Study of the Rate-Determining Step of Rh Catalyzed CO2 Reduction: Insight on the Hydrogen Assisted Molecular Dissociation
Abstract
1. Introduction
2. Results and Discussion
2.1. Clean Rh (100) Slab Characterization
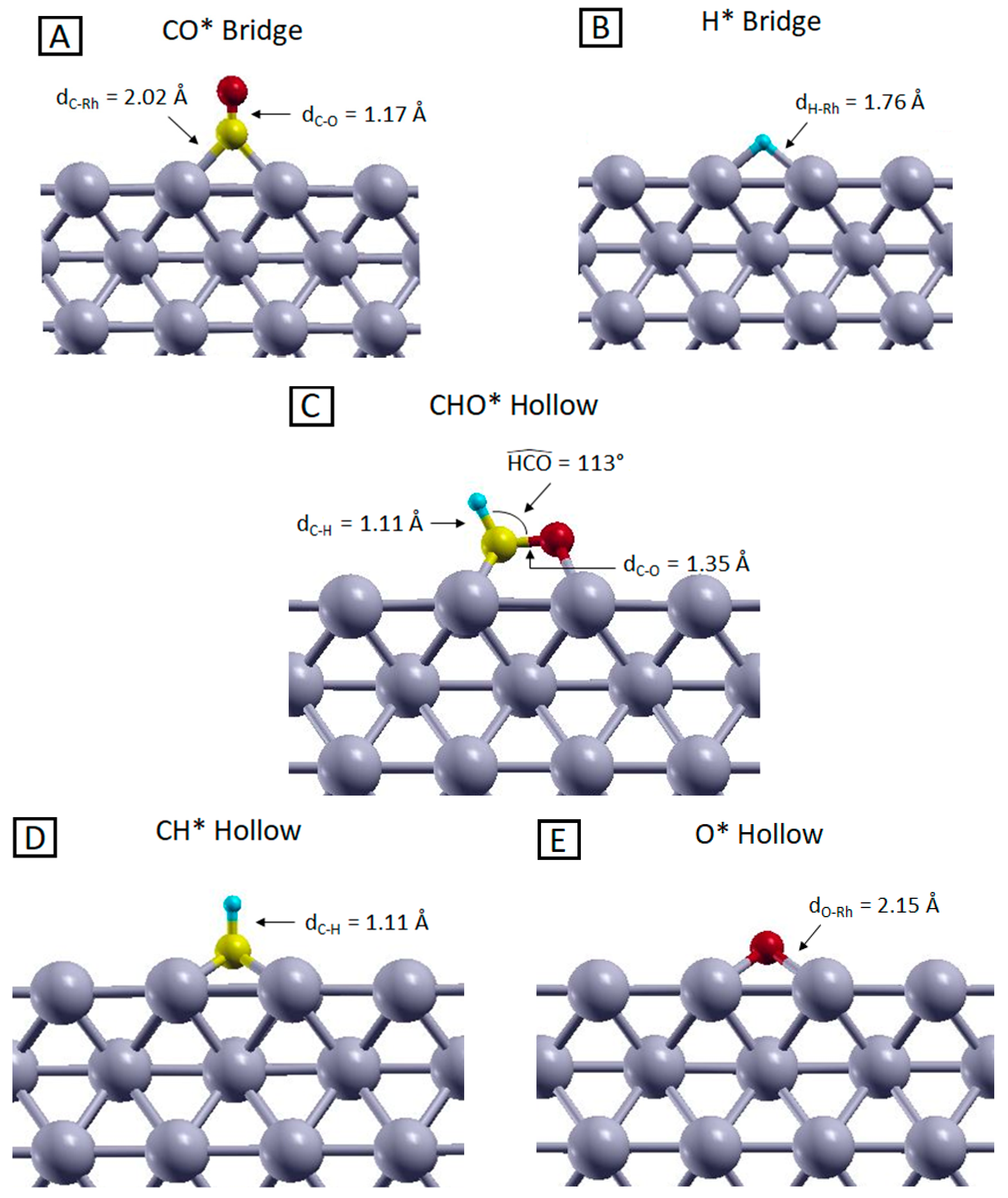
2.2. Description of the Adsorbates
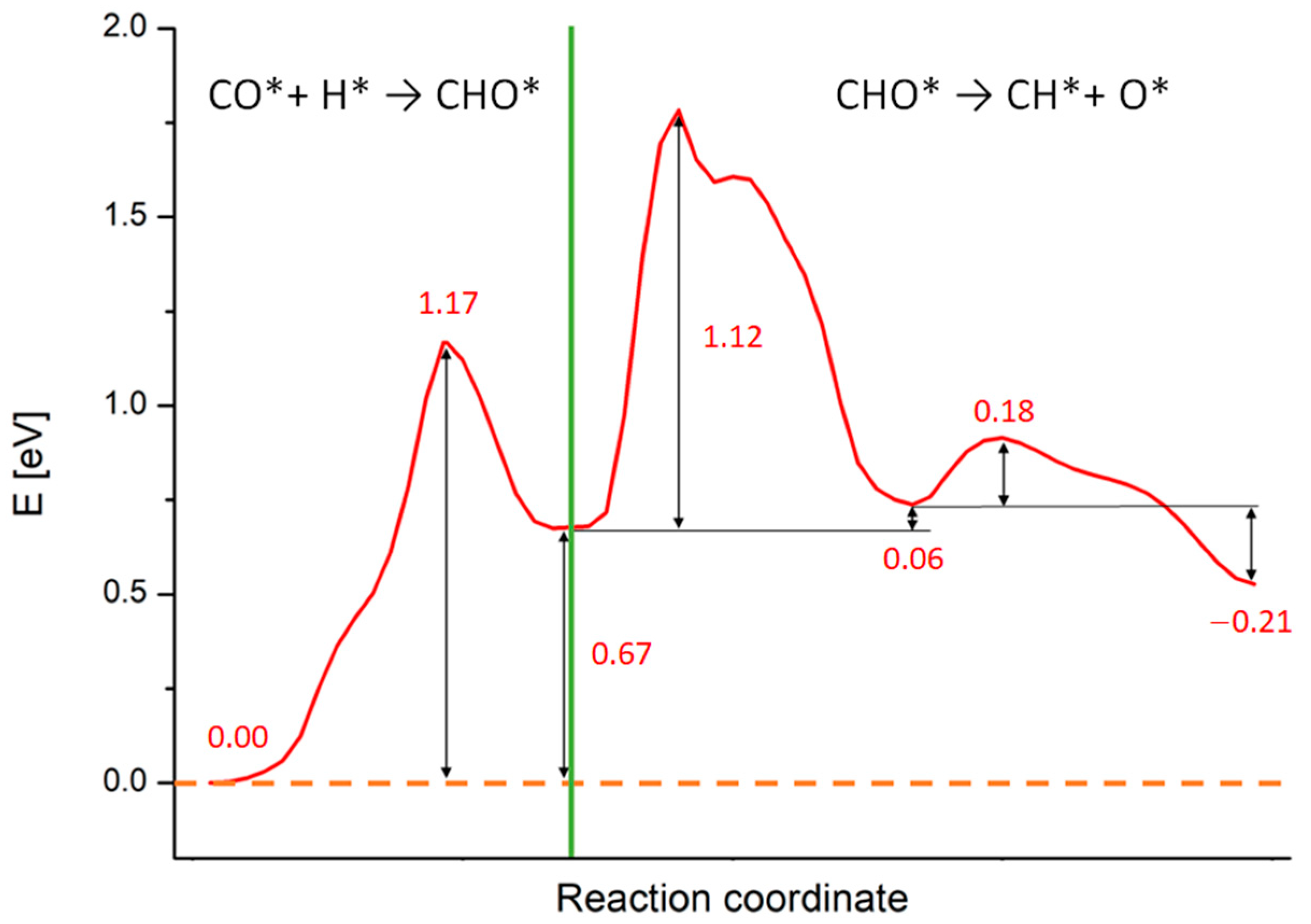
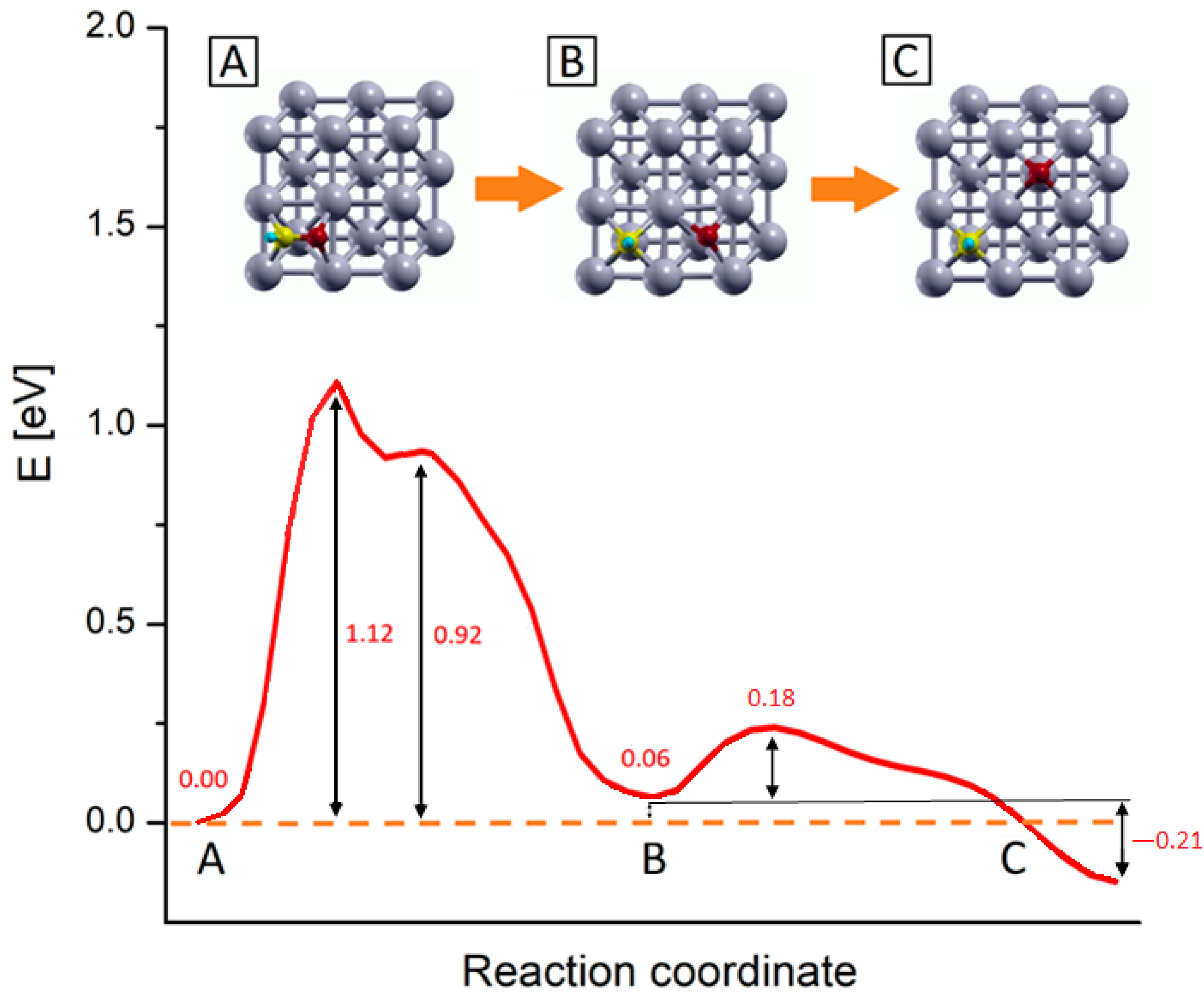
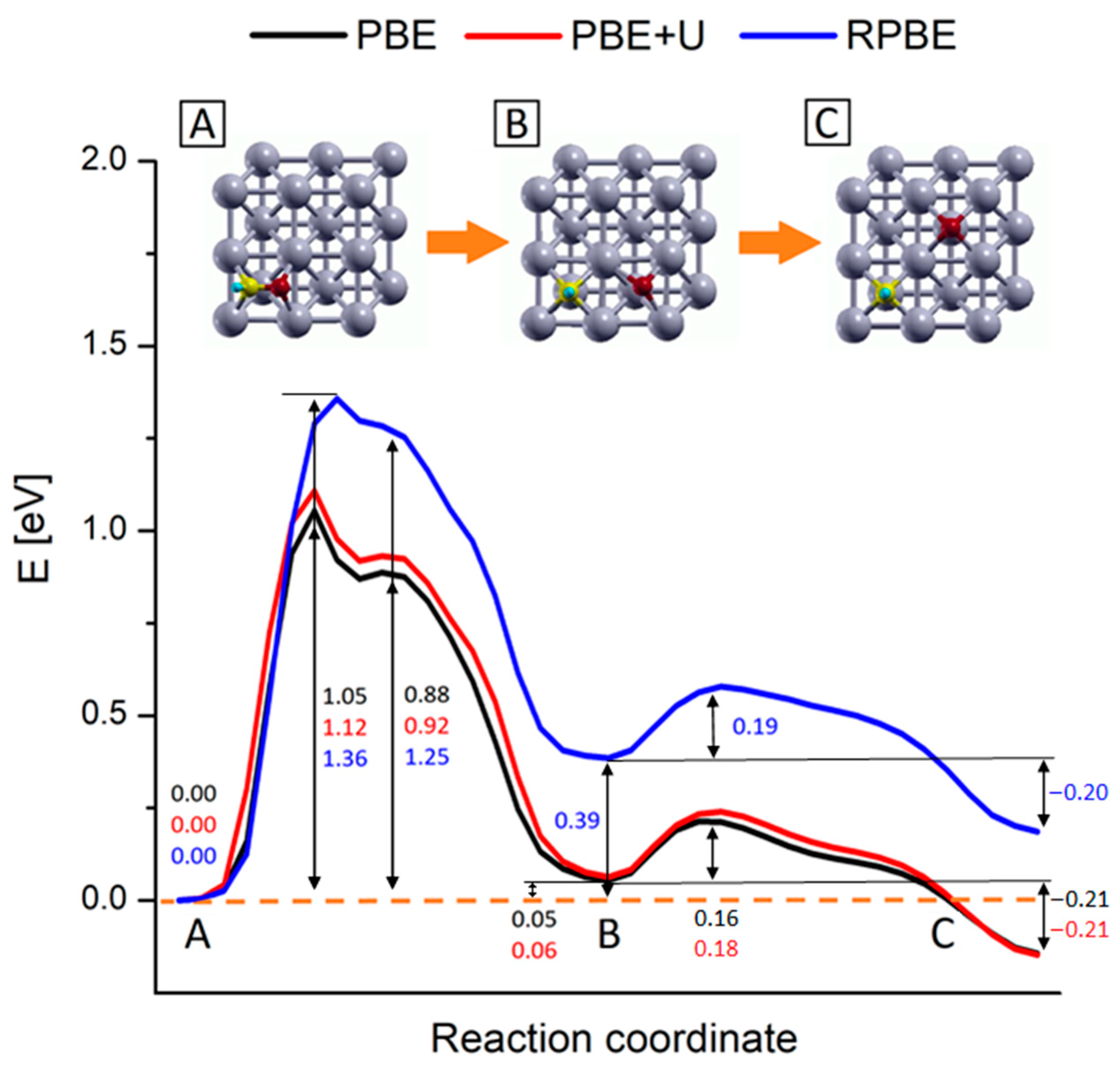
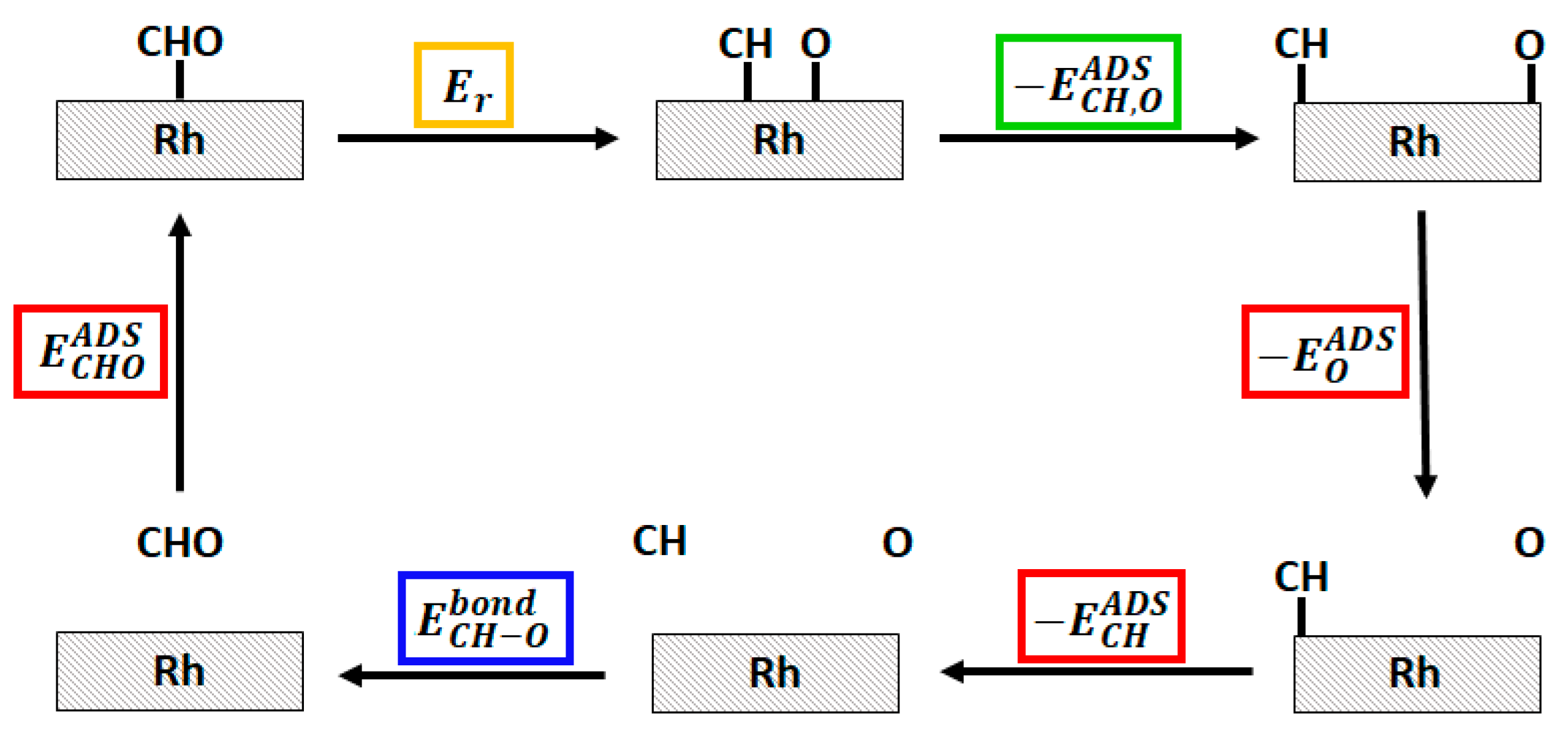
2.3. Study of the Methanation Rate-Determining Step
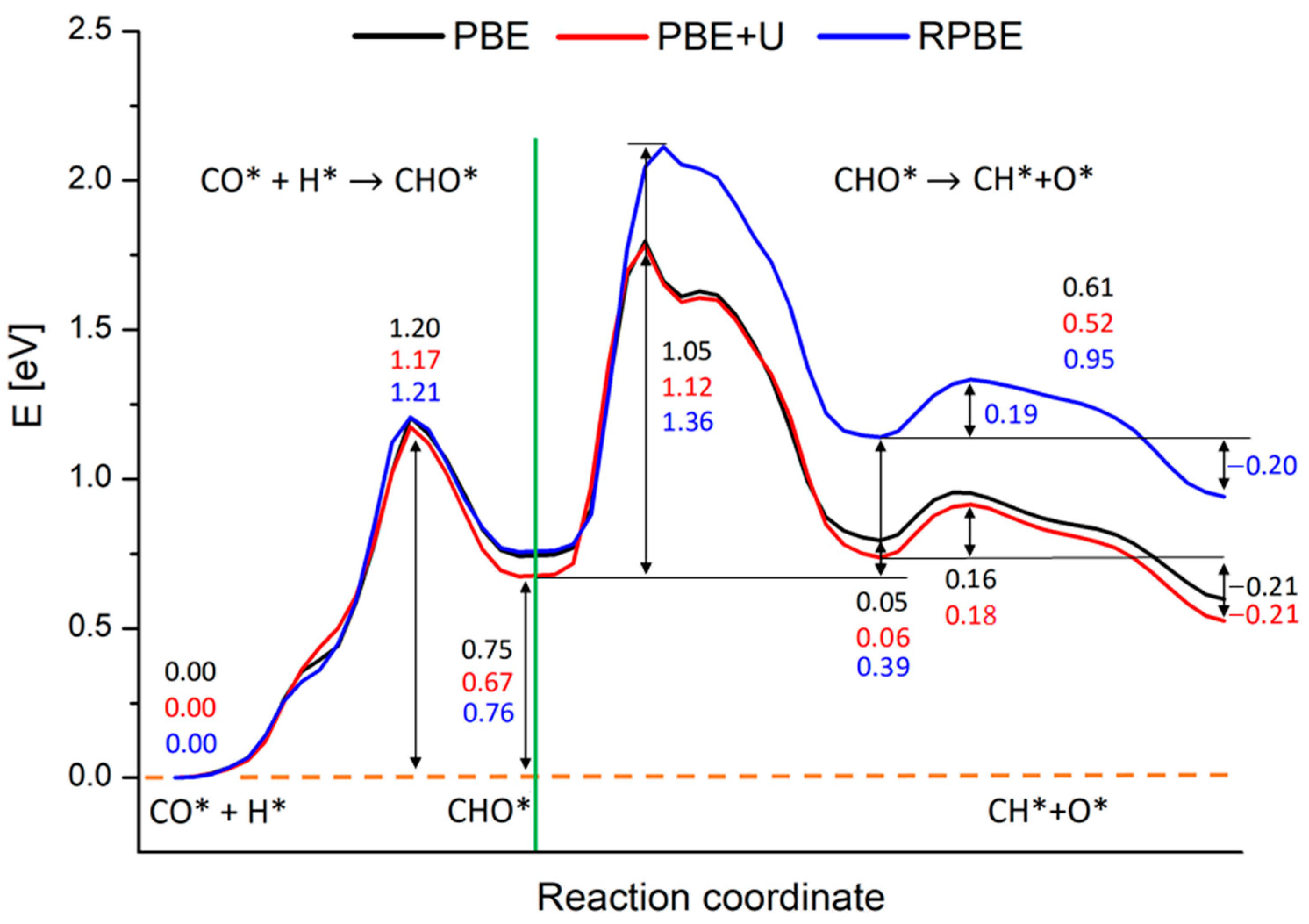
2.4. Comparison among Different Computational Techniques
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
- is the energy required to build a surface starting from an infinite bulk crystal:where is the total energy of the -layer slab and is the energy per atom of the infinite bulk. In this case, = 8. The ½ factor accounts for the presence of two symmetric surfaces for each slab.
- represents the energy required for extracting an electron from the system:where is the electrostatic potential calculated at the center of vacuum region and is the Fermi energy of the slab.
- : quantifies how much the superficial layers of the slab move with respect to their ideal position in the bulk crystal structure:where is the distance between layers i and j, and is the distance between the layers of the bulk.
References
- Intergovernmental Panel on Climate Change. IPCC’s Fifth Assessment Report; IPCC: Geneva, Switzerland, 2014.
- Vijayavenkataraman, S.; Iniyan, S.; Goic, R. A review of climate change, mitigation and adaptation. Renew. Sustain. Energy Rev. 2012, 16, 878–897. [Google Scholar] [CrossRef]
- Mebrahtu, C.; Krebs, F.; Abate, S.; Perathoner, S.; Centi, G.; Palkovits, R. CO2 Methanation: Principles and Challenges. In Studies in Surface Science and Catalysis; Elsevier B.V.: Amsterdam, The Netherlands, 2019; Volume 178, pp. 85–103. ISBN 9780444641274. [Google Scholar]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schlüter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation—From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Miao, B.; Ma, S.S.K.; Wang, X.; Su, H.; Chan, S.H. Catalysis mechanisms of CO2 and CO methanation. Catal. Sci. Technol. 2016, 6, 4048–4058. [Google Scholar] [CrossRef]
- Su, X.; Xu, J.; Liang, B.; Duan, H.; Hou, B.; Huang, Y. Catalytic carbon dioxide hydrogenation to methane: A review of recent studies. J. Energy Chem. 2016, 25, 553–565. [Google Scholar] [CrossRef]
- Erdőhelyi, A. Hydrogenation of carbon dioxide on supported rh catalysts. Catalysts 2020, 10, 155. [Google Scholar] [CrossRef]
- Kattel, S.; Liu, P.; Chen, J.G. Tuning Selectivity of CO2 Hydrogenation Reactions at the Metal/Oxide Interface. J. Am. Chem. Soc. 2017, 139, 9739–9754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, S.; Guo, W.; Hu, Y.; Huang, J.; Mulcahy, J.R.; Wei, W.D. Surface-Plasmon-Driven Hot Electron Photochemistry. Chem. Rev. 2018, 118, 2927–2954. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, X.; Zhang, D.; Su, N.Q.; Yang, W.; Everitt, H.O.; Liu, J. Product selectivity in plasmonic photocatalysis for carbon dioxide hydrogenation. Nat. Commun. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Li, X.; Everitt, H.O.; Liu, J. Confirming nonthermal plasmonic effects enhance CO2 methanation on Rh/TiO2 catalysts. Nano Res. 2019, 12, 1906–1911. [Google Scholar] [CrossRef]
- Kim, C.; Hyeon, S.; Lee, J.; Kim, W.D.; Lee, D.C.; Kim, J.; Lee, H. Energy-efficient CO2 hydrogenation with fast response using photoexcitation of CO2 adsorbed on metal catalysts. Nat. Commun. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Avanesian, T.; Gusmão, G.S.; Christopher, P. Mechanism of CO2 reduction by H2 on Ru(0001) and general selectivity descriptors for late-transition metal catalysts. J. Catal. 2016, 343, 86–96. [Google Scholar] [CrossRef]
- Dai, X.; Sun, Y. Reduction of carbon dioxide on photoexcited nanoparticles of VIII group metals. Nanoscale 2019, 11, 16723–16732. [Google Scholar] [CrossRef]
- Wang, J.; Kawazoe, Y.; Sun, Q.; Chan, S.; Su, H. The selectivity and activity of catalyst for CO hydrogenation to methanol and hydrocarbon: A comparative study on Cu, Co and Ni surfaces. Surf. Sci. 2016, 645, 30–40. [Google Scholar] [CrossRef]
- Sexton, B.A.; Somorjai, G.A. The hydrogenation of CO and CO2 over polycrystalline rhodium: Correlation of surface composition, kinetics and product distributions. J. Catal. 1977, 46, 167–189. [Google Scholar] [CrossRef]
- Castner, D.G.; Sexton, B.A.; Somorjai, G.A. Leed and Thermal Desorption Studies of Small Molecules (H2, O2, CO, CO2, NO, C2H4, C2H2 and C) Chemisorbed on the Rhodium (111) and (100) Surfaces. Surf. Sci. 1978, 71, 519–540. [Google Scholar] [CrossRef]
- van Tol, M.F.H.; Gielbert, A.; Nieuwenhuys, B.E. The adsorption and dissociation of CO2 on Rh. Appl. Surf. Sci. 1993, 67, 166–178. [Google Scholar] [CrossRef]
- Fisher, I.A.; Bell, A.T. A comparative study of CO and CO2 hydrogenation over Rh/SiO2. J. Catal. 1996, 162, 54–65. [Google Scholar] [CrossRef]
- Novák, É.; Fodor, K.; Szailer, T.; Oszkó, A.; Erdöhelyi, A. CO2 hydrogenation on Rh/TiO2 previously reduced at different temperatures. Top. Catal. 2002, 20, 107–117. [Google Scholar] [CrossRef]
- Jacquemin, M.; Beuls, A.; Ruiz, P. Catalytic production of methane from CO2 and H2 at low temperature: Insight on the reaction mechanism. Catal. Today 2010, 157, 462–466. [Google Scholar] [CrossRef]
- Karelovic, A.; Ruiz, P. Mechanistic study of low temperature CO2 methanation over Rh/TiO 2 catalysts. J. Catal. 2013, 301, 141–153. [Google Scholar] [CrossRef]
- Liu, X.; Sun, L.; Deng, W.Q. Theoretical Investigation of CO2 Adsorption and Dissociation on Low Index Surfaces of Transition Metals. J. Phys. Chem. C 2018, 122, 8306–8314. [Google Scholar] [CrossRef]
- Solymosi, F.; Pásztor, M. Analysis of the IR-spectral behavior of adsorbed CO formed in H2 + CO2 surface interaction over supported rhodium. J. Catal. 1987, 104, 312–322. [Google Scholar] [CrossRef]
- Henderson, M.A.; Worley, S.D. An Infrared Study of the Dissociation of Carbon Dioxide over Support Rhodium Catalyst. Surf. Sci. 1985, 149, L1–L6. [Google Scholar] [CrossRef]
- Claver, C. Rhodium Catalysis; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783319666631. [Google Scholar]
- Dietz, L.; Piccinin, S.; Maestri, M. Mechanistic insights into CO2 activation via reverse water—Gas shift on metal surfaces. J. Phys. Chem. C 2015, 119, 4959–4966. [Google Scholar] [CrossRef]
- Alfonso, D.R. Further theoretical evidence for hydrogen-assisted CO dissociation on Ru(0001). J. Phys. Chem. C 2013, 117, 20562–20571. [Google Scholar] [CrossRef]
- Zhang, S.T.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. Hydrogenation mechanism of carbon dioxide and carbon monoxide on Ru(0001) surface: A density functional theory study. RSC Adv. 2014, 4, 30241–30249. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, X.; Yu, Y. A comparative DFT study on the dehydrogenation of methanol on Rh(100) and Rh(110). Appl. Surf. Sci. 2018, 436, 268–276. [Google Scholar] [CrossRef]
- Zhu, Y.A.; Chen, D.; Zhou, X.G.; Yuan, W.K. DFT studies of dry reforming of methane on Ni catalyst. Catal. Today 2009, 148, 260–267. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jónsson, H. Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901–9904. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.; Hansen, L.B.; Nørskov, J.K. Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 7413–7421. [Google Scholar] [CrossRef]
- Tolba, S.A.; Gameel, K.M.; Ali, B.A.; Almossalami, H.A.; Allam, N.K. Density Functional Calculations—Recent Progresses of Theory and Application. Intech Open: London, UK, 2018; pp. 3–30. [Google Scholar] [CrossRef]
- Patra, A.; Bates, J.E.; Sun, J.; Perdew, J.P. Properties of real metallic surfaces: Effects of density functional semilocality and van der Waals nonlocality. Proc. Natl. Acad. Sci. USA 2017, 114, E9188–E9196. [Google Scholar] [CrossRef]
- Xie, J.; Scheffler, M. Structure and dynamics of Rh surfaces. Phys. Rev. B 1998, 57, 4768–4775. [Google Scholar] [CrossRef]
- Begley, A.M.; Kim, S.K.; Jona, F.; Marcus, P.M. Surface relaxation of Rh{001}. Phys. Rev. B 1993, 48, 12326–12329. [Google Scholar] [CrossRef]
- Derry, G.N.; Kern, M.E.; Worth, E.H. Recommended values of clean metal surface work functions. J. Vac. Sci. Technol. A 2015, 33, 060801. [Google Scholar] [CrossRef]
- Tyson, W.R. Surface energies of solid metals. Can. Metall. Q. 1975, 14, 307–314. [Google Scholar] [CrossRef]
- De Waele, S.; Lejaeghere, K.; Sluydts, M.; Cottenier, S. Error estimates for density-functional theory predictions of surface energy and work function. Phys. Rev. B 2016, 94, 235418. [Google Scholar] [CrossRef]
- Kose, R.; Brown, W.A.; King, D.A. Role of Lateral Interactions in Adsorption Kinetics: CO/Rh{100}. J. Phys. Chem. B 1999, 103, 8722–8725. [Google Scholar] [CrossRef]
- Jansen, M.M.M.; Gracia, J.; Nieuwenhuys, B.E.; Niemantsverdriet, J.W. Interactions between co-adsorbed CO and H on a Rh(100) single crystal surface. Phys. Chem. Chem. Phys. 2009, 11, 10009–10016. [Google Scholar] [CrossRef]
- De Jong, A.M.; Niemantsverdriet, J.W. The adsorption of CO on Rh(100): Reflection absorption infrared spectroscopy, low energy electron diffraction, and thermal desorption spectroscopy. J. Chem. Phys. 1994, 101, 10126–10133. [Google Scholar] [CrossRef]
- Kim, J.; Peebles, H.C.; White, J.M. Electron spectroscopic study of the interaction of coadsorbed CO and D2 on Rh(100) at low temperature. Surf. Sci. 1982, 114, 363–380. [Google Scholar] [CrossRef]
- Van Bavel, A.P.; Hopstaken, M.J.P.; Curulla, D.; Niemanstsverdriet, J.W.; Lukkien, J.J.; Hilbers, P.A.J. Quantification of lateral repulsion between coadsorbed CO and N on Rh(100) using temperature-programmed desorption, low-energy electron diffraction, and Monte Carlo simulations. J. Chem. Phys. 2003, 119, 524–532. [Google Scholar] [CrossRef]
- Baraldi, A.; Gregoratti, L.; Comelli, G.; Dhanak, V.R.; Kiskinova, M.; Rosei, R. CO adsorption and CO oxidation on Rh(100). Appl. Surf. Sci. 1996, 99, 1–8. [Google Scholar] [CrossRef]
- Hung, T.C.; Liao, T.W.; Liao, Z.H.; Hsu, P.W.; Cai, P.Y.; Lu, W.H.; Wang, J.H.; Luo, M.F. Dependence on size of supported Rh nanoclusters for CO adsorption. RSC Adv. 2016, 6, 3830–3839. [Google Scholar] [CrossRef]
- Nieskens, D.L.S.; Jansen, M.M.M.; Van Bavel, A.P.; Curulla-Ferré, D.; Niemantsverdriet, J.W. The influence of carbon on the adsorption of CO on a Rh(100) single crystal. Phys. Chem. Chem. Phys. 2006, 8, 624–632. [Google Scholar] [CrossRef]
- Nieskens, D.L.S.; Curulla-Ferré, D.; Niemantsverdriet, J.W. Atom-molecule interactions on transition metal surfaces: A DFT study of CO and several atoms on Rh(100), Pd(100) and Ir(100). ChemPhysChem 2006, 7, 1075–1080. [Google Scholar] [CrossRef]
- Liu, D.J. CO oxidation on Rh(100): Multisite atomistic lattice-gas modeling. J. Phys. Chem. C 2007, 111, 14698–14706. [Google Scholar] [CrossRef]
- Gajdoš, M.; Eichler, A.; Hafner, J. CO adsorption on close-packed transition and noble metal surfaces: Trends from ab initio calculations. J. Phys. Condens. Matter 2004, 16, 1141–1164. [Google Scholar] [CrossRef]
- Mason, S.E.; Grinberg, I.; Rappe, A.M. First-principles extrapolation method for accurate CO adsorption energies on metal surfaces. Phys. Rev. B 2004, 69, 161401. [Google Scholar] [CrossRef]
- Tan, L.; Huang, L.; Liu, Y.; Wang, Q. Detailed mechanism of the NO + CO reaction on Rh(100) and Rh(111): A first-principles study. Appl. Surf. Sci. 2018, 444, 276–286. [Google Scholar] [CrossRef]
- Kresse, G.; Gil, A.; Sautet, P. Significance of single-electron energies for the description of CO on Pt(111). Phys. Rev. B 2003, 68, 3–6. [Google Scholar] [CrossRef]
- Köhler, L.; Kresse, G. Density functional study of CO on Rh(111). Phys. Rev. B 2004, 70, 165405. [Google Scholar] [CrossRef]
- Efstathiou, A.M.; Bennett, C.O. Enthalpy and entropy of H2 Adsorption on Rh/Al2O3 measured by temperature-programmed desorption. J. Catal. 1990, 124, 116–126. [Google Scholar] [CrossRef]
- Kose, R.; Brown, W.A.; King, D.A. Calorimetric heats of dissociative adsorption for O2 on Rh{100}. Surf. Sci. 1998, 402–404, 856–860. [Google Scholar] [CrossRef]
- Panayotov, D.; Mihaylov, M.; Nihtianova, D.; Spassov, T.; Hadjiivanov, K. Spectral evidence for hydrogen-induced reversible segregation of CO adsorbed on titania-supported rhodium. Phys. Chem. Chem. Phys. 2014, 16, 13136–13144. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, F.; Zhao, X.; Wang, B.; Ling, L. First-Principles Study about the Effect of Coverage on H2 Adsorption and Dissociation over a Rh(100) Surface. J. Phys. Chem. C 2015, 119, 10355–10364. [Google Scholar] [CrossRef]
- Wang, B.; Song, L.; Zhang, R. The dehydrogenation of CH4 on Rh(111), Rh(110) and Rh(100) surfaces: A density functional theory study. Appl. Surf. Sci. 2012, 258, 3714–3722. [Google Scholar] [CrossRef]
- Wellendorff, J.; Silbaugh, T.L.; Garcia-Pintos, D.; Nørskov, J.K.; Bligaard, T.; Studt, F.; Campbell, C.T. A benchmark database for adsorption bond energies to transition metal surfaces and comparison to selected DFT functionals. Surf. Sci. 2015, 640, 36–44. [Google Scholar] [CrossRef]
- Lin, Y.Z.; Sun, J.; Lin, J.D.; Chen, H.B.; Liao, D.W.; Yi, J.; Liao, D.W.; Chen, H.B.; Liao, D.W. Energetics of chemisorption and conversion of methane on transition metal surfaces. J. Mol. Struct. THEOCHEM 2002, 587, 63–71. [Google Scholar] [CrossRef]
- Van Grootel, P.W.; Van Santen, R.A.; Hensen, E.J.M. Methane dissociation on high and low indices Rh surfaces. J. Phys. Chem. C 2011, 115, 13027–13034. [Google Scholar] [CrossRef]
- Van Grootel, P.W.; Hensen, E.J.M.; Van Santen, R.A. The CO formation reaction pathway in steam methane reforming by rhodium. Langmuir 2010, 26, 16339–16348. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Lv, C.Q.; Wang, G.C. Chemisorbed oxygen atom on the activation of C-H bond in methane: A Rh model study. RSC Adv. 2015, 5, 66221–66230. [Google Scholar] [CrossRef]
- Diao, Z.Y.; Hao, C.; Wang, Z.X.; Dong, C.C.; Pang, X.H. Adsorption, vibration, and diffusion of O atoms on Rh low-index and (711) stepped defective surfaces. J. Phys. Chem. B 2005, 109, 12467–12473. [Google Scholar] [CrossRef]
- Xing, B.; Pang, X.Y.; Wang, G.C. C-H bond activation of methane on clean and oxygen pre-covered metals: A systematic theoretical study. J. Catal. 2011, 282, 74–82. [Google Scholar] [CrossRef]
- Brown, W.A.; Kose, R.; King, D.A. Femtomole adsorption calorimetry on single-crystal surfaces. Chem. Rev. 1998, 98, 797–831. [Google Scholar] [CrossRef]
- Jiang, R.; Guo, W.; Li, M.; Zhu, H.; Zhao, L.; Lu, X.; Shan, H. Methanol dehydrogenation on Rh(111): A density functional and microkinetic modeling study. J. Mol. Catal. A Chem. 2011, 344, 99–110. [Google Scholar] [CrossRef]
- Solymosi, F.; Erdöhelyi, A. Methanation of CO2 on supported rhodium catalysts. Stud. Surf. Sci. Catal. 1981, 7, 1448–1449. [Google Scholar] [CrossRef]
- Zhang, Z.; Kladi, A.; Verykios, X.E. Effects of carrier doping on kinetic parameters of CO2 hydrogenation on supported rhodium catalysts. J. Catal. 1994, 148, 737–747. [Google Scholar] [CrossRef]
- Beuls, A.; Swalus, C.; Jacquemin, M.; Heyen, G.; Karelovic, A.; Ruiz, P. Methanation of CO2: Further insight into the mechanism over Rh/γ-Al2 O3 catalyst. Appl. Catal. B Environ. 2012, 113, 2–10. [Google Scholar] [CrossRef]
- Bao, J.L.; Truhlar, D.G. Variational transition state theory: Theoretical framework and recent developments. Chem. Soc. Rev. 2017, 46, 7548–7596. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef] [PubMed]
- Arblaster, J.W. Crystallographic properties of rhodium. Platin. Met. Rev. 1997, 41, 184–189. [Google Scholar]
- Head, J.D.; Zerner, M.C. A Broyden—Fletcher—Goldfarb—Shanno optimization procedure for molecular geometries. Chem. Phys. Lett. 1985, 122, 264–270. [Google Scholar] [CrossRef]
- Bengtsson, L. Dipole correction for surface supercell calculations. Phys. Rev. B 1999, 59, 12301–12304. [Google Scholar] [CrossRef]

| Method | σ [eV/atom] | |||
|---|---|---|---|---|
| PBE | −4.1 | 0.7 | 5.11 | 1.09 |
| RPBE | −1.6 | 0.3 | 4.97 | 0.96 |
| PBE (ref. [36]) | −4.1 | 0.5 | 5.12 | 1.26 |
| PBEsol (ref. [36]) | −3.6 | 0.8 | 5.38 | 1.34 |
| PW91 (ref. [37]) | −3.0 | −0.1 | 4.91 | 0.99 |
| Experiments (refs. [38,39,40]) | −1.16 ± 1.6 | 0 ± 1.6 | 5.3 ± 0.15 | 1.12 |
| Species | Adsorption Site | Eads PBE [eV] | Eads PBE+U [eV] | Eads RPBE [eV] | Eads Experimental [eV] |
|---|---|---|---|---|---|
| CO | Bridge | −1.997 | −1.413 | −1.529 | −1.40, ref. [43] |
| Top | −1.929 | −1.343 | −1.483 | ||
| Hollow | −1.864 | −1.292 | −1.420 | ||
| H | Bridge | −3.838 (−0.52) | −3.838 (−0.52) | −3.590 (−0.65) | (−0.52), ref. [57] |
| Top | − | − | − | ||
| Hollow | −3.823 (−0.51) | −3.823 (−0.51) | −3.584 (−0.65) | ||
| CHO | Bridge | −3.310 | −3.394 | −2.642 | - |
| Top | −2.991 | −3.085 | −2.333 | ||
| Hollow | −3.310 | −3.394 | −2.642 | ||
| CH | Bridge | - | - | - | - |
| Top | - | - | - | ||
| Hollow | −7.603 | −7.582 | −6.426 | ||
| O | Bridge | −6.206 (−4.29) | −6.509 (−4.45) | −5.153 (−2.41) | (−4.00), ref. [58] |
| Top | - | - | - | ||
| Hollow | −6.325 (−4.53) | −6.647 (−4.75) | −5.370 (−3.04) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanzan, M.; Marsili, M.; Corni, S. Study of the Rate-Determining Step of Rh Catalyzed CO2 Reduction: Insight on the Hydrogen Assisted Molecular Dissociation. Catalysts 2021, 11, 538. https://doi.org/10.3390/catal11050538
Vanzan M, Marsili M, Corni S. Study of the Rate-Determining Step of Rh Catalyzed CO2 Reduction: Insight on the Hydrogen Assisted Molecular Dissociation. Catalysts. 2021; 11(5):538. https://doi.org/10.3390/catal11050538
Chicago/Turabian StyleVanzan, Mirko, Margherita Marsili, and Stefano Corni. 2021. "Study of the Rate-Determining Step of Rh Catalyzed CO2 Reduction: Insight on the Hydrogen Assisted Molecular Dissociation" Catalysts 11, no. 5: 538. https://doi.org/10.3390/catal11050538
APA StyleVanzan, M., Marsili, M., & Corni, S. (2021). Study of the Rate-Determining Step of Rh Catalyzed CO2 Reduction: Insight on the Hydrogen Assisted Molecular Dissociation. Catalysts, 11(5), 538. https://doi.org/10.3390/catal11050538







