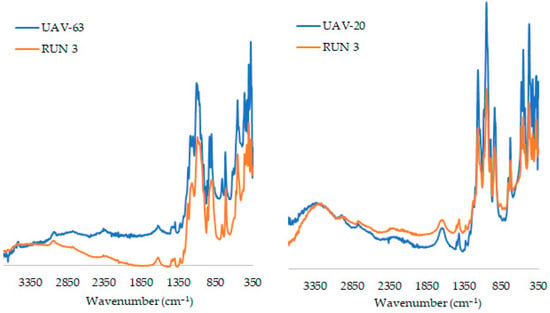
Figure 7.
ATR FT-IR spectra of the fresh (blue) and the used catalyst (orange) after three consecutive reactions Gly/Ace, at 55 °C, after 6 h. Catalysts [Eu2(H4nmp)2(H2O)2(SO4)]·H2O (UAV-63) and [La2(H3nmp)2(H2O)4]·4.5H2O (UAV-20).
A plausible mechanism of the Brønsted acid-catalyzed acetalization reaction is presented in Figure 8. A Brønsted acid site protonates the oxygen atom of the carbonyl group of acetone, which then suffers a nucleophilic attack from glycerol with the formation of an intermediate which, after elimination of water, gives a carbocation. The latter undergoes an intramolecular nucleophilic attack by one of the OH groups of glycerol [paths a) and b)] to give both the acetals. The catalyst is protonated [(path c)] and can be used in a new catalytic cycle or, after its recovery and washing, it can be reused.
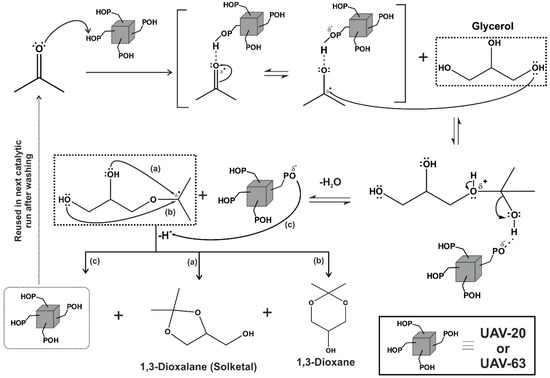
Figure 8.
Mechanistic proposal for the acetalization of glycerol with acetone in the presence of UAV-63 or UAV-20.
3. Materials and Methods
3.1. Reagents
All reagents and solvents were obtained from commercial sources and used as received without further purification. In synthesis of [Eu2(H4nmp)2(H2O)2(SO4)]·H2O (UAV-63) and of [La2(H3nmp)2(H2O)4]·4.5H2O (UAV-20), europium (III) oxide (>99.99%, Jinan Henghua Sci. & Tec. Co. Ltd., Jinan, China), lanthanum(III) oxide (>99.99%, Jinan Henghua Sci. & Tec. Co. Ltd., Jinan, China), nitrilo(trimethylphosphonic acid (H6nmp, N(CH2PO3H2)3, 97%, Fluka, Algés, Portugal) and sulfuric acid (H2SO4, 98% José Manuel Gomes dos Santos, Odivelas, Portugal) were used. For catalytic tests, glycerol (CMD Chemicals, ≥99.5%, Funchal, Portugal), acetone (Aldrich, 99%, Algés, Portugal), absolute ethanol anhydrous (Carlo-Erba, >99.9%, Barcelona, Spain), and 1,4-dioxane (Emplura, Algés, Portugal) as internal standard were used.
3.2. General Instrumentation
Powder X-Ray Diffraction (PXRD) data were collected at ambient temperature on an Empyrean PANalytical diffractometer, with a working wavelength of λ1 = 1.540598 Å and λ2 = 1.544426 Å (Cu Kα1,2 X-radiation), equipped with an PIXcel 1D detector and a flat-plate sample holder in a Bragg-Brentano para-focusing optics configuration (45 kV, 40 mA). Intensity data were collected by the step-counting method (step 0.01°), in continuous mode, in the 3.5° ≤ 2θ° ≤ 50° range.
Energy dispersive X-ray spectroscopy (EDS) was carried out on a high-resolution Hitachi SU-70 (Monocomp) working at 15 kV, employing a Sprit 1.9 EDS microanalysis system. Samples were prepared by deposition on aluminium sample holders followed by carbon coating using an Emitech K950X carbon evaporator.
Attenuated total reflectance (ATR) FT-IR spectra were measured on a Mattson-7000 infrared spectrophotometer equipped with a Specac Golden Gate Mk II ATR accessory with a diamond top plate and KRS-5 focusing lenses.
3.3. Catalyst Synthesis
[Eu2(H4nmp)2(H2O)2(SO4)]·H2O (UAV-63) was prepared by adapting the procedure previously reported [23]. A reactive mixture containing 0.1400 g (0.047 mmol) of nitrilo(trimethylphosphonic) acid (H6nmp) and 0.844 g (0.025 mmol) of Eu2O3 were dissolved in ca. 5 mL of distilled water and 500 μL of concentrated sulfuric acid in a 10 mL IntelliVent microwave reactor. The reaction was carried out on a CEM Focused Microwave Synthesis System Discover S-Class equipment, under constant magnetic stirring (controlled by the microwave equipment), using an irradiation power of 50 W at 90 °C for 20 minutes. A constant flow of air (pressure 20–30 psi) ensured the temperature inside the reactor. The resulting white microcrystalline powder was filtered off, washed with copious amounts of distilled water, and dried at room temperature. After drying, the powder was heated at 120 °C overnight.
[La2(H3nmp)2(H2O)4]·4.5H2O (UAV-20) was prepared as reported previously [17]. A reactive mixture containing 0.1422 g (0.047 mmol) of nitrilo(trimethylphosphonic) acid (H6nmp) and 0.808 g (0.025 mmol) of Eu2O3 were dissolved in ca. 5 mL of distilled water and 100 μL of concentrated sulfuric acid in a 10 mL IntelliVent microwave reactor. The reaction was carried out on a CEM Focused Microwave Synthesis System Discover S-Class equipment, under constant magnetic stirring (controlled by the microwave equipment), using an irradiation power of 50W at 60 °C for 1 minute. A constant flow of air (pressure 20–30 psi) ensured the temperature inside the reactor. The resulting white microcrystalline powder was filtered off, washed with copious amounts of distilled water, and dried at room temperature. After drying, the powder was heated at 120 °C overnight.
3.4. Catalytic Tests
The reactions of glycerol (Gly) with acetone (Ace) were carried out under air in a closed borosilicate vessel equipped with a valve and a PTFE-coated magnetic stirring bar (1500 rpm) and immersed in a thermostatically controlled liquid paraffin bath at 40 °C or at 55 °C. The batch reactors were loaded with the reactants in a 1:4 or 1:10 Gly/Ace molar ratio and 20 mg of catalyst [5 wt% catalyst relatively to Gly]. In a typical reaction 20 mg of catalyst, 0.4 g of Gly and 3 mL of acetone were used. For comparative purposes, H6nmp, La2O3, and Eu2O3 were also tested as putative catalysts. A blank test without catalyst was also carried out. A leaching test was performed in the same conditions as the typical catalytic reaction. After 3 hours of reaction the solid catalyst was separated from the mixture and the reaction was allowed to continue with the remaining filtrate. The solid catalyst was separated from the mixture by centrifugation at 3500 rpm and the supernatant liquid phase was passed through a Whatman Polydisc TF Chemical resistant in-line filter 0.45 μm PTFE membrane. After each test, the reactor was cooled to ambient temperature, 4 mL of ethanol and 4.34 mmol of 1,4-dioxane as internal standard were added, and the solid catalyst was separated by centrifugation at 3500 rpm. The evolution of the catalytic reactions was monitored by gas chromatography (GC) using a SCION apparatus equipped with a DBFFAP (Agilent, 30 m × 320 µm × 0,25 µm) column and a flame ionization detector (250 °C). The separation of the organic species was carried out with hydrogen as the carrier gas (constant flow rate of 30 mL min−1) using a temperature program for the column starting at 35 °C during 2 min and increasing to 60 °C at 10 °C/min, holding for 1 min, and heating up to 230 °C at 15 °C/min and holding for 10 min (the total runtime being 27 min), with the injector at 250 °C.
Individual experiments were performed for a given reaction time and the results reported are the mean values of three replicates (experimental error <5%, error bars in the figures were calculated using standard deviation procedures). Conversion was based on the limiting reactant, glycerol, and its quantification was based on a calibration curve. The product yields were calculated relative to the initial amount of the limiting reactant, glycerol, using the formula: 100 × [(molar concentration of product at time t)/(initial molar concentration of Gly)]. The catalyst was separated from the reaction mixture by centrifugation at 3500 rpm, washed with ethanol, dried at 80 °C overnight and reused for up to three 6 h batch runs of the Gly/Ace acetalization reaction at 55 °C. All recycling cycles were performed under the same initial experimental conditions.
For identification of products, 1.5 µL aliquots were injected in an Agilent 8860 GC System gas chromatograph with GC 5977B Network Mass Selective Detector operating at 70 eV at 250 °C, and equipped with a DBFFAP (Agilent, 30 m × 320 µm × 0.25 µm) column. GC-MS was equipped with an auto sampler with a splitless injector at 220 °C. The analyses were carried out with helium as the carrier gas (constant flow rate of 1.4 mL min−1) and using a temperature program for the column starting at 60 °C during 5 min and increasing to 225 °C at 15 °C/min, then holding for 30 min (total runtime 46 min). The system includes a Mass Selective Detector operating in Electron Ionization (EI) mode at 70 eV and scanning the mass range m/z 50–550 in a 1 s cycle in a full scan mode acquisition. The organic compounds were identified using the software Agilent MassHunter Qualitative10.0 (Agilent Technologies, Santa Clara, CA, USA), supported by NIST2014 mass spectral library.
4. Conclusions
The coordination polymers [Eu2(H4nmp)2(H2O)2(SO4)]·H2O (UAV-63, a 3D network) and [La2(H3nmp)2(H2O)4]·4.5H2O (UAV-20, a 1D chain) proved to be effective solid acid catalysts in the synthesis of solketal from glycerol and acetone, in the absence of a co-solvent. Both catalysts yield (2,2-dimethyl-1,3-dioxolan-4-yl)methanol (solketal) with very good selectivities, with only 2,2-dimethyl-1,3-dioxan-5-ol obtained as a small yield co-product. Raising the temperature from 40 °C to 55 °C increases the catalytic activity from 22% to 84% for UAV-63 and from 8% to 56% for UAV-20 after 6 h of reaction. Although the conversion of glycerol is lower over UAV-20 than UAV-63 in the first cycle (56% and 84%, respectively, at 6 h and 55 °C) this catalyst is stable upon reuse after washing and drying, exhibiting a similar conversion after three cycles. In contrast, UAV-63 slightly deactivates with increasing cycles (from 84% to 69%). Although the conversion after UAV-63 third cycle is still higher than UAV-20, in the presence of water the former partially transforms into the 2D layered material, [Eu2(H4nmp)2(H2O)3(SO4)]·2H2O. The decrease of conversion in successive catalytic cycles is ascribed to this in situ transformation. The performance of both UAV catalysts compared positively to that reported in the literature when compared to the only two crystalline CP/MOF-type materials studied for this reaction system, since no co-solvent is added here and the Cat/Gly ratio (%, w/w) is rather low, allowing high selectivity to solketal and high conversions of glycerol.
Author Contributions
I.C.M.S.S.-V. planned and executed all the catalytic studies, carried out the post-reaction characterization of the catalysts, and prepared the final draft of the manuscript. R.F.M. prepared and characterized the materials used as catalysts, read and corrected the manuscript. F.A.A.P. read and corrected the manuscript. J.R. critically read the paper and proposed changes and corrections. M.M.Q.S. was responsible for the conceptualization of the catalytic studies, read and corrected the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from PT national funds (FCT/MCTES, Fundação para a Ciência e Tecnologia and Ministério da Ciência, Tecnologia e Ensino Superior) through LAQV-REQUIMTE (UIDB/50006/2020) and CICECO-Aveiro Institute of Materials (UIDB/50011/2020 & UIDP/50011/2020). The position held by I.C.M.S.S.-V. (Ref. 197_97_ARH-2018) was funded by national funds (OE), through FCT, I.P., in the scope of the framework contract foreseen in the numbers 4, 5 and 6 of article 23 of the Decree-Law 57/2016 of 29 August, changed by Law 57/2017 of 19 July. R.F.M. gratefully acknowledges FCT for a Junior Research Position (CEECIND/00553/2017).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zahid, I.; Ayoub, M.; Abdullah, B.B.; Nazir, M.H.; Ameen, M.; Zulqarnain; Mohd Yusoff, M.H.; Inayat, A.; Danish, M. Production of Fuel Additive Solketal via Catalytic Conversion of Biodiesel-Derived Glycerol. Ind. Eng. Chem. Res. 2020, 59, 20961–20978. [Google Scholar] [CrossRef]
- Fatimah, I.; Sahroni, I.; Fadillah, G.; Musawwa, M.M.; Mahlia, T.M.I.; Muraza, O. Glycerol to Solketal for Fuel Additive: Recent Progress in Heterogeneous Catalysts. Energies 2019, 12, 2872. [Google Scholar] [CrossRef]
- Nda-Umar, U.I.; Ramli, I.; Taufiq-Yap, Y.H.; Muhamad, E.N. An overview of recent research in the conversion of glycerol into biofuels, fuel additives and other bio-based chemicals. Catalysts 2019, 9, 15. [Google Scholar] [CrossRef]
- Talebian-Kiakalaieh, A.; Amin, N.A.S.; Najaafi, N.; Tarighi, S. A review on the catalytic acetalization of bio-renewable glycerol to fuel additives. Front. Chem. 2018, 6, 1–25. [Google Scholar] [CrossRef]
- Rodrigues, A.; Bordado, J.C.; Dos Santos, R.G. Upgrading the glycerol from biodiesel production as a source of energy carriers and chemicals—A technological review for three chemical pathways. Energies 2017, 10, 1817. [Google Scholar] [CrossRef]
- Szori, M.; Giri, B.R.; Wang, Z.; Dawood, A.E.; Viskolcz, B.; Farooq, A. Glycerol carbonate as a fuel additive for a sustainable future. Sustain. Energy Fuels 2018, 2, 2171–2178. [Google Scholar] [CrossRef]
- Rahaman, M.S.; Phung, T.K.; Hossain, M.A.; Chowdhury, E.; Tulaphol, S.; Lalvani, S.B.; O’Toole, M.; Willing, G.A.; Jasinski, J.B.; Crocker, M.; et al. Hydrophobic functionalization of HY zeolites for efficient conversion of glycerol to solketal. Appl. Catal. A Gen. 2020, 592. [Google Scholar] [CrossRef]
- Mota, C.J.A.; Da Silva, C.X.A.; Rosenbach, N.; Costa, J.; Da Silva, F. Glycerin derivatives as fuel additives: The addition of glycerol/acetone ketal (solketal) in gasolines. Energy Fuels 2010, 24, 2733–2736. [Google Scholar] [CrossRef]
- Oliveira, P.A.; Souza, R.O.M.A.; Mota, C.J.A. Atmospheric pressure continuous production of solketal from the acid-catalyzed reaction of glycerol with acetone. J. Braz. Chem. Soc. 2016, 27, 1832–1837. [Google Scholar] [CrossRef]
- Catuzo, G.L.; Santilli, C.V.; Martins, L. Hydrophobic-hydrophilic balance of ZSM-5 zeolites on the two-phase ketalization of glycerol with acetone. Catal. Today 2020. [Google Scholar] [CrossRef]
- Antunes, M.M.; Mendes, R.F.; Almeida Paz, F.A.; Valente, A.A. Versatile coordination polymer catalyst for acid reactions involving biobased heterocyclic chemicals. Catalysts 2021, 11, 190. [Google Scholar] [CrossRef]
- Ferreira, P.; Fonseca, I.M.; Ramos, A.M.; Vital, J.; Castanheiro, J.E. Valorisation of glycerol by condensation with acetone over silica-included heteropolyacids. Appl. Catal. B Environ. 2010, 98. [Google Scholar] [CrossRef]
- Vicente, G.; Melero, J.A.; Morales, G.; Paniagua, M.; Martín, E. Acetalisation of bio-glycerol with acetone to produce solketal over sulfonic mesostructured silicas. Green Chem. 2010, 12. [Google Scholar] [CrossRef]
- Kowalska-Kus, J.; Held, A.; Frankowski, M.; Nowinska, K. Solketal formation from glycerol and acetone over hierarchical zeolites of different structure as catalysts. J. Mol. Catal. A Chem. 2017, 426, 205–212. [Google Scholar] [CrossRef]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Cui, H.; Zhang, J.; Zhang, L.; Su, C.Y. Applications of metal-organic frameworks in heterogeneous supramolecular catalysis. Chem. Soc. Rev. 2014, 43, 6011–6061. [Google Scholar] [CrossRef] [PubMed]
- Mendes, R.F.; Silva, P.; Antunes, M.M.; Valente, A.A.; Almeida Paz, F.A. Sustainable synthesis of a catalytic active one-dimensional lanthanide-organic coordination polymer. Chem. Commun. 2015, 51, 10807–10810. [Google Scholar] [CrossRef]
- Mendes, R.F.; Antunes, M.M.; Silva, P.; Barbosa, P.; Figueiredo, F.; Linden, A.; Rocha, J.; Valente, A.A.; Almeida Paz, F.A. A Lamellar Coordination Polymer with Remarkable Catalytic Activity. Chem. A Eur. J. 2016, 22, 13136–13146. [Google Scholar] [CrossRef] [PubMed]
- Mirante, F.; Mendes, R.F.; Almeida Paz, F.A.; Balula, S.S. High catalytic efficiency of a layered coordination polymer to remove simultaneous sulfur and nitrogen compounds from fuels. Catalysts 2020, 10, 731. [Google Scholar] [CrossRef]
- Gascon, J.; Corma, A.; Kapteijn, F.; Llabrés I Xamena, F.X. Metal organic framework catalysis: Quo vadis? ACS Catal. 2014, 4, 361–378. [Google Scholar] [CrossRef]
- Bakuru, V.R.; Churipard, S.R.; Maradur, S.P.; Kalidindi, S.B. Exploring the Brønsted acidity of UiO-66 (Zr, Ce, Hf) metal-organic frameworks for efficient solketal synthesis from glycerol acetalization. Dalt. Trans. 2019, 48, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, M.N.; Panchenko, V.N.; Khan, N.A.; Hasan, Z.; Prosvirin, I.P.; Tsybulya, S.V.; Jhung, S.H. Isostructural metal-carboxylates MIL-100(M) and MIL-53(M) (M: V, Al, Fe and Cr) as catalysts for condensation of glycerol with acetone. Appl. Catal. A Gen. 2017, 529, 167–174. [Google Scholar] [CrossRef]
- Mendes, R.F.; Almeida Paz, F.A. Dynamic breathing effect in metal-organic frameworks: Reversible 2D-3D-2D-3D single-crystal to single-crystal transformation. Inorganica Chim. Acta 2017, 460, 99–107. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).