Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications
Abstract
1. Introduction
2. Photocatalysis for Water Purification
2.1. Reactor Design
2.2. Photocatalytic Activity in Complex Water Matrices and Real Samples
2.2.1. Presence of Interfering Species and Real Water Matrices
2.2.2. Degradation of Pollutants at Low Concentration
2.3. Cost and Environmental Life-Cycle Assessment
3. Photocatalysis for Air Purification
3.1. Photocatalytic Reactors for Air Treatment
3.2. Tests in Realistic Conditions
3.3. Photocatalyst Deactivation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Uyguner-demirel, C.S.; Birben, N.C.; Bekbolet, M. Elucidation of background organic matter matrix effect on photocatalytic treatment of contaminants using TiO2: A review. Catal. Today 2017, 284, 202–214. [Google Scholar] [CrossRef]
- Bletsou, A.A.; Jeon, J.; Hollender, J.; Archontaki, E.; Thomaidis, N.S. Targeted and non-targeted liquid chromatography-mass spectrometric workflows for identification of transformation products of emerging pollutants in the aquatic environment. TrAC Trends Anal. Chem. 2015, 66, 32–44. [Google Scholar] [CrossRef]
- Antonopoulou, M.; Kosma, C.; Albanis, T.; Konstantinou, I. An overview of homogeneous and heterogeneous photocatalysis applications for the removal of pharmaceutical compounds from real or synthetic hospital wastewaters under lab or pilot scale. Sci. Total Environ. 2021, 765, 144163. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2020: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2020; Volume 5. [Google Scholar]
- Richardson, S.D.; Kimura, S.Y. Water analysis: Emerging contaminants and current issues. Anal. Chem. 2019, 81, 4645–4677. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of poly- and perfluoroalkyl substances (PFAS) from water by adsorption: Role of PFAS chain length, effect of organic matter and challenges in adsorbent regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef] [PubMed]
- Gar Alalm, M.; Ookawara, S.; Fukushi, D.; Sato, A.; Tawfik, A. Improved WO3 photocatalytic efficiency using ZrO2 and Ru for the degradation of carbofuran and ampicillin. J. Hazard. Mater. 2016, 302, 225–231. [Google Scholar] [CrossRef]
- Couto, C.F.; Santos, A.V.; Amaral, M.C.S.; Lange, L.C.; de Andrade, L.H.; Foureaux, A.F.S.; Fernandes, B.S. Assessing potential of nanofiltration, reverse osmosis and membrane distillation drinking water treatment for pharmaceutically active compounds (PhACs) removal. J. Water Process Eng. 2020, 33, 101029. [Google Scholar] [CrossRef]
- Zhang, S.; Pu, W.; Chen, A.; Xu, Y.; Wang, Y.; Yang, C.; Gong, J. Oxygen vacancies enhanced photocatalytic activity towards VOCs oxidation over Pt deposited Bi2WO6 under visible light. J. Hazard. Mater. 2020, 384, 121478. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; Lefevre, C.; Robert, D.; Keller, N. Highly robust La1-xTixFeO3 dual catalyst with combined photocatalytic and photo-CWPO activity under visible light for 4-chlorophenol removal in water. Appl. Catal. B Environ. 2020, 262, 118310. [Google Scholar] [CrossRef]
- Loeb, S.K.; Alvarez, P.J.J.; Brame, J.A.; Cates, E.L.; Choi, W.; Crittenden, J.; Dionysiou, D.D.; Li, Q.; Li-Puma, G.; Quan, X.; et al. The technology horizon for photocatalytic water treatment: Sunrise or sunset? Environ. Sci. Technol. 2019, 53, 2937–2947. [Google Scholar] [CrossRef]
- Serrà, A.; Philippe, L.; Perreault, F.; Garcia-Segura, S. Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Res. 2021, 188, 116543. [Google Scholar] [CrossRef] [PubMed]
- Hodges, B.C.; Cates, E.L.; Kim, J.H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Samy, M.; Ibrahim, M.G.; Fujii, M.; Diab, K.E.; Elkady, M.; Alalm, G. CNTs/MOF-808 painted plates for extended treatment of pharmaceutical and agrochemical wastewaters in a novel photocatalytic reactor. Chem. Eng. J. 2021, 406, 7. [Google Scholar] [CrossRef]
- Adhikari, S.; Selvaraj, S.; Kim, D.H. Construction of heterojunction photoelectrode via atomic layer deposition of Fe2O3 on Bi2WO6 for highly efficient photoelectrochemical sensing and degradation of tetracycline. Appl. Catal. B Environ. 2019, 244, 11–24. [Google Scholar] [CrossRef]
- Deng, F.; Lu, X.; Luo, Y.; Wang, J.; Che, W.; Yang, R.; Luo, X.; Luo, S.; Dionysiou, D.D. Novel visible-light-driven direct Z-scheme CdS/CuInS2 nanoplates for excellent photocatalytic degradation performance and highly-efficient Cr(VI) reduction. Chem. Eng. J. 2019, 361, 1451–1461. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, W.; Yao, Q.; Huang, L.; Chen, L.; Boury, B.; Chen, Z. Zn-free MOFs like MIL-53(Al) and MIL-125(Ti) for the preparation of defect-rich, ultrafine ZnO nanosheets with high photocatalytic performance. Appl. Catal. B Environ. 2019, 244, 719–731. [Google Scholar] [CrossRef]
- Janssens, R.; Mandal, M.K.; Dubey, K.K.; Luis, P. Slurry photocatalytic membrane reactor technology for removal of pharmaceutical compounds from wastewater: Towards cytostatic drug elimination. Sci. Total Environ. 2017, 599–600, 612–626. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, P.; Xu, P.; Hu, L.; Wang, X.; Qu, J.; Zhang, G. Submerged membrane photocatalytic reactor for advanced treatment of p-nitrophenol wastewater through visible-light-driven photo-Fenton reactions. Sep. Purif. Technol. 2021, 256, 117783. [Google Scholar] [CrossRef]
- Kumar, A.; Khan, M.; He, J.; Lo, I.M.C. Recent developments and challenges in practical application of visible-light-driven TiO2–based heterojunctions for PPCP degradation: A critical review. Water Res. 2020, 170, 115356. [Google Scholar] [CrossRef]
- European Parliament; Council of the European Union. Regolamento (CE) n. 1223/2009 del Parlamento Europeo e Del Consiglio del 30 Novembre 2009, Sui Prodotti Cosmetici (Testo Rilevante ai Fini del SEE); Council of the European Union: Brussels, Belgium, 2009. [Google Scholar]
- Cheng, X.; Wang, P.; Liu, H. Visible-light-driven photoelectrocatalytic degradation of diclofenac by N, S-TiO2/TiO2NTs photoelectrode: Performance and mechanism study. J. Environ. Chem. Eng. 2015, 3, 1713–1719. [Google Scholar] [CrossRef]
- Parrino, F.; Corsino, S.F.; Bellardita, M.; Loddo, V.; Palmisano, L.; Torregrossa, M.; Viviani, G. Sequential biological and photocatalysis based treatments for shipboard slop purification: A pilot plant investigation. Process Saf. Environ. Prot. 2019, 125, 288–296. [Google Scholar] [CrossRef]
- Deng, M.; Wu, X.; Zhu, A.; Zhang, Q.; Liu, Q. Well-dispersed TiO2 nanoparticles anchored on Fe3O4 magnetic nanosheets for efficient arsenic removal. J. Environ. Manag. 2019, 237, 63–74. [Google Scholar] [CrossRef]
- Martín-Sómer, M.; Pablos, C.; de Diego, A.; van Grieken, R.; Encinas, Á.; Monsalvo, V.M.; Marugán, J. Novel macroporous 3D photocatalytic foams for simultaneous wastewater disinfection and removal of contaminants of emerging concern. Chem. Eng. J. 2019, 366, 449–459. [Google Scholar] [CrossRef]
- Shephard, G.S.; Stockenström, S.; de Villiers, D.; Engelbrecht, W.J.; Wessels, G.F.S. Degradation of microcystin toxins in a falling film photocatalytic reactor with immobilized titanium dioxide catalyst. Water Res. 2002, 36, 140–146. [Google Scholar] [CrossRef]
- Rezaei, R.; Mohseni, M. Impact of pH on the kinetics of photocatalytic oxidation of 2,4-dichlorophenoxy acetic acid in a fluidized bed photocatalytic reactor. Appl. Catal. B Environ. 2017, 205, 92–98. [Google Scholar] [CrossRef]
- Khan, S.; Kim, J.; Sotto, A.; Van der Bruggen, B. Humic acid fouling in a submerged photocatalytic membrane reactor with binary TiO2-ZrO2 particles. J. Ind. Eng. Chem. 2015, 21, 779–786. [Google Scholar] [CrossRef]
- Rimoldi, L.; Meroni, D.; Falletta, E.; Pifferi, V.; Falciola, L.; Cappelletti, G.; Ardizzone, S. Emerging pollutant mixture mineralization by TiO2 photocatalysts. The role of the water medium. Photochem. Photobiol. Sci. 2017, 16, 60–66. [Google Scholar] [CrossRef]
- Awfa, D.; Ateia, M.; Fujii, M.; Yoshimura, C. Photocatalytic degradation of organic micropollutants: Inhibition mechanisms by different fractions of natural organic matter. Water Res. 2020, 174, 115643. [Google Scholar] [CrossRef]
- Ye, Y.; Bruning, H.; Liu, W.; Rijnaarts, H.; Yntema, D. Effect of dissolved natural organic matter on the photocatalytic micropollutant removal performance of TiO2 nanotube array. J. Photochem. Photobiol. A Chem. 2019, 371, 216–222. [Google Scholar] [CrossRef]
- Martínez-Montelongo, J.H.; Medina-Ramírez, I.E.; Romo-Lozano, Y.; Zapien, J.A. Development of a sustainable photocatalytic process for air purification. Chemosphere 2020, 257, 127236. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; Ivanez, J.; Robert, D.; Keller, N. Activity enhancement pathways in LaFeO3@TiO2 heterojunction photocatalysts for visible and solar light driven degradation of myclobutanil pesticide in water. J. Hazard. Mater. 2020, 400, 123099. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Khavar, A.H.C.; Moussavi, G.; Mahjoub, A.R.; Satari, M.; Abdolmaleki, P. Synthesis and visible-light photocatalytic activity of In,S-TiO2@rGO nanocomposite for degradation and detoxification of pesticide atrazine in water. Chem. Eng. J. 2018, 345, 300–311. [Google Scholar] [CrossRef]
- Garcia-Muñoz, P.; Fresno, F.; Lefevre, C.; Robert, D.; Keller, N. Synergy effect between photocatalysis and heterogeneous photo-Fenton catalysis on Ti-doped LaFeO3 perovskite for high efficiency light-assisted water treatment. Catal. Sci. Technol. 2020, 10, 1299–1310. [Google Scholar] [CrossRef]
- Fang, Z.; Hu, Y.; Cheng, J.; Chen, Y. Continuous removal of trace bisphenol A from water by high efficacy TiO2 nanotube pillared graphene-based macrostructures in a photocatalytically fluidized bed. Chem. Eng. J. 2019, 372, 581–589. [Google Scholar] [CrossRef]
- Rezaei, R.; Mohseni, M. Impact of natural organic matter on the degradation of 2, 4-dichlorophenoxy acetic acid in a fluidized bed photocatalytic reactor. Chem. Eng. J. 2017, 310, 457–463. [Google Scholar] [CrossRef]
- Surenjan, A.; Pradeep, T.; Philip, L. Application and performance evaluation of a cost-effective vis- LED based fluidized bed reactor for the treatment of emerging contaminants. Chemosphere 2019, 228, 629–639. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, J.; Zeng, G.; Cheng, W.; Hu, J. Photocatalytic membrane in water purification: Is it stepping closer to be driven by visible light? J. Memb. Sci. 2019, 584, 364–392. [Google Scholar] [CrossRef]
- Soliveri, G.; Sabatini, V.; Farina, H.; Ortenzi, M.A.; Meroni, D.; Colombo, A. Double side self-cleaning polymeric materials: The hydrophobic and photoactive approach. Colloids Surf. A Physicochem. Eng. Asp. 2015, 483, 285–291. [Google Scholar] [CrossRef]
- Fischer, K.; Grimm, M.; Meyers, J.; Dietrich, C.; Gläser, R.; Schulze, A. Photoactive microfiltration membranes via directed synthesis of TiO2 nanoparticles on the polymer surface for removal of drugs from water. J. Memb. Sci. 2015, 478, 49–57. [Google Scholar] [CrossRef]
- Lin, Y.F.; Tung, K.L.; Tzeng, Y.S.; Chen, J.H.; Chang, K.S. Rapid atmospheric plasma spray coating preparation and photocatalytic activity of macroporous titania nanocrystalline membranes. J. Memb. Sci. 2012, 389, 83–90. [Google Scholar] [CrossRef]
- Nunes, B.N.; Paula, L.F.; Costa, Í.A.; Machado, A.E.H.; Paterno, L.G.; Patrocinio, A.O.T. Layer-by-layer assembled photocatalysts for environmental remediation and solar energy conversion. J. Photochem. Photobiol. C Photochem. Rev. 2017, 32, 1–20. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, X.; Oakes, K.D.; Peng, P.; Zhou, Y.N.; Servos, M.R. Hydrothermal growth of free standing TiO2 nanowire membranes for photocatalytic degradation of pharmaceuticals. J. Hazard. Mater. 2011, 189, 278–285. [Google Scholar] [CrossRef]
- Nyamutswa, L.T.; Zhu, B.; Collins, S.F.; Navaratna, D.; Duke, M.C. Light conducting photocatalytic membrane for chemical-free fouling control in water treatment. J. Memb. Sci. 2020, 604, 118018. [Google Scholar] [CrossRef]
- Wu, X.-Q.; Shen, J.-S.; Zhao, F.; Shao, Z.-D.; Zhong, L.-B.; Zheng, Y.-M. Flexible electrospun MWCNTs/Ag3PO4/PAN ternary composite fiber membranes with enhanced photocatalytic activity and stability under visible-light irradiation. J. Mater. Sci. 2018, 53, 10147–10159. [Google Scholar] [CrossRef]
- Liu, T.; Wang, L.; Liu, X.; Sun, C.; Lv, Y.; Miao, R.; Wang, X. Dynamic photocatalytic membrane coated with ZnIn2S4 for enhanced photocatalytic performance and antifouling property. Chem. Eng. J. 2020, 379, 122379. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, Y.; Jiang, Y. Photocatalytic self-cleaning carbon nitride nanotube intercalated reduced graphene oxide membranes for enhanced water purification. Chem. Eng. J. 2019, 356, 915–925. [Google Scholar] [CrossRef]
- Fischer, K.; Schulz, P.; Atanasov, I.; Latif, A.A.; Thomas, I.; Kühnert, M.; Prager, A.; Griebel, J.; Schulze, A. Synthesis of high crystalline TiO2 nanoparticles on a polymer membrane to degrade pollutants from water. Catalysts 2018, 8, 376. [Google Scholar] [CrossRef]
- Behnajady, M.A.; Modirshahla, N.; Daneshvar, N.; Rabbani, M. Photocatalytic degradation of an azo dye in a tubular continuous-flow photoreactor with immobilized TiO2 on glass plates. Chem. Eng. J. 2007, 127, 167–176. [Google Scholar] [CrossRef]
- Cerrato, G.; Bianchi, C.L.; Galli, F.; Pirola, C.; Morandi, S.; Capucci, V. Micro-TiO2 coated glass surfaces safely abate drugs in surface water. J. Hazard. Mater. 2019, 363, 328–334. [Google Scholar] [CrossRef]
- Abdel-Maksoud, Y.; Imam, E.; Ramadan, A. TiO2 solar photocatalytic reactor systems: Selection of reactor design for scale-up and commercialization—Analytical review. Catalysts 2016, 6, 138. [Google Scholar] [CrossRef]
- Fouad, K.; Gar Alalm, M.; Bassyouni, M.; Saleh, M.Y. A novel photocatalytic reactor for the extended reuse of W–TiO2 in the degradation of sulfamethazine. Chemosphere 2020, 257, 127270. [Google Scholar] [CrossRef]
- Fouad, M.; Gar Alalm, M.; El-Etriby, H.K.; Boffito, D.C.; Ookawara, S.; Ohno, T.; Fujii, M. Visible-light-driven photocatalytic disinfection of raw surface waters (300–5000 CFU/mL) using reusable coated Ru/WO3/ZrO2. J. Hazard. Mater. 2021, 402, 123514. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M. MIL-53(Al)/ZnO coated plates with high photocatalytic activity for extended degradation of trimethoprim via novel photocatalytic reactor. Sep. Purif. Technol. 2020, 249, 117173. [Google Scholar] [CrossRef]
- Samy, M.; Ibrahim, M.G.; Gar Alalm, M.; Fujii, M.; Diab, K.E.; ElKady, M. Innovative photocatalytic reactor for the degradation of chlorpyrifos using a coated composite of ZrV2O7 and graphene nano-platelets. Chem. Eng. J. 2020, 395, 124974. [Google Scholar] [CrossRef]
- Stephan, B.; Ludovic, L.; Dominique, W. Modelling of a falling thin film deposited photocatalytic step reactor for water purification: Pesticide treatment. Chem. Eng. J. 2011, 169, 216–225. [Google Scholar] [CrossRef]
- Sun, F.; He, J.; Wu, P.; Zeng, Q.; Liu, C.; Jiang, W. Magnetic photocatalyst CoFe2O4-Ag2O with magnetic aggregation bed photocatalytic reactor for continuous photodegradation of methyl orange. Chem. Eng. J. 2020, 397, 125397. [Google Scholar] [CrossRef]
- Yu, H.; Song, L.; Hao, Y.; Lu, N.; Quan, X.; Chen, S.; Zhang, Y.; Feng, Y. Fabrication of pilot-scale photocatalytic disinfection device by installing TiO2 coated helical support into UV annular reactor for strengthening sterilization. Chem. Eng. J. 2016, 283, 1506–1513. [Google Scholar] [CrossRef]
- Malato, S.; Blanco, J.; Vidal, A.; Fernández, P.; Cáceres, J.; Trincado, P.; Oliveira, J.C.; Vincent, M. New large solar photocatalytic plant: Set-up and preliminary results. Chemosphere 2002, 47, 235–240. [Google Scholar] [CrossRef]
- Mueses, M.A.; Machuca-Martinez, F.; Li Puma, G. Effective quantum yield and reaction rate model for evaluation of photocatalytic degradation of water contaminants in heterogeneous pilot-scale solar photoreactors. Chem. Eng. J. 2013, 215–216, 937–947. [Google Scholar] [CrossRef]
- Plakas, K.V.; Sarasidis, V.C.; Patsios, S.I.; Lambropoulou, D.A.; Karabelas, A.J. Novel pilot scale continuous photocatalytic membrane reactor for removal of organic micropollutants from water. Chem. Eng. J. 2016, 304, 335–343. [Google Scholar] [CrossRef]
- Meroni, D.; Jiménez-Salcedo, M.; Falletta, E.; Bresolin, B.M.; Kait, C.F.; Boffito, D.C.; Bianchi, C.L.; Pirola, C. Sonophotocatalytic degradation of sodium diclofenac using low power ultrasound and micro sized TiO2. Ultrason. Sonochem. 2020, 67, 105123. [Google Scholar] [CrossRef] [PubMed]
- Aoudjit, L.; Martins, P.M.; Madjene, F.; Petrovykh, D.Y.; Lanceros-Mendez, S. Photocatalytic reusable membranes for the effective degradation of tartrazine with a solar photoreactor. J. Hazard. Mater. 2018, 344, 408–416. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Samy, M.; Ookawara, S.; Ohno, T. Immobilization of S-TiO2 on reusable aluminum plates by polysiloxane for photocatalytic degradation of 2,4-dichlorophenol in water. J. Water Process Eng. 2018, 26, 329–335. [Google Scholar] [CrossRef]
- Khaledi Maki, L.; Maleki, A.; Rezaee, R.; Daraei, H.; Yetilmezsoy, K. LED-activated immobilized Fe-Ce-N tri-doped TiO2 nanocatalyst on glass bed for photocatalytic degradation organic dye from aqueous solutions. Environ. Technol. Innov. 2019, 15, 100411. [Google Scholar] [CrossRef]
- Marinho, B.A.; Djellabi, R.; Cristóvão, R.O.; Loureiro, J.M.; Boaventura, R.A.R.; Dias, M.M.; Carlos, J.; Lopes, B.; Vilar, V.J.P. Intensification of heterogeneous TiO2 photocatalysis using an innovative micro-meso-structured-reactor for Cr (VI) reduction under simulated solar light. Chem. Eng. J. 2017, 318, 76–88. [Google Scholar] [CrossRef]
- Ateia, M.; Gar Alalm, M.; Awfa, D.; Johnson, M.S.; Yoshimura, C. Modeling the degradation and disinfection of water pollutants by photocatalysts and composites: A critical review. Sci. Total Environ. 2020, 698, 134197. [Google Scholar] [CrossRef]
- Ren, M.; Drosos, M.; Frimmel, F.H. Inhibitory effect of NOM in photocatalysis process: Explanation and resolution. Chem. Eng. J. 2018, 334, 968–975. [Google Scholar] [CrossRef]
- Zhao, H.; Li, G.; Tian, F.; Jia, Q.; Liu, Y.; Chen, R. g-C3N4 surface-decorated Bi2O2CO3 for improved photocatalytic performance: Theoretical calculation and photodegradation of antibiotics in actual water matrix. Chem. Eng. J. 2019, 366, 468–479. [Google Scholar] [CrossRef]
- Repousi, V.; Petala, A.; Frontistis, Z.; Antonopoulou, M.; Konstantinou, I.; Kondarides, D.I.; Mantzavinos, D. Photocatalytic degradation of bisphenol A over Rh/TiO2 suspensions in different water matrices. Catal. Today 2017, 284, 59–66. [Google Scholar] [CrossRef]
- Liu, S.; Lim, M.; Fabris, R.; Chow, C.W.K.; Drikas, M.; Korshin, G.; Amal, R. Multi-wavelength spectroscopic and chromatography study on the photocatalytic oxidation of natural organic matter. Water Res. 2010, 44, 2525–2532. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Han, R.; Luo, L.; Zhang, X.; Zhang, S. A novel strategy to evaluate the aromaticity degree of natural organic matter based on oxidization-induced chemiluminescence. Environ. Sci. Technol. 2020, 54, 4171–4179. [Google Scholar] [CrossRef] [PubMed]
- Gora, S.L.; Andrews, S.A. Removal of natural organic matter and disinfection byproduct precursors from drinking water using photocatalytically regenerable nanoscale adsorbents. Chemosphere 2019, 218, 52–63. [Google Scholar] [CrossRef]
- Drosos, M.; Ren, M.; Frimmel, F.H. The effect of NOM to TiO2: Interactions and photocatalytic behavior. Appl. Catal. B Environ. 2015, 165, 328–334. [Google Scholar] [CrossRef]
- Zhang, B.; Shan, C.; Wang, S.; Fang, Z.; Pan, B. Unveiling the transformation of dissolved organic matter during ozonation of municipal secondary effluent based on FT-ICR-MS and spectral analysis. Water Res. 2021, 188, 116484. [Google Scholar] [CrossRef]
- Schmidt, T.C. Recent trends in water analysis triggering future monitoring of organic micropollutants. Anal. Bioanal. Chem. 2018, 410, 3933–3941. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Singer, H.P.; Slobodnik, J.; Ipolyi, I.M.; Oswald, P.; Krauss, M.; Schulze, T.; Haglund, P.; Letzel, T.; Grosse, S.; et al. Non-target screening with high-resolution mass spectrometry: Critical review using a collaborative trial on water analysis. Anal. Bioanal. Chem. 2015, 407, 6237–6255. [Google Scholar] [CrossRef]
- Arcanjo, G.S.; Mounteer, A.H.; Bellato, C.R.; da Silva, L.M.M.; Brant Dias, S.H.; da Silva, P.R. Heterogeneous photocatalysis using TiO2 modified with hydrotalcite and iron oxide under UV—Visible irradiation for color and toxicity reduction in secondary textile mill effluent. J. Environ. Manag. 2018, 211, 154–163. [Google Scholar] [CrossRef]
- Rueda-Marquez, J.J.; Levchuk, I.; Fernández Ibañez, P.; Sillanpää, M. A critical review on application of photocatalysis for toxicity reduction of real wastewaters. J. Clean. Prod. 2020, 258, 120694. [Google Scholar] [CrossRef]
- Keen, O.; Bolton, J.; Litter, M.; Bircher, K.; Oppenländer, T. Standard reporting of Electrical Energy per Order (EEO) for UV/H2O2 reactors (IUPAC Technical Report). Pure Appl. Chem. 2018, 90, 1487–1499. [Google Scholar] [CrossRef]
- Benotti, M.J.; Stanford, B.D.; Wert, E.C.; Snyder, S.A. Evaluation of a photocatalytic reactor membrane pilot system for the removal of pharmaceuticals and endocrine disrupting compounds from water. Water Res. 2009, 43, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Miklos, D.B.; Remy, C.; Jekel, M.; Linden, K.G.; Drewes, J.E.; Hübner, U. Evaluation of advanced oxidation processes for water and wastewater treatment—A critical review. Water Res. 2018, 139, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Sadik, W.A.; El-Demerdash, A.-G.M.; Nashed, A.W.; Mostafa, A.A.; Hamad, H.A. Highly efficient photocatalytic performance of Cu2O@TiO2 nanocomposite: Influence of various inorganic oxidants and inorganic anions. J. Mater. Res. Technol. 2019, 8, 5405–5414. [Google Scholar] [CrossRef]
- Mu, D.; Addy, M.; Anderson, E.; Chen, P.; Ruan, R. A life cycle assessment and economic analysis of the Scum-to-Biodiesel technology in wastewater treatment plants. Bioresour. Technol. 2016, 204, 89–97. [Google Scholar] [CrossRef]
- Magdy, M.; Gar Alalm, M.; El-Etriby, H.K. Comparative life cycle assessment of five chemical methods for removal of phenol and its transformation products. J. Clean. Prod. 2021, 291, 125923. [Google Scholar] [CrossRef]
- Muñoz, I.; Peral, J.; Antonio Ayllón, J.; Malato, S.; Passarinho, P.; Domènech, X. Life cycle assessment of a coupled solar photocatalytic–biological process for wastewater treatment. Water Res. 2006, 40, 3533–3540. [Google Scholar] [CrossRef]
- Pesqueira, J.F.J.R.; Pereira, M.F.R.; Silva, A.M.T. A life cycle assessment of solar-based treatments (H2O2, TiO2 photocatalysis, circumneutral photo-Fenton) for the removal of organic micropollutants. Sci. Total Environ. 2021, 761, 143258. [Google Scholar] [CrossRef]
- Gar Alalm, M.; Tawfik, A.; Ookawara, S. Comparison of solar TiO2 photocatalysis and solar photo-Fenton for treatment of pesticides industry wastewater: Operational conditions, kinetics, and costs. J. Water Process Eng. 2015, 8, 55–63. [Google Scholar] [CrossRef]
- Navarro, S.; Fenoll, J.; Vela, N.; Ruiz, E.; Navarro, G. Photocatalytic degradation of eight pesticides in leaching water by use of ZnO under natural sunlight. J. Hazard. Mater. 2009, 172, 1303–1310. [Google Scholar] [CrossRef]
- Hay, S.O.; Obee, T.; Luo, Z.; Jiang, T.; Meng, Y.; He, J.; Murphy, S.C.; Suib, S. The viability of photocatalysis for air purification. Molecules 2015, 20, 1319–1356. [Google Scholar] [CrossRef]
- Nath, R.K.; Zain, M.F.M.; Jamil, M. An environment-friendly solution for indoor air purification by using renewable photocatalysts in concrete: A review. Renew. Sustain. Energy Rev. 2016, 62, 1184–1194. [Google Scholar] [CrossRef]
- Paz, Y. Photocatalytic treatment of air: From basic aspects to reactors. Adv. Chem. Eng. 2009, 36, 289–336. [Google Scholar]
- Peral, J.; Domènech, X.; Ollis, D.F. Heterogeneous photocatalysis for purification, decontamination and deodorization of air. J. Chem. Technol. Biotechnol. 1997, 70, 117–140. [Google Scholar] [CrossRef]
- Demeestere, K.; Dewulf, J.; Van Langenhove, H. Heterogeneous photocatalysis as an advanced oxidation process for the abatement of chlorinated, monocyclic aromatic and sulfurous volatile organic compounds in air: State of the art. Crit. Rev. Environ. Sci. Technol. 2007, 37, 489–538. [Google Scholar] [CrossRef]
- Escobedo, S.; de Lasa, H. Photocatalysis for air treatment processes: Current technologies and future applications for the removal of organic pollutants and viruses. Catalysts 2020, 10, 966. [Google Scholar] [CrossRef]
- Boyjoo, Y.; Sun, H.; Liu, J.; Pareek, V.K.; Wang, S. A review on photocatalysis for air treatment: From catalyst development to reactor design. Chem. Eng. J. 2017, 310, 537–559. [Google Scholar] [CrossRef]
- Paz, Y. Application of TiO2 photocatalysis for air treatment: Patents’ overview. Appl. Catal. B Environ. 2010, 99, 448–460. [Google Scholar] [CrossRef]
- Birnie, M.; Riffat, S.; Gillott, M. Photocatalytic reactors: Design for effective air purification. Int. J. Low Carbon Technol. 2006, 1, 47–58. [Google Scholar] [CrossRef]
- Costarramone, N.; Cantau, C.; Desauziers, V.; Pécheyran, C.; Pigot, T.; Lacombe, S. Photocatalytic air purifiers for indoor air: European standard and pilot room experiments. Environ. Sci. Pollut. Res. 2017, 24, 12538–12546. [Google Scholar] [CrossRef]
- Binas, V.; Venieri, D.; Kotzias, D.; Kiriakidis, G. Modified TiO2 based photocatalysts for improved air and health quality. J. Mater. 2017, 3, 3–16. [Google Scholar]
- De Niederhausern, S.; Bondi, M.; Bondioli, F. Self-cleaning and antibacteric ceramic tile surface. Int. J. Appl. Ceram. Technol. 2013, 10, 949–956. [Google Scholar] [CrossRef]
- Portela, R.; Tessinari, R.F.; Suarez, S.; Rasmussen, S.B.; Hernández-Alonso, M.D.; Canela, M.C.; Avila, P.; Sánchez, B. Photocatalysis for continuous air purification in wastewater treatment plants: From lab to reality. Environ. Sci. Technol. 2012, 46, 5040–5048. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Poon, C. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Pirola, C.; Stucchi, M.; Sacchi, B.; Cerrato, G.; Morandi, S.; Di Michele, A.; Carletti, A.; Capucci, V. A New Frontier of Photocatalysis Employing Micro-Sized TiO2: Air/Water Pollution Abatement and Self-Cleaning/Antibacterial Applications; Intech Open: London, UK, 2016; ISBN 9535124846. [Google Scholar]
- Bianchi, C.L.; Cerrato, G.; Bresolin, B.M.; Djellabi, R.; Rtimi, S. Digitally printed AgNPs doped TiO2 on commercial porcelain-grès tiles: Synergistic effects and continuous photocatalytic antibacterial activity. Surfaces 2020, 3, 11–25. [Google Scholar] [CrossRef]
- Cerrato, G.; Galli, F.; Boffito, D.C.; Operti, L.; Bianchi, C.L. Correlation preparation parameters/activity for microTiO2 decorated with SilverNPs for NOx photodegradation under LED light. Appl. Catal. B Environ. 2019, 253, 218–225. [Google Scholar] [CrossRef]
- Bianchi, C.L.; Cerrato, G.; Pirola, C.; Galli, F.; Capucci, V. Photocatalytic porcelain grés large slabs digitally coated with AgNPs-TiO2. Environ. Sci. Pollut. Res. 2019, 26, 36117–36123. [Google Scholar] [CrossRef]
- Zadi, T.; Azizi, M.; Nasrallah, N.; Bouzaza, A.; Maachi, R.; Wolbert, D.; Rtimi, S.; Assadi, A.A. Indoor air treatment of refrigerated food chambers with synergetic association between cold plasma and photocatalysis: Process performance and photocatalytic poisoning. Chem. Eng. J. 2020, 382, 122951. [Google Scholar] [CrossRef]
- Zhong, L.; Haghighat, F. Photocatalytic air cleaners and materials technologies–abilities and limitations. Build. Environ. 2015, 91, 191–203. [Google Scholar] [CrossRef]
- Farhanian, D.; Haghighat, F.; Lee, C.-S.; Lakdawala, N. Impact of design parameters on the performance of ultraviolet photocatalytic oxidation air cleaner. Build. Environ. 2013, 66, 148–157. [Google Scholar] [CrossRef]
- Destaillats, H.; Sleiman, M.; Sullivan, D.P.; Jacquiod, C.; Sablayrolles, J.; Molins, L. Key parameters influencing the performance of photocatalytic oxidation (PCO) air purification under realistic indoor conditions. Appl. Catal. B Environ. 2012, 128, 159–170. [Google Scholar] [CrossRef]
- Kiesgen de_Richter, R.; Ming, T.; Caillol, S. Fighting global warming by photocatalytic reduction of CO2 using giant photocatalytic reactors. Renew. Sustain. Energy Rev. 2013, 19, 82–106. [Google Scholar] [CrossRef]
- Horváth, E.; Rossi, L.; Mercier, C.; Lehmann, C.; Sienkiewicz, A.; Forró, L. Photocatalytic nanowires-based air filter: Towards reusable protective masks. Adv. Funct. Mater. 2020, 30, 2004615. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, J.; Feng, X.; Li, J.; Hao, Y.; Zhang, J.; Wang, H.; Yin, A.; Zhou, J.; Ma, X. Metal-organic frameworks with photocatalytic bactericidal activity for integrated air cleaning. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Filho, B.M.; Araujo, A.L.P.; Padrão, S.P.; Boaventura, R.A.R.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J.P. Effect of catalyst coated surface, illumination mechanism and light source in heterogeneous TiO2 photocatalysis using a mili-photoreactor for n-decane oxidation at gas phase. Chem. Eng. J. 2019, 366, 560–568. [Google Scholar] [CrossRef]
- Du, P.; Carneiro, J.T.; Moulijn, J.A.; Mul, G. A novel photocatalytic monolith reactor for multiphase heterogeneous photocatalysis. Appl. Catal. A Gen. 2008, 334, 119–128. [Google Scholar] [CrossRef]
- Romero-Vargas Castrillón, S.; De Lasa, H.I. Performance evaluation of photocatalytic reactors for air purification using computational fluid dynamics (CFD). Ind. Eng. Chem. Res. 2007, 46, 5867–5880. [Google Scholar] [CrossRef]
- Whyte, H.E. Evaluation of the Performance of Photocatalytic Systems for the Treatment of Indoor Air in Medical Environments. Ph.D. Thesis, Ecole Nationale Supérieure Mines-Télécom Atlantique, Nantes, France, 2018. [Google Scholar]
- Lyu, J.; Zhu, L.; Burda, C. Considerations to improve adsorption and photocatalysis of low concentration air pollutants on TiO2. Catal. Today 2014, 225, 24–33. [Google Scholar] [CrossRef]
- Thomson, C.G.; Lee, A.-L.; Vilela, F. Heterogeneous photocatalysis in flow chemical reactors. J. Org. Chem. 2020, 16, 1495–1549. [Google Scholar]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, X. Photocatalytic oxidation for indoor air purification: A literature review. Build. Environ. 2003, 38, 645–654. [Google Scholar] [CrossRef]
- Sannino, D.; Vaiano, V.; Ciambelli, P.; Eloy, P.; Gaigneaux, E.M. Avoiding the deactivation of sulphated MoOx/TiO2 catalysts in the photocatalytic cyclohexane oxidative dehydrogenation by a fluidized bed photoreactor. Appl. Catal. A Gen. 2011, 394, 71–78. [Google Scholar] [CrossRef]
- Cheng, Z.; Quan, X.; Xiang, J.; Huang, Y.; Xu, Y. Photocatalytic degradation of bisphenol A using an integrated system of a new gas-liquid-solid circulating fluidized bed reactor and micrometer Gd-doped TiO2 particles. J. Environ. Sci. 2012, 24, 1317–1326. [Google Scholar] [CrossRef]
- Ren, H.; Koshy, P.; Chen, W.-F.; Qi, S.; Sorrell, C.C. Photocatalytic materials and technologies for air purification. J. Hazard. Mater. 2017, 325, 340–366. [Google Scholar] [CrossRef]
- Van Dijk, V.H.A.; Simmelink, G.; Mul, G. The influence of water vapour on the photocatalytic oxidation of cyclohexane in an internally illuminated monolith reactor. Appl. Catal. A Gen. 2014, 470, 63–71. [Google Scholar] [CrossRef]
- Marinho, B.A.; Cristóvão, R.O.; Djellabi, R.; Loureiro, J.M.; Boaventura, R.A.R.; Vilar, V.J.P. Photocatalytic reduction of Cr (VI) over TiO2-coated cellulose acetate monolithic structures using solar light. Appl. Catal. B Environ. 2017, 203, 18–30. [Google Scholar] [CrossRef]
- Raupp, G.B.; Alexiadis, A.; Hossain, M.M.; Changrani, R. First-principles modeling, scaling laws and design of structured photocatalytic oxidation reactors for air purification. Catal. Today 2001, 69, 41–49. [Google Scholar] [CrossRef]
- Monteiro, R.A.R.; Miranda, S.M.; Rodrigues-Silva, C.; Faria, J.L.; Silva, A.M.T.; Boaventura, R.A.R.; Vilar, V.J.P. Gas phase oxidation of n-decane and PCE by photocatalysis using an annular photoreactor packed with a monolithic catalytic bed coated with P25 and PC500. Appl. Catal. B Environ. 2015, 165, 306–315. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Miranda, S.M.; Monteiro, R.A.R.; Martins, S.D.S.; Silva, A.M.T.; Faria, J.L.; Boaventura, R.A.R.; Vilar, V.J.P. Perchloroethylene gas-phase degradation over titania-coated transparent monoliths. Appl. Catal. B Environ. 2013, 140, 444–456. [Google Scholar] [CrossRef]
- Portela, R. Eliminación Fotocatalítica de H2S en Aire Mediante TiO2 Soportado Sobre Sustratos Transparentes en el UV-A; Universidad de Santiago de Compostela: A Coruña, Spain, 2009; ISBN 8478346104. [Google Scholar]
- Zacarías, S.M.; Manassero, A.; Pirola, S.; Alfano, O.M.; Satuf, M.L. Design and performance evaluation of a photocatalytic reactor for indoor air disinfection. Environ. Sci. Pollut. Res. 2020, 1–9. [Google Scholar] [CrossRef]
- Mo, J.; Zhang, Y.; Xu, Q.; Lamson, J.J.; Zhao, R. Photocatalytic purification of volatile organic compounds in indoor air: A literature review. Atmos. Environ. 2009, 43, 2229–2246. [Google Scholar] [CrossRef]
- Imoberdorf, G.E.; Cassano, A.E.; Alfano, O.M.; Irazoqui, H.A. Modeling of a multiannular photocatalytic reactor for perchloroethylene degradation in air. AIChE J. 2006, 52, 1814–1823. [Google Scholar] [CrossRef]
- Imoberdorf, G.E.; Cassano, A.E.; Irazoqui, H.A.; Alfano, O.M. Simulation of a multi-annular photocatalytic reactor for degradation of perchloroethylene in air: Parametric analysis of radiative energy efficiencies. Chem. Eng. Sci. 2007, 62, 1138–1154. [Google Scholar] [CrossRef]
- Van Walsem, J.; Verbruggen, S.W.; Modde, B.; Lenaerts, S.; Denys, S. CFD investigation of a multi-tube photocatalytic reactor in non-steady-state conditions. Chem. Eng. J. 2016, 304, 808–816. [Google Scholar] [CrossRef]
- Da Costa Filho, B.M.; Silva, G.V.; Boaventura, R.A.R.; Dias, M.M.; Lopes, J.C.B.; Vilar, V.J.P. Ozonation and ozone-enhanced photocatalysis for VOC removal from air streams: Process optimization, synergy and mechanism assessment. Sci. Total Environ. 2019, 687, 1357–1368. [Google Scholar] [CrossRef]
- Taranto, J.; Frochot, D.; Pichat, P. Combining cold plasma and TiO2 photocatalysis to purify gaseous effluents: A preliminary study using methanol-contaminated air. Ind. Eng. Chem. Res. 2007, 46, 7611–7614. [Google Scholar] [CrossRef]
- Maurer, D.L.; Koziel, J.A. On-farm pilot-scale testing of black ultraviolet light and photocatalytic coating for mitigation of odor, odorous VOCs, and greenhouse gases. Chemosphere 2019, 221, 778–784. [Google Scholar] [CrossRef]
- Sun, S.; Ding, J.; Bao, J.; Gao, C.; Qi, Z.; Li, C. Photocatalytic oxidation of gaseous formaldehyde on TiO2: An in situ DRIFTS study. Catal. Lett. 2010, 137, 239–246. [Google Scholar] [CrossRef]
- Ardizzone, S.; Bianchi, C.L.; Cappelletti, G.; Naldoni, A.; Pirola, C. Photocatalytic degradation of toluene in the gas phase: Relationship between surface species and catalyst features. Environ. Sci. Technol. 2008, 42, 6671–6676. [Google Scholar] [CrossRef]
- Weon, S.; Choi, W. TiO2 nanotubes with open channels as deactivation-resistant photocatalyst for the degradation of volatile organic compounds. Environ. Sci. Technol. 2016, 50, 2556–2563. [Google Scholar] [CrossRef]
- Walenta, C.A.; Kollmannsberger, S.L.; Kiermaier, J.; Winbauer, A.; Tschurl, M.; Heiz, U. Ethanol photocatalysis on rutile TiO2(110): The role of defects and water. Phys. Chem. Chem. Phys. 2015, 49, 22809–22814. [Google Scholar] [CrossRef]
- Piera, E.; Ayllón, J.A.; Doménech, X.; Peral, J. TiO2 deactivation during gas-phase photocatalytic oxidation of ethanol. Catal. Today 2002, 76, 259–270. [Google Scholar] [CrossRef]
- Djellabi, R.; Ghorab, F.M.; Nouacer, S.; Smara, A.; Khireddine, O. Cr(VI) photocatalytic reduction under sunlight followed by Cr(III) extraction from TiO2 surface. Mater. Lett. 2016, 176, 106–109. [Google Scholar] [CrossRef]
- Chen, P.; Cui, W.; Wang, H.; Dong, X.; Li, J.; Sun, Y.; Zhou, Y.; Zhang, Y.; Dong, F. The importance of intermediates ring-opening in preventing photocatalyst deactivation during toluene decomposition. Appl. Catal. B Environ. 2020, 272, 118977. [Google Scholar] [CrossRef]
- Ribeiro, B.M.B.; Fujimoto, T.M.; Bricio, B.G.M.; Doubek, U.L.R.; Tomaz, E. Gas-phase aromatic compounds degradation by a partially TiO2 coated photoreactor assisted with ozone. Process Saf. Environ. Prot. 2020, 135, 265–272. [Google Scholar] [CrossRef]

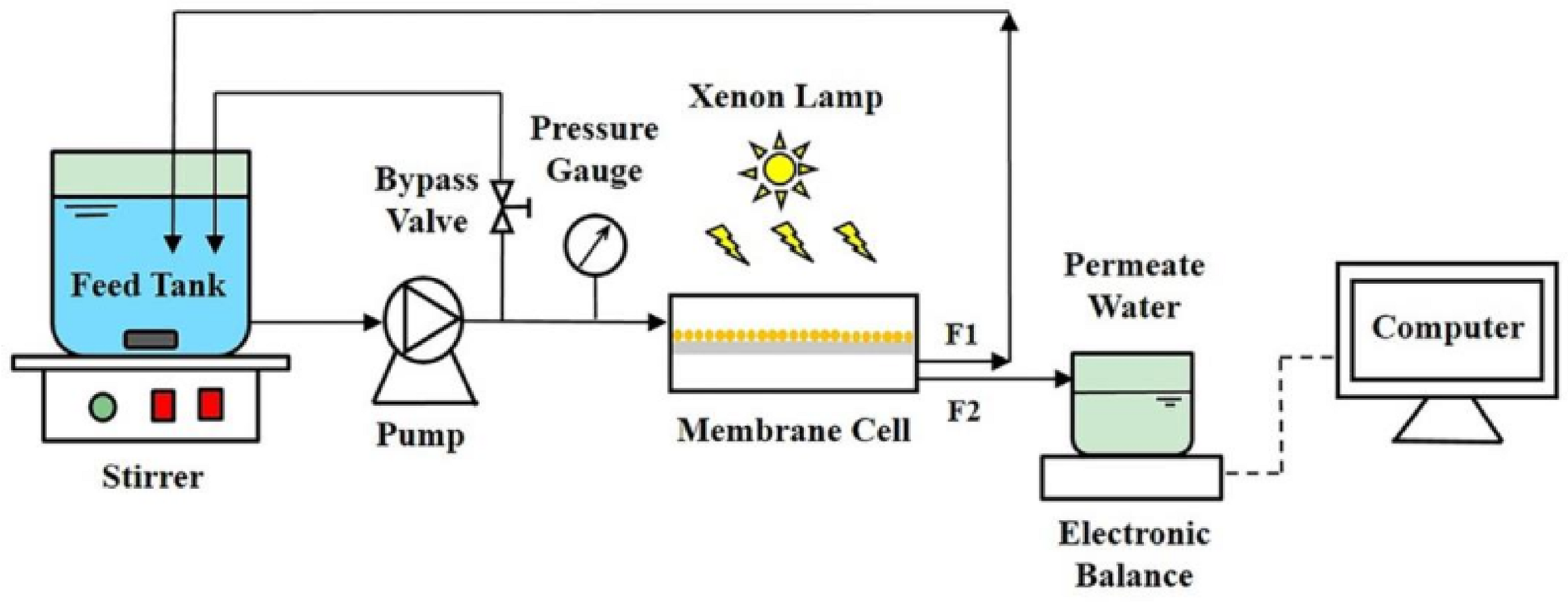
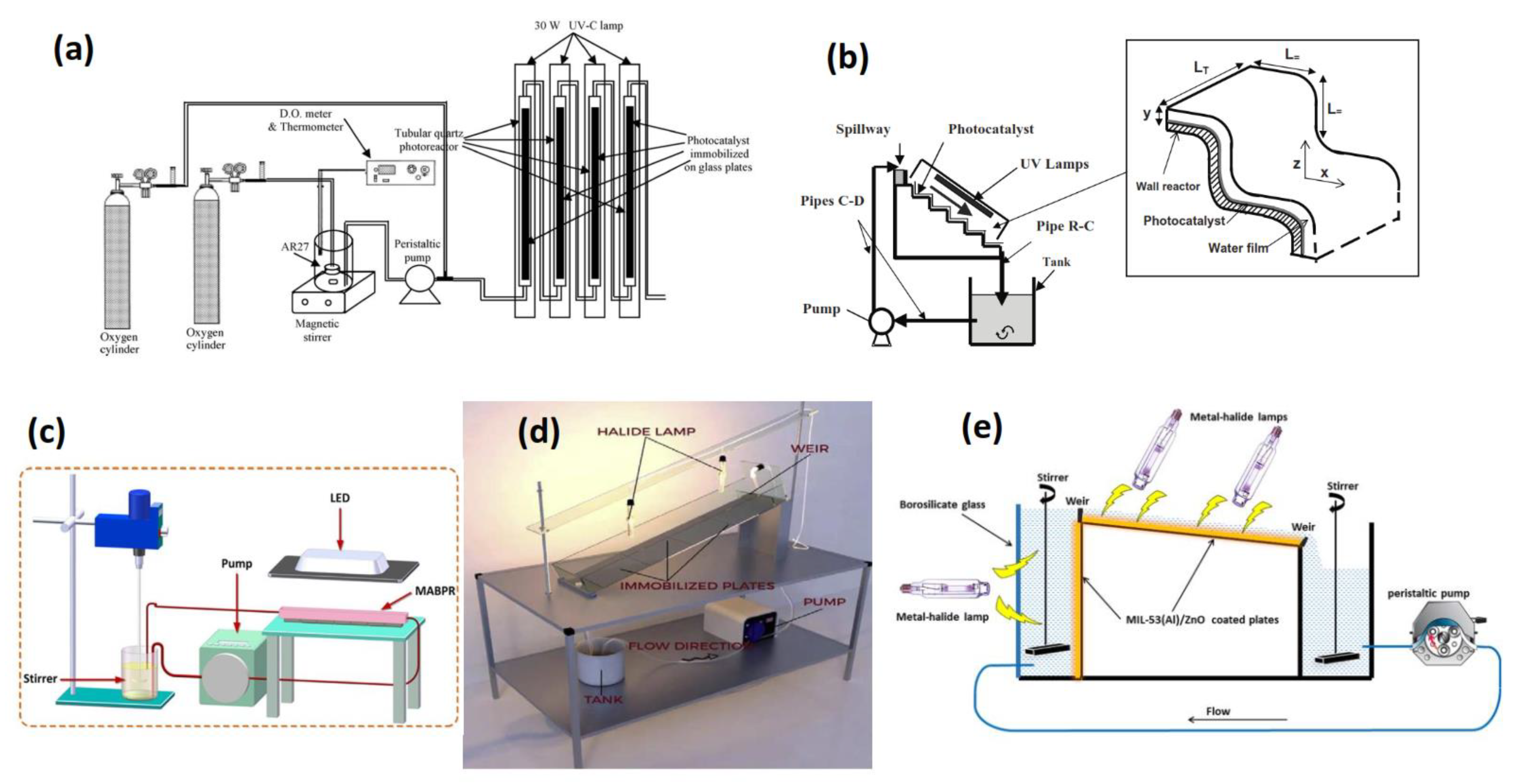
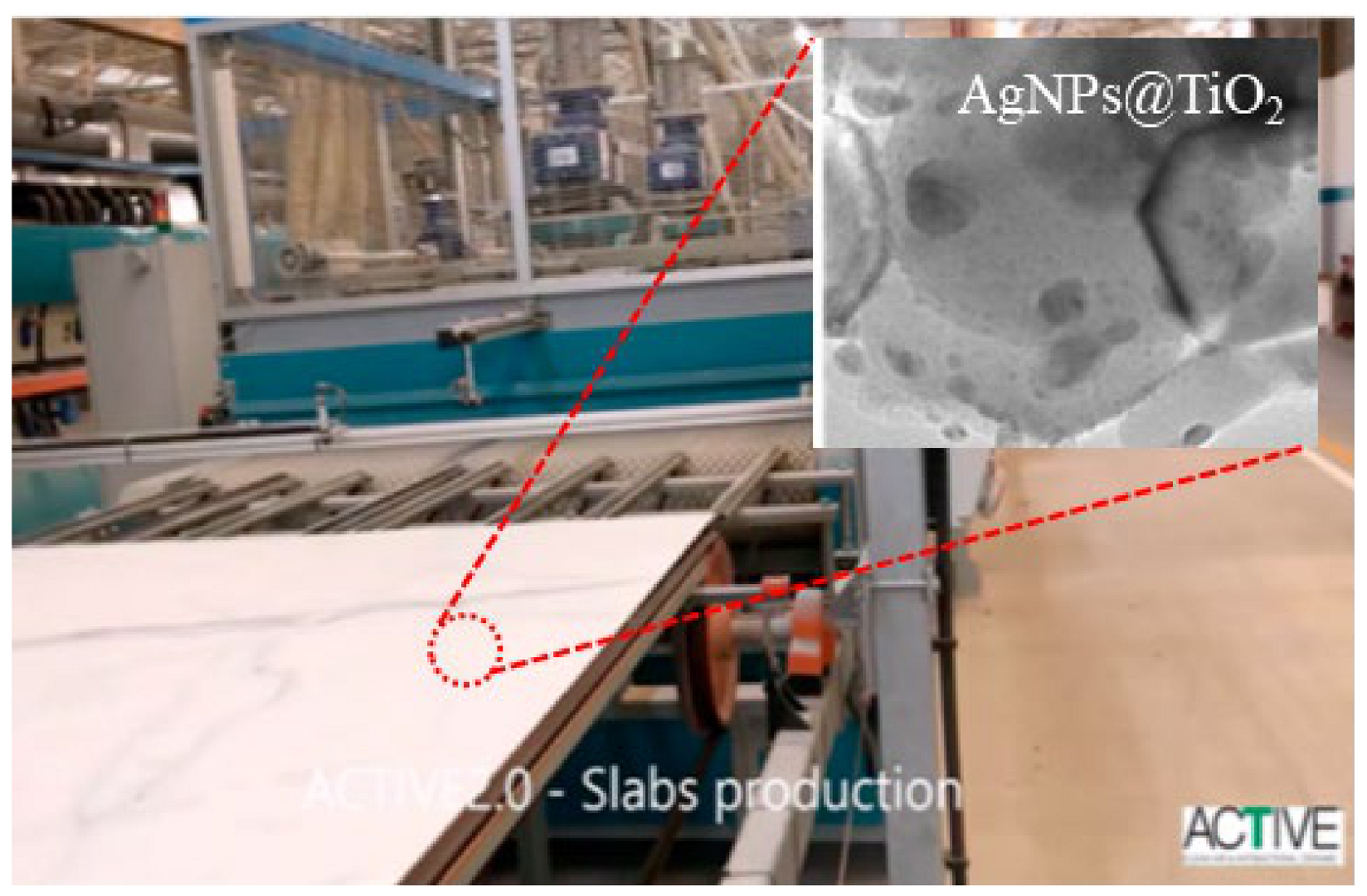
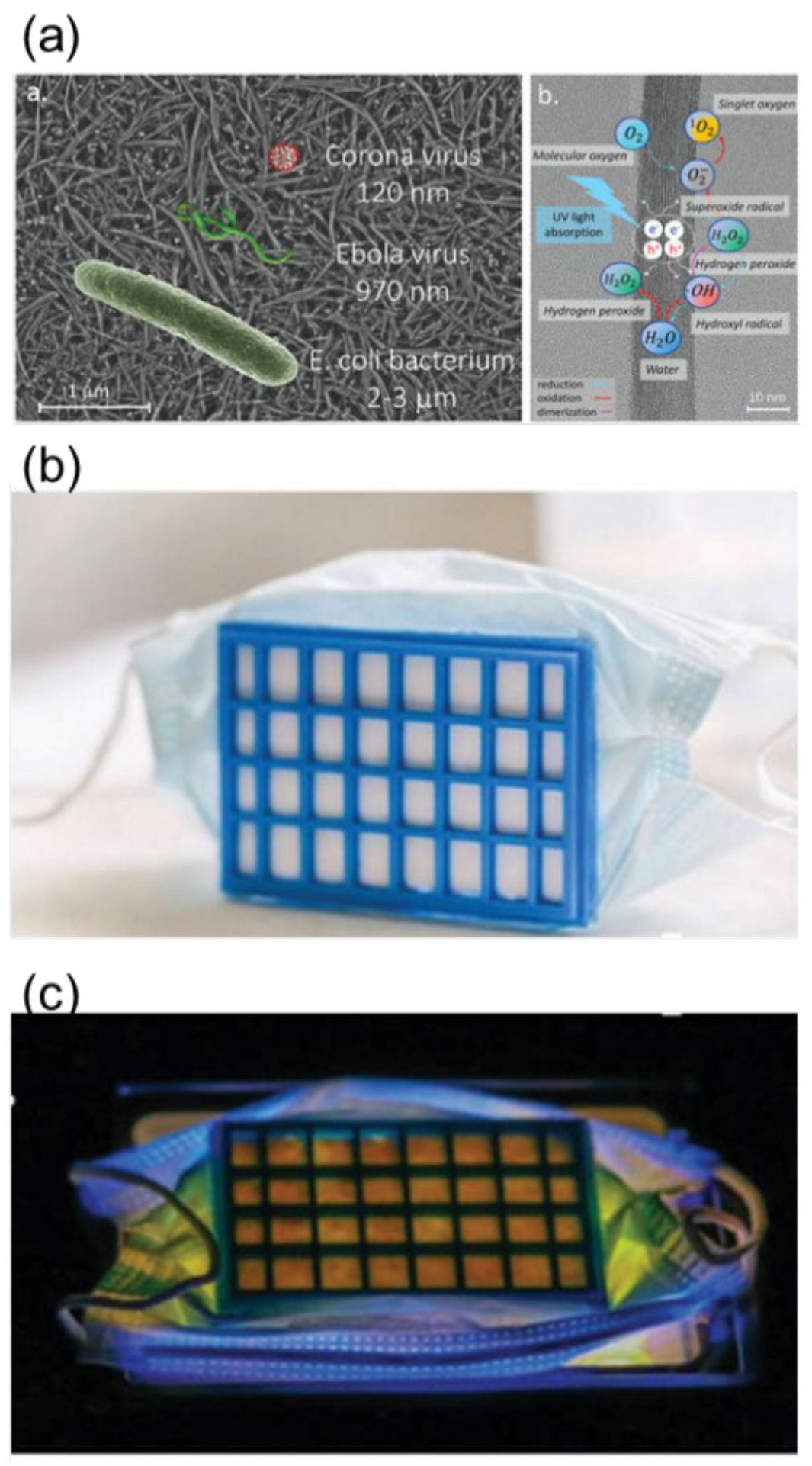
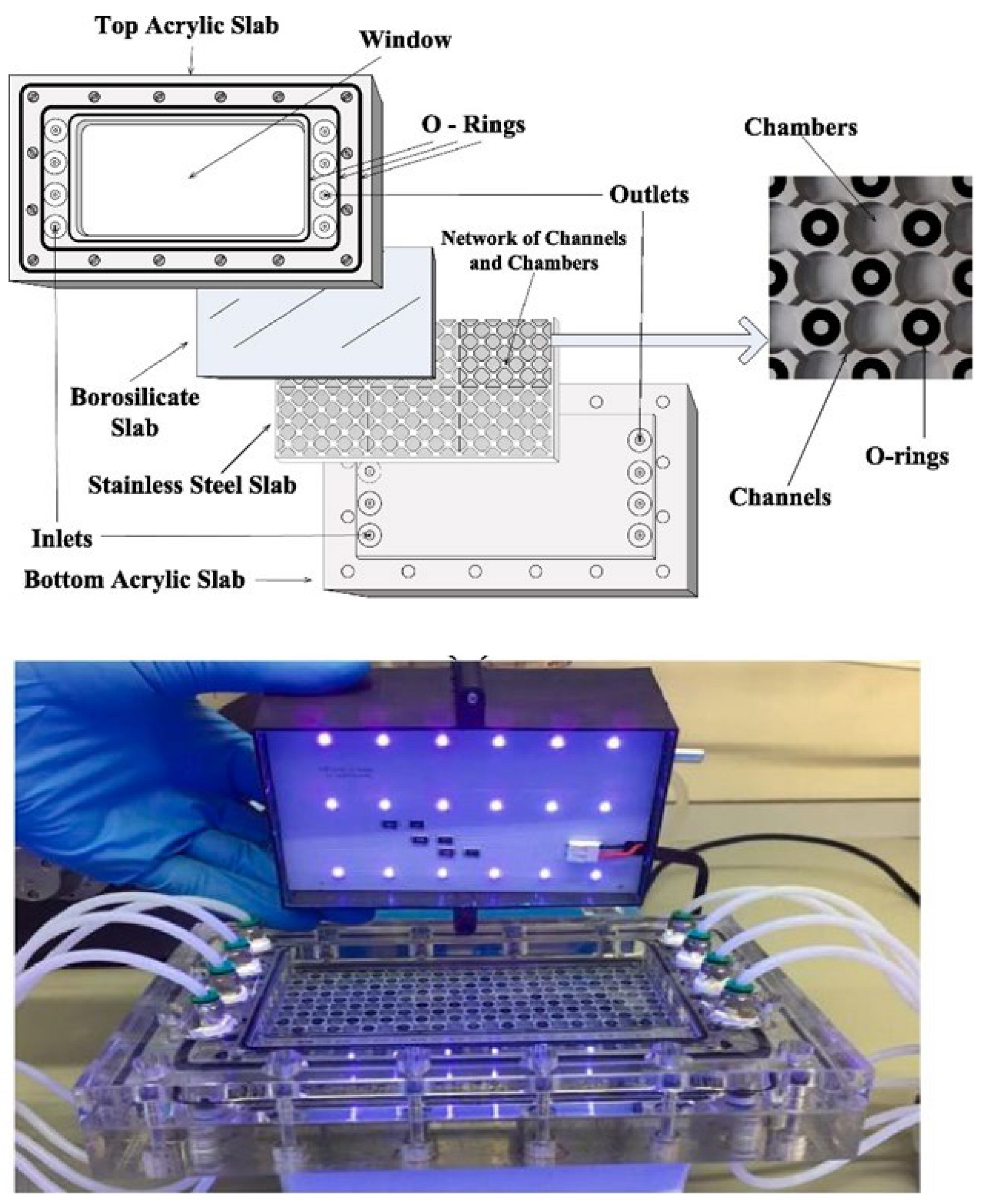
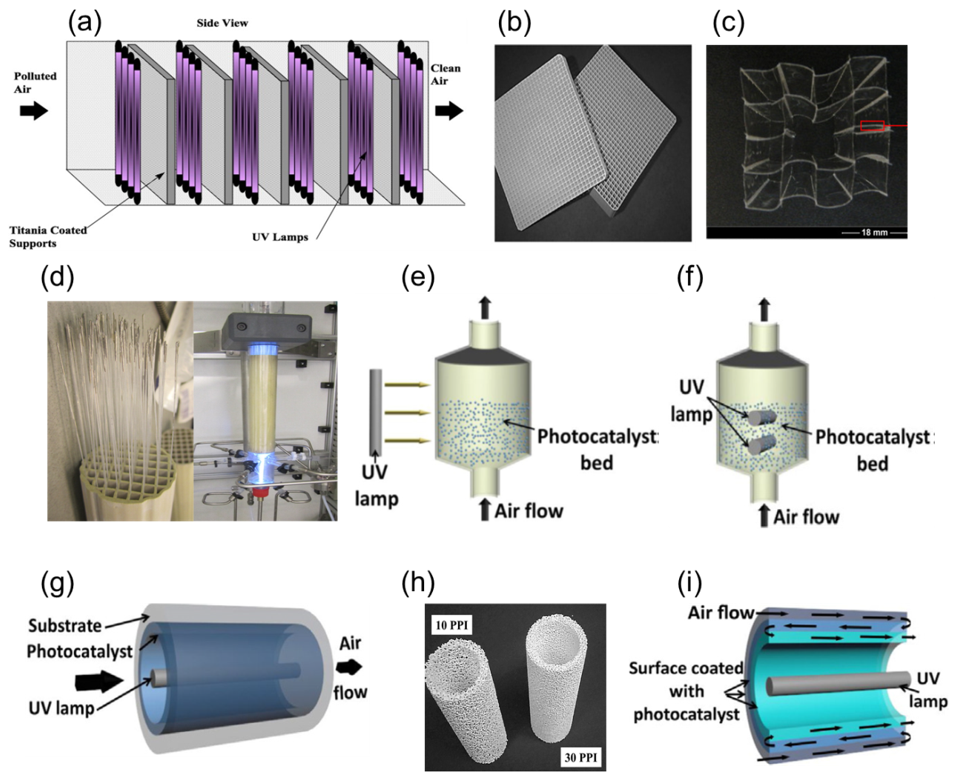
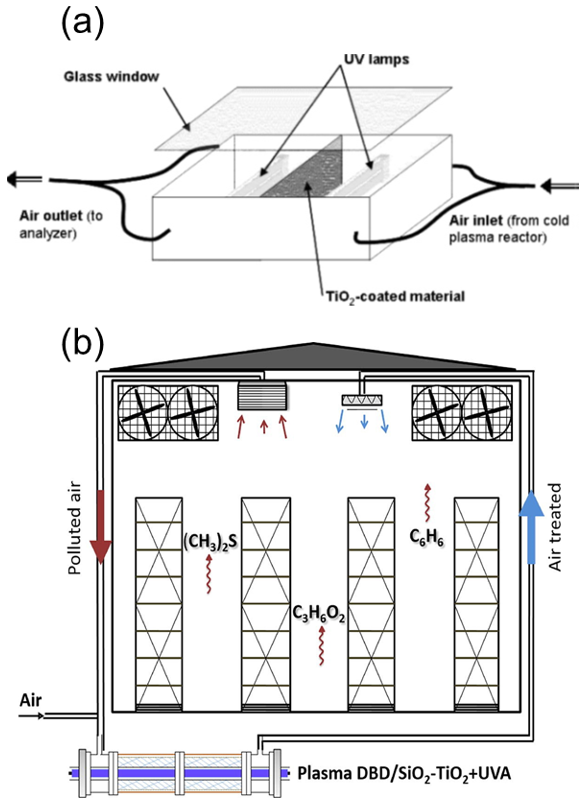
| Reactor Type | Photocatalyst | Pollutant | Irradiation Source | Max Degradation % | Reaction Time (min) | No of Reuse Cycles | Degradation % at Last Cycle | Reference |
|---|---|---|---|---|---|---|---|---|
| Fluidized bed reactor | TiO2 nanotubes/graphene | Bisphenol A | 350W Xe lamp | 100% * | 30 | 5 | 92% | [37] |
| Photocatalytic PVDF membrane | ZnIn2S4 | Fluvastatin | 500 W Xe lamp | 99.75% | 180 | 6 | 91.53% | [48] |
| Photocatalytic graphene oxide membrane | g-C3N4 | Rhodamine B | 300 W Xe lamp with 420 nm cut-off filter | 98.7% | 90 | - | - | [49] |
| Solar membrane reactor | TiO2 | Tartrazine | Natural solar light | 78% | 300 | 2 | 67% | [65] |
| Photocatalytic polymer membrane | TiO2 | Methylene blue | Solar lamp | 100% * | 40 | 9 | 100% * | [50] |
| Coated plate reactor | W-TiO2 | Sulfamethazine | 400 W metal-halide lamp | 100% * | 120 | 5 | 90.3% | [54] |
| Tubular continuous-flow with attached catalyst | TiO2 | Acid Red 27 | 30 W UV-C lamps | 100% * | - | - | - | [51] |
| Falling film reactor | TiO2 | Chlortoluron | 24 W UV lamp | 100% * | 250 | - | - | [58] |
| Submerged coated plate | Ru-WO3/ZrO2 | Multiple bacteria | 400 W metal-halide lamp | 100% * | 240 | 4 | 100% * | [55] |
| Submerged coated plate | S-TiO2 | 2,4-dichlorophenol | 400 W metal-halide lamp | 98% | 480 | 5 | 80% | [66] |
| Coated plate reactor | ZrV2O7/graphene nano-platelets | Chlorpyrifos | 400 W metal-halide lamp | 96.8% | 90 | 5 | 91% | [57] |
| Magnetic aggregation bed reactor | CoFe2O4-Ag2O | Methyl orange | 600 W LED lamp | 92% | 60 | 3 | 80% | [59] |
| Submerged coated plate | Fe-Cr-N-TiO2 | Direct blue 15 | 20 W LED lamp | 100% * | 60 | - | - | [67] |
| Submerged membrane reactor | Fe-ZnS/g-C3N4 | p-nitro-phenol | 500 W Xe lamp | 93.5% | 300 | - | - | [19] |
| Micro–meso-reactor | TiO2 | Cr(VI) | 1700 W Xe lamp | 100% * | 60 | 10 | 70% | [68] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alalm, M.G.; Djellabi, R.; Meroni, D.; Pirola, C.; Bianchi, C.L.; Boffito, D.C. Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts 2021, 11, 562. https://doi.org/10.3390/catal11050562
Alalm MG, Djellabi R, Meroni D, Pirola C, Bianchi CL, Boffito DC. Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts. 2021; 11(5):562. https://doi.org/10.3390/catal11050562
Chicago/Turabian StyleAlalm, Mohamed Gar, Ridha Djellabi, Daniela Meroni, Carlo Pirola, Claudia Letizia Bianchi, and Daria Camilla Boffito. 2021. "Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications" Catalysts 11, no. 5: 562. https://doi.org/10.3390/catal11050562
APA StyleAlalm, M. G., Djellabi, R., Meroni, D., Pirola, C., Bianchi, C. L., & Boffito, D. C. (2021). Toward Scaling-Up Photocatalytic Process for Multiphase Environmental Applications. Catalysts, 11(5), 562. https://doi.org/10.3390/catal11050562










